Abstract
Clean water and the increased use of renewable energy are considered to be two of the main goals in the effort to achieve a sustainable living environment. The fulfillment of these goals may include the use of solar-driven photocatalytic processes that are found to be quite effective in water purification, as well as hydrogen generation. H2 production by water splitting and photocatalytic degradation of organic pollutants in water both rely on the formation of electron/hole (e−/h+) pairs at a semiconducting material upon its excitation by light with sufficient photon energy. Most of the photocatalytic studies involve the use of TiO2 and well-suited model compounds, either as sacrificial agents or pollutants. However, the wider application of this technology requires the harvesting of a broader spectrum of solar irradiation and the suppression of the recombination of photogenerated charge carriers. These limitations can be overcome by the use of different strategies, among which the focus is put on the creation of heterojunctions with another narrow bandgap semiconductor, which can provide high response in the visible light region. In this review paper, we report the most recent advances in the application of TiO2 based heterojunction (semiconductor-semiconductor) composites for photocatalytic water treatment and water splitting. This review article is subdivided into two major parts, namely Photocatalytic water treatment and Photocatalytic water splitting, to give a thorough examination of all achieved progress. The first part provides an overview on photocatalytic degradation mechanism principles, followed by the most recent applications for photocatalytic degradation and mineralization of contaminants of emerging concern (CEC), such as pharmaceuticals and pesticides with a critical insight into removal mechanism, while the second part focuses on fabrication of TiO2-based heterojunctions with carbon-based materials, transition metal oxides, transition metal chalcogenides, and multiple composites that were made of three or more semiconductor materials for photocatalytic water splitting.
Keywords: TiO2 heterojunction, semiconductor coupling, water treatment, photocatalytic degradation, photocatalytic water splitting, H2 production
1. Introduction
Nowadays, accessible clean water and energy resources are among the highest priorities for sustainable economic growth and societal wellbeing. Water supports life and is a crucial resource for humanity; it is also at the core of natural ecosystems and climate regulation. Water stress is primarily a water quantity issue, but it also occurs as a consequence of a deterioration of water quality and a lack of appropriate water management [1]. Environmental problems that are associated with water pollution have been a persistently important issue over recent decades, correlated negatively with the health and ecosystem. Activities of the Water JPI’s Strategic Research and Innovation Agenda focus on, among others, new materials and processes, energy efficiency, thus supporting key enabling technologies for clean water and wastewater treatment [2]. EU Energy Strategies 2020, 2030, and 2050 set increasing standards for the reduction of greenhouse gas emissions by 20, 40, and 80–95%, respectively, which is achievable by significant investments in the development and application of new low-carbon and renewable energy technologies [3]. In light of increased energy demands and the need to reduce greenhouse gas emissions, the focus has been turned from the fossil fuels toward renewable energy resources and vectors: solar, wind, tides, waves, geothermal, biomass, biofuels, and hydrogen (H2) [4]. Alternative fuels are required to have as small environmental footprint, and be storable and economical, whereas H2 satisfies the first two conditions. The research over the last decades has been focused on fulfilling the third requirement, which triggers its production by solar energy, a largely available and intrinsically renewable energy resource, through water splitting. It should be emphasized that H2, as a fuel, possesses higher heat content than gasoline (per unit mass) [5].
The pioneering work of Fujishima and Honda [6] for H2 production by photoelectrochemical water splitting while using TiO2 photoanode and Pt cathode opened the potential possibilities for generating this energy vector, i.e., fuel, directly from water and solar energy. Works by Bard and Frank in 1977 [7], exhibiting photocatalytic oxidation of CN to CNO−, and by Ollis et al. [8], studying the photocatalytic degradation of organic contaminants in water, practically opened a new research field within new water purification technologies. H2 production by water splitting and photocatalytic degradation of organic pollutants in water both rely on the formation of electron/hole (e-/h+) pairs at a semiconducting material upon its excitation by light with sufficient photon energy [9,10,11,12]. These processes, which can be conducted under environmentally friendly and mild conditions, are economically viable, possessing a potential of becoming effective methods to produce clean energy and water, owing to their low-cost, long-term stability, and usage of solar energy [13].
A well-suited model catalyst for photocatalytic studies is TiO2. Its wide application has been promoted, due to: (i) high photocatalytic activity under the incident photon wavelength of 300 < λ <3 90 nm and (ii) multi-faceted functional properties, such as chemical and thermal stability, resistance to chemical breakdown, and attractive mechanical properties [14,15]. However, harvesting a broader spectrum of solar irradiation involves the lowering of the band gap of semiconducting material, whilst inhibiting the recombination of photogenerated charges. Strategies, including doping with non-metals, incorporation or deposition of noble metals (ions), and material engineering solutions that are based on composites formation using transition metals, carbon nanotubes, dye sensitizers, conductive polymers, graphene (oxide), and semiconducting materials, present viable solutions for set tasks [9,10,15,16]. It is of great importance to combine TiO2 with narrow band gap semiconductors with visible light response to obtain an effective composite for photocatalytic applications. The obtained synergistic effect between two or more semiconductors will then promote efficient charge separation, sufficient visible light response, and high photocatalytic performance. With the dramatic increase of the papers published related to these topics, a comprehensive review is desirable, providing a general overview on processes occurring while using TiO2-based heterojunction (semiconductor) systems for photocatalytic water purification and water splitting. Despite reviews focusing on TiO2–based semiconductor composites [17,18], those focusing on the removal of contaminants of emerging concern (CECs) are quite scarce. In addition, this review also summarizes TiO2-based nanocomposites for photocatalytic water splitting providing insight into effectiveness of a variety of materials groups representing the alternative for replacing the utilization of expensive, toxic, and non-abundant materials. The first part of the review focuses on TiO2–based heterojunction (semiconductor-semiconductor coupling) composites, being selected on the basis of band gap energies suitable to make heterojunctions with documented applications providing promising results in CECs treatment and stability of prepared materials, and also respecting their most recent applications for the photocatalytic degradation of CECs (i.e., demonstrating the current focus within the field), such as pharmaceuticals and pesticides, with critical insight into the pollutants removal mechanism. The second part targets the most recent achievements in the field of fabrication of TiO2-based heterojunctions with carbon based materials, transition metal oxides, transition metal chalcogenides, and multiple composites that were made of three or more semiconductor materials for photocatalytic water splitting.
2. Photocatalytic Water Treatment
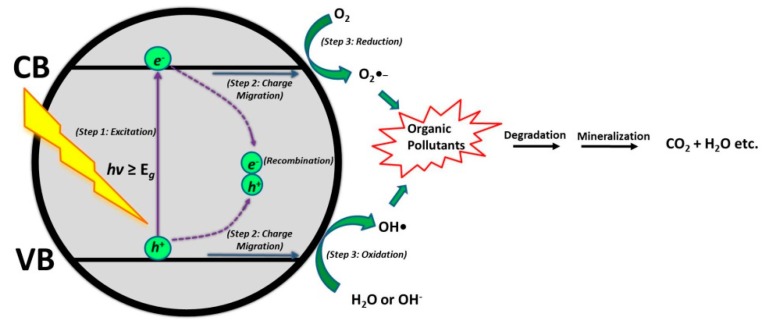
The general mechanism of semiconductor photocatalysis (Figure 1) is composed of three main steps: 1. e−/h+ pairs are generated on the surface of the semiconductor under illumination with the required wavelength or energy; then, 2.) photogenerated charges (i.e., e−/h+) migrate to the surface of the semiconductor; and lastly, 3.) e− and h+ induce redox reactions on the surface that facilitate destruction of organic pollutants [19,20]. As stressed above, TiO2 is still the most studied and widely used material for photocatalytic degradation reactions. However, TiO2 suffers from fast e−/h+ pair recombination and large band gap (Eg = 3.1–3.2 eV), which can only be excited under UV light irradiation. The strategies for improving these issues are provided above, while, among them, semiconductor-coupling presents a viable structure-properties engineering solution for the enhancement of TiO2 photocatalytic activity due to the simultaneously reduced e−/h+ recombination rate and enhanced visible light absorption [21].
Figure 1.
Photocatalytic reaction mechanism over semiconductor material.
Three main types of heterojunction architectures are reported for TiO2/semiconductor composites [22]. In Type I heterojunction, the conduction band (CB) of TiO2 is higher in energy (more negative potential) when compared to the CB of semiconductor 2 and the valence band (VB) of TiO2 is lower in energy (more positive potential) as compared to the VB of semiconductor 2 [23,24]. This leads to the accumulation of photogenerated h+ and e− in semiconductor 2. In Type II heterojunction (where TiO2 can be semiconductor 1 or 2), the CB of semiconductor 2 is higher than the CB position of semiconductor 1 leading to facile transfer of photogenerated e− from CB of semiconductor 2 to CB of semiconductor 1 [25]. Meanwhile, photogenerated h+ in VB of semiconductor 1 can travel to the VB of semiconductor 2, which facilitates efficient charge separation. Type III heterojunction (also known as broken gap situations) [26] shares the same charge transfer mechanism, like Type II heterojunction. In this case, the CB and VB of semiconductor 2 are higher than CB and VB of TiO2 [27,28]. These heterojunction types are explained in detail in the context of particular material combinations in the further text and graphically represented through Figures 2, 3 and 5–10.
2.1. Coupling of TiO2 with Metal Oxides
2.1.1. TiO2/WO3
Tungsten oxide (WO3), which is a visible light active photocatalyst with band gap of 2.4–2.8 eV, is a promising candidate for photocatalytic applications, due to its oxidative properties, nontoxicity, low cost, and stability in acidic solutions. In addition, WO3 directly matches the band positions of TiO2 to form a heterojunction (Type II Heterojunction) [29,30,31,32]. Several authors studied the application of TiO2/WO3 composites for the degradation of various CECs; either pesticides or pharmaceuticals (Table 1). Hence, Macias et al. [24] studied the photocatalytic degradation of herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) while using TiO2/WO3 composites under natural sunlight. They reported the rather high effectiveness of the studied system: 94.6% degradation of 2,4-D and 88.6% mineralization of overall organic content under two and four hours of natural sunlight irradiation, respectively.
Table 1.
Photocatalytic degradation of contaminants of emerging concern (CECs) over TiO2/WO3 composites.
| Catalyst | Target Pollutant | Initial Concentration/Working Volume ((mg L−1) /mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| TiO2 - WO3
(0.5 g/L ) |
2,4-dichlorophenoxy acetic acid | 50 (in 250 mL) |
Light Source: natural sunlight 11AM-4PM; pH = 4 |
120 min | 94.6 (TOC = 88.6) |
[24] |
| TiO2 - WO3
(0.6 g/L) |
Diclofenac | 25 (in 100 mL) |
Light Source: 400 W Metal Halide Lamp; pH = 5 |
240 min | TOC = 91 | [30] |
| TiO2 - WO3
(0.1 g/L) |
Amoxicillin | 100 (in 25,000 mL) |
CPC Reactor with accumulated energy 550,000 J/m2 |
NA | 64.4 (@ 550 kJ/m2) | [35] |
| (WO3/TiO2-C) (1.0 g/L) |
Diclofenac | 10 (in 300 mL) |
Light Source: 1500 W Xenon Lamp with filter(λ > 290 nm) ; pH = 7 |
NA | 100 (@ 250 kJ/m2) (TOC = 82.4 @ 400 kJ/m2) |
[32] |
| (WO3/TiO2-N) (1.0 g/L) |
Diclofenac | 10 | Light Source: 1500 W Xenon Lamp with ID65 solar filter; pH = 6.5 |
NA | 100 (@ 250 kJ/m2) (TOC = 100 @400 kJ/m2) |
[31] |
NA—not available.
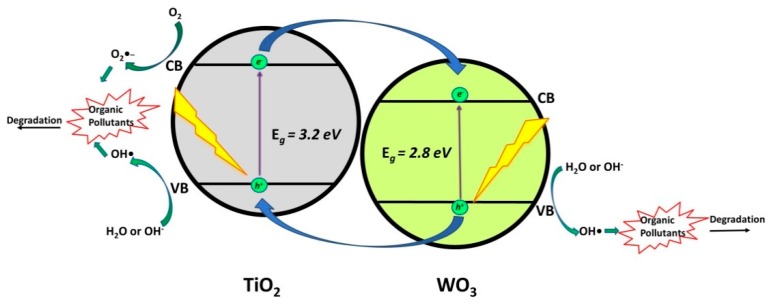
Besides, they studied the mechanisms that are responsible for forming reactive species within the system and, based on their findings, proposed that, upon forming e−/h+ pairs under solar irradiation, photogenerated e− from CB of TiO2 are transferred to CB of WO3. Consequently, W6+ was first reduced to W5+ on WO3 surface, while the W5+ ions are then oxidized to W6+ by adsorbed O2 producing superoxide anion radical (O2●‒). The photogenerated h+ in VB of WO3 are transferred to VB of TiO2 where they reacted with water (or hydroxyl ions, HO−) forming hydroxyl radicals (•OH) (Figure 2). The generated reactive oxygen species (ROS) promoted the degradation of 2,4-D and its intermediates, eventually yielding rather high mineralization extents, while their occurrence in the system was confirmed through tests with common scavenging agents (e.g., tert-butanol (TB) for •OH, formic acid (FA) for h+, and p-benzoquinone (BQ) for O2●‒) [24].
Figure 2.
Photocatalytic degradation mechanism over TiO2/WO3 composite.
The same composite type was used in the degradation of pharmaceuticals. Hence, Mugunthan et al. [30] treated diclofenac (DCF) while using TiO2/WO3 composites under 4 hrs of visible light irradiation and reported a maximum of 92% mineralization of overall organic content. They also elucidated the DCF degradation pathway by LC/MS measurements, which included C-N cleavage in the DCF molecule forming benzene-ring based intermediates at the first stage, and open-ring intermediates at the later stage, which were eventually mineralized. Such findings were quite similar to other studies employing •OH based processes in the degradation of DCF ([33,34]), thus implying the important role of formed ROS, primarily •OH, in the case of TiO2/WO3 solar driven photocatalysis as well. Arce-Sarria et al. [35] studied the performance of TiO2/WO3 composite for the degradation of another pharmaceutical, Amoxicillin (AMX), in pilot scale reactor, where they achieved 64.4% degradation.
Besides “pure” TiO2/WO3 composite, several authors studied the performance of its enriched analogues (Table 1). Hence, Cordero-García et al. [32] studied DCF degradation by WO3/C-doped TiO2 and reported 100% DCF degradation and 82.4% mineralization of the overall organic content under 250 kJ/m2 and 400 kJ/m2 of solar-accumulated energy, respectively. They also stated that the WO3/C-doped TiO2 composite showed superior photocatalytic activity for the complete degradation and mineralization of DCF when comparing to the pristine TiO2, used as benchmark material. Based on the findings on elucidated mechanisms within the studied composite and DCF degradation pathway provided, the authors concluded that the incorporation of elemental carbon to TiO2 crystal structure promoted the formation of a C2p-hybridized valence band that lowered the band gap of TiO2 by mixing with O2p orbitals. As a result, upon visible light irradiation, TiO2 generates e−/h+ pairs, where the photogenerated e− are promoted to the Ti 3d states (VB), thus reducing Ti4+ to Ti3+. Ti3+ can be easily oxidized by WO3 due to the differences in the reduction potential between TiO2 (−0.70 V vs NHE) and WO3 (−0.03 V vs NHE). Subsequently, W6+ traps photogenerated e− to form its reduced state W5+, while the redox reaction occurs further by returning to its original oxidation state in reaction with adsorbed O2 on the composite catalyst surface (similarly as discussed above in the case of “pure” TiO2/WO3), thus leading to improved charge separation and the formation of ROS, which contributed in DCF degradation and mineralization of formed intermediates. The same authors studied the degradation of DCF with another enhanced WO3/TiO2 composite (N-doped TiO2), and again reported high degradation and mineralization rates; 100% according to both indicators under 250 kJ/m2 and 400 kJ/m2 of solar-accumulated energy, respectively [31]. They stressed that the same mechanism that was responsible for the enhancement of photocatalyst activity in C-doped WO3/TiO2 composite [32] also improved the performance of N-doped WO3/TiO2 [31].
2.1.2. TiO2/Fe2O3
Iron oxide (α-Fe2O3) is a promising candidate for photocatalytic applications, due to its abundance, nontoxicity, low cost, stability in aqueous solutions (pH > 3), and narrow band gap (2.0–2.2 eV), which directly matches the band positions of TiO2 to form heterojunction (Type I Heterojunction) [23,36].
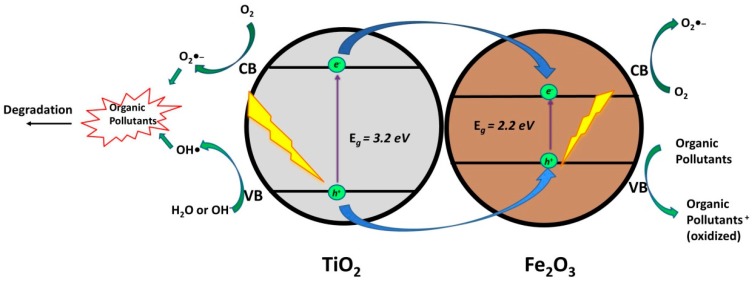
Several authors report the photocatalytic degradation of CECs using TiO2/Fe2O3 composites (Table 2). Hence, Mirmasoomi et al. [37] used TiO2/Fe2O3 as a catalyst for photocatalytic degradation of Diazinon (DZ). The authors reported an optimized system with maximum degradation of DZ equal to 95.07% within 45 min. under visible light irradiation. In another study by Moniz et al. [23], photocatalytic degradation of 2,4-D while using TiO2/Fe2O3 composites was investigated, reporting 100% 2,4-D degradation and 100% mineralization of overall organic content within 2 h and 3 h, respectively, using irradiation from a 300 W Xenon Lamp. The authors found out that, when compared to the benchmark TiO2 (P25), the TiO2/Fe2O3 composite shows superior photocatalytic activity. Based on photoluminescence and photocurrent studies, the TiO2/Fe2O3 composite exhibits enhanced separation of e−/h+ pairs due to the formed heterojunction. The proposed mechanism was supported with DFT studies, which firstly involved the transfer of photogenerated e− from TiO2 CB to Fe2O3 CB. In addition, Fe2O3 binds strongly with (dissolved) oxygen, thus aiding the photoelectron transfer. This in-situ second stage mechanism facilitates the facile migration of h+ from the VB of TiO2 [23]. Macías et al. [24] studied the same system, TiO2/Fe2O3 composites for photocatalytic degradation of 2,4-D, but while using natural sunlight. The authors reported 96.8% 2,4-D degradation and 90.0% mineralization of overall organic content under two hours and four hours, respectively. Contrary to the presented mechanism of Moniz et al. [23], Macias et al. [24] proposed that the incorporation of Fe2O3 causes the photogenerated e− in CB of TiO2 to be transferred to CB of Fe2O3, promoting the reduction of Fe3+ to Fe2+. Photogenerated h+ in VB of TiO2 are transferred to VB of Fe2O3, which leads to the regeneration of Fe3+ and avoids the recombination of e−/h+ pairs at TiO2 surface. In addition, Fe2O3 (Eg = 2.2 eV) can be excited by visible light irradiation producing photogenerated e−/h+ pairs. Photogenerated e− in CB of Fe2O3 can be transferred to O2 dissolved in water to form O2●‒, while photogenerated h+ in VB of Fe2O3 can facilitate generation of •OH eventually contributing to the degradation of present organics [24] (Figure 3). The formation of mentioned ROS and their involvement in degradation of targeted pollutant was confirmed through common scavenging tests using TB, FA, and BQ.
Table 2.
Photocatalytic degradation of CEC’s over TiO2/Fe2O3 composites.
| Catalyst | Target Pollutant | Initial Concentration/Working Volume ((mg L−1) /mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| TiO2/Fe2O3 (0.1 g/L ) |
Diazinon | 10 (in 300 mL) |
Light Source: 60 W Philips Visible lamp; pH = natural |
45 min | 95.07 | [37] |
| TiO2/Fe2O3 (10 mg) |
2,4-dichlorophenoxy acetic acid | 50 (in 100 mL) |
Light Source: 300 W Xenon Lamp; pH = natural |
120 min | 100 (TOC = 100 @ 150 min.) |
[23] |
| TiO2/Fe2O3 (0.5 g/L ) |
2,4-dichlorophenoxy acetic acid | 50 (in 250 mL) |
Light Source: natural sunlight 11AM-4PM; pH = 4 |
120 min | 96.8 (TOC =90 @ 240 min.) |
[24] |
| TiO2/Fe2O3 (70 mg) |
Oxytetracycline Hydrochloride | 60 (in 70 mL) |
Light Source: 300 W Iodine Tungsten Lamp; pH = 5.5 |
300 min | 75.6 | [38] |
| TiO2/Fe2O3 (1.0 g/L) |
Oxytetracycline | 60 | Light Source: 300 W Iodine Tungsten Lamp; pH = 5.5 |
300 min | ~80 | [39] |
| TiO2/Fe2O3/CNT (100 mg) |
Tetracycline | 20 (in 100mL) |
Light Source: 300 W Xenon Lamp; pH = natural |
90 min | 89.41 | [40] |
| TiO2-coated α-Fe2O3 core-shell (100 mg) |
Tetracycline Hydrochloride | 50 (in 200 mL) |
Light Source: 300 W Xenon Lamp (λ > 420 nm) ; pH = 5. 45 Oxidant = 120 µL (30% H2O2) |
90 min | 100 | [41] |
Figure 3.
Photocatalytic degradation mechanism over TiO2/Fe2O3 composite.
The photocatalytic degradation of the pharmaceutical tetracycline (TC) and its derivatives, such as oxytetracycline (OTC), using TiO2/Fe2O3 materials has also been reported. Hence, it was found out that, under visible light irradiation (λ = 400–750 nm), α-Fe2O3 was activated and generated e−/h+ pairs, and then photogenerated e− from CB of α-Fe2O3 moved to TiO2 trapping sites for atmospheric O2 to form O2●‒, which was proven to largely contribute to the degradation of OTC. On the other hand, the photogenerated h+ from VB of α-Fe2O3 primarily reacted with OH−, resulting in the generation of •OH, which also contributed to the degradation of OTC. When compared to TiO2 reference material, the TiO2/Fe2O3 composite exhibited an enhanced photocatalytic activity under visible light due to efficient e−/h+ separation, as stated above [38]. The same authors [38,39] also studied the degradation mechanism of OTC while using LC/MS TOF analysis and, based on the formed intermediates, established the OTC degradation pathway, and concluded that •OH mainly mediated degradation. Besides, “pure” TiO2/Fe2O3, enriched analogue with carbon nanotubes (CNTs) was also studied (Table 2). Hence, TiO2/Fe2O3/CNTs was used as the catalyst for photocatalytic degradation of TC, under visible light illumination [40]. It was found that the effectiveness of photocatalytic degradation of TC within 90 min. treatment using TiO2/Fe2O3/CNTs was almost twice higher when comparing to that achieved by benchmark TiO2; 89.41% and 47.64%, respectively. The authors attributed the improved photocatalytic efficiency to the presence of the CNT, which acted as a photogenerated e− acceptor, thereby suppressing e−/h+ recombination [40]. In another study, the core-shell structured α-Fe2O3 (with TiO2 shell of around 15 nm) exhibited 100% TC removal in 90 min. [41]. The degradation improvement was ascribed to the addition of H2O2 in the system, which generated more ROS than by the common photocatalytic mechanisms described above [41]. Hence, the contribution of H2O2 in such a system can be described through restraining e−/h+ recombination and increasing HO• generation in the system, as in Equation (1) [15]:
| (1) |
2.1.3. TiO2/Spinel Ferrite
Spinel ferrites (MFe2O4) are metal oxides, where M is a divalent ion (i.e., Mg2+, Ca2+, Sr2+, Ni2+, Zn2+, etc.), serving as promising candidates for photocatalytic applications due to their narrow band gap range (1.3–2.2 eV) and magnetic properties [42,43]. Spinel ferrites band positions match TiO2, thus possessing compatibility to form a heterojunction (Type II Heterojunction) [44,45,46,47].
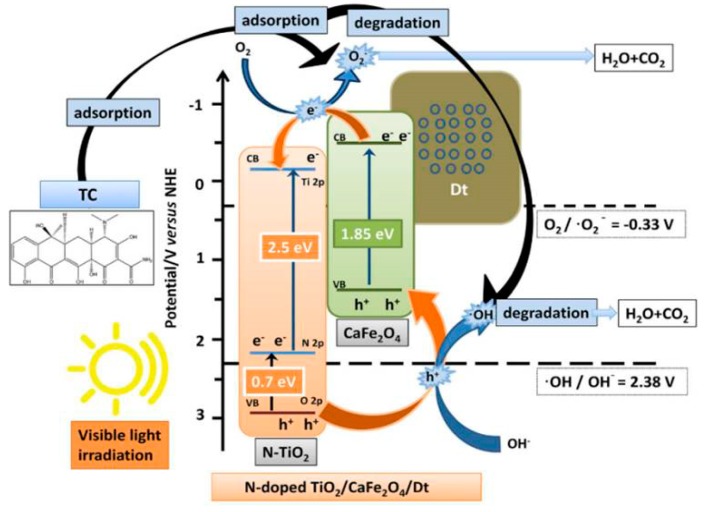
The literature provides applications of MFe2O4/TiO2 materials as photocatalysts in treatment of CECs, as in the case of previously discussed TiO2-based composites, however, it should be noted that authors within such composites used modified TiO2 (Table 3). Hence, Chen et al. [44] studied photocatalytic degradation of TC and its derivatives using N-doped TiO2/CaFe2O4/diatomite, and reported 91.7% removal of TC within 150 min. under visible light irradiation. The authors studied the composite stability and reusability; the results obtained after five cycles indicates that employed composite is rather stable, enabling 89.2% removal of TC. They also proposed the photocatalytic mechanism occurring within the composite; the excitation of both N-TiO2 and CaFe2O4 by visible light leads to the formation of e−/h+ pairs (Figure 4). The photogenerated e− in CB of N-TiO2 can directly react to adsorbed O2 generating O2●‒, while photogenerated h+ in VB of N-TiO2 directly react with H2O and OH− producing •OH. Simultaneously, photogenerated e− in CB of CaFe2O4 can undergo the same mechanism (i.e., reaction with O2 to produce O2●‒). In addition, the formed heterojunction helps the migration of e− from CB of CaFe2O4 to CB of N-TiO2, and h+ from VB of N-TiO2 to VB of CaFe2O4 (Figure 4). Such a transfer of charge carriers between the two semiconductors hinders the recombination process and enhances the photocatalytic activity of the composite, thus leading to more efficient generation of ROS (O2●‒ and •OH) [44]; the existence of formed ROS was confirmed through scavenging tests while using isopropyl alcohol (IPA), ammonium oxalate (AO), and BQ for •OH, h+ and O2●‒, respectively. Such behavior is confirmed by studying the degradation pathway of TC; it was found that the TC intermediates match those that formed through radical driven reactions undergoing in the first step demethylation and hydroxylation. The second step considered the removal of functional groups (amino, hydroxyl, and methyl) and further ring opening reactions that are mainly mediated by photogenerated h+, yielding small fragments that were eventually mineralized [44]. Such a pathway confirmed the dual role of photogenerated h+, as a promotor •OH generation and as sites for the direct oxidation of adsorbed organics. There are several other studies investigating the application of different MFe2O4/TiO2 materials (N-doped TiO2/SrFe2O4 diatomite [46]; Ce/N-co-doped TiO2/NiFe2O4/ diatomite and ZnFe2O4/TiO2 [47]) for the photocatalytic degradation of CECs, such as TC, OTC, and bisphenol A (BPA). Interestingly, the same mechanisms responsible for charge transfer and consequent generation of ROS were reported, regardless of the different M type within the spinel ferrite part of composite and/or TiO2 (non-doped or doped with metal and/or non-metal ions).
Table 3.
Photocatalytic degradation of CEC’s over TiO2/MFe2O4 composites.
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1) /mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) |
reference |
|---|---|---|---|---|---|---|
| N-TiO2/ CaFe2O4 /diatomite (2.0 g/L) |
Tetracycline | 10 (in 200 mL) |
Light Source: 150 W Xenon Lamp with UV light filter | 150 min | 91.7 (TOC =~80 @ 2h) |
[44] |
| N-TiO2/ SrFe2O4 /diatomite (2.0 g/L) |
Tetracycline | 10 (in 200 mL) |
Light Source: 150 W Xenon Lamp with UV light filter | 150 min | 92 (TOC = ~80 @ 2h) |
[46] |
| Ce/N co-doped TiO2 / NiFe2O4 diatomite (0.5 g/L) |
Tetracycline | 20 (in 200 mL) |
Light Source: 150 W Xenon Lamp with UV light filter | 180 min | 98.2 (TOC = ~95) |
[45] |
| ZnFe2O4 / TiO2
(1.0 g/L) |
Bisphenol A | 10 (in 200 mL) |
Light Source: 300 W Xenon Lamp pH= 7 |
30 min | 100 | [47] |
Figure 4.
Proposed mechanism for the tetracycline (TC) photodegradation process using N-doped TiO2/CaFe2O4/ diatomite [44].
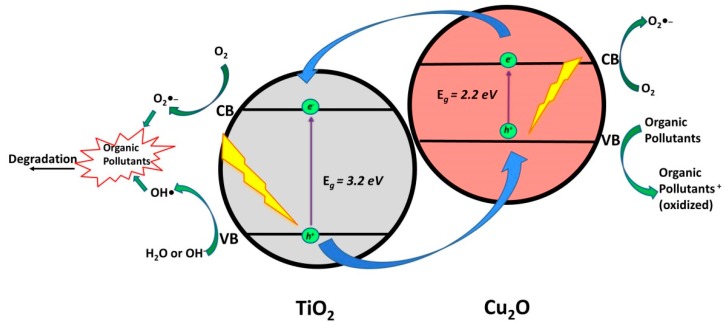
2.1.4. TiO2/Cu2O
Cu2O, a p-type semiconductor (Eg = 2.0–2.2 eV), is also a good candidate for making heterojunctions with TiO2 (Type II Heterojunction). Hence, the photocatalytic degradation of various CECs (TC [48], and tetrabromodiphenyl ethers [49]) using TiO2/Cu2O composite materials was reported under solar light irradiation (Table 4). Based on the findings in the mentioned studies, the photocatalytic mechanism of TiO2/Cu2O under solar light illumination involves the activation of both Cu2O and TiO2 to generate e−/h+ pairs (Figure 5). Photogenerated e− in CB of Cu2O then can migrate to CB of TiO2 and, along with photogenerated e− in CB of TiO2, react with O2 to form O2•_. Simultaneously, photogenerated h+ in VB of Cu2O can be directly involved in the oxidation of adsorbed organics, while photogenerated h+ in VB of TiO2 can directly oxidize adsorbed organics or react with H2O (i.e., OH−) and generate •OH. Besides, these h+ can also directly migrate to the VB of Cu2O, thus leading to effective charge separation that improves the overall photocatalytic activity of the composite [48].
Table 4.
Photocatalytic degradation of CEC’s over TiO2/Cu2O composites.
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1) /mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| Cu2O-TiO2 supported palygorskite (1.0 g/L) |
Tetracycline Hydrochloride | 30 (in 50mL) |
Light Source: 500 Xe Lamp; pH = 8.7 |
240 min | 88.81 | [48] |
| TiO2-Cu2O film | Tetrabromodiphenyl Ethers | 5 (in 100 mL) |
Light Source: 300 W Xenon Lamp; pH = natural solvent CH3OH:H2O (50:50 v/v) |
150 min | 90 | [49] |
Figure 5.
Photocatalytic degradation mechanism over TiO2/Cu2O composite.
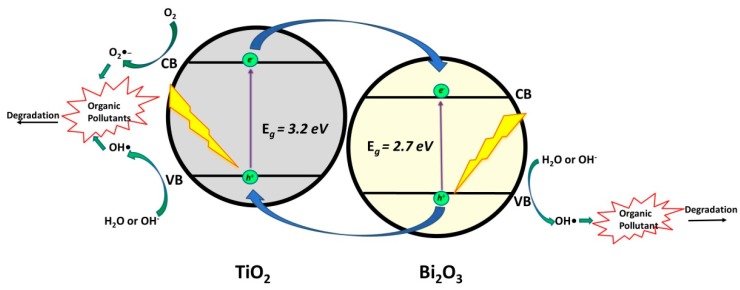
2.1.5. TiO2/Bi2O3
Bi2O3, a semiconductor with band gap range in the visible region (2.5–2.8 eV), is also a good candidate for making heterojunctions with TiO2 (Type II Heterojunction). Studies including its application in photocatalytic degradation of CECs (quinalphos [50] and ofloxacin [51]) under solar light irradiation (Table 5) revealed the occurring photocatalytic mechanism. Both of the composite phases can be activated under solar irradiation generating e−/h+ pairs (Figure 6). Accordingly, photogenerated h+ in VB of TiO2 are involved in the production of •OH (through reactions with H2O, i.e., OH−) as of e−/h+ pairs. In addition, h+ in VB of Bi2O3 can be transferred to VB of TiO2 that contributes to the direct oxidation of adsorbed organics or the generation of •OH [51].
Table 5.
Photocatalytic degradation of CEC’s over TiO2/Bi2O3 composites.
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1) /mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| Bi2O3–TiO2 (50 mg) |
Quinalphos | 25 (in 50 mL) |
Light Source: Visible light with 1.56µmol/m2/s; pH = 8 | 100 min | 92 | [50] |
| Bi2O3–TiO2 (0.5 g/L) |
Ofloxacin | 25 | Light Source: 70.3 K lux; pH = 7 |
120 min | 92 | [51] |
Figure 6.
Photocatalytic degradation mechanism over TiO2/Bi2O3 composite.
2.2. Coupling of TiO2 with Metal Sulfides
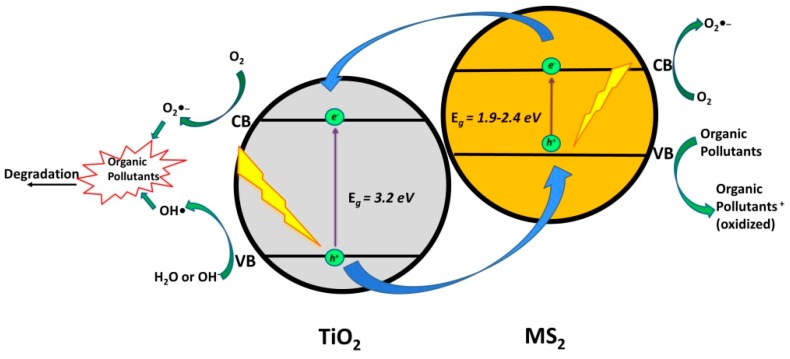
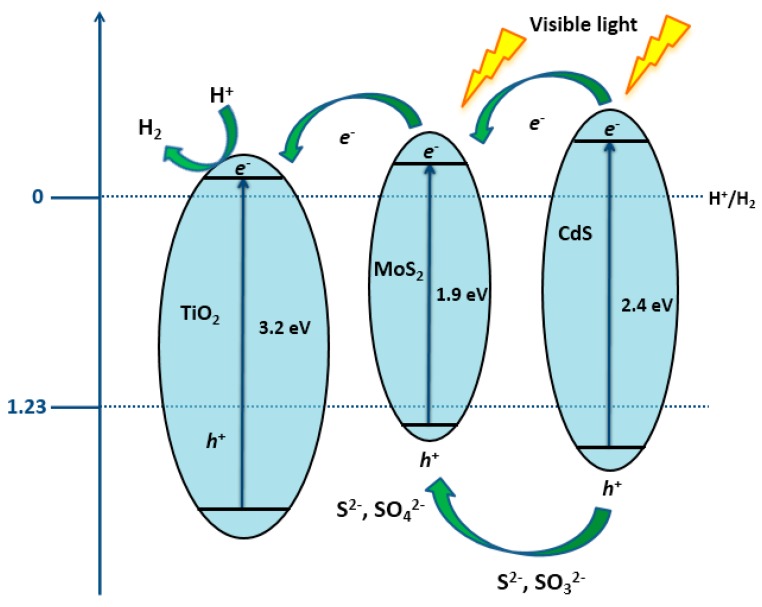
Cadmium sulfide (CdS), a metal sulfide semiconductor with a visible light range band gap (Eg = 2.1–2.4 eV), has been proven to be compatible with TiO2, due to its higher position of CB than that of TiO2 (Type II Heterojunction) (Figure 7) [25,52]. However, one should be aware that its application can lead to adverse effects due to its instability, resulting in the leaching of toxic Cd2+ during treatment [53]. Although its CB and VB positions are thermodynamically favorable for photocatalytic application, CdS as a photocatalytic material faces serious problems. Next to the above-mentioned promotion of toxic effects, issues, like poor stability due to photocorrosion and limited separation efficiency of photogenerated charge carriers, do not speak in favor of CdS application [54,55]. Photocorrosion is not only related to the photogenerated h+ in semiconductor itself that oxidizes S2– and release Cd2+ to the solution, but also with newly formed O2, where higher solubility in water leads to more dramatic levels of photocorrosion of CdS [54,56]. However, CdS was widely investigated in photocatalytic purposes, even in recent studies that focused on the degradation of CECs (ofloxacin, ciprofloxacin, tetracycline, and 17α-ethynylestradiol), where it was used in various forms (nano-rods, nano-belts) [25,52,57,58] (Table 6). Generally, upon visible light illumination, CdS is excited and generates the e−/h+ pair, where photogenerated e− in CB of CdS migrates to CB of TiO2 and is consumed in reactions with O2 to produce O2●‒, while h+ remain in the VB of CdS.
Figure 7.
General photocatalytic degradation mechanism over TiO2/MS (M = Cd or Cu) or MS2 (M = Mo and Sn) composite.
Table 6.
Photocatalytic degradation of CEC’s over TiO2 /Metal sulfide composites.
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) |
Reference |
|---|---|---|---|---|---|---|
| CdS –TiO2 (50 mg) |
Tetracycline Hydrochloride | 50 (in 50mL) |
Light Source: 500 W Xenon Lamp with filter (λ > 400 nm); pH = natural | 480 min | 87.0 | [58] |
| Au-CdS/TiO2 nanowire (20 mg) |
Ciprofloxacin | 20 | Average solar light intensity = 100, 000 | 60 min | 99 | [57] |
| CdS/TiO2 (450 mg) |
Ofloxacin | 10 (in 100mL) |
Light Source: 85 W Oreva bulb with 4150 lumens (λ = 450-650 nm); pH = natural | 180 min | 86 | [52] |
| CdS nano-rod/TiO2 nano-belt ( 0.50 g/L) |
17α-ethynylestradiol | 3 (in 10 mL) |
Light Source: 500 W Xenon Lamp with filter (λ > 420 nm); pH = natural | 120 min | 92 | [25] |
| CuS/TiO2 nanobelts | Enrofloxacin | 5 (in 35 mL) |
Light Source: 35 W Xenon Lamp; pH = natural | 120 min | 85.5 (TOC = 27.7) |
[59] |
| Au-CuS-TiO2 nanobelts | Oxytetracycline | 5 ( in 35 mL) |
Light Source: 35 W Xenon Lamp; pH = natural | 60 min | 96 (TOC = 68) |
[60] |
| MoS2 /TiO2
(25 mg/L) |
Acetaminophen | 302 | Light Source: Sunlight; pH = natural |
25 min | 40 | [64] |
| N,S co-doped TiO2 @MoS2 (0.98g/L) |
Diclofenac | 0.15 ( in 100 mL) |
Light Source: 60 W LED lamp; pH = 5.5 | 150 min | 98 | [61] |
| TiO2/SnS2 films | 17β-estradiol | 1.36 (in 90 mL) |
Light Source: 450 W Xenon Arc Lamp | 90 min | 51.0 | [66] |
| TiO2/SnS2 films | Diclofenac | 31.8 ( in 90mL) |
Light Source: 450 W Xenon Arc Lamp; pH = 4 | 60 min | 76.21 | [67] |
Copper sulfide (CuS), which is another metal sulfide semiconductor with narrow band gap of 2.0 eV, has also been reported to be coupled with TiO2 (Type II Heterojunction) [59,60]. Jiang et al. [59] reported a 85.5% degradation of enrofloxacin and 27.7% mineralization of overall organic content using immobilized CuS/TiO2 nanobelts (Table 6). They elucidated the mechanisms occurring in the composite upon excitation by solar irradiation. Hence, such broad wavelengths excited both composite phases (CuS and TiO2) and resulted in e−/h+ pairs, while the transfer of charges was analogous, as in the case of the CdS/TiO2 composite. Photogenerated e− in CB (−0.33 eV) of CuS underwent transfer to CB (−0.19 eV) of TiO2 and were consumed in reactions with O2 forming O2●‒. Photogenerated h+ in VB of CuS remained there and present potential active sites for direct degradation of organics that were adsorbed at the CuS surface, since they cannot be involved in generation of •OH due to too high energy band positioning. On the other hand, photogenerated h+ in VB of TiO2 can directly react with adsorbed organics and OH− generating •OH. Chen et.al [60], incorporated Au nanoparticles to CuS/TiO2 nanobelts structure to enhance the photocatalytic degradation ability of the composite by capturing e− and, consequently, suppressing the recombination of photogenerated charges. As a result, they obtained 96% degradation of OTC and 68% mineralization of the overall organic content within one hour under artificial sunlight illumination. Accordingly, the mechanism of such enriched CuS/TiO2 composite involves, besides the above discussed mechanism, the path considering the transfer of e− to Au, which leads to enhanced charge separation, thus delaying recombination. In such a case scenario, photogenerated h+ would have higher probability to react either with adsorbed organics or with HO‒ in order to generate •OH (exclusively those in VB of TiO2), thus contributing to the overall system efficiency. The involvement of formed ROS into reaction mechanisms for OTC degradation was confirmed by scavenging tests using TB, AO, and BQ.
Molybdenum disulfide (MoS2), a two-dimensional (2D) layered metal chalcogenide with an indirect band gap of 1.1 eV and 1.9 eV direct band gap in monolayered form, with unique structure, low-cost, high thermal stability, and electrostatic integrity, is also a suitable candidate for forming heterojunction with TiO2 (Type II Heterojunction) [61,62,63]. Hence, Kumar et al. [64] reported its application in the photocatalytic degradation of paracetamol. Furthermore, Irandost et al. [61] applied the modified MoS2/TiO2 composite (they used N,S-co-doped TiO2) in the photocatalytic degradation of DCF under visible LED lamp irradiation (Table 6). Hence, the synergistic effect of dopants in TiO2 promoted its visible light activity, yielding the formation of e−/h+ pairs in both composite phases. The mechanism of charge formation and consequent transfer was similar, as described above for CuS/TiO2, which was excited by solar irradiation. Hence, photogenerated e− in CB of N,S-co doped TiO2 and CB of MoS2 were able to undergo reactions with O2 forming O2●‒, while h+ in VB of TiO2 promoted •OH formation in reactions with HO‒ and provide the direct oxidation of adsorbed organics, while, again, h+ in MoS2 were able to do only the latter. The importance of •OH and h+ in DCF degradation was confirmed by trapping agents used in scavenging tests: TB and potassium iodide (KI), respectively.
Tin sulfide (SnS2), which is a metal sulfide semiconductor with band gap of 2.2 eV [65], has also been reported to be coupled with TiO2 (Type II Heterojunction) [66,67]. Hence, Kovačić et al. [66] reported improved the degradation of 17β-estradiol (E2), for 51%, using SnS2/TiO2 when comparing to the benchmark material (P25) TiO2 under solar irradiation. A similar improvement was obtained by comparing performances of the same materials in the case of DCF degradation [67] (Table 6). The reason for such improvement relies on the potential of photogenerated e− in CB of SnS2 to migrate to CB of TiO2, while h+ remained at the VB of SnS2. In such case, the efficient separation of charges is achieved, thus facilitating the improved redox reactions, enabling effective degradation of adsorbed organics directly on the surface by h+, in spite of the limited ability of such a composite to generate •OH. Accordingly, the adsorption has been shown as an important step in the effectiveness of the SnS2/TiO2 composite. Kovačić et al. [67] utilized DFT calculations to study the surface interaction of polar compounds (DCF) and non-polar compounds (memantine) at SnS2/TiO2 composite and found that DCF was more efficiently degraded due to much higher adsorption ability in comparison to memantine, which is one of its structure feature limitations (amine functionality).
2.3. Coupling of TiO2 with Silver- Based Semiconductors
Silver Phosphate (Ag3PO4), a promising semiconductor with narrow band gap (Eg ≥ 2.4 eV), showed good photocatalytic performance in the degradation of organic pollutants under visible light irradiation [68,69]. Namely, Ag3PO4 exhibits a quantum efficiency of up to 90% [68] and it can absorb wavelengths that are shorter than ~530 nm [69]. Despite the qualities of Ag3PO4 as a potential photocatalyst, it still suffers from limitations, such as photocorrosion, small but not negligible solubility in water (Ksp = 1.6 × 10−16), and particle agglomeration upon synthesis [70]. To overcome these limitations, constructing a heterojunction between Ag3PO4 and a compatible semiconductor has attracted attention due to the increase in charge separation and production of more ROS [71]. The positions of VB and CB in TiO2 directly match the Ag3PO4 band positions, thus providing the compatibility to form a heterojunction.
Hence, Wang et al. [72] investigated the performance of TiO2 nanotubes/Ag3PO4 quantum dots for the degradation of TC under visible light illumination, and reported a high removal rate within a short treatment period; 90% TC removal within 8 min (Table 7).
Table 7.
Photocatalytic degradation of CEC’s over TiO2/Silver-Based Semiconductor composites.
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| Ti3+ -doped TiO2 nanotubes/ Ag3PO4 quantum dots (0.5 g/L) |
Tetracycline | 10 (NA) |
Light Source: 400 W Xenon Lamp; pH = natural |
8 min | 90 | [72] |
| TiO2 nanotube/ Ag3PO4 nanoparticles (40 mg) |
Ciprofloxacin | 10 (in 40 mL) |
Light Source: 300 W Xenon Lamp | 60 min | 85.3 | [73] |
| TiO2-x / Ag3PO4
(100 mg) |
Bisphenol A | 10 (in 100 mL) |
Light Source: 500 W Xenon Lamp with filter (λ = 420 nm); pH = natural |
16 min | 95 | [74] |
| Ag2O/ TiO2 quantum dots (0.25 g/L) |
Levofloxacin | 10 (in 100 mL) |
Light Source: 85 W Oreva CFL (4150 lumens) (λ = 380–700 nm) pH=4 |
90 min | 81 | [27] |
| Ag2O /TiO2 –zeolite (50 mg) |
Norfloxacin | 5 (in 100 mL) |
Light Source: 35 W Xenon Lamp | 60 min | 98.7 (TOC = 83.1) |
[28] |
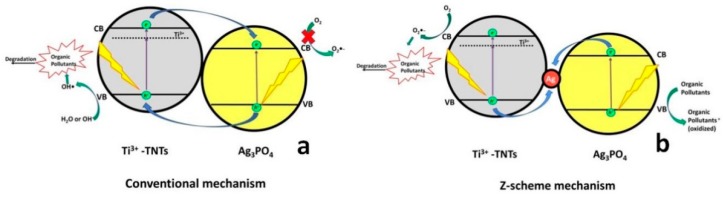
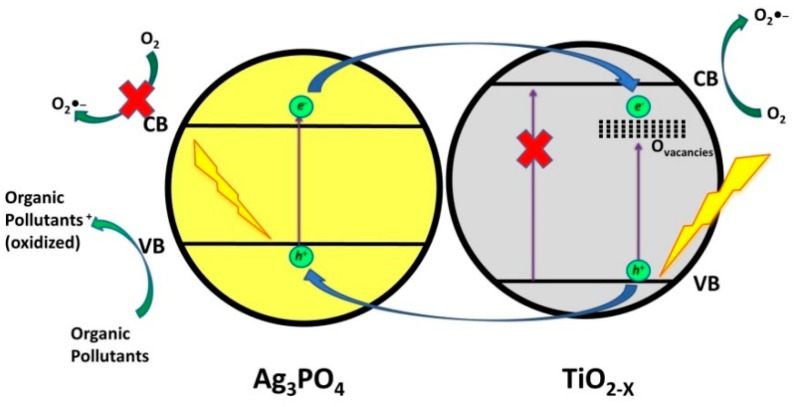
The conventional heterojunction transfer mechanism (Figure 8a) explains that the photogenerated h+ in the composite would be promoted from the VB of Ag3PO4 to VB of Ti3+-doped TiO2 nanotubes, where can react with H2O or HO− forming •OH. Simultaneously, photogenerated e− from the Ti3+-doped TiO2 nanotubes CB can react with O2 forming O2●‒ or may transfer to the CB of Ag3PO4. However, O2●‒ are not formed in Ag3PO4, due to the fact that the position of its CB is lower than the standard reduction potential of O2●‒/O2. Wang et al. [72] concluded that TC was primarily degraded by O2●− and photogenerated h+ based on the results of the conducted electron trapping experiments. Accordingly, they have extended the study by proposing a Z-scheme heterojunction transfer mechanism (Figure 8b). Under this mechanism, Ag(0) acts a recombination center, “collecting” photogenerated e− from CB of Ag3PO4, where they undergo recombination with the photogenerated h+ from VB of Ti3+-doped TiO2 nanotubes. In such case, photogenerated h+ on VB of Ag3PO4 might participate in the direct oxidation reactions with adsorbed organics, while the photogenerated e− in the CB of Ti3+-doped TiO2 nanotubes can be involved in forming desired ROS, O2●‒, thus contributing to the enhanced performance of composite photocatalyst. Du et al. [73] applied analogue TiO2/Ag3PO4 composite employing TiO2 nanotube arrays for the degradation of ciprofloxacin (CIP) under solar irradiation and reported that 85.3% removal of CIP within 60 min. was facilitated through the above-mentioned mechanisms. Furthermore, Liu et al. [74] reported 95% degradation of BPA in 16 min. using TiO2−X/Ag3PO4 under visible light irradiation (Table 7). They reported that both composite phases, TiO2−X and Ag3PO4, were excited and generated e−/h+ pairs. Hence, photogenerated h+ in VB of TiO2−X are promoted to VB of Ag3PO4 and contributed to the direct oxidation of adsorbed organics, similarly as reported in the study by Wang et al. [72]. Photogenerated e− from the CB of Ag3PO4 are transferred to oxygen vacancies (Vo) of TiO2 and contributed in reactions with adsorbed O2 generating O2●‒ (Figure 9). They also investigated the role of these species in the degradation of BPA and found, based on monitoring BPA degradation pathway by LC/MS analysis, that intermediates are formed through two pathways: 1) hydroxylation, through reactions with O2●‒ yielding BPA-o-catechol; and, 2) direct oxidation by h+ forming isopropenylphenol and phenol, which was further oxidized by h+ yielding hydroquinone and its dehydrated form benzoquinone.
Figure 8.
Photocatalytic mechanisms Ti3+-TNTs/Ag3PO4 (a) conventional heterojunction, and (b) Z-scheme heterojunction.
Figure 9.
Photocatalytic reaction mechanism of TiO2−X/Ag3PO4 under visible light irradiation.
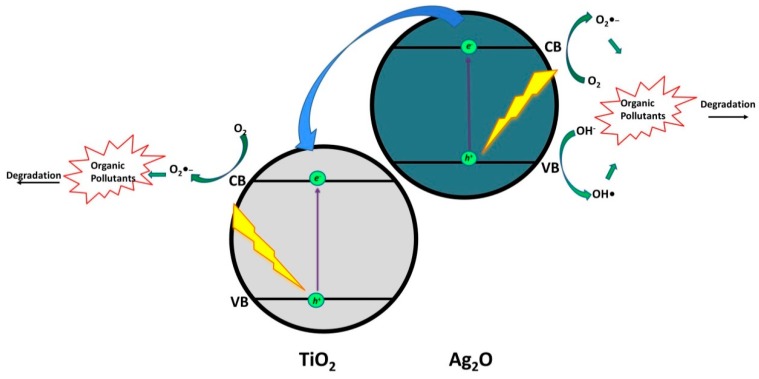
Silver oxide (Ag2O), a visible light active photocatalyst with band gap of 1.2 eV, is another silver-based compound with semiconducting properties. Based on the band positions (VB and CB), it represents a promising matching candidate to form heterojunctions with TiO2 (Type III Heterojunction). Hence, photocatalytic degradation of levofloxacin (LEV) using Ag2O/TiO2 quantum dots is reported with the maximum of 81% LEV degradation within 90 min. of visible light irradiation [27]. Based on the proposed mechanism under visible light illumination (Figure 10), upon excitation of Ag2O, e−/h+ pairs are formed, whereas TiO2 is not activated due to its wide band gap. Photogenerated e− in the CB of Ag2O were transferred to CB of TiO2 and involved in reactions with adsorbed O2 forming O2●‒ that participated in LEV degradation. In addition, photogenerated h+ in VB of Ag2O yielded the formation of •OH, through reactions with OH−, and participated in LEV degradation as well. The authors employed LC-MS analysis to elucidate LEV degradation pathway and, as such, establish the role of formed ROS. Hence, parent compound LEV underwent decarboxylation of the acetyl group; hydroxylation resulting in the formation of quinolone moieties; demethylation and the subsequent addition of hydrogen atom generating modifications at piperazine ring; while successive •OH attack resulted in multi-hydroxylated intermediates. Such findings confirmed the dominant role of •OH in LEV degradation.
Figure 10.
Photocatalytic degradation mechanism over TiO2/Ag2O composite.
In another study, Gou et al. [28] investigated the application of Ag2O/TiO2/zeolite composite for solar-driven degradation of norfloxacin (NOR) (Table 7). Besides high effectiveness (98.7% NOR degradation and 83.1% mineralization of organic content within 60 min. treatment), they elucidated the NOR degradation pathway, involving in the initial stage decarboxylation, defluorination or hydroxylation of parent compound (NOR), which confirmed the involvement of both formed ROS (O2●‒ and •OH).
2.4. Coupling of TiO2 with Graphene and Graphene-Like Materials
2.4.1. TiO2/Graphene Composites
Graphene is a zero bandgap semiconductor with a sheet-like structure (i.e., it is considered as a 2D monolayer material) consisting of sp2 hybridized carbon atoms with excellent thermal conductivity, optical transmittance, high mechanical strength, large surface area (2600 m2/g), and appreciable charge carrier transport [75]. Under light illumination, it can achieve a reverse saturation state with high density (~ 1013 cm2) of hot electrons above the Fermi level, which can be used as a powerful agent in redox reactions [76]. It was also found that the incorporation of graphene-based materials (i.e., graphene oxide and its reduced form; GO and rGO, respectively) with TiO2 might suppress e−/h+ pairs recombination. As such, TiO2/graphene-based composites were employed in the photocatalytic degradation of CECs (Table 8).
Table 8.
Photocatalytic degradation of CEC’s over TiO2/Semiconductor/graphene composites.
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) |
Reference |
|---|---|---|---|---|---|---|
| TiO2/ WO3/GO (2 mg) |
Bisphenol A | 20 ( in 50 mL) |
Light Source: sunlight Ph = 7 |
7 h | 93.2 | [77] |
| Graphene-WO3 /TiO2 nanotube (photoelectrodes ) |
Dimethyl Phthalate | 10 (in 40 mL) |
Light Source: 150W Xe lamps |
120 min | 75.9 | [78] |
| TiO2 /ZnO/GO (0.5 g/L) |
Bisphenol A | 10 (in 50 mL) |
Light Source: 18 UV lamps (λ =365 nm) ;Visible metal halide lamps(λ = 400–800 nm) pH = 6 | 120 min. (UV) 180 min. (Vis) |
99.7 (UV) 94.9 (Vis) |
[79] |
| TiO2 /ZnO/GO (0.5 g/L) |
Ibuprofen | 10 (in 50 mL) |
Light Source: 18 UV lamps (λ = 365 nm) ;Visible metal halide lamps(λ = 400–800 nm) pH = 6 | 120 min. (UV) 180 min. (Vis) |
98.5 (UV) 79.6 (Vis) |
[79] |
| TiO2 /ZnO/GO (0.5 g/L) |
Flurbiprofen | 10 ( in 50 mL) |
Light Source: 18 UV lamps (λ=365 nm) ;Visible metal halide lamps(λ= 400–800 nm) pH= 6 | 120 min. (UV) 180 min. (Vis) |
98.1(UV) 82.2 (Vis) |
[79] |
| ZnFe2O4/rGO/TiO2 (0.1 g) |
Fulvic Acid | 20 (in 50 mL) |
Light Source: 300 W (λ=420 nm); Vol H2O2 = 0.8 mL, pH= 7 | 180 min | 95.4% | [80] |
| TiO2 /MoS2 /rGO (0.5 g/L) |
Bisphenol A | 10 (in 50 mL) |
Light Source: 20 W (λ = 254 nm); |
300 min | 62.4 | [81] |
| TiO2/BiVO4/rGO | Tetracycline | 10 µg/L (NA) |
Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 96.2 | [82] |
| TiO2/BiVO4/rGO | Chlorotetracycline | 10 µg/L (NA) |
Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 97.5 | [82] |
| TiO2/BiVO4/rGO | Oxytetracycline | 10 µg/L (NA) |
Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 98.7 | [82] |
| TiO2/BiVO4/rGO | Doxycycline | 10 µg/L (NA) |
Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 99.6 | [82] |
2.4.2. TiO2/Semiconductor/Graphene Composites
As described in above sections, the coupling of TiO2 with other semiconductors promotes efficient charge transfer, eventually yielding improved photocatalytic activity. However, in most cases, the recombination is still an existing issue that needs to be suppressed. Such a double effect can be obtained by combining composite concept involving two semiconductors (even “pure” TiO2, which cannot be active under visible light) with graphene-based materials. For instance, Hao et al. [77] reported 93.2% degradation of BPA in seven hours of sunlight irradiation while using the TiO2/WO3/GO composite. The mechanism occurring in such combined composite involved the excitation of both TiO2 and WO3 under solar light irradiation (TiO2 utilized UV-A fraction), yielding the generation e−/h+ in both semiconductors. Hence, photogenerated e− in CB of TiO2 can directly react with absorbed O2, producing O2●−, or it can be transferred to CB of WO3, and then further migrate to GO enhancing charge separation. Since the amount of adsorbed O2 is quite limited, the tendency of e− to recombine with h+ is rather favored; ~90% of pairs recombine rapidly after excitation [14]. Hence, the charge separation represents an important factor in the evaluation of photocatalyst performance. Accordingly, in the case of effective separation and recombination suppression, as in the case with GO, photogenerated h+ in VB of activated composite components, e.g., of TiO2 and WO3 in the case of TiO2/WO3/GO, can be involved in a larger amount, either directly or indirectly (through formation of •OH) in the degradation of present organics. It should be noted that, in composites with two semiconductors, GO could also act as redox site, attracting photogenerated e− and h+, thus promoting improved surface migration of charges [77]. Table 8 summarizes several works regarding TiO2/semiconductor/GO composites employed for the degradation of CECs with analogous mechanism, as mentioned above.
2.4.3. TiO2/g-C3N4
Graphitic carbon nitride (g-C3N4), a two-dimensional, metal-free polymeric π-conjugated semiconductor material, which has attracted a lot of attention [83,84,85,86,87,88,89,90,91] since the pioneering work of Wang et al. [92] in 2009, due to its high stability, visible light response with the bandgap of 2.7 eV and non-toxicity [93], thus representing a viable candidate to be applied in photocatalytic water treatment [80], has certainly been one of the most investigated photocatalysts inside carbon-based nanomaterials. It can be easily synthesized through the direct pyrolysis of nitrogen-rich precursors, such as melamine, cyanamide, dicyandiamide, and urea, but its practical application and principle drawback is low specific surface area and high rate of electron-hole recombination [83,94,95]. Therefore, g-C3N4 modification to address shortcomings are needed, e.g., as an excellent candidate to form heterojunction with TiO2 (Type II Heterojunction), due to their matched band positions (VB and CB). Hence, several studies employing g-C3N4/TiO2 were focused on photocatalytic degradation of CECs (Table 9). For instance, Yang et al. [96] reported 88.1% degradation of CIP within 180 min. under visible light irradiation. The authors ascribed the improved photocatalytic activity to multiple effects: (i) an increase in the surface area of the composite; (ii) good dispersity of TiO2 in g-C3N4 enabling the intimately coupling of composite phases; and, (iii) extension of light absorption of the composite due to low band gap of g-C3N4. Trapping experiments that were conducted revealed that photogenerated h+ were the major reactive site involved in CIP degradation.
Table 9.
Photocatalytic degradation of CEC’s over TiO2 /g-C3N4 composites.
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) |
Experimental Conditions | Reaction Time | Removal Extent (%) |
Reference |
|---|---|---|---|---|---|---|
| g-C3N4/TiO2 (30 mg) |
Ciprofloxacin | 10 (in 80 mL) |
Light Source: 300 W Xe Lamp with filter (λ > 400 nm) pH = natural |
180 min | 88.1 | [96] |
| g-C3N4/TiO2 (30 mg) |
Acyclovir | 10 (in 100 mL) |
Light Source: 300 W Xe Lamp with filter (λ > 420 nm) pH = natural |
90 min | 100 | [97] |
| mpg-C3N4/TiO2 (membrane) |
Sulfamethoxazole | 10 (in 50 mL) |
Light Source: 300 W Xe Lamp pH = natural Flow rate = 13 mL/min. Membrane flux = 918 L /m2 h |
1800 min | 69 | [98] |
| TiO2@g-C3N4 core-shell (100 mg) |
Tetracycline | 20 (in 100 mL) |
Light Source: Xenon Lamp with full spectrum pH = natural |
9 min | (2.2 mg/min.) | [99] |
| g-C3N4 –shielding polyester/ TiO2
(130 mg) |
sulfaquinoxaline | 2 × 10−5 mol/L (30 mL) |
Light Source: Q-Sun Xe-1 test, pH = 7 |
90 min | 97 | [101] |
| g-C3N4 –shielding polyester/ TiO2
(130 mg) |
thiamethoxam | 2 × 10−5 mol/L (30 mL) |
Light Source: Q-Sun Xe-1 test, pH = 7 |
180 min | ~95 | [101] |
| g-C3N4/TiO2/kaolinite (200 mg) |
Ciprofloxacin | 10 (in 100 mL) |
Light Source: Ave. light intensity =90 mW/cm2 ; Xe Lamp with filter (λ > 400 nm), pH = natural | 240 min | 92 | [100] |
| S-Ag/ TiO2 @ g-C3N4
(0.20 g/L) |
Triclosan | 10 (in 100 mL) |
Light Source: 250 W Xe Lamp with filter (λ > 420 nm), pH = 7.8 | 60 min | 92.3 (Detoxification Efficiency= 64.3± 3.9) |
[102] |
| Co-TiO2 @g-C3N4 (5 mg ; 2 × 2 cm2 membranes) |
Tetracycline Hydrochloride | 20 (in 10 mL) |
Light Source: 300 W Xe Lamp with filter (λ > 420 nm), pH = 7 | 60 min. | 90.8 | [103] |
| D35-TiO2/g-C3N4 (0.5g/L) |
Bisphenol A | 10 (in 100 mL) |
Light Source: 300 W Metal Halide pH = 7, Oxidant = 2mM Persulfate |
15 min | 100 (TOC= 50) |
[104] |
| C dots decorated g-C3N4/ TiO2 (1.0 g/L) |
Enrofloxacin | 4 ( in 50 mL) |
Light Source: 350 W Xe Lamp with filter (λ > 420 nm) pH = natural |
60 min | 91.6 |
[105] |
| graphene quantum dots/ Mn-N-TiO2 /g-C3N4 (45 mg) |
Ciprofloxacin | 10 (in 80 mL) |
Light Source: 300 W Xe Lamp (320 ≤λ ≤ 780 nm), pH = 7 |
120 min | 89 | [107] |
| graphene quantum dots/ Mn-N-TiO2 /g-C3N4 (45 mg) |
Diethyl Phthalate | 10 (in 80mL) |
Light Source: 300 W Xe Lamp (320 ≤ λ ≤ 780 nm), pH = 7 |
120 min | 70.4 | [107] |
| MoS2 supported TiO2/g-C3N4
(30 mg) |
Atrazine | 10 (in 100 mL) |
Light Source: 500 W Xe Lamp (λ > 420 nm), pH = 7 |
300 min | 86.5 | [108] |
| WO3–TiO2 @g-C3N4 | Acetylsalicylate | 10 (in 100 mL) |
Light Source: 500 W Metal Halide pH = natural |
90 min | 98 | [109] |
| WO3–TiO2 @g-C3N4 | Methyl-theobromine | 10 (in 100 mL) |
Light Source: 500 W Metal Halide pH = natural |
90 min | 97 | [109] |
In another study, Li et al. [97] reported the 100% degradation of Acyclovir in 90 min. using g-C3N4/TiO2 under visible light irradiation. However, after seven hours of continuous irradiation, any TOC removal was not noticed, implying the formation of rather recalcitrant intermediates with high resistance to degradation by ROS that formed within the studied system. Trapping experiments for formed reactive species elucidated that g-C3N4/TiO2 under visible light irradiation only produced h+ and O2●‒, and not the most reactive •OH, explaining limited oxidation capability and none TOC removal in the case of acyclovir degradation. This significant contribution proves that the use of g-C3N4/TiO2 under visible light irradiation must undergo careful laboratory tests regarding the susceptibility of targeted organics and their intermediates to degradation by h+ and O2●‒ prior to considering real scale application [97].
Several studies also showed that the tailoring of composite morphology promotes improved photocatalytic efficiency. For instance, Yu et al. [98] prepared a mesoporous g-C3N4/TiO2 that was applied to polysulfone ultrafiltration membranes for sulfamethoxazole (SMX) removal. It was found that mpg-C3N4/TiO2 exhibit 69% SMX degradation within 30 hours of sunlight irradiation. On the other hand, TiO2 nanosheets with exposed facets (001) (core)-g-C3N4 (shell) composite exhibit a higher degradation rate of 2.2 mg/min., which is 36% faster when compared to TiO2 and g-C3N4 physically-mixed composite. The improved effect is ascribed to the close interaction of TiO2 and g-C3N4 core-shell structure, whereas, in physically mixed composite the formed heterojunction is random and non-uniform [99].
The use of support materials, such as clays [100] and polymers [101], has been also utilized for improved adsorption capacity and the stability of g-C3N4/TiO2 composites. For instance, Chen et al. [101] used g-C3N4–shielding polyester fiber (PET)/TiO2 for photocatalytic degradation of sulfaquinoxaline and thiamethoxam. Interestingly, the composite removal efficiency for sulfaquinoxaline reached 97%, after 10 consequent cycles. Furthermore, the introduction of kaolinite with g-C3N4/TiO2 improved the surface area and adsorption capacity of the composite, leading to 92% degradation of CIP in 240 min. of visible light irradiation [100].
An additional approach considers doping of metals and non-metals in TiO2, enhancing its light absorption capacity from UV absorption to visible light absorption. Thus, incorporating doped TiO2 with g-C3N4 structures has also attracted great attention for the degradation of CECs. For instance, S-Ag/TiO2 @g-C3N4 [102] was employed for the degradation of Triclosan (TS) and yielded 92.3% degradation of TS within 60 min. under visible light irradiation. Song et al. [103] fabricated a nanofibrous Co-TiO2 coated with g-C3N4, which was applied to TC removal; the authors reported a consistent stability of composite photocatalyst during five consecutive cycles.
Besides doping, sensitization with dyes [104] and carbon dots [105] was also found to enhance the light absorption capacity of g-C3N4/TiO2 composite. For example, D35 organic dye was applied next to g-C3N4/TiO2 and it was found that the light absorption range was enhanced up to 675 nm [104]. On the other hand, Su et al. [105] studied the application of C dots decorated/g-C3N4/TiO2 for the degradation of enrofloxacin under visible light and assigned the observed enhancement to the upconversion photoluminescence properties of C dots, which convert near-infrared light wavelength into visible light wavelength [106]. As effective solutions for improving g-C3N4/TiO2 performance, the incorporation of graphene quantum dots [107] and another semiconductor (i.e., MoS2 [108], WO3 [109]) is also reported; such systems resulted in enhanced separation of charges and the suppression of their recombination, thus leading to improved photocatalytic activity in the degradation of CECs.
3. Photocatalytic Water Splitting
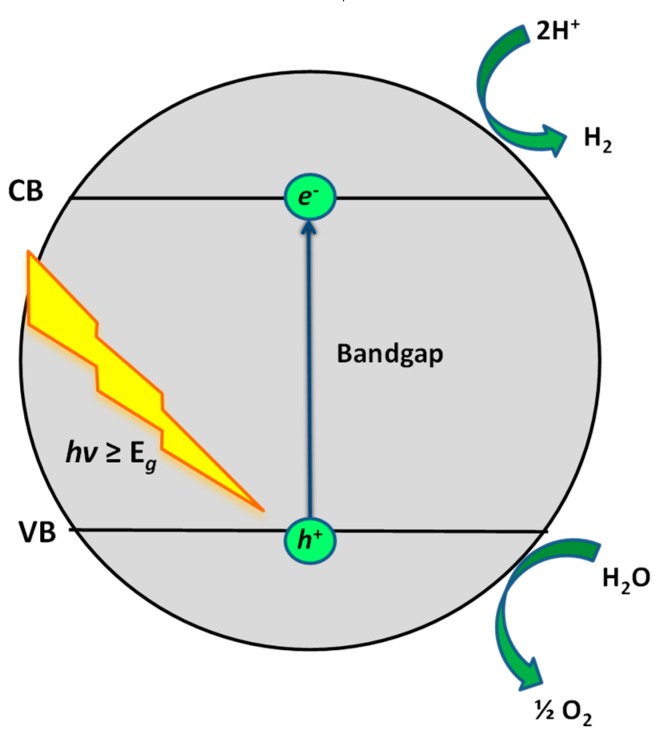
Photocatalytic water splitting implies a non-spontaneous process, where the light photons are used to break the water molecules assisted by a photocatalyst, which generates photoexcited charge carriers, i.e., e−/h+ pairs, delivering them to the solid-liquid interface, where the redox half-reactions of water oxidation and reduction are catalyzed [110,111], analogously, as described above for photocatalytic water treatment. The difference in water splitting is that photogenerated charges (i.e., e− and h+) need to react with H+ as the electron acceptor adsorbed on the photocatalyst surface or within the surrounding electrical double layer of the charged particles in order to generate H2 [112], instead of O2 generating O2●−, as in photocatalytic water treatment (Figure 1). The donors are the same; H2O, however, desired the product of such reaction is O2. Figure 11 shows the principal mechanism of photocatalytic water splitting with the use of TiO2 semiconductor nanoparticle. The VB and CB of semiconductor or their composites have to have favorable positions in order to enable occurrence of such reactions.
Figure 11.
The principle photocatalytic water splitting mechanism over illuminated TiO2 nanoparticle.
Generally speaking, there are two competitive processes that occur inside the photocatalyst and affect H2 evolution. Similarly as in the case of water purification, first is the charge recombination process. Such a process reduces the excited charges for >90%, as mentioned above [14]; according to some authors, even less than 1% of photoexcited charge carriers are able to participate in the photo-redox reactions forming H2 [111]. Such a negative tendency can be improved by controlling the recombination rate [75], as also described in detail in the case of the composite materials used for water purification. The second process is the separation of photogenerated charge carriers that favor H2 evolution, also mentioned above in the case of water purification, but here with more important role [111].
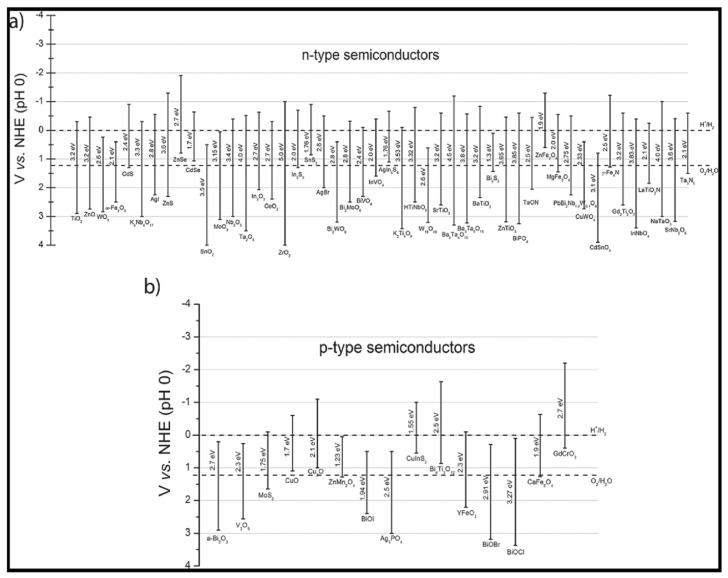
The positions of CB and VB define the redox potential of photogenerated charge carriers. A CB minimum (CBmin) that is smaller than 0 V vs. standard hydrogen electrode (SHE) is required for H2 generation, while the maximum of VB (VBmax) has to be higher than O2/H2O reduction potential, by definition, in order to enable O2 evolution [112]. As mentioned above, H2 generation through this process is non-spontaneous, needing the standard Gibbs free energy change of +237 kJ/mol or 1.23 eV, and to accomplish water splitting under visible light irradiation, the bandgap of the photocatalyst should be more than 1.23 eV and less than 3.0 eV [111]. The electronic structures of diverse semiconductors fulfill the necessary conditions for the water splitting reaction, as can be seen from Figure 12.
Figure 12.
Valence band (VB) and conduction band (CB) band positions of various (a) n-type semiconductors; (b) p-type semiconductors [111].
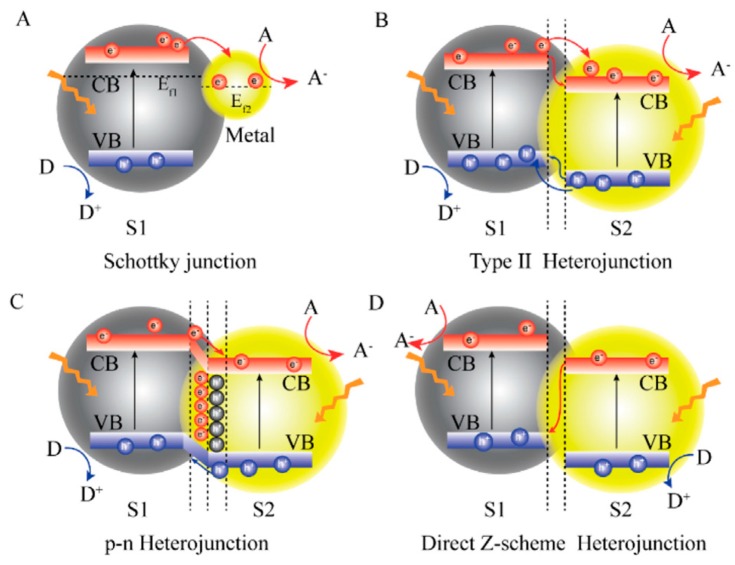
Within the scope of this review are recent achievements in TiO2-heterojunction systems for photocatalytic H2 generation. It is important to explain the separation mechanisms of charge carriers that occur in such hybrid materials: (i) Schottky junctions—photogenerated e− migration from semiconductor to metal surface due to a higher work function of metal than those of semiconductor, thus forming a Schottky junction (Figure 13a); (ii) Type II heterojunction (represented in details in the case of water purification) (Figure 13b); and, (iii) p-n Heterojunction—supply of an additional electric field to accelerate the charge carrier transfer (Figure 13c); and, (iv) Direct Z-scheme heterojunction—e− in the CB of second semiconductor recombined with the photogenerated h+ in the VB of the first semiconductor, leaving the photogenerated e− in first semiconductor and the photogenerated h+ in second semiconductor for photocatalysis (Figure 13d) [112].
Figure 13.
Separation mechanisms of charge carriers in hybrid materials: (A) Schottky junction; (B) Type II Heterojunction; (C) p-n Heterojunction; and, (D) Direct Z-scheme Heterojunction [112].
The process efficiency is determined through the Quantum yield (QY) and Apparent Quantum Yield (AQY), as described with Equations (2) and (3) [93]. The overall quantum yield is predicted to be higher than the apparent one since the number of absorbed photons is usually less than that of incident photons [111].
| (2) |
| (3) |
H2 generation can also be realized in the presence of sacrificial agents, which, in this case, serve as electron donors that accept photogenerated h+ of the VB, thus enhancing the separation of photogenerated charge carriers, which results in higher quantum efficiency [113]. Alcohols are generally used as a h+ scavenger, and the more α-H atoms the alcohol has, the higher H2 production rate is achieved due to more efficient consumption of h+ in the photoreaction. The number of α-H atoms in the alcohols can serve as the reference when selecting an appropriate scavenger for photocatalytic reaction [112].
After briefly providing the basic principles to fully understand the H2 evolution through photocatalytic water splitting, following sections are more focused on the recent achievements in fabrication and the evaluation of different TiO2-based heterojunctions with different families of materials, including carbon-based, transition metal oxides and chalcogenides, and multiple-based composites consisting of three or more semiconductor materials for H2 generation.
3.1. Carbon-Based/TiO2
Among a variety of materials that are selected for the preparation of TiO2-based nanocomposites to increase their photocatalytic efficiency, nanostructured carbon materials, such as carbon nanotubes and graphene family nanomaterials (e.g., GO, rGO, g-C3N4), are of particular interest [114]. The advantages, such as chemical stability, structural diversity with prominent light-absorptive, and electron transport properties, make them promising materials for use in photocatalytic H2 generation by the water splitting processes [115].
3.1.1. TiO2/g-C3N4
The advantages and limitations of g-C3N4 are already mentioned above in the case of water treatment. The limitations referring to low light utilization efficiency and insufficient surface area can be easily broken by the preparation of 2D nanomaterials, especially g-C3N4 nanosheets (CNNS) [84]. The self-assembly method of construction 2D/2D TiO2/CNNS heterojunction composites achieved a hydrogen evolution rate (HER) of 350 µmol/h/g under visible light, in comparison with the produced H2 with the use of pure TiO2 nanosheets (20 µmol/h/g) and g-C3N4 nanosheets (130 µmol/h/g) [85].
Liu et al. recorded another use of CNNS [84], who synthesized partially reduced TiO2−x through NaBH4 treatments with the formation of an additional mid-gap band state (Ti3+ and oxygen vacancies—Ovs) to extend absorption edge. The implementation of novel design tactic in the form of a protective carbon layer that was coated onto TiO2−x/CNNS hetero-junction photocatalyst enhanced the photocatalytic efficiency. The H2 evolution was tested under visible and simulated solar light with the use of triethanolamine (TEOA) as a sacrificial agent and Pt as a co-catalyst. In the case of visible light irradiation, the highest HER was 417.24 µmol/h/g, while, under AM 1.5 irradiation, the obtained amount was 1830.93 µmol/h/g. The enhanced photocatalytic activity that was ascribed to the formation of Ti3+ defects was also noticed with the use of g-C3N4/Ti3+-doped TiO2 Z-scheme system that was synthesized via the polycondensation of urea with TiO2, followed by hydrogenation treatment [86]. UV-Vis diffuse reflectance spectroscopy, X-ray photoelectron spectroscopy (XPS), and electron paramagnetic resonance (EPR) have shown that hydrogenation treatment conferred Ti3+ defect states that were below the CBmin of TiO2 and improved the visible light absorption of the composite with the obtained HER of 1938 µmol/h/g under solar light.
Although special efforts are being made to synthesize noble-metal free nanocomposites, there is still widespread use of Pt as a co-catalyst in H2 evolution reactions. Except for the already mentioned TiO2−x/CNNS photocatalyst [84], TiO2/g-C3N4 composites with the use of photodeposited Pt as co-catalyst reached HER of 4128 µmol/h/g [87] and 1041 µmol/h/g [83] under solar and visible light irradiation, respectively. Pan et al. [88] also exhibited a high HER of 13800 µmol/h/m2 by the use of Pt as a co-catalyst with g-C3N4/TiO2 nanofilm. Enhanced activity is also attributed to the use of a magnetic-driven rotating frame, which was developed to enhance the mass transfer process during the photocatalytic reaction.
The charge transfer efficiency between TiO2/g-C3N4 composite can be enhanced by the doping of different heteroatoms, like C and K atoms. Hence, Zou et al. [89] synthesized C-doped TiO2@g-C3N4 core-shell hollow nanospheres with enhanced visible-light photocatalytic activity for H2 evolution of 35.6 µmol/h/g, which was 22.7 and 10.5 times higher than that of C-TiO2 and g-C3N4. The structure of TiO2 hollow spheres resulted in the reflection of light within the interior cavity, thus increasing the utilization of the light energy. Ma et al. [90] prepared a series of K intercalated g-C3N4 modified TiO2 nanobelts with enhanced light absorption, transfer efficiency, and H2 evolution efficiency of 50 µmol/h, which is 6.4 times greater than that of pristine g-C3N4. The use of carbon atoms in the form of carbon quantum dots (CQDs) as electron reservoirs improves the efficiency of separating the photogenerated charge carriers. CQDs present an important class of carbon materials since their discovery in 2004 by Xu et al. [116], with varying sizes in the range of 1–10 nm. They are good materials for photocatalytic applications due to features, like superiority in chemical stability and low toxicity [117]. Pan et al. synthesized he 2D carbon quantum dots modified porous g-C3N4/TiO2 nano-heterojunction [91] and reached 6.497 µmol/h/g of produced H2 with the full spectrum absorption.
3.1.2. TiO2-G/GO/rGO
Following above-mentioned hot-electron mechanism, which can promote redox reactions, Lu et al. explored 3D graphene materials (3DG) coupled with TiO2 [76] for efficient photocatalytic H2 production under UV-visible light. TiO2/3DG with a 5 wt.% graphene loading that was annealed at 650 °C exhibited the highest H2 evolution rate of 1205 µmol/h/g.
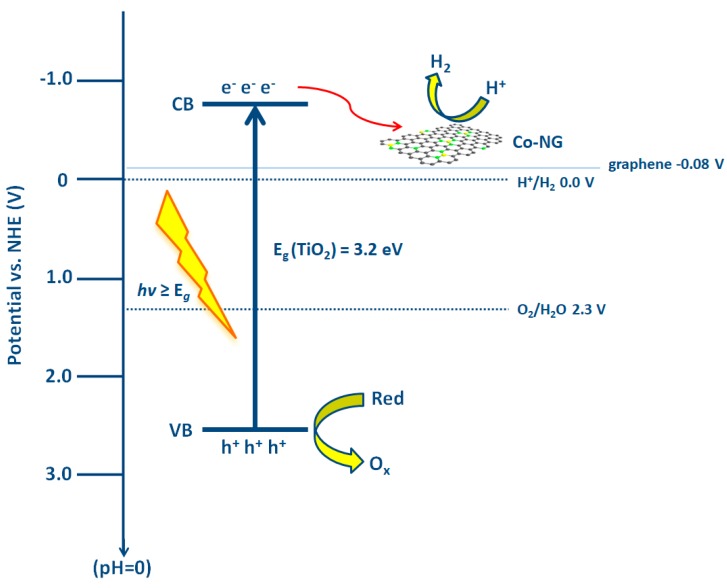
Yi et al. [118] synthesized a composite in which TiO2 nanobelts were supported by N-doped graphene (NG) coordinated with a single Co atom to replace noble metals with a cost-effective photocatalyst. Under simulated solar irradiation (Figure 14), e−/h+ pairs are formed. The transfer of photogenerated e− from the CB of TiO2 to Co-NG was energetically favorable since the Fermi energy level of graphene (−0.08V vs. NHE) is lower than the CB of TiO2 (−0.39 V vs. NHE). NG, with a large specific surface area, acted as “freeway” for e− transportation, delivering e− from TiO2 to Co single-atom, where they were trapped catalyzing H+ reduction to form H2 due to lower the overpotential needed for Co-NG when comparing to that of NG. Co-NG/TiO2 showed HER of 677.44 µmol/h/g under the illumination of AM 1.5 G simulated sunlight.
Figure 14.
Schematic diagram of proposed photocatalytic mechanism in the CO-NG/TiO2 system.
GO/TiO2 nanocomposites have recently been used for H2 production via photocatalytic water splitting under visible light through the formation of Ti-O-C bonding by unpaired π electron of GO with TiO2 surface [114,119]. GO acts as an e− acceptor, promoting the separation of the photogenerated e−/h+ pairs in TiO2. These nanocomposites can be synthesized by photo assisted reduction via mixing or sonication and by sol-gel [114]. Hernández-Majalca et al. [114] enhanced the synthesis for the GO-TiO2 nanocomposite using photoassisted anchoring and modifying GO oxidation method through the use of microwaves. The obtained nonporous product had a specific surface area of 45 m2/g and absorption onset of 477 nm, which made it active under visible light. Finally, the photocatalytic activity of the nanocomposite was enhanced towards the production of H2, reaching 6500 mol/g in 8 h, which was much higher amount when comparing to that obtained by TiO2-P25 (460 mol/g) at the same irradiation time.
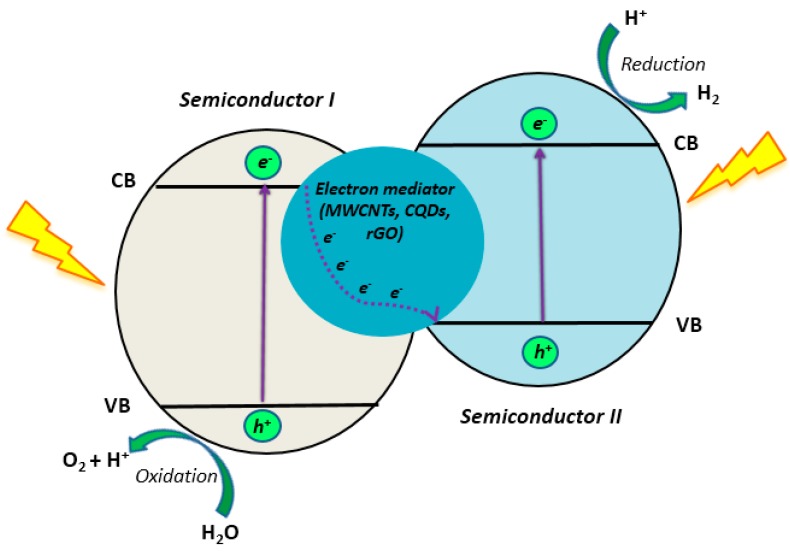
The reduced form of GO, rGO, is a two-dimensional carbon material with the role of an electron mediator that is much superior in chemical stability and morphological diversity than GO [120]. Iwase et al. published the very first report using rGO as a e− mediator in 2011 [121]. Since then, a number of published works were recorded for the use of rGO-based composites in photocatalytic H2 production [75,122,123]. Recent achievements in the synthesis of TiO2/rGO composites for the purpose of H2 generation include work from Reedy et al. [75] and Samal et al. [122]. They obtained rather high HERs while using TiO2/rGO composites under solar and visible light: 24880 µmol/h/g and 2700 µmol/h/g of produced H2, respectively. Ida et al. undertook further investigation on TiO2/rGO composites [123], managing to enhance the photocatalytic activity of the obtained composite by the simultaneous doping of nitrogen on TiO2 and rGO. The following values for the HER are obtained: TiO2 (1585 µmol/h/g) < N-TiO2 (6179 µmol/h/g) < TiO2/RGO (12244 µmol/h/g) < N-TiO2/N-RGO (15028 µmol/h/g).
3.1.3. TiO2/CNT
Recently, TiO2/carbon nanotubes (CNT) have been of great interest due to their high-quality active sites, large specific surface area, and retention of charge recombination, where CNTs can act as a p-type semiconductor, having a role as a powerful electron sink [119]. The coupling of CNT with TiO2 forms an advanced nanocomposite with enhanced quantum efficiency that forms heterojunction acting as an impurity by forming Ti-O-C or Ti-C defects that enable visible light absorption and, consequently, the creation of e−/h+ pairs and hindering e−/h+ recombination [124]. CNTs, such as single-wall carbon nanotubes (SWCNTs) and multiwall carbon nanotubes (MWCNTs), have attracted much interest due to their unique chemical, electrical, and optical properties [125]. Olowoyo et al. [124] prepared a series of TiO2 nanoparticles that were modified with MWCNTs by a combined sonothermal-hydrothermal method. The synthesized photocatalysts were examined for water splitting under batch conditions at different pH ranges. The highest rate of H2 yield, amounting 69.41 µmol/h/g, was obtained using 2 wt.% CNT-TiO2 under visible light at pH 2. Hence, the acidic medium improved the photocatalytic feasibility of the system due to a higher concentration of H+ ions, serving as the reactants, thus increasing the reaction rate.
Bellamkonda et al. [126] used a different approach in synthesizing CNT-G-TiO2 composites and prepared nanocomposites via the solution-based method, in which nanocrystalline anatase TiO2 was grown onto graphene nanosheets and carbon nanotubes. Spectroscopic and photocatalytic studies revealed that graphene acts as an electron reservoir, while the role of CNTs is to prevent the restacking of graphene nanosheets and provide additional electron transport channels, thereby suppressing the recombination rate of e−/h+ pairs in the obtained composite. The combination of all these factors resulted in increasing the HER from 19000 µmol/h/g (obtained by anatase TiO2) to 22000 µmol/h/g (obtained by G-TiO2), and finally to 29000 µmol/h/g (obtained by CNT-G-TiO2), which is 8-fold higher than obtained by the commercial TiO2 (Degussa P25).
The photocatalytic performance of TiO2 under visible light can be promoted by coupling both MWCNTs and SWCNTs, as presented by Umer et al. [125]. Such an effect occurs due to their dual natural behavior, such as reducing rapid recombination of e−/h+ pairs and providing support in harvesting visible light. The maximum H2 evolution rate of 5486 µmol/h/g was achieved over MWCNT/TiO2/SWCNT, which is 1.24– and 1.42–fold higher than using single CTN-TiO2 composites (SWCNT/TiO2 and MWCNT/TiO2, respectively).
3.2. Transition-Metal Oxides/TiO2
Excellent chemical stability has opened the possibility of the application of transition metal oxides (TMO) in the field of clean energy production. The above displayed Figure 12 contains main TMOs, like p-type (CuO, V2O5) and n-type (TiO2, WO3, MoO3, ZnO, Fe2O3) semiconductors that are used in photocatalytic H2 production with pertaining VB and CB energy levels. Visible-light driven TMOs with narrow band gaps are highly desired. The most used materials within this group, such as CuO, Fe2O3, and WO3, have the bandgap energies that allow for them to be active in the visible light region, but the low energy levels of CB position disable them from consuming photoinduced electrons in reactions yielding H2. By changing the morphologies of desired components and co-doping with different elements, their CB and VB edges can be shifted toward a H2 reduction and O2 oxidation potential [127].
Some TMOs, specifically WO3, have been loaded with a different co-catalyst, like Rh, to effectively produce H2 from water, to control the desired morphology in the form of nanorods, nanotubes, and nanowires. Camposeco et al. [128] focused on the use of Rh-WO3 photocatalyst that was supported on TiO2 nanotubes (Rh-WO3/NT) for H2 production via the water splitting process. WO3 alone cannot take part in H2 production since the CB energy level of WO3 is lower than H2 reduction potential. However, by loading with Rh nanoparticles, the enhancement in H2 production was noticed. An analysis of energy band levels for the VB and CB that were determined by UV-Vis results and XPS spectra showed that the presence of WO3 and Rh in the titanate nanotubes simultaneously shift the VBmax and CBmin, thus reducing the bandgap of titanate nanotubes. 0.5 wt.% Rh– 3 wt.% WO3/NT nanocomposite under visible light irradiation yielded HER of 87 μmol/h, while 3 wt.% WO3/NT showed much lower effectiveness (only 13 μmol/h).
In another work, Ren et al. [129] constructed cooperative Schottky and p-n (SPN) heterojunction by forming a NiO/Ni/TiO2 heterostructure that showed a narrower band gap, higher photocurrent density, and ability to absorb light in the visible region. High HER is obtained for the observed composite, amounting 4653 μmol/h/g, which is approx. 2.3 times higher than obtained with NiO/TiO2 with a p-n junction (2059 μmol/h/g), under visible light irradiation and by the use of Pt as a co-catalyst.
A representative example of TMOs is also hematite (Fe2O3), already referred above to as promising visible light active photocatalyst for water treatment. The coupling of Fe2O3 with TiO2 efficiently inhibits the recombination of photogenerated charge carriers and enhances the absorption of solar light [130]. By the use of thermal decomposition of FeCl3 and TiCl4 as precursors, Bhagya et al. [113] synthesized the Fe2O3-TiO2 composite and investigated its photocatalytic activity for H2 production under the influence of different proton sources. Besides focusing on the use of TMOs for improving photocatalytic efficiency, this work also highlights the influence of different sacrificial agents as electron donors that consume photogenerated h+, yielding H2 production. Under simulated solar irradiation, a very high H2 rate of 880 μmol/h, with an apparent quantum efficiency of 19.39%, is achieved while using Fe2O3-TiO2 and diethylamine hydrogen chloride (DAH), which is much higher than that obtained in the case without DAH (323 μmol/h). Madhumitha et al. [130] also explored the influence of different sacrificial reagents on H2 production under a visible light source. With the optimization of parameters, i.e., catalyst dosage, flow rate, incident light irradiation, and type of sacrificial agent, they achieved the maximum of HER, amounting 2700 μmol/h. The increase in photoactivity was attributed to the effective charge transfer from TiO2 to Fe2O3 and the use of EDTA, which suppressed the recombination of photogenerated charge carriers.
Easy preparation, environmental friendliness, and good re-utilization enable the wide use of ZnO/TiO2-based composites. Xie et al. [131] achieved high rates of H2 evolution while using ZnO/TiO2 composites with Pt as a co-catalyst. Under visible light irradiation, 2150 µmol/h/g of H2 is achieved. Additionally, high carrier mobility can be achieved by the use of ZnO in the form of quantum dots (QDs), which presents ideal heavy-metal free “green” modification of TiO2 composites. Chen et al. obtained the fabrication of ZnO QDs decorated TiO2 nanowires via a facile calcination method [132]. They used the obtained composite under solar irradiation next to Pt as a co-catalyst and achieved HER of 313.5 µmol/h.
The use of TMO QDs is also recorded in the work of Liu et al. [133], who decorated two-dimensional TiO2 nanobelts with zero-dimensional Co3O4 quantum dots. When compared with bulk materials, 0D Co3O4 QDs have attracted considerable attention due to their small size (<10 nm), providing a large specific surface area with more active sites and shorter charges transport paths. Due to the decoration of Co3O4 QDs, the bandgap of the obtained composite was also narrowed, and in application next to Pt as co-catalyst, they obtained a rather high HER of 1735.1 μmol/h/g. In comparison, Zhang et al. [134], with the use of bulk p-Co3O4/n-TiO2, achieved a smaller HER of 8.16 μmol/h/g.
Among the TMOs that appear as promising candidates for coupling with TiO2, CuO is one of such, especially due to its narrow bandgap (1.4–1.6 eV) and the promotion of effective charge separation [5,135], as already reported as promising water treatment photocatalyst. Hasan et al. [5] conducted solar H2 production using a TiO2/CuO nanofiber composite that was synthesized by electrospinning technique. Fabricated nanofibers were annealed in different atmospheres to determine the crystalline phase and photocatalytic performance. For the nanofibers that were crystallized in the anatase phase, EPR and XRD analysis referred to the substitution of some Ti4+ ions by Cu2+ ions, leading to the formation of some defects below the CB of TiO2, which led to a narrow band gap, yielding enhanced HER in the amount of 2715 µmol/h/g. For comparison, without annealing in a different atmosphere, Wang et al. [135] only achieved 47 µmol/h/g of produced H2 while using the TiO2/CuO composite that was irradiated by solar light. Other oxidation states of Cu inside the oxides are investigated for coupling with TiO2, such as Cu2O [136]. With all of the benefits, such as environmental compatibility, high visible light activity and earth abundance, wider applications of Cu2O in water splitting are still limiting, since the redox potential of monovalent copper lies within its band gap, thus photogenerated charge carriers thermodynamically prefer the transformation of Cu2O into CuO and Cu, rather than to be used in redox reactions with water constituents forming H2 [136]. Wei et al. [136] stabilized the Cu2O by modulating the defects in faceted Cu2O/TiO2 heterostructures to suppress this disproportionation process. Hence, Cu2O was arranged onto 101-faceted TiO2 and it was found that oxygen vacancies in {101}-faceted TiO2 can create a unique channel for Z-scheme charge transfer in Cu2O/TiO2 heterostructures. Composite that was obtained by such an approach showed the maximum HER of 32600 µmol/h/g, with a quantum efficiency of 53.5% at an irradiation wavelength of 350 nm.
3.3. Transition Metal Chalcogenides-TiO2
As already mentioned, various techniques have been applied to modify the TiO2 photocatalysts with a purpose of wider application in the field of solar-driven H2 production through water splitting. Sensitization with narrow bandgap semiconductors was found to be an effective method for enhancing all of the deficiencies that occur during the sole use of TiO2. Regarding an appropriate band gaps, another group of semiconductors with great application potential in photocatalytic H2 generation are transition metal chalcogenides. Recently, CdS is one of the most studied materials [54,55,137,138,139,140]; however, the toxic effects due to potential leaching of Cd2+ have to be strongly considered, followed by others (MoS2, ZnS, ZnSe, CdSe) indicated in Figure 12, as mentioned in part related to water treatment [127].
3.3.1. TiO2/CdS
CdS is the most important chalcogenides semiconductor as a hydrogen production catalyst due to its narrow bandgap (2.4 eV), which enables its visible light response [56]. Its drawbacks described above in Section 2.2. can be alleviated; susceptibility to photocorrosion can be suppressed by the use of sacrificial agents (sodium sulfite/sulfide) that effectively consume photogenerated h+, while the limited separation efficiency of photogenerated charge carriers can be solved either by using CdS in the form of QDs due to a shorter transportation path or by incorporating CdS onto support materials, such as TiO2 [55,56].
Rao et al. [137] synthesized CdS/TiO2 core/shell nanorods with tunable shell thickness to minimize charge carriers recombination and limit photocorrosion. The investigation of photocatalytic activity performed under UV-vis light irradiation confirmed that optimized concentration of sacrificial agents (0.3 M Na2S and Na2SO4 aqueous solution), shell thickness of 6.3 nm, and solution pH of 8.0 enhance the H2 production rate of 5791 mL/h/g. Du et al. [138], who fabricated pyramid-like CdS nanoparticles that were grown on porous TiO2, obtained the same type of composite, but with the different morphology. Under UV-vis irradiation and without noble-metal co-catalysts, 5 mol% CdS-TiO2 achieved an H2 production rate of 1048.7 μmol/h/g, which is almost six times and 1.5 times higher than that of pure TiO2 and CdS, respectively. Table 10 provides further examples of TiO2/CdS-based composites with their respective photocatalytic activities for H2 production.
Table 10.
The photocatalytic performance of H2 generation in some related TiO2/CdS-based nanocomposites.
| Photocatalyst | Light Source | HER | Reference |
|---|---|---|---|
| CdS/Pt/TiO2 film | 300 W Xe lamp | 3.074 µmol/h/g | [54] |
| CdS/Pt/TiO2 nanosheets | 350 W Xe arc lamp | 265 µmol/h | [141] |
| CdS/Pt/TiO2 nanotubes | 300 W Xe lamp | 402 µmol/h | [142] |
| CdS/TiO2 nanotubes | 350 W Xe lamp | 2585 µL/h/g | [143] |
| CdS-Ti-MCM-48-21-25 | 300 W Xe lamp | 2726 µmol/h | [139] |
3.3.2. TiO2/CuS
CuS has emerged as an alternative co-catalyst for H2 production, which is abundant, cheap, and nonhazardous. With its VB and CB at positions of −1.56 eV vs. NHE, and −0.09 eV, CuS shows low reflectance in the visible and relatively high reflectance in the near-infrared region, which makes it a good candidate for solar energy absorption [144]. Chandra et al. [144] investigated the photocatalytic activity for H2 generation of synthesized CuS/TiO2 (CT) heterostructured nanocomposite under UV-vis and only visible light. Under irradiation, the highest HER of 12362 μmol/h/g was achieved while using 0.4 mol.% CuS/TiO2, while only 155 μmol/h/g of H2 was produced under visible light and comparable conditions.
In comparison, Dang et al. [145] produced a series of CuxS (x = 1 or 2) co-modified TiO2 nanocomposites while using a one-step precipitation approach. The EDS, XPS, and XRF results confirmed the existence of three different phases: CuS, Cu2S, and TiO2. The results have shown that CuS and Cu2S dual co-catalysts under simulated solar light exhibited high H2 production of 5620 µmol/h/g, which is about 58 times higher than that of the unloaded TiO2. The enhancement in H2 production can be contributed to the co-deposition of CuS and Cu2S onto the TiO2 nanoparticle surface that efficiently extended visible light absorption and facilitated the separation of charge carriers. The CuxS/TiO2 composite showed high stability; after three consecutive cycles the photocatalytic efficiency for H2 production decreased for only 11.7%.
3.3.3. TiO2/MoS2
MoS2 represents two-dimensional transition metal dichalcogenide (TMD) that can be prepared into ultrathin-layered structures and, thus, participates in the H2 evolution reaction as an effective non-noble metal alternative [117,146]. Without the use of any sacrificial agent and co-catalyst, Huang et al. [146] observed photocatalytic H2 generation while using MoS2 quantum dots@TiO2 nanotube arrays nanocomposite in pure water under visible light. The photocatalytic activity was influenced by the amount of MoS2 QDs coated on TiO2 NTAs. Hence, the maximum of 53.9 μmol/cm2/h of H2 was produced, which was ascribed to the decreased bandgap and the surface plasmonic properties of the obtained composite promoting charge carrier separation and the absorption capacity to visible light. Du et al. [63] grew in situ two transition metal chalcogenides—MoS2 and CdS—on porous TiO2 by using the sol-gel method, followed by the calcination and hydrothermal method. Under visible light irradiation and without the use of noble metals as the co-catalyst, 3% MoS2-CdS-TiO2 produced 4146 μmol/h/g of H2. In this ternary composite (Figure 15), the porous structure of TiO2 accepts generated e− from CdS and provides surface area for H2 production, while MoS2, as a conductive medium, enabled the transfer of e− between CB of CdS and TiO2, simultaneously inhibiting the photocorrosion of CdS as h+ collector.
Figure 15.
The proposed photocatalytic mechanism in MoS2-CdS-TiO2 photocatalyst.
3.4. Multiple TiO2-Based Composites
This section is dedicated to multiple ternary and quaternary TiO2-based composites. Recently, significant emphasis was laid on the formation of Z-scheme structured photocatalysts. In such materials, redox reactions occur in each semiconductor, allowing for the combination of a semiconductor with strong reduction power with another semiconductor with strong oxidation power [93].
All of the solid Z-scheme heterojunctions are usually composed of two photocatalysts and an electron mediator, which enable efficient H2 production through the synergistic action between two isolated photosystems and electron mediator cleverly arranged in a nano-platform [147]. Reversible redox couples (e.g., IO3−/I−, Fe3+/Fe2+) are usually applied as electron mediators in the Z-scheme system. Solid electron mediators are more suitable for the application. Noble metals (Au, Ag) and carbon-based materials (MWCNT, rGO, CQDs) are commonly used as solid electron mediators for photocatalytic H2 generation [148].
Ng et al. [147], who synthesized Zn0.5Cd0.5S-MWCNT-TiO2 ternary nanocomposite, where MWCNT acted as an electron mediator, fabricated a solid Z-scheme system. The obtained material efficiently suppressed charge recombination and promoted water reduction, achieving HER of 21.9 µmol/h. Furthermore, Liu et al. [149] have used carbon quantum dots (QDs) as an electron mediator between TiO2 and Zn0.5Cd0.5S film and achieved 38740 µmol/h/m2 of produced H2 under solar light. Lv et al. investigated the use of rGO as an electron mediator [120], synthesizing sandwich-like TiO2/rGO/LaFeO3 ternary heterostructure, which, under solar light, obtained HER of 893 µmol/h/g, which is almost 3.2, 14.4, and 11.4 times superior to the direct Z-scheme components TiO2/LaFeO3 composite, pure TiO2, and LaFeO3, respectively. The photocatalytic mechanism of H2 production is the same for all three above mentioned solid electron mediators: MWCNT, rGO, and CQDs (Figure 16). Upon excitation by light, e−/h+ are formed in Z-scheme semiconducting components, depending on the ability of each component to absorb emitted light. Hence, photogenerated e− from semiconductor I can be easily recombined with h+ from semiconductor II through the electron mediator, leaving more oxidative holes and reductive electrons to participate in the redox reactions in the corresponding active sites [148].
Figure 16.
The type Z-heterojunction with the use of different solid electron mediators (multiwall carbon nanotubes (MWCNTs), carbon quantum dots (CQDs), rGO).
In Z-type heterojunction, noble metals can also perform the role of electron mediators. Zou et al. performed the construction of g-C3N4/Au/C-TiO2 hollow spheres with Au nanoparticles (NPs) as the electron mediator [150]. The as-prepared composite showed high HER of 129 µmol/h/g under visible light, which can be attributed to the efficient charge separation in the constructed Z-scheme system, the broadened visible-light response range, owing to the surface plasmon resonance (SPR) effects on Au nanoparticles, and the hollow structure of C-TiO2 that gives photocatalyst unique properties of low density and high light-harvesting efficiency. In another work presented by Yang et al. [151], Au NPs were applied as a solid electron mediator in the ternary urchin-like ZnIn2S4-Au-TiO2 nanocomposite, which, at an optimal ratio of 24 wt.% Au NPs and 60 wt.% ZnIn2S4, achieved HER of 186.3 µmol/h/g under solar light irradiation.
Table 11 lists other multiple composites, except ones with the Z-scheme heterojunction that are already mentioned, as well as reaction conditions in the photocatalytic system and the obtained hydrogen evolution rates.
Table 11.
Multiple TiO2-based composites for the use in photocatalytic hydrogen generation.
| Photocatalyst | Reaction Conditions | HER | Reference |
|---|---|---|---|
| CdSQDs/WC/TiO2 | Photocatalyst dispersed in 20 vol.% lactic acid as an electron donor under visible light. | 624.9 µmol/h/ | [55] |
| ZnO/ZnCr2O4@TiO2-NTA | Photocatalyst dispersed in aqueous methanol solution under simulated solar light. | 1680 µmol/cm2 | [152] |
| F-TiO2/CdSe-DETA | Photocatalyst was dispersed in a mixed solution of Na2S and Na2SO3 as a sacrificial agents under visible light with the use of Pt as a cocatalyst. | 12381 µmol/h/g | [153] |
| g-C3N4/TiO2/RGO | 5 mg of the g-C3N4-TiO2/RGO nano-composite was dispersed in 50 mL glycerol-water solution under UV-vis light. | 19610 µmol/h/g | [13] |
| CDS/CNF/Pt-TiO2 | Photocatalyst was dispersed in a mixed solution of Na2S and Na2SO3 as a sacrificial agents under visible light. | 16.34 µmol for 3 h | [154] |
| F-TiO2/CdS-DETA, Pt as a cocatalyst | 50 mg of the photocatalyst was dispersed in 100 mL of mixed aqueous solution containing 0.35 mg/L Na2S and 0.25 mg/L Na2SO3 with the use of Pt as a cocatalyst. |
5342.86 µmol/h/g | [155] |
| CdS@TiO2@Au | 20 mg of the photocatalyst was dispersed in 40 ml of aqueous solution containing 0.1 M Na2S and 0.1 M Na2SO3 as the sacrificial agents under visible light. |
1720 µmol/h/g | [156] |
| TiO2-Au-CdS | 0.1 g of the sample was immersed in an aqueous solution containing 0.1 M Na2S and 0.1 M Na2SO3 as the sacrificial agents under visible light. | 1810 µmol/h/g | [157] |
| N-TiO2/g-C3N4@NixP | 50 mg photocatalyst was suspended in a 100 mL solution containing 10 vol.% triethanolamine (TEOA) under 300 W Xe lamp irradiation. | 5438 µmol/h/g | [158] |
| TiO2/CdS/CNT | 0.1 g of photocatalyst was dispersed in solution containing 70 mL of distilled water or seawater and 30 mL of sacrificial agent. The photocatalyst were irradiated using three visible-light sunlamps of each 100 W and UV lamp of 8 W. | 3502 µmol/h from pure water and 1373 µmol/h from seawater | [159] |
| WS2/ C-TiO2/ g-C3N4 | 50 mg of photocatalyst was added in to 80 mL TEOA aqueous solution with the use of Pt as a cocatalyst. | 17726 for DI water and 29978 µmol/g for seawater | [148] |
| TiO2/La2O2CO3/rGO | 0.05 g of powder catalyst was dispersed in 80 Ml ethylene-glycol water (5/95, v/v) solution under UV-vis light. | 583 µmol/h | [160] |
| TiO2-Cu@C | Photocatalyst was dispersed in methanol aqueous solution under UV-vis light. | 3911 µmol/g/h | [161] |
4. Conclusions
TiO2-based photocatalytic technology represents a promising up-coming technology for both renewable energy generation and water purification applications. It is necessary to work on developing TiO2-based semiconductor materials, which are active under broader spectrum of solar light, overcoming the indicated disadvantages of the solely TiO2 utilization, since TiO2 as a photocatalytic material cannot be used alone due to its limitations such as activity only in UV light region and rapid recombination of photogenerated charge carriers.
TiO2-semiconductor coupling offers promising results in water purification, particularly for the degradation and mineralization of CECs. However, it is necessary to evaluate the toxicities of degradation intermediates of CEC to check the real efficiency of such composites. Furthermore, the immobilization of TiO2–Semiconductor composites to photocatalytic reaction membranes must be envisaged for further upscale opportunities.
Great progress has also been recorded in the development of TiO2-based heterojunction for the application in solar-driven photocatalytic hydrogen generation. By morphological control of the obtained composites, higher H2 generation, as well as better light harvesting can be achieved. It is noticed that still a great deal of research is being conducted by the use of different noble-metal co-catalysts and sacrificial agents, which, although increasing the efficiency of the process, reduces its environmental friendliness and increases the performance cost. Accordingly, to allow for the practical deployment of such units, as well as commercialization, it is necessary to produce cost-effective systems that will consider economic impact assessment, including operation cost and energy consumption, and that will not require the use of costly co-catalysts and other substances that promote system performance.
Author Contributions
Conceptualization, H.K., F.F., and A.L.B.; writing—original draft preparation, K.P., F.M.d.R., H.K.; writing—review and editing, M.K., U.L.Š., D.D.D., F.F. and A.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by projects: “Nano-sized Solar-active Catalysts for Environmental Technologies” (NaSCEnT, IP-2018-01-1982), Croatian Science Foundation, and “Water Purification and Energy Conversion using Novel Composite Materials and Solar Irradiation” (KK.01.1.1.04.0001), European Structural and Investment Funds (Croatia)
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.European Commission Directive 2000/60/EC of the European Parliament and of the Council, of 23 October 2000. Off. J. Eur. Communities. 2000;327:1–72. [Google Scholar]
- 2.Water JPI. [(accessed on 12 December 2019)]; Available online: http://www.waterjpi.eu/mapping-agenda/strategic-re.
- 3.European Commission Energy Strategy. [(accessed on 12 December 2019)]; Available online: https://ec.europa.eu/energy/en/topics/energy-strategy.
- 4.Martin M. Alternative Energy Sources and Technologies. Springer; Basel, Switzerland: 2016. [Google Scholar]
- 5.Hasan M.M., Allam N.K. Unbiased spontaneous solar hydrogen production using stable TiO2-CuO composite nanofiber photocatalysts. RSC Adv. 2018;8:37219–37228. doi: 10.1039/C8RA06763E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujishima A., Honda K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 7.Frank S.N., Bard A.J. Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at TiO2. J. Am. Chem. Soc. 1977;99:303–304. doi: 10.1021/ja00443a081. [DOI] [Google Scholar]
- 8.Turchi C.S., Ollis D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990;122:178–192. doi: 10.1016/0021-9517(90)90269-P. [DOI] [Google Scholar]
- 9.Chong M.N., Jin B., Chow C.W.K., Saint C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010;44:2997–3027. doi: 10.1016/j.watres.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Pelaez M., Nolan N.T., Pillai S.C., Seery M.K., Falaras P., Kontos A.G., Dunlop P.S.M., Hamilton J.W.J., Byrne J.A., O’Shea K., et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012;125:331–349. doi: 10.1016/j.apcatb.2012.05.036. [DOI] [Google Scholar]
- 11.Jafari T., Moharreri E., Amin A.S., Miao R., Song W., Suib S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules. 2016;21:900. doi: 10.3390/molecules21070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing D., Guo L., Zhao L., Zhang X., Liu H., Li M., Shen S., Liu G., Hu X., Zhang X., et al. Efficient solar hydrogen production by photocatalytic water splitting: From fundamental study to pilot demonstration. Int. J. Hydrogen Energy. 2010;35:7087–7097. doi: 10.1016/j.ijhydene.2010.01.030. [DOI] [Google Scholar]
- 13.Hafeez H.Y., Lakhera S.K., Bellamkonda S., Rao G.R., Shankar M.V., Bahnemann D.W., Neppolian B. Construction of ternary hybrid layered reduced graphene oxide supported g-C3N4-TiO2 nanocomposite and its photocatalytic hydrogen production activity. Int. J. Hydrogen Energy. 2018;43:3892–3904. doi: 10.1016/j.ijhydene.2017.09.048. [DOI] [Google Scholar]
- 14.Schneider J., Matsuoka M., Takeuchi M., Zhang J., Horiuchi Y., Anpo M., Bahnemann D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014;114:9919–9986. doi: 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- 15.Pichat P. Photocatalysis and Water Purification: From Fundamentals to Recent Applications. Wiley; Weinheim, Germany: 2013. [Google Scholar]
- 16.Fagan R., McCormack D.E., Dionysiou D.D., Pillai S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016;42:2–14. doi: 10.1016/j.mssp.2015.07.052. [DOI] [Google Scholar]
- 17.Qi K., Cheng B., Yu J., Ho W. A review on TiO2 -based Z-scheme photocatalysts. Chin. J. Catal. 2017;38:1936–1955. doi: 10.1016/S1872-2067(17)62962-0. [DOI] [Google Scholar]
- 18.Calvete M.J.F., Piccirillo G., Vinagreiro C.S., Pereira M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coord. Chem. Rev. 2019;395:63–85. doi: 10.1016/j.ccr.2019.05.004. [DOI] [Google Scholar]
- 19.Tong H., Ouyang S., Bi Y., Umezawa N., Oshikiri M., Ye J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012;24:229–251. doi: 10.1002/adma.201102752. [DOI] [PubMed] [Google Scholar]
- 20.Pasternak S., Paz Y. On the similarity and dissimilarity between photocatalytic water splitting and photocatalytic degradation of pollutants. ChemPhysChem. 2013;14:2059–2070. doi: 10.1002/cphc.201300247. [DOI] [PubMed] [Google Scholar]
- 21.Wang H., Zhang L., Chen Z., Hu J., Li S., Wang Z., Liu J., Wang X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014;43:5234–5244. doi: 10.1039/C4CS00126E. [DOI] [PubMed] [Google Scholar]
- 22.Moniz S.J.A., Shevlin S.A., Martin D.J., Guo Z.X., Tang J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015;8:731–759. doi: 10.1039/C4EE03271C. [DOI] [Google Scholar]
- 23.Moniz S.J.A., Shevlin S.A., An X., Guo Z.X., Tang J. Fe2O3-TiO2 nanocomposites for enhanced charge separation and photocatalytic activity. Chem. A Eur. J. 2014;20:15571–15579. doi: 10.1002/chem.201403489. [DOI] [PubMed] [Google Scholar]
- 24.Macías-Tamez R., Villanueva-Rodríguez M., Ramos-Delgado N.A., Maya-Treviño L., Hernández-Ramírez A. Comparative Study of the Photocatalytic Degradation of the Herbicide 2,4-D Using WO3/TiO2 and Fe2O3/TiO2 as Catalysts. Water Air Soil Pollut. 2017;228:379. doi: 10.1007/s11270-017-3560-9. [DOI] [Google Scholar]
- 25.Luo L., Long J., Zhao S., Dai J., Ma L., Wang H., Xia L., Shu L., Jiang F. Effective visible-light-driven photocatalytic degradation of 17α-ethynylestradiol by crosslinked CdS nano-rod/TiO2 (B) nano-belt composite. Process Saf. Environ. Prot. 2019;130:77–85. doi: 10.1016/j.psep.2019.08.001. [DOI] [Google Scholar]
- 26.Marschall R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014;24:2421–2440. doi: 10.1002/adfm.201303214. [DOI] [Google Scholar]
- 27.Kaur A., Salunke D.B., Umar A., Mehta S.K., Sinha A.S.K., Kansal S.K. Visible light driven photocatalytic degradation of fluoroquinolone levofloxacin drug using Ag2 O/TiO2 quantum dots: A mechanistic study and degradation pathway. New J. Chem. 2017;41:12079–12090. doi: 10.1039/C7NJ02053H. [DOI] [Google Scholar]
- 28.Gou J., Ma Q., Deng X., Cui Y., Zhang H., Cheng X., Li X., Xie M., Cheng Q. Fabrication of Ag2O/TiO2-Zeolite composite and its enhanced solar light photocatalytic performance and mechanism for degradation of norfloxacin. Chem. Eng. J. 2017;308:818–826. doi: 10.1016/j.cej.2016.09.089. [DOI] [Google Scholar]
- 29.Dong P., Hou G., Xi X., Shao R., Dong F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano. 2017;4:539–557. doi: 10.1039/C6EN00478D. [DOI] [Google Scholar]
- 30.Mugunthan E., Saidutta M.B., Jagadeeshbabu P.E. Visible light assisted photocatalytic degradation of diclofenac using TiO2-WO3 mixed oxide catalysts. Environ. Nanotechnol. Monit. Manag. 2018;10:322–330. [Google Scholar]
- 31.Cordero-García A., Turnes Palomino G., Hinojosa-Reyes L., Guzmán-Mar J.L., Maya-Teviño L., Hernández-Ramírez A. Photocatalytic behaviour of WO3/TiO2-N for diclofenac degradation using simulated solar radiation as an activation source. Environ. Sci. Pollut. Res. 2017;24:4613–4624. doi: 10.1007/s11356-016-8157-0. [DOI] [PubMed] [Google Scholar]
- 32.Cordero-García A., Guzmán-Mar J.L., Hinojosa-Reyes L., Ruiz-Ruiz E., Hernández-Ramírez A. Effect of carbon doping on WO3/TiO2 coupled oxide and its photocatalytic activity on diclofenac degradation. Ceram. Int. 2016;42:9796–9803. doi: 10.1016/j.ceramint.2016.03.073. [DOI] [Google Scholar]
- 33.Pérez-Estrada L.A., Malato S., Gernjak W., Agüera A., Thurman E.M., Ferrer I., Fernández-Alba A.R. Photo-fenton degradation of diclofenac: Identification of main intermediates and degradation pathway. Environ. Sci. Technol. 2005;39:8300–8306. doi: 10.1021/es050794n. [DOI] [PubMed] [Google Scholar]
- 34.Salaeh S., Juretic Perisic D., Biosic M., Kusic H., Babic S., Lavrencic Stangar U., Dionysiou D.D., Loncaric Bozic A. Diclofenac removal by simulated solar assisted photocatalysis using TiO2-based zeolite catalyst; mechanisms, pathways and environmental aspects. Chem. Eng. J. 2016;304:289–302. doi: 10.1016/j.cej.2016.06.083. [DOI] [Google Scholar]
- 35.Arce-Sarria A., Machuca-Martínez F., Bustillo-Lecompte C., Hernández-Ramírez A., Colina-Márquez J. Degradation and loss of antibacterial activity of commercial amoxicillin with TiO2/WO3-assisted solar photocatalysis. Catalysts. 2018;8:222. doi: 10.3390/catal8060222. [DOI] [Google Scholar]
- 36.Mishra M., Chun D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015;498:126–141. doi: 10.1016/j.apcata.2015.03.023. [DOI] [Google Scholar]
- 37.Mirmasoomi S.R., Mehdipour Ghazi M., Galedari M. Photocatalytic degradation of diazinon under visible light using TiO2/Fe2O3 nanocomposite synthesized by ultrasonic-assisted impregnation method. Sep. Purif. Technol. 2017;175:418–427. doi: 10.1016/j.seppur.2016.11.021. [DOI] [Google Scholar]
- 38.Li R., Liu J., Jia Y., Zhen Q. Photocatalytic degradation mechanism of oxytetracyclines using Fe2O3-TiO2 nanopowders. J. Nanosci. Nanotechnol. 2017;17:3010–3015. doi: 10.1166/jnn.2017.13076. [DOI] [Google Scholar]
- 39.Li R., Jia Y., Wu J., Zhen Q. Photocatalytic degradation and pathway of oxytetracycline in aqueous solution by Fe2O3-TiO2 nanopowder. RSC Adv. 2015;5:40764–40771. doi: 10.1039/C5RA04540A. [DOI] [Google Scholar]
- 40.Lu C., Guan W., Zhang G., Ye L., Zhou Y., Zhang X. TiO2 /Fe2O3/CNTs magnetic photocatalyst: A fast and convenient synthesis and visible-light-driven photocatalytic degradation of tetracycline. Micro Nano Lett. 2013;8:749–752. doi: 10.1049/mnl.2013.0428. [DOI] [Google Scholar]
- 41.Zheng X., Fu W., Kang F., Peng H., Wen J. Enhanced photo-Fenton degradation of tetracycline using TiO2 -coated α-Fe2O3 core–shell heterojunction. J. Ind. Eng. Chem. 2018;68:14–23. doi: 10.1016/j.jiec.2018.07.024. [DOI] [Google Scholar]
- 42.Casbeer E., Sharma V.K., Li X.Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012;87:1–14. doi: 10.1016/j.seppur.2011.11.034. [DOI] [Google Scholar]
- 43.Garcia-Muñoz P., Fresno F., de la Peña O’Shea V.A., Keller N. Ferrite Materials for Photoassisted Environmental and Solar Fuels Applications. Top. Curr. Chem. 2020;378:6. doi: 10.1007/s41061-019-0270-3. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Wu Q., Wang J., Song Y. The fabrication of magnetic recyclable nitrogen-doped titanium dioxide/calcium ferrite/diatomite heterojunction nanocomposite for improved visible-light-driven degradation of tetracycline. J. Chem. Technol. Biotechnol. 2019;94:2702–2712. doi: 10.1002/jctb.6082. [DOI] [Google Scholar]
- 45.Chen Y., Liu K. Fabrication of magnetically recyclable Ce/N co-doped TiO2/NiFe2O4/diatomite ternary hybrid: Improved photocatalytic efficiency under visible light irradiation. J. Alloys Compd. 2017;697:161–173. doi: 10.1016/j.jallcom.2016.12.153. [DOI] [Google Scholar]
- 46.Wu Q., Zhang Z. The fabrication of magnetic recyclable nitrogen modified titanium dioxide/strontium ferrite/diatomite heterojunction nanocomposite for enhanced visible-light-driven photodegradation of tetracycline. Int. J. Hydrogen Energy. 2019;44:8261–8272. doi: 10.1016/j.ijhydene.2019.01.145. [DOI] [Google Scholar]
- 47.Nguyen T.B., Doong R.A. Heterostructured ZnFe2O4/TiO2 nanocomposites with a highly recyclable visible-light-response for bisphenol a degradation. RSC Adv. 2017;7:50006–50016. doi: 10.1039/C7RA08271A. [DOI] [Google Scholar]
- 48.Shi Y., Yang Z., Wang B., An H., Chen Z., Cui H. Adsorption and photocatalytic degradation of tetracycline hydrochloride using a palygorskite-supported Cu2O-TiO2 composite. Appl. Clay Sci. 2016;119:311–320. doi: 10.1016/j.clay.2015.10.033. [DOI] [Google Scholar]
- 49.Hu Z., Wang X., Dong H., Li S., Li X., Li L. Efficient photocatalytic degradation of tetrabromodiphenyl ethers and simultaneous hydrogen production by TiO2-Cu2O composite films in N2 atmosphere: Influencing factors, kinetics and mechanism. J. Hazard. Mater. 2017;340:1–15. doi: 10.1016/j.jhazmat.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Sud D., Syal A. Investigations on the Phase Transformation, Optical Characteristics, and Photocatalytic Activity of Synthesized Heterostructured Nanoporous Bi2O3–TiO2. J. Chin. Chem. Soc. 2016;63:776–783. doi: 10.1002/jccs.201600099. [DOI] [Google Scholar]
- 51.Sood S., Mehta S.K., Sinha A.S.K., Kansal S.K. Bi2O3/TiO2 heterostructures: Synthesis, characterization and their application in solar light mediated photocatalyzed degradation of an antibiotic, ofloxacin. Chem. Eng. J. 2016;290:45–52. doi: 10.1016/j.cej.2016.01.017. [DOI] [Google Scholar]
- 52.Kaur A., Umar A., Anderson W.A., Kansal S.K. Facile synthesis of CdS/TiO2 nanocomposite and their catalytic activity for ofloxacin degradation under visible illumination. J. Photochem. Photobiol. A Chem. 2018;360:34–43. doi: 10.1016/j.jphotochem.2018.04.021. [DOI] [Google Scholar]
- 53.Kušić H., Leszczynska D. Altered toxicity of organic pollutants in water originated from simultaneous exposure to UV photolysis and CdSe/ZnS quantum dots. Chemosphere. 2012;89:900–906. doi: 10.1016/j.chemosphere.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Ning X., Li J., Yang B., Zhen W., Li Z., Tian B., Lu G. Inhibition of photocorrosion of CdS via assembling with thin film TiO2 and removing formed oxygen by artificial gill for visible light overall water splitting. Appl. Catal. B Environ. 2017;212:129–139. doi: 10.1016/j.apcatb.2017.04.074. [DOI] [Google Scholar]
- 55.Pan Y.X., Zhou T., Han J., Hong J., Wang Y., Zhang W., Xu R. CdS quantum dots and tungsten carbide supported on anatase-rutile composite TiO2 for highly efficient visible-light-driven photocatalytic H2 evolution from water. Catal. Sci. Technol. 2016;6:2206–2213. doi: 10.1039/C5CY01634G. [DOI] [Google Scholar]
- 56.Zhu R., Yang R., Hu L., Chen B. Preparation of Z-Scheme system of CdS-RGO-BiVO4 and its activity for hydrogen production. Int. J. Hydrogen Energy. 2019;44:25119–25128. doi: 10.1016/j.ijhydene.2019.06.129. [DOI] [Google Scholar]
- 57.Kandi D., Behera A., Martha S., Naik B., Parida K.M. Quantum confinement chemistry of CdS QDs plus hot electron of Au over TiO2 nanowire protruding to be encouraging photocatalyst towards nitrophenol conversion and ciprofloxacin degradation. J. Environ. Chem. Eng. 2019;7:102821. doi: 10.1016/j.jece.2018.102821. [DOI] [Google Scholar]
- 58.Li W., Ding H., Ji H., Dai W., Guo J., Du G. Photocatalytic degradation of tetracycline hydrochloride via a CdS-TiO2 heterostructure composite under visible light irradiation. Nanomaterials. 2018;8:415. doi: 10.3390/nano8060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang Y., Zhang M., Xin Y., Chai C., Chen Q. Construction of immobilized CuS/TiO2 nanobelts heterojunction photocatalyst for photocatalytic degradation of enrofloxacin: Synthesis, characterization, influencing factors and mechanism insight. J. Chem. Technol. Biotechnol. 2019;94:2219–2228. [Google Scholar]
- 60.Chen Q., Wu S., Xin Y. Synthesis of Au-CuS-TiO2 nanobelts photocatalyst for efficient photocatalytic degradation of antibiotic oxytetracycline. Chem. Eng. J. 2016;302:377–387. doi: 10.1016/j.cej.2016.05.076. [DOI] [Google Scholar]
- 61.Irandost M., Akbarzadeh R., Pirsaheb M., Asadi A., Mohammadi P., Sillanpää M. Fabrication of highly visible active N, S co-doped TiO2@MoS2 heterojunction with synergistic effect for photocatalytic degradation of diclofenac: Mechanisms, modeling and degradation pathway. J. Mol. Liq. 2019;291:111342. doi: 10.1016/j.molliq.2019.111342. [DOI] [Google Scholar]
- 62.Wu L., Shi S., Li Q., Zhang X., Cui X. TiO2 nanoparticles modified with 2D MoSe2 for enhanced photocatalytic activity on hydrogen evolution. Int. J. Hydrogen Energy. 2019;44:720–728. doi: 10.1016/j.ijhydene.2018.10.214. [DOI] [Google Scholar]
- 63.Du J., Wang H., Yang M., Zhang F., Wu H., Cheng X., Yuan S., Zhang B., Li K., Wang Y., et al. Highly efficient hydrogen evolution catalysis based on MoS2/CdS/TiO2 porous composites. Int. J. Hydrogen Energy. 2018;43:9307–9315. doi: 10.1016/j.ijhydene.2018.03.208. [DOI] [Google Scholar]
- 64.Kumar N., Bhadwal A.S., Mizaikoff B., Singh S., Kranz C. Electrochemical detection and photocatalytic performance of MoS2/TiO2 nanocomposite against pharmaceutical contaminant: Paracetamol. Sens. Bio-Sens. Res. 2019;24:100288. doi: 10.1016/j.sbsr.2019.100288. [DOI] [Google Scholar]
- 65.Deng L., Liu H., Gao X., Su X., Zhu Z. SnS2/TiO2 nanocomposites with enhanced visible light-driven photoreduction of aqueous Cr(VI) Ceram. Int. 2016;42:3808–3815. doi: 10.1016/j.ceramint.2015.11.043. [DOI] [Google Scholar]
- 66.Kovacic M., Kopcic N., Kusic H., Bozic A.L. Solar driven degradation of 17β-estradiol using composite photocatalytic materials and artificial irradiation source: Influence of process and water matrix parameters. J. Photochem. Photobiol. A Chem. 2018;361:48–61. doi: 10.1016/j.jphotochem.2018.05.015. [DOI] [Google Scholar]
- 67.Kovacic M., Papac J., Kusic H., Karamanis P., Loncaric Bozic A. Degradation of polar and non-polar pharmaceutical pollutants in water by solar assisted photocatalysis using hydrothermal TiO2-SnS2. Chem. Eng. J. 2019;382:122826. doi: 10.1016/j.cej.2019.122826. [DOI] [Google Scholar]
- 68.Yan T., Guan W., Li W., You J. Ag3PO4 photocatalysts loaded on uniform SiO2 supports for efficient degradation of methyl orange under visible light irradiation. RSC Adv. 2014;4:37095–37099. doi: 10.1039/C4RA06135G. [DOI] [Google Scholar]
- 69.Yi Z., Ye J., Kikugawa N., Kako T., Ouyang S., Stuart-Williams H., Yang H., Cao J., Luo W., Li Z., et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010;9:559–564. doi: 10.1038/nmat2780. [DOI] [PubMed] [Google Scholar]
- 70.Ren J., Chai Y., Liu Q., Zhang L., Dai W.L. Intercorrelated Ag3PO4 nanoparticles decorated with graphic carbon nitride: Enhanced stability and photocatalytic activities for water treatment. Appl. Surf. Sci. 2017;403:177–186. doi: 10.1016/j.apsusc.2017.01.172. [DOI] [Google Scholar]
- 71.Meng S., Ning X., Zhang T., Chen S.F., Fu X. What is the transfer mechanism of photogenerated carriers for the nanocomposite photocatalyst Ag3PO4/g-C3N4, band-band transfer or a direct Z-scheme? Phys. Chem. Chem. Phys. 2015;17:11577–11585. doi: 10.1039/C5CP01523E. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Yu H., Lu Y., Qu J., Zhu S., Huo M. A nano-composite comprised of Ti3+ -doped TiO2 nanotubes and Ag3PO4 quantum dots with enhanced photocatalytic activity under visible light. Mater. Lett. 2019;240:35–38. doi: 10.1016/j.matlet.2018.12.075. [DOI] [Google Scholar]
- 73.Du J., Ma S., Yan Y., Li K., Zhao F., Zhou J. Corn-silk-templated synthesis of TiO2 nanotube arrays with Ag3PO4 nanoparticles for efficient oxidation of organic pollutants and pathogenic bacteria under solar light. Colloids Surf. A Physicochem. Eng. Asp. 2019;572:237–249. doi: 10.1016/j.colsurfa.2019.04.018. [DOI] [Google Scholar]
- 74.Liu J., Xie F., Li R., Li T., Jia Z., Wang Y., Wang Y., Zhang X., Fan C. TiO2-x /Ag3PO4 photocatalyst: Oxygen vacancy dependent visible light photocatalytic performance and BPA degradative pathway. Mater. Sci. Semicond. Process. 2019;97:1–10. doi: 10.1016/j.mssp.2019.03.002. [DOI] [Google Scholar]
- 75.Police A.K.R., Chennaiahgari M., Boddula R., Vattikuti S.V.P., Mandari K.K., Chan B. Single-step hydrothermal synthesis of wrinkled graphene wrapped TiO2 nanotubes for photocatalytic hydrogen production and supercapacitor applications. Mater. Res. Bull. 2018;98:314–321. doi: 10.1016/j.materresbull.2017.10.034. [DOI] [Google Scholar]
- 76.Lu Y., Ma B., Yang Y., Huang E., Ge Z., Zhang T., Zhang S., Li L., Guan N., Ma Y., et al. High activity of hot electrons from bulk 3D graphene materials for efficient photocatalytic hydrogen production. Nano Res. 2017;10:1662–1672. doi: 10.1007/s12274-016-1390-5. [DOI] [Google Scholar]
- 77.Hao X., Li M., Zhang L., Wang K., Liu C. Photocatalyst TiO2/WO3/GO nano-composite with high efficient photocatalytic performance for BPA degradation under visible light and solar light illumination. J. Ind. Eng. Chem. 2017;55:140–148. doi: 10.1016/j.jiec.2017.06.038. [DOI] [Google Scholar]
- 78.Wang G., Chen Q., Xin Y., Liu Y., Zang Z., Hu C., Zhang B. Construction of graphene-WO3/TiO2 nanotube array photoelectrodes and its enhanced performance for photocatalytic degradation of dimethyl phthalate. Electrochim. Acta. 2016;222:1903–1913. doi: 10.1016/j.electacta.2016.11.182. [DOI] [Google Scholar]
- 79.Bilgin Simsek E., Kilic B., Asgin M., Akan A. Graphene oxide based heterojunction TiO2–ZnO catalysts with outstanding photocatalytic performance for bisphenol-A, ibuprofen and flurbiprofen. J. Ind. Eng. Chem. 2018;59:115–126. doi: 10.1016/j.jiec.2017.10.014. [DOI] [Google Scholar]
- 80.Feng J., Wang Y., Hou Y., Li L. Hierarchical structured ZnFe2O4@RGO@TiO2 composite as powerful visible light catalyst for degradation of fulvic acid. J. Nanopart. Res. 2017;19:178. doi: 10.1007/s11051-017-3842-6. [DOI] [Google Scholar]
- 81.Luo L.j., Li J., Dai J., Xia L., Barrow C.J., Wang H., Jegatheesan J., Yang M. Bisphenol A removal on TiO2–MoS2–reduced graphene oxide composite by adsorption and photocatalysis. Process Saf. Environ. Prot. 2017;112:274–279. doi: 10.1016/j.psep.2017.04.032. [DOI] [Google Scholar]
- 82.Wang W., Han Q., Zhu Z., Zhang L., Zhong S., Liu B. Enhanced photocatalytic degradation performance of organic contaminants by heterojunction photocatalyst BiVO4/TiO2/RGO and its compatibility on four different tetracycline antibiotics. Adv. Powder Technol. 2019;30:1882–1896. doi: 10.1016/j.apt.2019.06.006. [DOI] [Google Scholar]
- 83.Alcudia-Ramos M.A., Fuentez-Torres M.O., Ortiz-Chi F., Espinosa-González C.G., Hernández-Como N., García-Zaleta D.S., Kesarla M.K., Torres-Torres J.G., Collins-Martínez V., Godavarthi S. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. Ceram. Int. 2020;46:38–45. doi: 10.1016/j.ceramint.2019.08.228. [DOI] [Google Scholar]
- 84.Liu C., Wu P., Wu J., Hou J., Bai H., Liu Z. Effective protect of oxygen vacancies in carbon layer coated black TiO2-x/CNNS hetero-junction photocatalyst. Chem. Eng. J. 2019;359:58–68. doi: 10.1016/j.cej.2018.11.117. [DOI] [Google Scholar]
- 85.Yang Y., Li X., Lu C., Huang W. G-C3N4 Nanosheets Coupled with TiO2 Nanosheets as 2D/2D Heterojunction Photocatalysts Toward High Photocatalytic Activity for Hydrogen Production. Catal. Lett. 2019;140:2930–2939. doi: 10.1007/s10562-019-02805-8. [DOI] [Google Scholar]
- 86.Kong L., Zhang X., Wang C., Xu J., Du X., Li L. Ti3+ defect mediated g-C3N4 /TiO2 Z-scheme system for enhanced photocatalytic redox performance. Appl. Surf. Sci. 2018;448:288–296. doi: 10.1016/j.apsusc.2018.04.011. [DOI] [Google Scholar]
- 87.Wang J., Wang G., Wang X., Wu Y., Su Y., Tang H. 3D/2D direct Z-scheme heterojunctions of hierarchical TiO2 microflowers/g-C3N4 nanosheets with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon. 2019;149:618–626. doi: 10.1016/j.carbon.2019.04.088. [DOI] [Google Scholar]
- 88.Pan C., Jia J., Hu X., Fan J., Liu E. In situ construction of g-C3N4 /TiO2 heterojunction films with enhanced photocatalytic activity over magnetic-driven rotating frame. Appl. Surf. Sci. 2018;430:283–292. doi: 10.1016/j.apsusc.2017.07.189. [DOI] [Google Scholar]
- 89.Zou Y., Shi J.W., Ma D., Fan Z., Lu L., Niu C. In situ synthesis of C-doped TiO2@g-C3N4 core-shell hollow nanospheres with enhanced visible-light photocatalytic activity for H2 evolution. Chem. Eng. J. 2017;322:435–444. doi: 10.1016/j.cej.2017.04.056. [DOI] [Google Scholar]
- 90.Ma J., Zhou W., Tan X., Yu T. Potassium ions intercalated into g-C3N4-modified TiO2 nanobelts for the enhancement of photocatalytic hydrogen evolution activity under visible-light irradiation. Nanotechnology. 2018;29:215706. doi: 10.1088/1361-6528/aab564. [DOI] [PubMed] [Google Scholar]
- 91.Pan J., You M., Chi C., Dong Z., Wang B., Zhu M., Zhao W., Song C., Zheng Y., Li C. The two dimension carbon quantum dots modified porous g-C3N4/TiO2 nano-heterojunctions for visible light hydrogen production enhancement. Int. J. Hydrogen Energy. 2018;43:6586–6593. doi: 10.1016/j.ijhydene.2018.02.067. [DOI] [Google Scholar]
- 92.Wang X., Maeda K., Thomas A., Takanabe K., Xin G., Carlsson J.M., Domen K., Antonietti M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009;8:76–80. doi: 10.1038/nmat2317. [DOI] [PubMed] [Google Scholar]
- 93.Corredor J., Rivero M.J., Rangel C.M., Gloaguen F., Ortiz I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019;94:3049–3063. doi: 10.1002/jctb.6123. [DOI] [Google Scholar]
- 94.Wen J., Xie J., Chen X., Li X. A review on g-C3N4 -based photocatalysts. Appl. Surf. Sci. 2017;391:72–123. doi: 10.1016/j.apsusc.2016.07.030. [DOI] [Google Scholar]
- 95.Huang H., He M., Yang X., Tian Z., Hu J., Wen B. One-pot hydrothermal synthesis of TiO2/RCN heterojunction photocatalyst for production of hydrogen and rhodamine B degradation. Appl. Surf. Sci. 2019;493:202–211. doi: 10.1016/j.apsusc.2019.07.013. [DOI] [Google Scholar]
- 96.Yang Z., Yan J., Lian J., Xu H., She X., Li H. g-C3N4/TiO2 Nanocomposites for Degradation of Ciprofloxacin under Visible Light Irradiation. ChemistrySelect. 2016;1:5679–5685. doi: 10.1002/slct.201600861. [DOI] [Google Scholar]
- 97.Li G., Nie X., Gao Y., An T. Can environmental pharmaceuticals be photocatalytically degraded and completely mineralized in water using g-C3N4/TiO2 under visible light irradiation?-Implications of persistent toxic intermediates. Appl. Catal. B Environ. 2016;180:726–732. doi: 10.1016/j.apcatb.2015.07.014. [DOI] [Google Scholar]
- 98.Yu S., Wang Y., Sun F., Wang R., Zhou Y. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation. Chem. Eng. J. 2018;337:183–192. doi: 10.1016/j.cej.2017.12.093. [DOI] [Google Scholar]
- 99.Wang W., Fang J., Shao S., Lai M., Lu C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017;217:57–64. doi: 10.1016/j.apcatb.2017.05.037. [DOI] [Google Scholar]
- 100.Li C., Sun Z., Zhang W., Yu C., Zheng S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018;220:272–282. doi: 10.1016/j.apcatb.2017.08.044. [DOI] [Google Scholar]
- 101.Chen Y., Lu W., Shen H., Gu Y., Xu T., Zhu Z., Wang G., Chen W. Solar-driven efficient degradation of emerging contaminants by g-C3N4-shielding polyester fiber/TiO2 composites. Appl. Catal. B Environ. 2019;258:117960. doi: 10.1016/j.apcatb.2019.117960. [DOI] [Google Scholar]
- 102.Xie X., Chen C., Wang X., Li J., Naraginti S. Efficient detoxification of triclosan by a S-Ag/TiO2@g-C3N4 hybrid photocatalyst: Process optimization and bio-toxicity assessment. RSC Adv. 2019;9:20439–20449. doi: 10.1039/C9RA03279G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song J., Wu X., Zhang M., Liu C., Yu J., Sun G., Si Y., Ding B. Highly flexible, core-shell heterostructured, and visible-light-driven titania-based nanofibrous membranes for antibiotic removal and E. coil inactivation. Chem. Eng. J. 2020;379:122269. doi: 10.1016/j.cej.2019.122269. [DOI] [Google Scholar]
- 104.Yang L., Bai X., Shi J., Du X., Xu L., Jin P. Quasi-full-visible-light absorption by D35-TiO2/g-C3N4 for synergistic persulfate activation towards efficient photodegradation of micropollutants. Appl. Catal. B Environ. 2019;256:117759. doi: 10.1016/j.apcatb.2019.117759. [DOI] [Google Scholar]
- 105.Su Y., Chen P., Wang F., Zhang Q., Chen T., Wang Y., Yao K., Lv W., Liu G. Decoration of TiO2/g-C3N4 Z-scheme by carbon dots as a novel photocatalyst with improved visible-light photocatalytic performance for the degradation of enrofloxacin. RSC Adv. 2017;7:34096–34103. doi: 10.1039/C7RA05485H. [DOI] [Google Scholar]
- 106.Wang F., Chen P., Feng Y., Xie Z., Liu Y., Su Y., Zhang Q., Wang Y., Yao K., Lv W., et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017;207:103–113. doi: 10.1016/j.apcatb.2017.02.024. [DOI] [Google Scholar]
- 107.Nie Y.C., Yu F., Wang L.C., Xing Q.J., Liu X., Pei Y., Zou J.P., Dai W.L., Li Y., Suib S.L. Photocatalytic degradation of organic pollutants coupled with simultaneous photocatalytic H2 evolution over graphene quantum dots/Mn-N-TiO2/g-C3N4 composite catalysts: Performance and mechanism. Appl. Catal. B Environ. 2018;227:312–321. doi: 10.1016/j.apcatb.2018.01.033. [DOI] [Google Scholar]
- 108.Jo W.K., Adinaveen T., Vijaya J.J., Sagaya Selvam N.C. Synthesis of MoS2 nanosheet supported Z-scheme TiO2/g-C3N4 photocatalysts for the enhanced photocatalytic degradation of organic water pollutants. RSC Adv. 2016;6:10487–10497. [Google Scholar]
- 109.Tahir M.B., Sagir M., Shahzad K. Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. J. Hazard. Mater. 2019;363:205–213. doi: 10.1016/j.jhazmat.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 110.Schubert J.S., Popovic J., Haselmann G.M., Nandan S.P., Wang J., Giesriegl A., Cherevan A.S., Eder D. Immobilization of Co, Mn, Ni and Fe oxide co-catalysts on TiO2 for photocatalytic water splitting reactions. J. Mater. Chem. A. 2019;7:18568–18579. doi: 10.1039/C9TA05637H. [DOI] [Google Scholar]
- 111.Patil S.B., Basavarajappa P.S., Ganganagappa N., Jyothi M.S., Raghu A.V., Reddy K.R. Recent advances in non-metals-doped TiO2 nanostructured photocatalysts for visible-light driven hydrogen production, CO2 reduction and air purification. Int. J. Hydrogen Energy. 2019;44:13022–13039. doi: 10.1016/j.ijhydene.2019.03.164. [DOI] [Google Scholar]
- 112.Yang Q., Dong L., Su R., Hu B., Wang Z., Jin Y., Wang Y., Besenbacher F., Dong M. Nanostructured heterogeneous photo-catalysts for hydrogen production and water splitting: A comprehensive insight. Appl. Mater. Today. 2019;17:159–182. doi: 10.1016/j.apmt.2019.07.016. [DOI] [Google Scholar]
- 113.Bhagya T.C., Krishnan A., Arunima Rajan S., Ameen Sha M., Sreelekshmy B.R., Jineesh P., Shibli S.M.A. Exploration and evaluation of proton source-assisted photocatalyst for hydrogen generation. Photochem. Photobiol. Sci. 2019;18:1716–1726. doi: 10.1039/C9PP00119K. [DOI] [PubMed] [Google Scholar]
- 114.Hernández-Majalca B.C., Meléndez-Zaragoza M.J., Salinas-Gutiérrez J.M., López-Ortiz A., Collins-Martínez V. Visible-light photo-assisted synthesis of GO-TiO2 composites for the photocatalytic hydrogen production. Int. J. Hydrogen Energy. 2019;44:12381–12389. doi: 10.1016/j.ijhydene.2018.10.152. [DOI] [Google Scholar]
- 115.Wang D., Saleh N.B., Sun W., Park C.M., Shen C., Aich N., Peijnenburg W.J.G.M., Zhang W., Jin Y., Su C. Next-Generation Multifunctional Carbon–Metal Nanohybrids for Energy and Environmental Applications. Environ. Sci. Technol. 2019;53:7265–7287. doi: 10.1021/acs.est.9b01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu X., Ray R., Gu Y., Ploehn H.J., Gearheart L., Raker K., Scrivens W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004;126:12736–12737. doi: 10.1021/ja040082h. [DOI] [PubMed] [Google Scholar]
- 117.Rao V.N., Reddy N.L., Kumari M.M., Cheralathan K.K., Ravi P., Sathish M., Neppolian B., Reddy K.R., Shetti N.P., Prathap P., et al. Sustainable hydrogen production for the greener environment by quantum dots-based efficient photocatalysts: A review. J. Environ. Manag. 2019;248:109246. doi: 10.1016/j.jenvman.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 118.Yi L., Lan F., Li J., Zhao C. Efficient Noble-Metal-Free Co-NG/TiO2 Photocatalyst for H2 Evolution: Synergistic Effect between Single-Atom Co and N-Doped Graphene for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2018;6:12766–12775. doi: 10.1021/acssuschemeng.8b02001. [DOI] [Google Scholar]
- 119.Rabin N.N., Ohmagari H., Islam M.S., Karim M.R., Hayami S. A procession on photocatalyst for solar fuel production and waste treatment. J. Incl. Phenom. Macrocycl. Chem. 2019;94:263–281. doi: 10.1007/s10847-019-00889-8. [DOI] [Google Scholar]
- 120.Lv T., Wang H., Hong W., Wang P., Jia L. In situ self-assembly synthesis of sandwich-like TiO2/reduced graphene oxide/LaFeO3 Z-scheme ternary heterostructure towards enhanced photocatalytic hydrogen production. Mol. Catal. 2019;475:110497. doi: 10.1016/j.mcat.2019.110497. [DOI] [Google Scholar]
- 121.Iwase A., Ng Y.H., Ishiguro Y., Kudo A., Amal R. Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. J. Am. Chem. Soc. 2011;133:11054–11057. doi: 10.1021/ja203296z. [DOI] [PubMed] [Google Scholar]
- 122.Samal A., Das D.P. Transfiguring UV light active “metal oxides” to visible light active photocatayst by reduced graphene oxide hypostatization. Catal. Today. 2018;300:124–135. doi: 10.1016/j.cattod.2017.03.052. [DOI] [Google Scholar]
- 123.Ida S., Wilson P., Neppolian B., Sathish M., Karthik P., Ravi P. Ultrasonically aided selective stabilization of pyrrolic type nitrogen by one pot nitrogen doped and hydrothermally reduced Graphene oxide/Titania nanocomposite (N-TiO2/N-RGO) for H2 production. Ultrason. Sonochem. 2019;57:62–72. doi: 10.1016/j.ultsonch.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 124.Olowoyo J.O., Kumar M., Jain S.L., Babalola J.O., Vorontsov A.V., Kumar U. Insights into Reinforced Photocatalytic Activity of the CNT-TiO2 Nanocomposite for CO2 Reduction and Water Splitting. J. Phys. Chem. C. 2019;123:367–378. doi: 10.1021/acs.jpcc.8b07894. [DOI] [Google Scholar]
- 125.Umer M., Tahir M., Azam M.U., Tahir B., Jaffar M.M., Alias H. Montmorillonite dispersed single wall carbon nanotubes (SWCNTs)/TiO2 heterojunction composite for enhanced dynamic photocatalytic H2 production under visible light. Appl. Clay Sci. 2019;174:110–119. doi: 10.1016/j.clay.2019.03.029. [DOI] [Google Scholar]
- 126.Bellamkonda S., Thangavel N., Hafeez H.Y., Neppolian B., Ranga Rao G. Highly active and stable multi-walled carbon nanotubes-graphene-TiO2 nanohybrid: An efficient non-noble metal photocatalyst for water splitting. Catal. Today. 2019;321:120–127. doi: 10.1016/j.cattod.2017.10.023. [DOI] [Google Scholar]
- 127.Haque F., Daeneke T., Kalantar-zadeh K., Ou J.Z. Two-Dimensional Transition Metal Oxide and Chalcogenide-Based Photocatalysts. Nano-Micro Lett. 2018;10:23. doi: 10.1007/s40820-017-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Camposeco R., Castillo S., Rodriguez-González V., Hinojosa-Reyes M., Medina-Álvares M.I., Mejía-Centeno I. Promotional effect of Rh nanoparticles on WO3/TiO2 titanate nanotube photocatalysts for boosted hydrogen production. J. Photochem. Photobiol. A Chem. 2018;353:114–121. doi: 10.1016/j.jphotochem.2017.11.014. [DOI] [Google Scholar]
- 129.Ren X., Gao P., Kong X., Jiang R., Yang P., Chen Y., Chi Q., Li B. NiO/Ni/TiO2 nanocables with Schottky/p-n heterojunctions and the improved photocatalytic performance in water splitting under visible light. J. Colloid Interface Sci. 2018;530:1–8. doi: 10.1016/j.jcis.2018.06.071. [DOI] [PubMed] [Google Scholar]
- 130.Madhumitha A., Preethi V., Kanmani S. Photocatalytic hydrogen production using TiO2 coated iron-oxide core shell particles. Int. J. Hydrogen Energy. 2018;43:3946–3956. doi: 10.1016/j.ijhydene.2017.12.127. [DOI] [Google Scholar]
- 131.Xie M.Y., Su K.Y., Peng X.Y., Wu R.J., Chavali M., Chang W.C. Hydrogen production by photocatalytic water-splitting on Pt-doped TiO2–ZnO under visible light. J. Taiwan Inst. Chem. Eng. 2017;70:161–167. doi: 10.1016/j.jtice.2016.10.034. [DOI] [Google Scholar]
- 132.Chen Q., Tong R., Chen X., Xue Y., Xie Z., Kuang Q., Zheng L. Ultrafine ZnO quantum dot-modified TiO2 composite photocatalysts: The role of the quantum size effect in heterojunction-enhanced photocatalytic hydrogen evolution. Catal. Sci. Technol. 2018;8:1296–1303. doi: 10.1039/C7CY02310C. [DOI] [Google Scholar]
- 133.Liu J., Ke J., Li Y., Liu B., Wang L., Xiao H., Wang S. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2018;236:396–403. doi: 10.1016/j.apcatb.2018.05.042. [DOI] [Google Scholar]
- 134.Zhang Q., Hai Z., Jian A., Xu H., Xue C., Sang S. Synthesis of p-Co3O4/n-TiO2 nanoparticles for overall water splitting under visible light irradiation. Nanomaterials. 2016;6:138. doi: 10.3390/nano6080138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang L., Zhang X., Gao H., Hu J., Mao J., Liang C., Zhang P., Shao G. 3D CuO network supported TiO2 nanosheets with applications for energy storage and water splitting. Sci. Adv. Mater. 2016;8:1256–1262. doi: 10.1166/sam.2016.2714. [DOI] [Google Scholar]
- 136.Wei T., Zhu Y.-N., An X., Liu L.-M., Cao X., Liu H., Qu J. Defect Modulation of Z-Scheme TiO2 /Cu2O Photocatalysts for Durable Water Splitting. ACS Catal. 2019;9:8346–8354. doi: 10.1021/acscatal.9b01786. [DOI] [Google Scholar]
- 137.Rao V.N., Reddy N.L., Kumari M.M., Ravi P., Sathish M., Neppolian B., Shankar M.V. Synthesis of titania wrapped cadmium sulfide nanorods for photocatalytic hydrogen generation. Mater. Res. Bull. 2018;103:122–132. doi: 10.1016/j.materresbull.2018.03.030. [DOI] [Google Scholar]
- 138.Du J., Wang H., Yang M., Li K., Zhao L., Zhao G., Li S., Gu X., Zhou Y., Wang L., et al. Pyramid-like CdS nanoparticles grown on porous TiO2 monolith: An advanced photocatalyst for H2 production. Electrochim. Acta. 2017;250:99–107. doi: 10.1016/j.electacta.2017.08.042. [DOI] [Google Scholar]
- 139.Peng R., Wu C.M., Baltrusaitis J., Dimitrijevic N.M., Rajh T., Koodali R.T. Solar hydrogen generation over CdS incorporated in Ti-MCM-48 mesoporous materials under visible light illumination. Int. J. Hydrogen Energy. 2016;41:4106–4119. doi: 10.1016/j.ijhydene.2016.01.040. [DOI] [Google Scholar]
- 140.Zhao D., Yang C.F. Recent advances in the TiO2/CdS nanocomposite used for photocatalytic hydrogen production and quantum-dot-sensitized solar cells. Renew. Sustain. Energy Rev. 2016;54:1048–1059. doi: 10.1016/j.rser.2015.10.100. [DOI] [Google Scholar]
- 141.Qi L., Yu J., Jaroniec M. Preparation and enhanced visible-light photocatalytic H2- production activity of CdS-sensitized Pt/TiO2 nanosheets with exposed (001) facets. Phys. Chem. Chem. Phys. 2011;13:8915–8923. doi: 10.1039/c1cp20079h. [DOI] [PubMed] [Google Scholar]
- 142.Li C., Yuan J., Han B., Jiang L., Shangguan W. TiO2 nanotubes incorporated with CdS for photocatalytic hydrogen production from splitting water under visible light irradiation. Int. J. Hydrogen Energy. 2010;35:7073–7079. doi: 10.1016/j.ijhydene.2010.01.008. [DOI] [Google Scholar]
- 143.Zhang Y.J., Yan W., Wu Y.P., Wang Z.H. Synthesis of TiO2 nanotubes coupled with CdS nanoparticles and production of hydrogen by photocatalytic water decomposition. Mater. Lett. 2008;62:3846–3848. doi: 10.1016/j.matlet.2008.04.084. [DOI] [Google Scholar]
- 144.Chandra M., Bhunia K., Pradhan D. Controlled Synthesis of CuS/TiO2 Heterostructured Nanocomposites for Enhanced Photocatalytic Hydrogen Generation through Water Splitting. Inorg. Chem. 2018;57:4524–4533. doi: 10.1021/acs.inorgchem.8b00283. [DOI] [PubMed] [Google Scholar]
- 145.Dang H., Cheng Z., Yang W., Chen W., Huang W., Li B., Shi Z., Qiu Y., Dong X., Fan H. Room-temperature synthesis of CuxS (x = 1 or 2) co-modified TiO2 nanocomposite and its highly efficient photocatalytic H2 production activity. J. Alloys Compd. 2017;709:422–430. doi: 10.1016/j.jallcom.2017.03.177. [DOI] [Google Scholar]
- 146.Wang Q., Huang J., Sun H., Ng Y.H., Zhang K.Q., Lai Y. MoS2 Quantum Dots@TiO2 Nanotube Arrays: An Extended-Spectrum-Driven Photocatalyst for Solar Hydrogen Evolution. ChemSusChem. 2018;11:1708–1721. doi: 10.1002/cssc.201800379. [DOI] [PubMed] [Google Scholar]
- 147.Ng B.J., Putri L.K., Tan L.L., Pasbakhsh P., Chai S.P. All-solid-state Z-scheme photocatalyst with carbon nanotubes as an electron mediator for hydrogen evolution under simulated solar light. Chem. Eng. J. 2017;316:41–49. doi: 10.1016/j.cej.2017.01.054. [DOI] [Google Scholar]
- 148.Yang C., Qin J., Rajendran S., Zhang X., Liu R. WS2 and C-TiO2 Nanorods Acting as Effective Charge Separators on g-C3N4 to Boost Visible-Light Activated Hydrogen Production from Seawater. ChemSusChem. 2018;11:4077–4085. doi: 10.1002/cssc.201801819. [DOI] [PubMed] [Google Scholar]
- 149.Liu E., Xu C., Jin C., Fan J., Hu X. Carbon quantum dots bridged TiO2 and Cd0.5Zn0.5S film as solid-state Z-scheme photocatalyst with enhanced H2 evolution activity. J. Taiwan Inst. Chem. Eng. 2019;97:316–325. doi: 10.1016/j.jtice.2019.02.027. [DOI] [Google Scholar]
- 150.Zou Y., Shi J.W., Ma D., Fan Z., Niu C., Wang L. Fabrication of g-C3N4/Au/C-TiO2 Hollow Structures as Visible-Light-Driven Z-Scheme Photocatalysts with Enhanced Photocatalytic H2 Evolution. ChemCatChem. 2017;9:3752–3761. doi: 10.1002/cctc.201700542. [DOI] [Google Scholar]
- 151.Yang G., Ding H., Chen D., Feng J., Hao Q., Zhu Y. Construction of urchin-like ZnIn2S4-Au-TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018;234:260–267. doi: 10.1016/j.apcatb.2018.04.038. [DOI] [Google Scholar]
- 152.Zhang L., Huang Y., Dai C., Liang Q., Yang P., Yang H., Yan J. Constructing ZnO/ZnCr2O4@TiO2 -NTA Nanocomposite for Photovoltaic Conversion and Photocatalytic Hydrogen Evolution. J. Electron. Mater. 2019;48:1724–1729. doi: 10.1007/s11664-019-06927-y. [DOI] [Google Scholar]
- 153.Ke X., Zhang J., Dai K., Liang C. Construction of flourinated-TiO2 nanosheets with exposed {001} facets/CdSe-DETA nanojunction for enhancing visible-light-driven photocatalytic H2 evolution. Ceram. Int. 2020;46:866–876. doi: 10.1016/j.ceramint.2019.09.044. [DOI] [Google Scholar]
- 154.Kim Y.K., Lim S.K., Park H., Hoffmann M.R., Kim S. Trilayer CdS/carbon nanofiber (CNF) mat/Pt-TiO2 composite structures for solar hydrogen production: Effects of CNF mat thickness. Appl. Catal. B Environ. 2016;196:216–222. doi: 10.1016/j.apcatb.2016.05.045. [DOI] [Google Scholar]
- 155.Dai K., Lv J., Zhang J., Zhu G., Geng L., Liang C. Efficient Visible-Light-Driven Splitting of Water into Hydrogen over Surface-Fluorinated Anatase TiO2 Nanosheets with Exposed {001} Facets/Layered CdS-Diethylenetriamine Nanobelts. ACS Sustain. Chem. Eng. 2018;6:12817–12826. doi: 10.1021/acssuschemeng.8b02064. [DOI] [Google Scholar]
- 156.Yuan W., Zhang Z., Cui X., Liu H., Tai C., Song Y. Fabrication of Hollow Mesoporous CdS@TiO2@Au Microspheres with High Photocatalytic Activity for Hydrogen Evolution from Water under Visible Light. ACS Sustain. Chem. Eng. 2018;6:13766–13777. doi: 10.1021/acssuschemeng.8b01787. [DOI] [Google Scholar]
- 157.Zhao H., Wu M., Liu J., Deng Z., Li Y., Su B.L. Synergistic promotion of solar-driven H2 generation by three-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl. Catal. B Environ. 2016;184:182–190. doi: 10.1016/j.apcatb.2015.11.018. [DOI] [Google Scholar]
- 158.Wu M., Zhang J., Liu C., Gong Y., Wang R., He B., Wang H. Rational Design and Fabrication of Noble-metal-free NixP Cocatalyst Embedded 3D N-TiO2/g-C3N4 Heterojunctions with Enhanced Photocatalytic Hydrogen Evolution. ChemCatChem. 2018;10:3069–3077. doi: 10.1002/cctc.201800197. [DOI] [Google Scholar]
- 159.Sampath S., Sellappa K. Visible-light-driven photocatalysts for hydrogen production by water splitting. Energy Sources Part A Recover. Util. Environ. Eff. 2020;42:719–729. doi: 10.1080/15567036.2019.1602194. [DOI] [Google Scholar]
- 160.Azam M.U., Tahir M., Umer M., Tahir B., Shehzad N., Siraj M. In-situ synthesis of TiO2/La2O2CO3/rGO composite under acidic/basic treatment with La3+/Ti3+ as mediators for boosting photocatalytic H2 evolution. Int. J. Hydrogen Energy. 2019;44:23669–23688. doi: 10.1016/j.ijhydene.2019.07.085. [DOI] [Google Scholar]
- 161.Zhu J., Zhang M., Xiong J., Yan Y., Li W., Cheng G. Electrostatically assembled construction of ternary TiO2-Cu@C hybrid with enhanced solar-to-hydrogen evolution employing amorphous carbon dots as electronic mediator. Chem. Eng. J. 2019;375:121902. doi: 10.1016/j.cej.2019.06.030. [DOI] [Google Scholar]