Short abstract
There have been numerous reports regarding the occurrence of keratitis in patients with soft contact lenses, but few reports in patients with rigid gas permeable contact lenses. To the best of our knowledge, the occurrence of infection associated with three species of pathogens has never been reported. Here, we describe a patient who exhibited refractory painless keratitis caused by three pathogens (Staphylococcus epidermidis, Acanthamoeba, and herpes simplex virus) and summarize similar reports published at multiple centers worldwide, with the aim of providing guidance for clinicians who might encounter mixed-type corneal infections. We describe the results of many ophthalmologic and laboratory investigations, which guided our treatment selection. We achieved good treatment efficacy, such that the patient exhibited a corrected visual acuity of 20/20 in the affected eye after a series of treatments, including curettage of ulcer lesions. Corneal infections caused by multiple pathogens are challenging in clinical practice. This summary of our experience in patient diagnosis and treatment can help clinicians to achieve a favorable prognosis in treatment of future patients.
Keywords: Multiple species infection, keratitis, painless, rigid gas permeable lenses, ulcer, cornea, curettage, Staphylococcus epidermidis, Acanthamoeba, herpes simplex virus
Introduction
Wearing contact lenses increases the risk of corneal trauma, which may lead to the development of keratitis, a serious eye disease that can result in blindness. Keratitis has been widely reported in patients with soft contact lenses, but rarely in patients wearing rigid gas permeable contact lenses.1,2 To the best of our knowledge, the occurrence of infection associated with three species of pathogens has never been reported. This report describes the treatment of a patient who exhibited mixed triple corneal infection, as well as similar cases previously reported in the literature, to discuss appropriate treatment for these types of complex corneal infections.
Case report
Presentation and initial treatment
A 25-year-old man who wore orthokeratological rigid gas permeable contact lenses (i.e., Ortho-K lenses) sought treatment after experiencing symptoms of right eye conjunctival hyperemia, photophobia, tearing, and foreign body sensation for 3 weeks; he had a sharp decline in Snellen visual acuity to 20/50, but reported no pain. At the onset of symptoms, he presented to his local hospital for evaluation; slit lamp examination showed conjunctival hyperemia and a mild opacity in the upper cornea of the right eye; confocal microscopy showed no abnormalities. Thus, the patient was asked to discontinue using Ortho-K lenses; he was prescribed tobramycin eye drops (four doses per day, one drop per dose), acyclovir eye drops (four doses per day, one drop per dose), and basic fibroblast growth factor eye drops (three doses per day, one drop per dose).
One week later, he returned to the local hospital due to aggravated photophobia and tearing. Snellen visual acuity in his right eye was 20/200; slit lamp examination showed the presence of a thickened nerve ending in the nasal cornea of the right eye. The medication regimen was then adjusted to cyclosporine eye drops (four doses per day, one drop per dose)—the local ophthalmologist presumably intended to suppress the immune inflammatory response without the use of glucocorticoids—and acyclovir eye drops (six doses per day, one drop per dose).
After 5 days, a follow-up slit lamp examination showed only slight improvement, while confocal microscopy revealed no obvious abnormalities. Intraocular pressure in the right eye was 23 mmHg, and Snellen visual acuity had diminished to hand motion; therefore, the patient was referred to another hospital. At the referral hospital, slit lamp examination of the right eye indicated obvious conjunctival hyperemia, diffuse edema of the corneal matrix with a foggy opacity, and white radial infiltration of the temporal corneal stroma (Figure 1a); it also revealed a large amount of keratic precipitates beneath the cornea, while the anterior chamber and lens were clear.
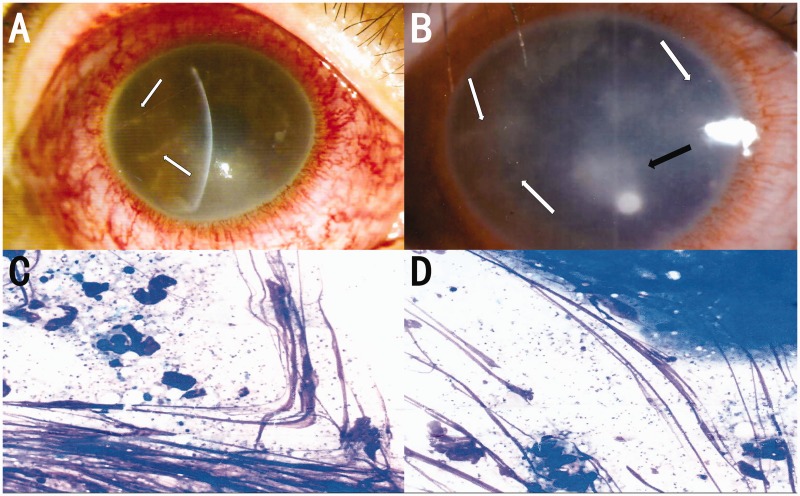
Figure 1.
(a) Slit lamp photographs show severe conjunctival hyperemia and radial perineuritis (white arrow). (b) Slit lamp photographs show aggregating radial perineuritis (white arrow) and paracentral corneal ulcer (black arrow). (c and d) Initial corneal scraping and culture show Staphylococcus epidermidis under 1000× magnification.
Transfer and further treatment
The patient was admitted to our hospital approximately 2 weeks after his initial presentation to his local hospital, with Snellen visual acuity of hand motion in the right eye. Slit lamp examination results were normal in the left eye. The right eye exhibited a grayish-white cornea; full-thickness edema; diffuse foggy opacity in the matrix; a 1.5-mm × 1-mm corneal ulcer on the side of the pupil (Figure 1b); peripheral epithelial defects; nasal, inferior, and temporal pseudodendritic nerve fiber infiltration; visible superior temporal vacuoles of multiple sizes (average diameter of 0.2 mm); fuzzy anterior chamber; and diffuse corneal fluorescein staining. Confocal microscopy revealed edema of the corneal stroma, aggregation of Langerhans cells, and infiltration of inflammatory cells, as well as multiple highly reflective structures similar to pine needles and several trophozoite manifestations (Figure 2a–c). Given that the patient had a corneal ulcer, corneal scraping and pathogen culture were performed. Gram staining, microbial isolation and identification, and pathogen culture of the ulcer scraping indicated the presence of Staphylococcus epidermidis (Figure 1c, d), without other observable pathogens. Moreover, the presence of herpes simplex virus-1 was confirmed by the presence of specific bands at approximately 180 bp after corneal scrapings were assessed by polymerase chain reaction (PCR) and gel electrophoresis.
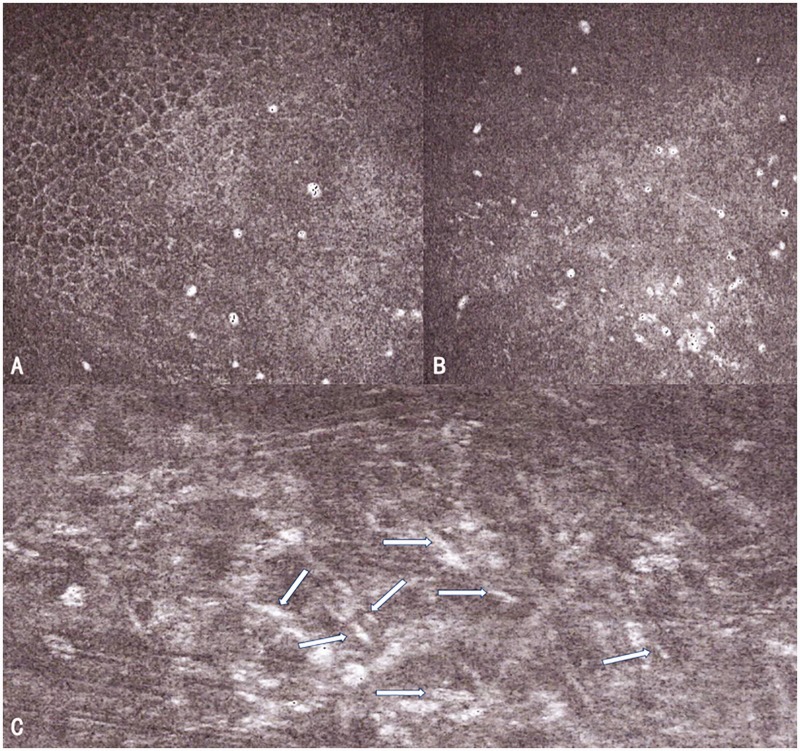
Figure 2.
Confocal microscopy images, taken when the patient was initially admitted to our hospital, show several trophozoite manifestations (a and b) and many highly reflective structures similar to pine needles (white arrow in c) in the deep stromal layer.
Based on the results of laboratory examinations, as well as the patient’s long history of wearing rigid gas permeable lenses (>6 hours per night) and the sign of an exposed nerve fiber at the periphery of the cornea, we made several modifications to the patient’s treatment. First, we discontinued the use of acyclovir and cyclosporine eye drops; second, we initiated ganciclovir treatment (four doses per day, two drops per dose) to control herpes simplex keratitis; third, we initiated gatifloxacin ophthalmic gel (four doses per day) [due to its superior effects against Gram-positive bacteria and its increased strength compared with that of levofloxacin3] to control staphylococcal infection; fourth, we initiated 0.05% chlorhexidine (24 doses per day, one drop per dose) and 0.02% polyhexamethylene biguanide eye drops (dosage and frequency identical to those of chlorhexidine) to control Acanthamoeba keratitis.
Follow-up treatment and resolution
Two weeks after his initial admission to our hospital, the patient returned for a follow-up examination. Slit lamp examination showed a grayish-white cornea, full-thickness lesions, localized ulceration, disappearance of superior temporal vacuoles, localized corneal stroma infiltration, and extensive keratic precipitates. Corneal fluorescence was present in a strip staining manner. Confocal microscopy indicated that only some circular highly reflective manifestations (later determined to be residual suspicious representations of trophozoites) were occasionally observed in the superficial stromal layer, without any other obvious pathogens. We concluded that the patient’s bacterial infection had improved; therefore, we advised the patient to continue ganciclovir gel at the same frequency and dose, while changing the frequency of gatifloxacin ophthalmic gel application to twice per day and the frequencies of 0.05% chlorhexidine and 0.02% polyhexamethylene biguanide to every 2 hours. We also prescribed compound tropicamide (twice per day) to control iris adhesion.
Four weeks after his initial admission to our hospital, the patient returned for another follow-up exam. Snellen visual in the right eye was 20/50; slit lamp examination showed that the cornea was less hazy, sparse keratic precipitates, corneal stroma exhibited sheet-shaped opacity, and central-region fluorescein staining was present. Confocal microscopy showed that the epithelium had already been repaired and that a scar had formed in the superficial stromal layer. Another corneal scraping and culture was performed; the results showed no observable pathogens. Considering that the patient's corneal stroma showed opacity, whereas confocal microscopy showed no abnormalities, we prescribed loteprednol etabonate ophthalmic suspension (two doses per day, one drop per dose); this treatment can effectively inhibit the development of capillaries, thereby alleviating the tissue damage caused by edema infiltration and inflammation, reducing scar and neovascularization formation, and promoting recovery from corneal inflammatory symptoms. We also changed the frequency of gatifloxacin gel to once daily in the evening; we changed the frequencies of chlorhexidine and polyhexamethylene biguanide to six times daily, while maintaining the doses and frequencies of compound tropicamide eye drops and ganciclovir gel to prevent stromal layer necrosis.
Approximately 5 weeks after the patient’s initial admission to our hospital, re-examination of his right eye showed Snellen visual acuity of 30/50, intraocular pressure of 17.8 mmHg, mixed hyperemia, relatively clear cornea, some stromal cloudiness, improved neuritis lesions, and sparse keratic precipitates. Confocal microscopy showed spotted keratic precipitates in the endothelial layer and revealed no typical pathogen. Because of the treatment effectiveness, we changed the frequencies of polyhexamethylene biguanide and chlorhexidine to every 6 hours; we changed loteprednol etabonate ophthalmic suspension to once daily and ganciclovir gel to twice daily, while maintaining the dose and frequency of gatifloxacin to prevent hormone-related infections.
Seven weeks after the patient’s initial admission to our hospital, re-examination of his right eye showed Snellen visual acuity of 20/50, intraocular pressure of 18.6 mmHg, mixed congestion, increasing corneal clarity, minimal stromal cloudiness, and considerably improved neuritis lesions. Therefore, we discontinued the polyhexamethylene biguanide treatment and tapered the chlorhexidine to once daily; however, we maintained all other treatment regimens.
Approximately 10 weeks after the patient’s initial admission to our hospital, re-examination of his right eye showed Snellen visual acuity of 30/50, which could be corrected to 20/25. We discontinued treatment with chlorhexidine and loteprednol etabonate ophthalmic suspension. The patient continued follow-up for 1 month and showed a corrected Snellen visual acuity of 20/20 in the right eye, which was regarded as clinical cure. The patient achieved good visual acuity and exhibited no further active inflammation; thus, he was considered to be clinically cured.
Discussion
The treatment of double microbial infection-derived keratitis has been widely reported,4–6 with infection mostly caused by two fungal species, a mixture of fungal and bacterial pathogens, or a mixture of a fungus and Acanthamoeba; in contrast, keratitis caused by triple infection has been extremely rare, constituting only 1.16% of all reported cases of corneal infection.7 The largest retrospective study of such infections thus far was conducted by Ray et al.,7 who reported that the isolated microorganisms consisted of only two species (bacterial and/or fungal), including two bacterial species (e.g., Neisseria meningitidis and Pseudomonas aeruginosa). An analysis of keratitis pathogens with a large sample size, conducted by Fernandes et al.,8 included only patients with two pathogen species (i.e., two bacterial species or a mixture of bacterial and fungal species), which cannot facilitate therapeutic guidance for mixed infections caused by multiple (≥3) pathogens of different types.
Here, we have described a patient who was simultaneously infected with three types of pathogens: amoeba, virus, and bacterium. The patient initially presented with some nonspecific symptoms, such as conjunctival hyperemia, photophobia, and tearing; however, his symptoms of corneal irritation showed progressive aggravation within 1 week. Moreover, characteristics of dendritic keratitis emerged, which indicated that herpes simplex keratitis was the most likely diagnosis. Therefore, the ophthalmologist at the local hospital insisted on acyclovir and cyclosporine treatment. In addition, the patient developed a rapidly aggravated peripheral corneal infiltration,9 which may have also led to the suspicion of secondary autoimmune-related keratitis caused by viral infection; this might have supported the cyclosporine treatment prescribed by the local hospital. After the patient was admitted to our hospital, we strongly suspected that he had multimicrobial keratitis with Acanthamoeba infection, given the signs of radioneuritis and ineffectiveness of the previous antiviral treatment regimen, as well as his long history of wearing Ortho-K contact lenses. Finally, confocal microscopy, corneal scraping examination, and PCR amplification findings confirmed our hypothesis.
For the treatment of Acanthamoeba keratitis, it is typically recommended that patients receive medication at least nine times per day for 3 to 4 weeks.10 For our patient, the results of laboratory examination supported our hypothesis; thus, to prevent the aggravation of endophthalmitis and loss of vision in the affected eye, we used the recommended treatment dose 24 times per day at the start of treatment. We then slowly tapered the dose, similar to a cocktail treatment, to avoid ocular surface damage while achieving a good therapeutic outcome.
Clinical practice points
Based on this report and the outcomes of treatment previously reported in the literature, critical points regarding treatment of corneal infection caused by three different types of pathogens are as follows:
As in the present report, individuals who wear contact lenses are more vulnerable to multimicrobial keratitis. The lens care liquid can be contaminated by residual proteins in the lenses, as well as by bacteria or fungi from the environment. The presence of residual protein in the contact lens care solution is conducive to the growth and reproduction of bacteria and fungi. Eventually, a large number of bacteria and fungi can provide a source of nutrition for Acanthamoeba.11,12
The diagnosis of Acanthamoeba keratitis cannot be entirely reliant on laboratory test results because confocal microscopy, the preferred diagnostic method, is not sufficiently sensitive to detect deep stromal lesions.13 The acquisition of a medical history is equally important, especially when the patient has a unique personal history, such as wearing contact lenses and/or an unhygienic environment. Clinicians should be aware of the potential for such infections and implement appropriate treatment, if necessary, with or without support from laboratory tests [previously regarded as the gold standard14] or typical signs (e.g., absence of severe eye pain and annular infiltration in this patient).
It is more difficult to diagnose viral infection, especially in patients with radioneuritis and pseudodendritic changes, as observed in our patient; these findings are indistinguishable from Acanthamoeba keratitis. Viral infections are typically accompanied by a history of upper respiratory tract infections (although this may not occur in some patients) or decreased immunity; they are also generally unilateral.15,16 Our patient did not demonstrate typical symptoms and manifested only posterior corneal deposits and swollen endothelial cells; therefore, we could only make the diagnosis of herpes simplex keratitis after PCR analysis of corneal scrapings. Empirically, antiviral drugs are used throughout the course of treatment because amoebic infection tends to undermine the barrier function of the cornea, such that it becomes more vulnerable to viral infection. Steroids should not be used prematurely, especially at an early stage of multipathogenic corneal infection caused by wearing contact lenses;5,7 otherwise, trophozoite regeneration can be promoted and latent viruses activated within the confinement ring.17 In this regard, cortisol should be applied after the pathogens are fully controlled.
When a multipathogenic infection (e.g., including Acanthamoeba) is highly suspected, anti-amoebic treatment should be implemented first because Acanthamoeba is closely associated with inflammation, and the Acanthamoeba-derived local immune response can facilitate the growth of other pathogenic species (e.g., bacteria and viruses).18,19 Notably, it is better to treat simultaneously if all pathogens have already been identified. However, when the corneal epithelium is deficient, precautions are needed regarding the dose of 0.05% chlorhexidine because its ocular surface toxicity can prolong the healing time of the corneal epithelium, thereby reducing the natural barrier function of the cornea.
For our patient, corneal scraping and culture were performed because of the emergence of a corneal ulcer at an early stage of the disease; the results confirmed bacterial infection. Multiple authors have shown that bacteria causing mixed keratitis are generally sensitive to the fluoroquinolone class of antibiotics,5,7,20,21 which was also confirmed in this case. Our first choice was gatifloxacin because—in addition to the above factors—its ability to permeate into the eyes is much greater than that of the commonly used levofloxacin; moreover, its half-life is longer than that of moxifloxacin.22 We instructed the patient to continue gatifloxacin until the end of topical glucocorticosteroid therapy to prevent the secondary growth of other bacteria.
After viral infection, anterior uveitis can be triggered in the cornea. To prevent formation of a posterior synechia, we prescribed 5 mg/mL compound tropicamide eye drops (twice per day); these were continued until the end of loteprednol treatment (a type of glucocorticosteroid) and contributed to the good corrected vision outcome of our patient.
When treating triple corneal infection, a full course of drug treatment with the full dose must be performed; an average treatment time of more than 28 days has been reported previously5,7,23 and was used for treatment of our patient.
Given the financial situation of our patient, we did not perform reverse transcriptase quantitative PCR after herpes simplex virus PCR to detect whether the herpes simplex virus DNA was indicative of residual antigen or active infection. Thus, if future patients exhibit similar clinical manifestations, reverse transcriptase quantitative PCR should be considered when economic circumstances permit. Furthermore, S. epidermidis might be a contaminant from the skin; however, as demonstrated by Khan et al.,24 immediate initiation of antibiotic prophylaxis plays a key role in eradicating potential bacterial strains. As a result, our treatment regimen facilitated a good outcome for the patient.
Conclusion
To the best of our knowledge, this is the first report and review regarding mixed corneal infection caused by three types of pathogens in the context of wearing Ortho-K lenses. The success of treatment in our patient has implications for the treatment of future corneal coinfections with ≥3 species.
Acknowledgements
We thank American Journal Experts for language editing.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethical approval and consent to participate
The need for ethical approval was waived, as this is a report of clinical practice and does not constitute biomedical research. Reporting is consistent with all ethical requirements.
Patient consent for publication
Informed consent was obtained from the patient for publication of this case report. All identifying information was removed from this manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Xuguang Sun https://orcid.org/0000-0003-1176-4279
References
- 1.Agarwal R, Gagrani M, Mahajan Aet al. Fulminant Sphingomonas paucimobilis keratitis: case study and review of literature. BMJ Case Rep 2019; 12; e231642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobia-Acquah E, Akowuah PK, Antwi-Adjei EKet al. Contact lens complications among wearers in Ghana. Cont Lens Anterior Eye 2019. Nov 27. doi: 10.1016/j.clae.2019.11.003. (Epub ahead of print). [DOI] [PubMed]
- 3.Grasela DM. Clinical pharmacology of gatifloxacin, a new fluoroquinolone. Clin Infect Dis 2000; 31: S51–S58. [DOI] [PubMed] [Google Scholar]
- 4.Hazarika M, Pai HV, Khanna Vet al. Rare case of polymicrobial keratitis with Balantidium coli. Cornea 2016; 35: 1665–1667. [DOI] [PubMed] [Google Scholar]
- 5.Lim BX, Koh VTC, Ray M. Microbial characteristics of post-traumatic infective keratitis. Eur J Ophthalmol 2018; 28: 13–18. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi Y, Eguchi H, Toibana Tet al. Polymicrobial sclerokeratitis caused by Scedosporium apiospermum and Aspergillus cibarius. Cornea 2014; 33: 875–877. [DOI] [PubMed] [Google Scholar]
- 7.Ray M, Nigel LCS, Tan AM. Triple infection keratitis. Eye Contact Lens 2014; 40: 123–126. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes M, Vira D, Dey Met al. Comparison between polymicrobial and fungal keratitis: clinical features, risk factors, and outcome. Am J Ophthalmol 2015; 160: 873–881. [DOI] [PubMed] [Google Scholar]
- 9.Bang SP, Jun JH. Acute exacerbation of staphylococcal catarrhal infiltration associated with treatment for Pseudomonas aeruginosa keratitis. Medicine 2018; 97: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karsten E, Watson SL, Foster LJ. Diversity of microbial species implicated in keratitis: a review. Open Ophthalmol J 2012; 6: 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arshad M, Carnt N, Tan Jet al. Water exposure and the risk of contact lens-related disease. Cornea 2019; 38: 791–797. [DOI] [PubMed] [Google Scholar]
- 12.Balczun C, Scheid PL. Detection of Balamuthia mandrillaris DNA in the storage case of contact lenses in Germany. Parasitol Res 2016; 115: 2111–2114. [DOI] [PubMed] [Google Scholar]
- 13.Kwok PW, Kam KW, Jhanji Vet al. Painless Acanthamoeba keratitis with normal vision. Optom Vis Sci 2017; 94: 432–435. [DOI] [PubMed] [Google Scholar]
- 14.Daas L, Viestenz A, Schnabel PAet al. Confocal microscopy as an early relapse marker for Acanthamoeba keratitis. Clin Anat 2018; 31: 60–63. [DOI] [PubMed] [Google Scholar]
- 15.Gulmez Sevim D, Gumus K, Cavanagh HD. Corneal pseudodendritic lesions masquerading as herpetic keratitis in a patient with tyrosinemia type I. Eye Contact Lens 2017; 43: e7–e9. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira Mendes J, Monteiro T. Pseudodendritic ulcer. J Fr Ophtalmol 2017; 40: 341–342. [DOI] [PubMed] [Google Scholar]
- 17.Robert PY. Clinical and diagnostic developments in corneal herpes. J Fr Ophtalmol 2004; 27: 524–527. [DOI] [PubMed] [Google Scholar]
- 18.Neelam S, Niederkorn JY. Pathobiology and Immunobiology of Acanthamoeba keratitis: insights from animal models. Yale J Biol Med 2017; 90: 261–268. [PMC free article] [PubMed] [Google Scholar]
- 19.Knickelbein JE, Kovarik J, Dhaliwal DKet al. Acanthamoeba keratitis: a clinicopathologic case report and review of the literature. Hum Pathol 2013; 44: 918–922. [DOI] [PubMed] [Google Scholar]
- 20.Ali NA, Reddy SC. Bilateral simultaneous infectious keratitis secondary to contact lens wear: an unusual case report with rare organisms. Eye Contact Lens 2007; 33: 338–340. [DOI] [PubMed] [Google Scholar]
- 21.Wu AL, Yeh LK, Ma DHet al. Clinical characteristics of Stenotrophomonas maltophilia keratitis. Cornea 2016; 35: 795–800. [DOI] [PubMed] [Google Scholar]
- 22.Hotta F, Eguchi H, Nishimura Ket al. A super-infection in the cornea caused by Stemphylium, Acremonium, and alpha Streptococcus. Ann Clin Microbiol Antimicrob 2017; 16: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim NC, Lim DKA, Ray M. Polymicrobial versus monomicrobial keratitis: a retrospective comparative study. Eye Contact Lens 2013; 39: 348–354. [DOI] [PubMed] [Google Scholar]
- 24.Khan AM, Larson B, Noth Jet al. Microbial cultures of the microkeratome blade immediately after flap construction in laser in situ keratomileusis. J Cataract Refr Surg 2008; 34: 842–845. [DOI] [PubMed] [Google Scholar]