Abstract
The phylloplane is an important habitat for yeasts and these yeasts may have antagonistic activities against pathogens and could be used as biocontrol agents. To investigate rice phylloplane yeasts, 282 strains were isolated from 89 rice leaf samples and identified as 15 known yeast species in the phylum Ascomycota and 35 known and two potential new species in the phylum Basidiomycota. The majority of rice phylloplane yeasts belonged to the phylum Basidiomycota. The evaluation of antagonistic activities of 83 yeast strains against rice pathogenic fungi Pyricularia oryzae, Rhizoctonia solani, Fusarium moniliforme, Helminthosporium oryzae and Curvularia lunata revealed that 14 strains inhibited these pathogens. Among the antagonistic strains, Torulaspora indica DMKU-RP31, T. indica DMKU-RP35 and Wickerhamomyces anomalus DMKU-RP25 inhibited all rice pathogens tested, and the production of volatile organic compounds, fungal cell wall degrading enzymes and biofilm were the possible antagonistic mechanisms against all rice pathogens tested in vitro. These yeast strains were evaluated for controlling rice sheath blight caused by R. solani in rice plants in the greenhouse and were found to suppress the disease by 60.0–70.3%, whereas 3% validamycin suppressed by 83.8%. Therefore, they have potential for being developed to be used as biocontrol agents for rice sheath blight.
Keywords: phylloplane yeast, antagonistic yeast, rice pathogenic fungi, rice sheath blight, biological control
1. Introduction
The term phylloplane refers to the parts of plants above ground and dominated by leaves and is an important habitat for microorganisms [1]. Growth of phylloplane microorganisms depends on organic compounds secreted from the plant itself and organic compounds from external sources [2,3]. The phylloplane of plants in both temperate and tropical regions has been reported to be colonized by yeasts belonging to both phyla, Ascomycota and Basidiomycota, although the majority of the strains are in Basidiomycota [4,5,6,7,8].
Rice is one of the most widely produced and consumed staple foods in the world, especially in Asia [9]. In Thailand the rice cultivation area in the year 2017/2018 was approximately 59.2 million hectares, with a production of 24.9 million tons (http://www.oae.go.th/view/1/). The rice species cultivated in Thailand is commonly known as Asian rice (Oryza sativa L.). One of the major causes of decreases in rice production is diseases caused by pathogenic fungi. The major rice diseases caused by fungal pathogens in Thailand are blast (caused by P. oryzae), sheath blight (caused by R. solani), bakanae (caused by F. moniliforme), brown spot (caused by H. oryzae) and dirty panicle (caused by Cu. lunata and H. oryzae) (Rice Department, Ministry of Agriculture and Cooperatives of Thailand, 2014). Rice sheath blight disease causes yield losses of 25%–35% of Thai rice production [10]. This disease is the second most important rice disease worldwide [11].
The management of rice diseases caused by fungi is mainly based on the use of the chemical fungicides such as Carbendazim®, Validamycin®, Propiconazole® and Mancozeb® [12,13,14]. However, the use of chemical fungicides is not a long-term solution and is becoming less acceptable due to increasing residues, toxicity to non-target organisms and other health and environmental hazards [15]. Biological control is an environmentally friendly alternative approach to plant disease management. In the last two decades, biological control based on using antagonistic yeast has been demonstrated. Antagonistic yeasts have been sought for use as biological control agents for plant and post-harvest diseases. For example, Papiliotrema (Cryptococcus) flavescens and Sporobolomyces roseus, reduced lesion density and necrosis of red stalk rot of maize caused by Colletotrichum graminicola when applied as a mixture to maize plants [16], Saccharomyces cerevisiae, Candida albicans and Candida sake were reported to significantly reduce the powdery mildew and Cercospora leaf spot diseases on sugar beet [17] and Rhodotorula (Rhodosporidium) kratochvilovae and Papiliotrema (Cryptococcus) laurentii UM108 suppressed the powdery mildew disease on wheat [18].
Antagonistic yeasts use both direct and indirect mechanisms to inhibit plant pathogens, including production of volatile organic compounds, cell wall degrading enzymes, siderophores and biofilm as well as competition of nutrients, and phosphate and zinc oxide solubilization [18,19,20,21,22,23,24,25]. Volatile organic compounds (VOCs) are substances with a low molecular weight (lower than 300 Da), low polarity and high vapor pressure [26]. Emission of VOCs by antagonistic yeasts have proven to be one of the important direct antagonistic mechanisms against pathogenic fungi. Candida intermedia was found to produce VOCs that inhibited mycelium growth of Botrytis cinerea [27]. Candida maltosa NP9 emitted VOCs that inhibited spore germination of Aspergillus brasiliensis [28]. Sporidiobolus pararoseus was found to produce VOCs that effectively inhibited both the conidial germination and the mycelial growth of B. cinerea [29]. Hua et al. [30] reported that VOCs produced by Wickerhamomyces anomalus WRL-076 inhibited growth and aflatoxin production of Aspergillus flavus. Secretion of fungal cell wall degrading enzymes, especially β- 1, 3-glucanase and chitinase, by antagonistic yeasts is one of the direct antagonistic activities against pathogenic fungi. These enzymes hydrolyze polymeric compounds in the fungal cell wall, and this directly suppresses activities and/or induces death. Candida oleophila, Meyerozyma guilliermondii and Pichia membranifaciens were reported to produce β- 1, 3-glucanase and chitinase, which kill pathogenic fungi [22]. M. guilliermondii produced β-1,3-glucanases and chitinase, and higher production of both enzymes was found when cultivated in a medium supplemented with cell wall fragments of Colletotrichum capsici [31]. P. membranifaciens suppressed the growth of B. cinerea through the production of β-1,3-glucanases [32]. Meyerozyma caribbica showed a high antagonistic potential against Colletotrichum gloeosporioides in mango through the production of hydrolytic enzymes such as chitinase and β-1,3-glucanase [23]. Siderophores are secondary metabolites which are low molecular weight compounds with a high affinity for iron. When siderophore producing antagonistic microorganisms are applied in the agricultural field, they suppress the pathogens’ growth or reduce their metabolisms by competition for iron, resulting in a decrease in the pathogens [33]. Rhodotorula glutinis has been shown to produce rhodotorulic acid, a hydroxamate type siderophore, which suppresses various plant pathogenic fungi [34]. This antagonistic yeast species was also reported to reduce B. cineria spore germination and disease caused by this pathogenic fungus in biocontrol experiments on apple fruit [35]. Biofilm is a group of microbial cells embedded within a matrix of extracellular polymeric substance produced by the cells, which adhere to a surface. Biofilm formation on a plant will protect the plant from destruction by pathogens and is related to the competition for nutrients on the surface of the plant [23,36]. In addition, yeasts cells in biofilm can destroy fungal pathogens by secretion of fungal cell-wall degrading enzymes during the adhesion process [36]. Competition for nutrients between antagonists and pathogenic fungi is among the direct antagonistic mechanisms. This mechanism is based on the ability of antagonistic yeasts to rapidly colonize and multiply on the plant surface and subsequently to compete with the pathogens for nutrients and space [24,31,37]. Reduced efficiency of W. anomalus and S. cerevisiae against Pichia digitatum in orange was obtained from this mechanism [38]. Tian et al. [39] reported that Metschnikowia pulcherrima could compete for nutrients under in vivo condition when glucose was added to wounds on mango fruits. Some microorganisms are capable of solubilizing insoluble essential elements such as phosphate and zinc oxide in the environment to their soluble forms, which are subsequently uptaken by plants [21].
This work aimed to study yeasts in the phylloplane of rice, to evaluate the antagonistic activities and mechanisms against rice pathogenic fungi of the rice phylloplane yeasts and to evaluate the efficacy of the selected antagonistic yeasts for controlling the rice sheath blight disease in rice plants in a greenhouse.
2. Materials and Methods
2.1. Rice Leaf Collection and Phylloplane Yeast Isolation
Eighty-nine samples of green and undamaged leaves of rice were collected at random from rice fields in nine provinces in Thailand between December 2011 and March 2012 (Table 1). Each sample was a composite sample consisting of many leaves collected from different rice plants in a small area. Each sample was put in a plastic bag and transferred to the laboratory in an ice box. The samples were stored at 8 °C and yeast isolation was initiated within 5 days.
Table 1.
Rice leaf samples collected in Thailand and the number of yeast strain isolated from the phylloplane.
| Province | District | Location | Sampling Month and Year | No. of Samples | No. of Strains |
|---|---|---|---|---|---|
| Chachoengsao | Ban Pho | 13°35′46.0″N 01°04′56.8″E | Dec 2011 | 1 | 3 |
| Bang Pakong | 13°29′46.3″N 00°57′14.5″E | Dec 2011 | 1 | 1 | |
| Bang Khla | 13°41′11.5″N 01°04′13.3″E | Dec 2011 | 2 | 8 | |
| Mueang Chachoengsao | 13°43′53.0″N 00°59′22.8″E | Dec 2011 | 1 | 6 | |
| Phanom Sarakham | 13°45′54.1″N 01°19′42.5″E | Dec 2011 | 2 | 7 | |
| Ratchasan | 13°48′45.8″N 01°16′54.9″E | Dec 2011 | 1 | 2 | |
| Chai Nat | Manorom | 15°20′37.9″N 00°08′52.0″E | Mar 2012 | 3 | 7 |
| Mueang Chai Nat | 15°13′28.7″N 00°05′45.7″E | Mar 2012 | 3 | 7 | |
| Kanchanaburi | Phanom Thuan | 14°09′08.7″N 99°40′48.2″E | Jan 2012 | 4 | 14 |
| Nakhon Nayok | Ban Na | 14°15′34.0″N 01°01′51.4″E | Dec 2011 | 4 | 8 |
| Mueang Nakhon Nayok | 14°15′29.3″N 01°13′04.8″E | Dec 2011 | 1 | 2 | |
| Pak Phli | 14°19′34.6″N 01°21′47.7″E | Feb 2012 | 4 | 6 | |
| Nakhon Pathom | Bang Len | 14°01′55.8″N 00°09′08.6″E | Jan 2012 | 3 | 7 |
| Don Tum | 13°58′00.6″N 00°04′13.7″E | Jan 2012 | 1 | 3 | |
| Kamphaeng Saen | 14°04′53.0″N 99°57′02.3″E | Jan 2012 | 8 | 29 | |
| Nakhon Sawan | Mueang Nakhon Sawan | 15°45′10.4″N 00°07′38.4″E | Mar 2012 | 2 | 11 |
| Phayuha Khiri | 15°30′33.9″N 00°09′51.0″E | Mar 2012 | 1 | 2 | |
| Nonthaburi | Bang Bua Thong | 13°55′56.9″N 00°24′37.1″E | Feb 2012 | 1 | 10 |
| Sai Noi | 14°01′04.3″N 00°18′55.7″E | Jan 2012 | 8 | 36 | |
| Prachin Buri | Mueang Prachin Buri | 14°08′44.1″N 01°22′55.1″E | Dec 2011 | 2 | 6 |
| Si Mahosot | 13°55′01.6″N 01°24′23.2″E | Dec 2011 | 2 | 5 | |
| Suphan Buri | Bang Pla Ma | 14°23′02.7″N 00°08′46.8″E | Mar 2012 | 7 | 20 |
| Doem Bang Nang Buat | 14°52′00.9″N 00°09′29.9″E | Mar 2012 | 1 | 1 | |
| Don Chedi | 14°39′53.3″N 99°57′59.0″E | Jan 2012 | 3 | 9 | |
| Mueang Suphan Buri | 14°26′57.4″N 00°03′44.0″E | Jan 2012 | 7 | 23 | |
| Song Phi Nong | 14°13′02.3″N 99°58′44.9″E | Jan 2012 | 4 | 12 | |
| U Thong | 14°26′27.5″N 99°52′39.5″E | Jan 2012 | 12 | 37 |
Yeast were isolated from the rice phylloplane by a plating of leaf washings as described by Surussawadee et al. [40]. Three grams of leaf from all the collected leaves in each sample were suspended in 50 mL of 0.85% sodium chloride solution in a 250 mL Erlenmeyer flask and shaken on a rotary shaker at room temperature (27 ± 3 °C) and 150 rpm for 1 h. An aliquot of 100 µL of the washing solution was then spread on yeast extract malt extract (YM) agar (3 g/L yeast extract, 3 g/L malt extract, 5 g/L peptone, 10 g/L glucose and 20 g/L agar), supplemented with 250 mg/L sodium propionate and 200 mg/L chloramphenicol and incubated at 25 °C until yeast colonies appeared. The colonies of different morphologies were selected and purified by streaking on YM agar. The purified yeast strains were suspended in YM broth supplemented with 150 g/L glycerol and maintained at 80 °C.
2.2. Yeast Identification
Yeasts were identified by analysis of the D1/D2 region of the large subunit (LSU) rRNA gene sequence similarity, and in some instances, the internal transcribed spacer (ITS) region sequence similarity was also analyzed. The sequence of the D1/D2 region of the LSU rRNA gene and the ITS region were determined from PCR products amplified from genomic DNA extracted from yeast cells following the methods previously described by Limtong et al. [41]. Amplification of the D1/D2 of the LSU rRNA gene was carried out using PCR with the forward primer NL1 (5′-GCATATCAATAAGCG GAGGAAAAG-3′) and the reverse primer NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) [42]. The ITS region was amplified with the forward primer ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and the reverse primer ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR products were checked using agarose gel electrophoresis and purified by using the HiYield Gel/PCR Fragment Extraction Kit (RBC Bioscience). The purified products were sequenced commercially by Macrogen (Geumcheon -gu, Seoul, Korea) using the primers NL1 and NL4 for the D1/D2 region and the primers ITS1 and ITS4 for the ITS region. The sequences were compared pairwise using a BLAST search [43]. For identification of ascomycetous yeasts, strains showing greater than 1% nucleotide substitutions in the D1/D2 region were considered to be different species and strains with 0–3 nucleotide differences were treated as conspecific species [42]. For basidiomycetous yeast identification, strains differing by two or more nucleotide substitutions were considered to represent different species [44]. When possible, complete ITS sequences were also analyzed in order to confirm D1/D2-based identifications. When necessary, the “potential new species” designation was used.
Phylogenetic analysis based on the sequences of the D1/D2 region of the LSU rRNA gene was used for confirming yeast identification with pairwise sequence similarity analysis. The nucleotide sequences of the type strains of the related species were obtained from NCBI (www.ncbi.nih.gov) databases. The sequences of yeast strains were aligned with their related species using MUSCLE [45] provided with the MEGA version 7.0 software package. A phylogenetic tree was constructed from the evolutionary distance data using the general time reversible (GTR) model and the maximum likelihood analysis performed with MEGA 7.0 [46] The confidence level of the clade was estimated using bootstrap analysis (1000 replicates). Yeast sequence data have been submitted to the GenBank database.
2.3. Selection of Antagonistic Yeasts Capable of Antagonize Fungi Cause Rice Diseases
The antagonistic activities of 83 strains of rice phylloplane yeast against five rice pathogenic fungi (Table 2) were determined by dual cultivation of yeast and pathogenic fungi on a potato dextrose agar (PDA; 4.0 g/L potato, infusion from (solids), 20.0 g/L glucose and 15.0 g/L agar-agar) dish following the method of Rosa et al. [21] with slight modification. The rice pathogenic fungus and yeast were grown side by side on PDA agar in a Petri dish. A loop of an active yeast culture (24 h at 25°C on yeast extract malt extract (YM) agar) was inoculated near the edge of the dish and then incubated at 25 °C for 2 days. A 5 mm diameter disk of actively growing fungal mycelium from a 7-day-old culture on PDA was cut with a cork borer and inoculated at the opposite edge. A PDA dish inoculated with only the pathogenic fungi was used as a control. Dishes inoculated with R. solani were incubated at 25 °C for 3 days, while those inoculated with the other plant pathogenic fungi were incubated at 25 °C for 7 days. Three replications were performed. The pathogenic fungal growth reduction was determined by measuring the radius of the fungal colony cultured with yeast compared with the control.
Table 2.
Rice pathogenic fungi used in this study.
Inhibition of fungal growth (%) = [(R1-R2)/R1] × 100.
R1 = radius of fungal colony cultured alone; R2 = radius of fungal colony cultured with yeast.
2.4. Determination of Antagonistic Mechanisms of Antagonistic Yeasts In Vitro
2.4.1. Production of Antifungal Volatile Organic Compounds
Production of VOCs against plant pathogenic fungi was measured using the double dishes assay technique as described by Di Francesco et al. [47] with slight modification. An aliquot (100 µL) of the antagonist yeast cell suspension of 108 cells/ mL was spread on a PDA dish. After 48 h, the cover of the dish was removed and the bottom with yeast inoculation was inverted and placed upside down on the bottom of a PDA dish inoculated with a fungal mycelial plug (5 mm in diameter). The two bottom dishes were sealed with a double layer of parafilm and incubated at 25 °C for 7 days. The control was a PDA dish inoculated with only the pathogenic fungus. Three replications were performed. The fungal growth inhibition was calculated with the flowing formula:
Inhibition of mycelial growth (%) = [(D1-D2)/D1] × 100.
D1 = diameters of fungal colony cultured alone.
D2 = diameter of fungal colony cultured with yeast.
2.4.2. Production of β-Glucanase and Chitinase
The production of the two fungal cell wall lytic enzymes, β-glucanase and chitinase, by the antagonistic yeasts was determined by cultivation in potato dextrose broth (PDB). A loop of active yeast cells (24 h at 25 °C on YM agar) was inoculated to 50 mL PDB in a 250 mL Erlenmeyer flask and incubated on a rotary shaker at 150 rpm for 5 days. The culture broth was collected, and cells were separated by centrifuging at 10,000 g for 5 min. The supernatant was analyzed for β-glucanase and chitinase activity. Three replications were performed.
To determine β -1, 3-glucanase activity, the colorimetric quantification of glucose (reducing sugar) released from laminarin was used. The enzymatic reaction was performed by mixing 200 µL of the cell free culture broth, 50 µL of sodium acetate buffer (0.1 M, pH 5.0) and 250 µL of laminarin (4 mg/mL), incubated at 37 °C for 1 h, and the reducing sugar concentration was determined using the method of Miller [48]. Briefly, 250 µL of dinitrosalicylic acid reagent was added to 250 µL of the enzymatic reaction mixture and incubated at 100 °C for 5 min, and the absorption was measured with a spectrophotometer at 540 nm. The enzymatic activity was expressed in U/mL, in which one unit of activity (U) was defined as 1 µg of reducing sugars released from laminarin per minute under the assay conditions.
Chitinase activity was evaluated by estimating the release of N-acetyl glucosamine (NAG) from the substrate colloidal chitin. A volume of 100 µL of the cell free culture broth was mixed with 200 µL of Mcllvaine buffer (50 mM citric acid, 100 mM sodium phosphate, pH 6.0) and 100 µL of 0.01% w/v colloidal chitin (prepared in Mcllvaine buffer). After incubation at 37 °C for 15 min, the reducing sugar concentration was determined using the method of Miller [48], the same as used to determine the reducing sugar concentration in investigation of b-1, 3-glucanase activity, except that the absorption was measured at 575 nm. The enzymatic activity was expressed in U/mL, in which one unit of activity (U) was defined as 1 µg of reducing sugars released from colloidal chitin per minute under the assay conditions.
Colloidal chitin was prepared as described by Khan et al. [49]. Chitin powder (40 g) was dissolved in 500 mL of concentrated hydrochloric acid and continuously stirred at 4°C for 1 h. The hydrolyzed chitin was washed several times with distilled water to completely remove acid and hence bring it to pH 6–7. Then the colloidal chitin was filtered through Whatman filter paper No.1. The colloidal chitin was collected, frozen in liquid nitrogen for lyophilization and stored at −20 °C until use.
2.4.3. Competition of Nutrients
To determine the effect of nutrient concentration on the pathogenic fungal mycelia growth reduction by the antagonistic yeast, the method of Zhang et al. [37] with slight modification was used. The dual cultivation of yeast and the pathogenic fungi was performed, the same as described previously except that PDA with different nutrient concentrations, namely, standard nutrient concentration (39 g/L PDA powder), half of the standard nutrient concentration (19.5 g/L PDA powder), one-fourth of the standard of nutrient concentration (9.7 g/L PDA powder), and one-tenth of the standard nutrient concentration (3.9 g/L PDA powder) were used. Three replications were performed. The radius of pathogenic fungal colony cultivated alone and that of a fungal colony cultivated with yeast was determined after 3 days at 25 °C. Inhibition of fungal growth was calculated as described previously.
2.4.4. Phosphate and Zinc Oxide Solubilization
To determine phosphate and zinc oxide solubilization, Pikovskaya’s agar [50] and zinc oxide agar [51] were used. A yeast cell suspension was prepared by mixing a 2-day-old culture grown on YM agar with sterile normal saline and adjusting the suspension to an optical density at 600 nm (OD600) of 0.10. The yeast cell suspension (5 µL) was dropped onto the surface of Pikovskaya’s agar or zinc oxide agar in a dish and incubated at 25 °C for 5 days. The halo zone diameter and the colony diameter were then measured. Three replications were performed. The phosphate or zinc oxide solubilization efficiency (SE) was calculated as a ratio of the halo zone diameter and the colony diameter.
2.4.5. Siderophore Production
Investigation of siderophore production by the selected antagonistic yeast was carried out by cultivation on chrome azurol S (CAS) blue agar in a Petri dish [52] A yeast cell suspension was prepared by mixing a loop full of a 2-day-old culture grown on YM agar in 3 mL of 0.85% sterile normal saline and adjusting the suspension to an optical density at 600 nm (OD600) of 0.10. The yeast cell suspension (10 µl) was dropped onto the surface of CAS blue agar and incubated at 25 °C for 10 days, in the dark. Three replications were performed. The medium color change (from blue to purple or yellow) around the colony indicated siderophore production.
2.4.6. Biofilm Formation
The antagonistic yeast strains were assessed for biofilm formation using the method described by Růžička et al. [53] with slight modification. A yeast cell suspension was prepared by mixing a 2-day-old culture grown on PDA with sterile water to reach an optical density at 600 nm (OD600) of 0.5. The yeast suspension (20 µL) was inoculated into each well of a 96-well microtiter plate containing 180 µL PDB, and the microtiter plate was incubated for 48 h at 25 °C. The negative control was a well containing only PDB. Three replications were performed. After incubation, the wells were emptied, rinsed with water and air-dried at room temperature. The adherent biofilm layer was stained with an aqueous solution of 1% (w/v) violet crystal for 20 min, rinsed with water and air-dried. The stained biofilm layer was eluted from each well with 200 µL of ethanol, and the absorbance (A) of each well was measured at 620 nm. Biofilm formation was considered as positive in a well where mean A of the treatment was higher than the mean A of the negative control.
2.5. Controlling of Rice Sheath Blight Disease in Rice Plants in the Greenhouse by the Selected Antagonistic Yeasts
The antagonistic yeast strains that strongly inhibited mycelium growth of R. solani DOAC 1406 in dual cultivation were selected for determination of their ability to control rice sheath blight disease in rice plants in the greenhouse.
An antagonistic yeast cell suspension was prepared using the method of Spadaro et al. [54] with slight modification. The selected yeast strain was cultured in 50 mL of yeast extract peptone dextrose (YPD) broth (10 g/L yeast extract, 20 g/L peptone and 20 g/L glucose) in a 250 mL Erlenmeyer flask and incubated on a rotary shaker at 150 rpm and 25 °C for 24 h. Cells were collected using centrifugation at 5000 g for 10 min, re-suspended in sterile Ringer’s solution and quantified with a haemacytometer to reach a concentration of 108 cells/mL.
Pathogenic fungus was cultivated on sterile rice straw. The rice straw was cut into pieces (1 × 2 cm), 15 g of the cut rice straw was put in a clean plastic bag (20 × 30 cm), and 15 mL distilled water was added to make the moisture content approximately 50%. The open end of the plastic bag was narrowed and closed with a cotton plug to make a closed system. The rice straw in the plastic bag was sterilized using autoclave at 121.5 °C for 15 min. After cooling, R. solani was inoculated by using 3 plugs (0.5 mm diameter) of a 3-day-old culture grown on PDA cut from the edge of a colony and incubated at 28 ± 2 °C for 14 days. The infested rice straw (5 g) was put on rice straw paper (20 × 7 cm) before use.
A greenhouse experiment was conducted in rice plants grown in pots using complete randomized design (CRD) with five replications. Chai Nat 1 rice cultivar, which is susceptible to rice sheath blight disease, was used. Rice seeds were sterilized in 10% Clorox solution for 1 min, rinsed three times with sterile distilled water and soaked in sterile distilled water for 24 h for imbibition prior to the germination trial [9]. Sterile seeds were grown in sterile loam soil in a plastic nursery basket for 20 days in a greenhouse and watered daily. One 20-day-old rice seedling was transplanted into a pot (22.5 cm diameter and 22 cm height) containing 5 kg of sterile loam soil. Tap water was added to the pots such that the water level was 3 cm above the soil surface, and that water level was kept constant by adding water daily. Twenty mL of a selected antagonistic yeast cell suspension was sprayed on the sheath of the rice plant. One hour after yeast inoculation, the rice straw paper containing the infested rice straw (5 g) was inoculated at 5 cm above water level on the sheath of a rice plant (45 days old) [55]. Five days after pathogenic fungus inoculation, the rice straw paper was removed. The fungicide, 3% (w/v) validamycin, was used for comparison. A rice plant inoculated with pathogenic fungi without any treatment was used as a positive control. Spraying of all treatments was repeated at 5 and 10 days after pathogenic fungus inoculation. Five replications were performed. The sheath blight disease symptoms were observed, and lesion height was measured 15 days after pathogenic fungus inoculation. The disease incidence and disease suppression of the treatment and control were calculated using the following formulae [56]:
Sheath blight disease incidence (%)
= (Average sheath blight lesion height/Average plant height) × 100.
Sheath blight disease suppression (%)
= [(Incidence of positive control-Incidence of treatment)/ Incidence of positive control)] × 100.
2.6. Yeast Population and Development of Sheath Blight Lesion on Rice Plants
To examine yeast population and development of sheath blight lesion on rice plants during controlling rice sheath blight in the greenhouse experiment, T. indica DMKU-RP31 was used. The experiment was conducted by inoculation of the selected antagonistic yeast and R. solani DOAC 1406, as described in Section 2.5. Yeast population and disease lesion development on rice sheath 3, 4 and 5 day(s) after the first spraying, 0 (1 h), 2, 4 and 5 day(s) after the second spraying, and 0 (1 h), 2, and 5 day(s) after third spraying were investigated. Three replicates were performed.
The yeast population on a rice sheath was determined using a swab test. The predetermined area of rice sheath surface was swapped using the sterile cotton swab. The cotton swab was then placed into a test tube containing sterile Ringer’s solution and shaken for 30–45 s to remove yeast cells from the cotton swab. Enumeration of the yeast population was carried out using the method of Nix et al. [57] with slight modifications. The washing solution was spread onto the surface of YM agar supplemented with 250 mg/L sodium propionate and 200 mg/L chloramphenicol. The inoculated plate was incubated at 25 °C for 48 h and the yeast colonies were counted. The population of yeast was expressed as colony forming units (CFU) per square centimeter (cm2) of rice sheath surface. The development of sheath blight lesion was measured as the lesion height.
2.7. Statistical Analysis
The data were subjected to analyses of variance (ANOVA) using IBM® SPSS statistics software version 22 (Armonk, New York, United States) for Windows. Statistical significance was evaluated using Duncan’s multiple range test (DMRT) and the significance level of p < 0.05 was considered as being significantly different.
3. Results
3.1. Rice Phylloplane Yeast Isolation and Identification
From 89 rice leaf samples collected from nine provinces in Thailand, 282 yeast strains, each representing a different morphology in individual sample, were obtained (Table 1). All yeast strains were identified by sequence similarity and phylogenetic analysis of the D1/D2 region of the LSU rRNA gene and, in some instances, by the ITS region as well. Forty-four strains were identified to be 15 known yeast species belonging to the subphylum Saccharomycotina, phylum Ascomycota (Table 3, Table S1, Figures S1 and S2). The known ascomycetous yeast species belonged to nine genera: Blastobotrys (one species), Candida (five species), Debaryomyces (one species), Hyphopichia (one species), Kodamaea (one species), Meyerozyma (two species), Torulaspora (one species), Wickerhamomyces (two species) and Yamadazyma (one species). A total of 238 strains were found to belong to the phylum Basidiomycota consisting of 15 known and one potential new species in the subphylum Agaricomycotina, 11 known and a potential new species in the subphylum Pucciniomycotina and nine known species in the subphylum Ustilaginiomycotina (Table 3, Tables S1 and S2, Figures S1 and S2). Among the strains belonging to the phylum Basidiomycota, 229 strains were identified to be 35 known species of yeasts and yeast-like fungi in 16 genera. These were Hannaella (four species), Papiliotrema (seven species), Saitozyma (one species), Trichosporon (three species), Occultifur (one species), Rhodotorula (four species), Sakaguchia (one species), Sporobolomyces (four species), Sporidiobolus (one species), Symmetrospora (one species), Dirkmeia (one species), Jaminaea (one species), Kalmanozyma (one species), Moesziomyces (three species), Pseudozyma (two species) and Ustilago (one species). In addition, three strains were found to be two potential new species in genus Rhodotorula (two strains) and Vishniacozyma (one strain). Among the species obtained, strains of Moesziomyces antarcticus were found in 55 rice leaf samples (61.8%) followed by strains of Dirkmeia churashimaensis, found in 36 rice leaf samples (40.4%) (Table 3).
Table 3.
Yeast species and strains belonging to the phyla Ascomycota and Basidiomycota in rice phylloplane.
| Taxa | No. of Strains | Frequency of Occurrence (%) c | No. of Strains Evaluated for Antagonistic Activity |
|---|---|---|---|
| Phylum Ascomycota, Subphylum Saccharomycotina | |||
| Blastobotrys arbuscular | 1 | 1.1 | |
| Candida diddensiae | 1 | 1.1 | |
| Candida maltosa | 2 | 2.2 | 2 |
| Candida parapsilosis | 6 | 6.7 | |
| Candida tropicalis | 4 | 4.5 | 2 |
| Candida wangnamkhiaoensis | 1 | 1.1 | |
| Debaryomyces nepalensis | 1 | 1.1 | 1 |
| Hyphopichia burtonii | 1 | 1.1 | |
| Kodamaea ohmeri | 7 | 7.9 | 7 |
| Meyerozyma caribbica | 11 | 12.4 | 11 |
| Meyerozyma guilliermondii | 2 | 2.2 | 1 |
| Torulaspora indica | 2 | 2.2 | 2 |
| Wickerhamomyces anomalus | 3 | 3.4 | 2 |
| Wickerhamomyces edaphicus | 1 | 1.1 | |
| Yamadazyma epiphylla a | 1 | 1.1 | |
| Phylum Basidiomycota, Subphylum Agaricomycotina | |||
| Hannaella sinensis | 4 | 4.5 | |
| Hannaella siamensis b | 7 | 7.9 | 2 |
| Hannaella pagnoccae | 1 | 1.1 | 1 |
| Hannaella phetchabunensis | 2 | 2.2 | |
| Papiliotrema aspenensis | 4 | 4.5 | |
| Papiliotrema flavescens | 7 | 7.9 | |
| Papiliotrema japonica | 15 | 16.9 | 5 |
| Papiliotrema laurentii | 2 | 2.2 | |
| Papiliotrema nemorosus | 1 | 1.1 | 1 |
| Papiliotrema rajasthanensis | 5 | 5.6 | |
| Papiliotrema siamense | 3 | 3.4 | |
| Saitozyma flava | 2 | 2.2 | 2 |
| Trichosporon asahii | 1 | 1.1 | |
| Trichosporon asteroides | 1 | 1.1 | |
| Trichosporon insectorum | 1 | 1.1 | |
| Potential new species closest to Vishniacozyma taibaiensis | 1 | 1.1 | |
| Phylum Basidiomycota, Subphylum Pucciniomycotina | |||
| Occultifur plantarum | 2 | 2.2 | 2 |
| Rhodotorula mucilaginosa | 9 | 10.1 | 4 |
| Rhodotorula paludigena | 7 | 7.9 | 1 |
| Rhodotorula taiwanensis | 24 | 27.0 | 12 |
| Rhodotorula toruloides | 2 | 2.2 | 2 |
| Potential new species closest to Rhodotorula toruloides | 2 | 2.2 | 2 |
| Sakaguchia oryzae | 5 | 5.6 | 3 |
| Sporobolomyces blumeae | 14 | 15.7 | 1 |
| Sporobolomyces carnicolor | 4 | 4.5 | |
| Sporobolomyces nakasei | 1 | 1.1 | |
| Sporidiobolus pararoseus | 7 | 7.9 | 3 |
| Symmetrospora vermiculata | 2 | 2.2 | 1 |
| Phylum Basidiomycota, Subphylum Ustilaginomycotina | |||
| Dirkmeia churashimaensis | 36 | 40.4 | 7 |
| Jaminaea angkoriensis | 3 | 3.4 | |
| Kalmanozyma vetiver | 1 | 1.1 | |
| Moesziomyces antarcticus | 55 | 61.8 | 5 |
| Moesziomyces aphidis | 1 | 1.1 | |
| Moesziomyces parantarcticus | 1 | 1.1 | 1 |
| Pseudozyma alboarmeniaca | 2 | 2.2 | |
| Pseudozyma hubeiensis | 1 | 1.1 | |
| Ustilago siamensis | 2 | 2.2 | |
3.2. Selection of Antagonistic Yeasts Capable of Antagonizing Fungi Causing Rice Diseases
Antagonistic activity of rice phylloplane yeast strains against rice pathogenic fungi, namely, F. moniliforme DOAC 1224, H. oryzae DOAC 2293, R. solani DOAC 1406, Cu. lunata DOAC 2313 and P. oryzae, were investigated. In this study we used 80 rice phylloplane yeast strains, consisting of 26 strains in eight species in Ascomycota and 54 strains in 18 species in Basidiomycota (Table 3). The results showed that only 14 yeast strains of five species (Kodamaea ohmeri, M. caribbica, M. guilliermondii, Torulaspora indica and W. anomalus) belonging to the phylum Ascomycota could inhibit growth of rice pathogenic fungi; none of the strains in the phylum Basidiomycota inhibited any of the species of rice pathogenic fungi. Among antagonistic strains, three yeast strains, namely, T. indica DMKU-RP31, T. indica DMKU-RP35 and W. anomalus DMKU-RP25, inhibited all five rice pathogenic fungal species. The other strains inhibit one to three species of rice pathogenic fungi (Table 4). Among these antagonistic yeast strains, two strains of T. indica (DMKU-RP31 and DMKU-RP35) showed the strongest inhibition against all rice pathogenic fungal species except Cu. lunata DOAC 2313, whereby these two yeast strains showed equal inhibition to that of K. ohmeri DMKU-RP233.
Table 4.
Growth inhibition of rice pathogenic fungi by yeasts on potato dextrose agar (PDA) at 25 °C for 7 days.
| Yeast | Growth Inhibition by Yeast (%) a | ||||
|---|---|---|---|---|---|
| Cu. lunata DOAC 2313 | F. moniliforme DOAC 1224 | H. oryzae DOAC 2293 | R. solani DOAC 1406 | P. oryzae | |
| Kodamaea ohmeri | |||||
| DMKU-RP06 | 48.3 ± 1.4d | 23.3 ± 5.73cd | 0 | 0 | 0 |
| DMKU-RP18 | 0 | 20.0 ± 3.57e | 0 | 0 | 0 |
| DMKU-RP24 | 0 | 16.7 ± 4.72f | 0 | 0 | 45.9 ± 6.5c |
| DMKU-RP34 | 0 | 25.1 ± 2.31c | 0 | 0 | 0 |
| DMKU-RP44 | 0 | 23.2 ± 3.89cd | 0 | 0 | 0 |
| DMKU-RP57 | 44.7 ± 1.5e | 46.6 ± 2.53a | 0 | 0 | 0 |
| DMKU-RP233 | 63.1 ± 0.5a | 23.3 ± 4.75cd | 0 | 0 | 38.8 ± 7.8d |
| Meyerozyma caribbica | |||||
| DMKU-RP07 | 38.0 ± 0.6 | 23.3 ± 3.27cd | 0 | 0 | 0 |
| DMKU-RP55 | 0 | 25.0 ± 2.9c | 59.8 ± 0.86bc | 0 | 33.5 ± 7.9d |
| Meyerozyma guilliermondii | |||||
| DMKU-RP26 | 0 | 15.2 ± 2.6g | 0 | 0 | 43.4 ± 8.7c |
| Torulaspora indica | |||||
| DMKU-RP31 | 62.0 ± 1.7ab | 46.6 ± 3.2a | 64.1 ± 0.7a | 86.3 ± 0.9a | 62.6 ± 4.4a |
| DMKU-RP35 | 61.0 ± 0.9ab | 46.6 ± 3.2a | 64.9 ± 1.5a | 85.4 ± 0.8a | 62.2 ± 4.6a |
| Wickerhamomyces anomalus | |||||
| DMKU-RP04 | 50.3 ± 1.7c | 30.0 ± 2.0b | 48.5 ± 2.9d | 0 | 0 |
| DMKU-RP25 | 59.1 ± 1.0b | 29.9 ± 2.1b | 60.2 ± 1.1b | 79.7 ± 0.5b | 55.7 ± 6.6b |
a Inhibition (%) = (radius of control fungal colony-Radius of fungal colony grow with yeast)/radius of control fungal colony × 100. Each value represents a mean “±” standard deviation (SD). In the same column, data followed by different lower-case letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05.
3.3. Antagonistic Mechanisms of Antagonistic Yeasts
3.3.1. Production of Antifungal Volatile Organic Compounds
The 14 antagonistic yeast strains were tested for their production of antifungal VOCs capable of pathogenic fungal growth inhibition using double Petri dish assays. The results revealed that most of the strains produced VOCs inhibiting growth of the rice pathogenic fungi (Table 5). Among the strains tested, T. indica DMKU-RP31 and T. indica DMKU-RP35 were capable of producing VOCs that showed the strongest growth inhibition of Cu. lunata DOAC 2313 (60.2 and 59.6%), F. moniliforme DOAC 1224 (50.9 and 51.2%), P. oryzae (91.9%) and R. solani DOAC 1406 (94.1%), whereas VOCs produced by W. anomalus DMKU-RP25 also inhibited growth of R. solani DOAC 1406 by 94.1%. In addition, VOCs produced by T. indica DMKU-RP31 and W. anomalus DMKU-RP25 revealed the highest (49.3%) inhibition of H. oryzae DOAC 2293 among the five pathogens tested.
Table 5.
Production of antifungal volatile organic compounds and competition of nutrients of the antagonistic yeasts against rice pathogenic fungi.
| Rice Pathogenic Fungus and Antagonistic Yeast | Growth Inhibition by VOCs (%) a | Growth Inhibition in Different Nutrient Competition b | ||||
|---|---|---|---|---|---|---|
| A c | B d | C e | D f | Sum | ||
| Cu. lunata DOAC 2313 | ||||||
| K. ohmeri DMKU-RP06 | 15.2 ± 2.0d | 48.3a | 18.8b | 6.3c | 0d | - |
| K. ohmeri DMKU-RP57 | 35.1 ± 0.5b | 44.7a | 15.1b | 0c | 0c | - |
| K. ohmeri DMKU-RP233 | 19.4 ± 0.3cd | 31.4a | 7.5b | 0c | 0c | - |
| M. caribbica DMKU-RP07 | 32.1 ± 0.6bc | 38.0a | 17.2b | 0c | 0c | - |
| T. indica DMKU-RP31 | 60.2 ± 0.3a | 62.1a | 38.4b | 6.9c | 0d | - |
| T. indica DMKU-RP35 | 59.6 ± 0.9a | 61.0a | 38.3b | 4.4c | 0d | - |
| W. anomalus DMKU-RP04 | 15.7 ± 1.1d | 50.3a | 22.8b | 6.1c | 0d | - |
| W. anomalus DMKU-RP25 | 23.8 ± 1.2c | 59.1a | 21.1b | 6.1c | 0d | - |
| F. moniliforme DOAC 1224 | ||||||
| K. ohmeri DMKU-RP06 | 6.8 ± 1.3e | 23.3a | 0b | 0b | 0b | - |
| K. ohmeri DMKU-RP18 | 25.3 ± 1.2c | 20.0a | 6.9b | 0c | 0c | - |
| K. ohmeri DMKU-RP24 | 17.6 ± 6.9d | 16.7a | 10.1b | 0c | 0c | - |
| K. ohmeri DMKU-RP34 | 20.2 ± 0.5cd | 25.1a | 8.7b | 0c | 0c | - |
| K. ohmeri DMKU-RP44 | 23.3 ± 0.7cd | 23.2a | 8.6b | 2.3c | 0d | - |
| K. ohmeri DMKU-RP57 | 0f | 46.6a | 25.6b | 11.9c | 0d | - |
| K. ohmeri DMKU-RP233 | 22.1 ± 1.1cd | 23.3a | 5.8b | 0c | 0c | - |
| M. caribbica DMKU-RP07 | 6.4 ± 1.9e | 23.3a | 6.8b | 0c | 0d | - |
| M. caribbica DMKU-RP55 | 0f | 25.0a | 11.6b | 1.6c | 0d | - |
| M. guilliermondii DMKU-RP26 | 0f | 15.2a | 3.8b | 0c | 0c | - |
| T. indica DMKU-RP31 | 50.9 ± 0.5a | 46.6a | 28.1b | 11.1c | 0d | |
| T. indica DMKU-RP35 | 51.2 ± 1.2a | 46.6a | 24.7b | 5.9c | 0d | - |
| W. anomalus DMKU-RP04 | 41.1 ± 1.4b | 30.0a | 10.7b | 0c | 0c | - |
| W. anomalus DMKU-RP25 | 35.2 ± 2.5b | 30.0a | 20.0b | 5.6c | 0d | - |
| H. oryzae DOAC 2293 | ||||||
| M. caribbica DMKU-RP55 | 0d | 59.8a | 44.4b | 23.5c | 0d | - |
| T. indica DMKU-RP31 | 49.3 ± 0.5a | 64.1a | 47.3b | 25.8c | 0d | - |
| T. indica DMKU-RP35 | 31.5 ± 0.6b | 64.9a | 47.2b | 24.6c | 0d | - |
| W. anomalus DMKU-RP04 | 21.5 ± 0.4c | 48.5a | 37.9b | 23.8c | 0d | - |
| W. anomalus DMKU-RP25 | 49.3 ± 0.5a | 60.2a | 46.6b | 25.6c | 0d | - |
| R. solani DOAC 1406 | ||||||
| T. indica DMKU-RP31 | 94.1 ± 0.0a | 86.3a | 80.7b | 0c | 0c | - |
| T. indica DMKU-RP35 | 94.1 ± 0.0a | 85.4a | 80.3b | 0c | 0c | - |
| W. anomalus DMKU-RP25 | 94.1 ± 0.0a | 79.7a | 73.3b | 0c | 0c | - |
| P. oryzae | ||||||
| K. ohmeri DMKU-RP24 | 9.8 ± 1.0d | 36.0a | 10.6b | 0c | 0c | - |
| K. ohmeri DMKU-RP233 | 73.3 ± 1.1b | 27.8a | 13.8b | 0c | 0c | - |
| M. caribbica DMKU-RP55 | 8.1 ± 0.4d | 21.4a | 0b | 0b | 0b | - |
| M. guilliermondii DMKU-RP26 | 7.2 ± 0.7d | 33.3a | 10.3b | 0c | 0c | - |
| T. indica DMKU-RP31 | 91.9 ± 0.0a | 55.8a | 26.7b | 0c | 0c | - |
| T. indica DMKU-RP35 | 91.9 ± 0.0a | 55.3a | 26.7b | 0c | 0c | - |
| W. anomalus DMKU-RP25 | 52.2 ± 1.0c | 47.7a | 17.0b | 0c | 0c | - |
a Inhibition (%) = (diameter of control fungal colony - diameter of fungal colony grow with yeast/ diameter of control fungal colony) × 100; each value represents a mean “±” standard deviation (SD). In the same column, for each rice pathogenic fungus tested, data followed by the different lower-case letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05. b Inhibition (%) = (radius of control fungal colony - radius of fungal colony grow with yeast/radius of control fungal colony) × 100; each value represents a mean “±” standard deviation (SD). In the same row, data followed by the different lower-case letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05. c A: standard nutrient concentration (39 g/L PDA powder). d B: half of standard nutrient concentration (19.5 g/L PDA powder). e C: one-fourth of standard nutrient concentration (9.7 g/L PDA powder). f D: one-tenth of standard nutrient concentration (3.9 g/L PDA powder).
3.3.2. Production of β-Glucanase and Chitinase
Production of β-glucanase and chitinase of 14 antagonistic yeast strains was determined by cultivation in PDB at 25 °C for 5 days. The results revealed that 12 strains produced small amounts (0.2–27.1 mU/mL) of β-glucanase, with K. ohmeri DMKU-RP34 producing the greatest amount of β-glucanase (Table 6). Eight antagonistic yeast strains produced small amounts (2.0–249.2 mU/mL) of chitinase; K. ohmeri DMKU-RP233 produced the largest amount of chitinase.
Table 6.
Production of β-glucanase and chitinase, phosphate and zinc oxide solubilization, siderophore production, and biofilm formation of the antagonistic yeasts.
| Antagonistic Yeast | Enzyme Activities (mU/mL) | SE a | Siderophore Production b | Biofilm Formation | ||||
|---|---|---|---|---|---|---|---|---|
| Glucanase | Chitinase | Ca3(PO)4 | ZnO | A c | A value d | Sum e | ||
| K. ohmeri DMKU-RP06 | 0 | 25.1 ± 0.5 | 0 | 0 | 0 | 0.1664 ± 0.03 | 2.2 | + |
| K. ohmeri DMKU-RP18 | 0.2 ± 0.0 | 0 | 0 | 0 | 0 | 0.4848 ± 0.06 | 6.5 | + |
| K. ohmeri DMKU-RP24 | 4.6 ± 0.9 | 0 | 0 | 0 | 0 | 0.5614 ± 0.07 | 7.5 | + |
| K. ohmeri DMKU-RP34 | 27.1 ± 2.5 | 2.0 ± 0.4 | 0 | 0 | 0 | 0.3535 ± 0.02 | 4.7 | + |
| K. ohmeri DMKU-RP44 | 0 | 0 | 0 | 0 | 0 | 0.4905 ± 0.03 | 6.6 | + |
| K. ohmeri DMKU-RP57 | 11.0 ± 2.1 | 249.2 ± 39.6 | 0 | 0 | 0 | 0.1505 ± 0.02 | 2.0 | + |
| K. ohmeri DMKU-RP233 | 17.8 ± 2.2 | 0 | 0 | 0 | 0 | 0.1188 ± 0.01 | 1.6 | + |
| M. caribbica DMKU-RP07 | 4.7 ± 0.3 | 88.4 ± 5.9 | 0 | 0 | 0 | 0.1616 ± 0.01 | 2.2 | + |
| M. caribbica DMKU-RP55 | 0.6 ± 0.2 | 0 | 0 | 0 | 0 | 0.1351 ± 0.02 | 1.8 | + |
| M. guilliermondii DMKU-RP26 | 4.6 ± 1.2 | 0 | 0 | 0 | 0 | 0.0746 ± 0.07 | 1.0 | - |
| T. indica DMKU-RP31 | 1.7 ± 0.4 | 35.2 ± 3.5 | 1.2 | 1.2 | 0 | 0.5407 ± 0.06 | 7.3 | + |
| T. indica DMKU-RP35 | 2.2 ± 0.3 | 166.8 ± 5.7 | 1.2 | 1.2 | 0 | 0.4252 ± 0.03 | 5.7 | + |
| W. anomalus DMKU-RP04 | 4.2 ± 0.3 | 109.8 ± 9.4 | 1.0 | 1.0 | 3.0 | 0.2967 ± 0.01 | 4.0 | + |
| W. anomalus DMKU-RP25 | 1.8 ± 0.2 | 107.5 ± 7.0 | 1.0 | 1.2 | 2.9 | 0.2121 ± 0.02 | 2.8 | + |
a Solubilization efficiency (SE) = diameter of the halo zone (cm)/diameter of the colony (cm); b diameter of holo zone (cm). c The absorbance of the biofilm layer stained with violet crystal solution measured at 620 nm (average ± SD). d Average absorbance of samples as a portion of Ac (control); e Interpretation of biofilm formation: A ≤ Ac, no biofilm formation; Ac < A, biofilm formation. The optical density cut-off value (Ac) = 0.0745.
3.3.3. Competition for Nutrients and Space
The effect of nutrient concentration on the antagonistic activity of the antagonistic yeast strains against the five stains of rice pathogenic fungi was examined using dual culture on PDA with different nutrient concentrations. The results demonstrated that all the antagonistic strains showed the highest inhibition of the mycelium growth of rice pathogenic fungi when dual cultured on standard PDA with standard nutrient concentration (39 g/L PDA powder), and the reduction of growth inhibition was observed when half of the standard nutrient concentration was used, and most of the antagonistic yeast strains failed to inhibit the pathogenic fungal growth when one-fourth and one-tenth of the standard nutrient concentration were used (Table 5).
3.3.4. Phosphate and Zinc Oxide Solubilization
Phosphate and zinc oxide solubilizing activity of all antagonistic yeasts determined on Pikovskaya’s agar and zinc oxide agar showed that only four strains grew and produced halo zones around colonies. The phosphate and zinc oxide solubilization efficiency (SE) units were calculated to be 1.0–1.2 (Table 6).
3.3.5. Siderophore Production
Determination of the siderophore production of the antagonistic yeast strains on chrome azurol sulfonate (CAS) agar dishes revealed that only two strains of W. anomalus (DMKU-RP04 and DMKU-RP25) grew well and formed orange halo zones (2.9–3.0 cm) around the colony (Table 6). This indicated that these two antagonistic yeast strains produced siderophores.
3.3.6. Biofilm Formation
Biofilm formation of the selected antagonistic yeast strains was determined based on absorbance values obtained from the negative control (A620 = 0.0745). The results indicated that 13 strains formed biofilms and M. guilliermondii DMKU-RP26 did not form biofilm (Table 6).
3.4. Controlling of Rice Sheath Blight Disease in Rice Plants in the Greenhouse by the Selected Antagonistic Yeasts
Three antagonistic yeast strains, namely T. indica DMKU-RP31, T. indica DMKU-RP35 and W. anomalus DMKU-RP25, which inhibited growth of R. solani DOAC 1406, the cause of sheath blight disease, by 86.3, 85.4 and 79.7%, respectively, were selected and tested for control of rice sheath blight disease in rice plants in the greenhouse. Forty-five-day-old rice seedlings were sprayed with a cell suspension of one of the three antagonistic yeast strains or a chemical fungicide, 3% validamycin. Fifteen days after pathogenic fungal inoculation, rice sheath blight disease symptoms were observed and lesion height was measured. The result revealed that T. indica DMKU-RP31, T. indica DMKU-RP35 and W. anomalus DMKU-RP25 suppressed sheath blight disease by 70.3%, 66.0% and 66.4%, respectively (Table 7, Figure 1). On the other hand, 3% validamycin suppressed this disease by 83.8%, which was higher than any of the three antagonistic yeast strains.
Table 7.
Efficacy of the antagonistic yeasts in suppressing of rice sheath blight disease caused by R. solani DOAC 1406 in rice plants grown in pots in the greenhouse.
| Treatment | Plant Height (cm) a | Lesion Height (cm) b | Disease Incidence (%) | Disease Suppression (%) |
|---|---|---|---|---|
| Control (negative control) | 94.0 ± 2.9a | 0d | 0d | 0 |
| R. solani (positive control) | 92.0 ± 2.6a | 23.8 ± 1.6a | 25.9 ± 2.3a | 0 |
| R. solani + T. indica DMKU-RP31 | 93.0 ± 4.1a | 7.2 ± 1.4b | 7.7 ± 1.4b | 70.3 |
| R. solani + T. indica DMKU-RP35 | 93.2 ± 2.3a | 8.3 ± 1.5b | 8.8 ± 1.4b | 66.0 |
| R. solani + W. anomalus DMKU-RP25 | 92.6 ± 3.0a | 8.1 ± 1.0b | 8.7 ± 0.8b | 66.4 |
| R. solani + 3% Validamycin | 93.4 ± 3.5a | 3.9 ± 0.5c | 4.2 ± 0.6c | 83.8 |
Each value represents a mean “±” standard deviation (SD). In the same column, data followed by the different lower-case letters are significantly different according to Duncan’s multiple range test at p≤0.05. a Plant height was an average height from five rice plants (measured above ground to the tallest leaf). b Lesion height was an average lesion height from five rice plants; in each plant the height of all lesions was measured and averaged.
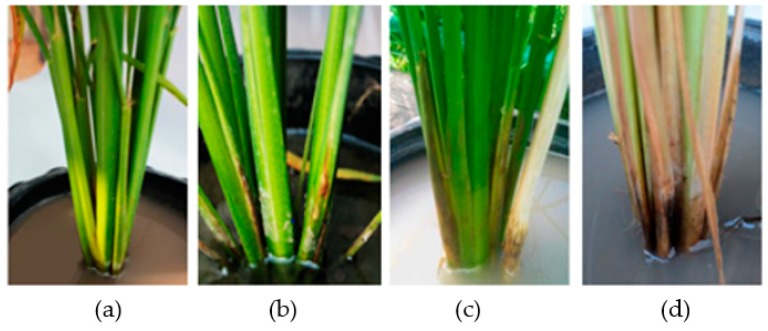
Figure 1.
Rice sheath blight disease lesions 15 days after R. solani DOAC 1406 inoculation. (a) Negative control: rice plant sprayed with distilled water, (b) rice plant inoculated with R. solani DOAC 1406 and sprayed with 3% validamycin, (c) rice plant inoculated with R. solani DOAC 1406 and sprayed with cell suspension of T. indica DMKU-RP31 and (d) positive control: rice plant inoculated with R. solani DOAC 1406 without any treatment.
3.5. Yeast Population and Development of Sheath Blight Lesion on Rice Plant
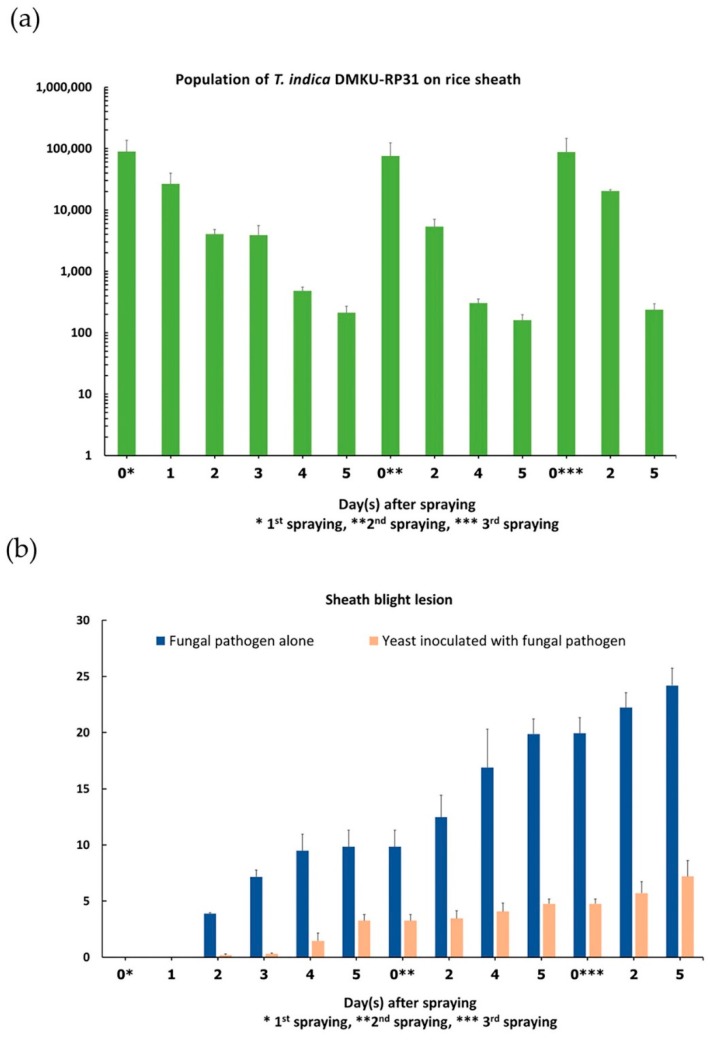
The population of T. indica DMKU-RP31 on the rice sheath surface during controlling rice sheath blight in a greenhouse experiment was examined and the results are shown in Figure 2a. The highest population of T. indica DMKU-RP31 (7.32x104 CFU/cm2) was observed at 0 day after spraying of yeast cell suspension (108 cells/mL) for 1 h, and then the population continuously decreased and reached the lowest (2.58 × 10 CFU/cm2) at 5 days. The second and third sprayings of yeast cell suspension (108 cells/mL) resulted in increasing of the yeast population to 7.29 × 105 and 8.74 × 105 CFU/cm2, respectively, after spraying for 1 h. However, the population continuously decreased again to 2.51 × 10 and 2.52 × 10 CFU/cm2, respectively, at 5 days.
Figure 2.
Yeast population and sheath blight diseases development. (a) Population of T. indica DMKU-RP31, (b) rice sheath blight lesion height.
The development of sheath blight lesion when inoculated with R. solani DOAC 1406 alone and with T. indica DMKU-RP31 was observed by measuring the lesion height. The results showed that the first lesion was observed at 2 days after inoculation of pathogenic fungus and continued to increase in both cases. However, inoculation of the antagonistic yeast with the pathogenic fungus resulted in lower lesion height than when inoculated with the pathogenic fungus alone (Figure 2b).
4. Discussion
In this work we isolated rice phylloplane yeasts by plating of leaf washing using YM agar and obtained a higher number of yeast strains in phylum Basidiomycota (84.4%) than in the phylum Ascomycota (15.6%). This result is in accordance with previous reports when the same isolation technique, the plating of leaf washings, was used to isolate yeast from sugarcane phylloplane for a diversity study in Brazil [4] and in Thailand [60]. Both investigations reported a majority of basidiomycetous yeast strains and a smaller number of ascomycetous strains. In the present study, Moesziomyces antarcticus was found to be present in as many as 55 rice leaf samples. Our result agrees well with the result of Nasanit et al. [7] that M. antarcticus was the most frequently detected species in the rice phylloplane when a culture-independent method was used to assess yeast diversity. Among 50 species obtained from rice phylloplane, ten species (C. tropicalis, D. nepalensis, M. antarcticus, M. aphidis P. flavescens, P. laurentii, P. rajasthanensis, R. taiwanensis and Sp. blumeae) were also detected when an enrichment isolation technique was used [61].
Various yeast species in both phyla have been reported to have antagonistic activity against plant pathogenic fungi. Examples of ascomycetous yeast species were C. oleophila, C. sake, Hanseniaspora uvarum, K. ohmeri, Metschnikowia fructicola, M. guilliermondii, S. cerevisiae and Torulaspora globosa, and examples of basidiomycetous yeast specieswere Cryptococcus albidus, P. laurentii and Sp. pararoseus [21,25,62,63,64,65]. In the present study, only 83 yeast strains out of 282 strains with active growing were evaluated for their antagonistic activities. This was because many yeast strains showed very weak growth or lost their viability after preservation at −80 °C for many months. The results revealed that 14 strains of five yeast species were capable of inhibiting growth of one to five rice pathogenic fungi, namely Cu. lunata, F. moniliforme, H. oryzae, P. oryzae and R. solani. Among the inhibiting species, T. indica and W. anomalus inhibited all five rice pathogenic fungi. The results indicated that among microflora associated with rice phylloplane, some antagonistic yeasts that have potential to control rice diseases caused by fungi are present. In this study, only ascomycetous yeast species were capable of antagonizing these fungal pathogens of rice. Some antagonistic yeast species found in the present study have been reported previously. M. guilliermondii could control the chilli anthracnose fungus after harvesting [31]. K. ohmeri revealed growth inhibition of Penicillium expansum, a postharvest pathogenic fungus [62]. W. anomalus was reported for its biocontrol activity against Alternaria alternata, Aspergillus carbonarius, Botrytis cinerea, Monilinia fructicola and P. digitatum [66]. Among Torulaspora species, only T. globose was previously reported for its biocontrol activity against Colletotrichum sublineolum in sorghum [21]. This is the first report on the strong antagonistic activity of T. indica strains against pathogenic fungi causing rice diseases, namely Cu. lunata, F. moniliforme, H. oryzae, P. oryzae and R. solani
Direct and indirect antagonistic mechanisms of the antagonistic yeast strains obtained in this study were evaluated. This study showed that VOC production played a role in the antagonistic activity of T. indica, W. anomalus and K. ohmeri against Cu. lunata, F. moniliforme, P. oryzae and R. solani but not H. oryzae. Our findings agree with those reports that emission of VOCs by antagonistic yeasts have proven to be one of the important direct antagonistic mechanisms against pathogenic fungi [27,28,29,30]. In this study, some yeast strains produced the fungal cell wall lytic enzymes β-1, 3-glucanase (12 strains) and chitinase (six strains) in liquid medium, although with relatively low activities. Therefore, the production of β-1,3-glucanase and chitinase seems to be one of the direct antagonistic mechanisms of the antagonistic yeast for inhibition of pathogenic fungi used in this study, which is same as that observed in various antagonistic yeast such as C. oleophila, M. guilliermondii and P. membranifaciens [22,31]. Competition for nutrients between antagonists and pathogenic fungi is among the direct antagonistic mechanisms. This mechanism is not easy to demonstrate on plants because it is difficult to control the other mechanisms [67]. In this study, we tested this mechanism in vitro on PDA with different nutrient concentration. The concentration used in the tests could be different from that available in plants. Unfortunately, we did not check the concentration of nutrients in the rice sheath. The results of the present study showed that the highest fungal mycelial growth inhibition was observed when standard nutrient concentration was used and the efficacy of fugal mycelial growth inhibition decreased when cultured at lower nutrient concentrations. These results could be interpreted to mean that nutrient competition was not a mechanism of these antagonistic yeasts against rice pathogenic fungi. Our study revealed that all of the antagonistic yeasts, except M. guilliermondii DMKU-RP26, showed the ability to form biofilms when grown in PDB. This ability could be one of the direct antagonistic mechanisms of these antagonistic yeasts. In the present study, we found that only the strains of W. anomalus produced siderophores. Therefore, this could be one of the antagonistic mechanisms by which W. anomalus controls the tested rice pathogenic fungi. In this study, strains of T. indica and W. anomalus showed phosphate and zinc oxide solubilizing activities. Our result appeared to be the same as that of T. globosa which exhibited phosphate solubilization to promote plant growth and be one of the biocontrol mechanisms [21]. Our results indicated that for T. indica and W. anomalus, VOC production was the major mechanism, whereas production of β -1,3-glucanase and chitinase, biofilm formation, and phosphate and zinc oxide solubilization were hypothesized as possible additional mechanisms. On the other hand, in the other yeast species, such as K. ohmeri, M. caribbica and M. guilliermondii, the antagonistic activity may result from the production of VOCs, β -1,3-glucanase and chitinase and biofilm, however, they did not have the ability to solubilize phosphate and zinc oxide. The antagonistic mechanisms determined in vivo should be further studied.
In this study, the antagonistic yeast cell concentration used in controlling rice sheath blight was 108 cells/mL, which was the same as that used by Rosa et al. [21] to evaluate the efficacy of T. globosa in controlling anthracnose in sorghum caused by fungus. They reported that this concentration significantly reduced the anthracnose of sorghum. Recently, Khunamwong et al. [63] reported that it was possible to significantly suppress rice sheath blight disease in a greenhouse by spraying a 108 cells/mL of W. anomalus DMKU-CE52 and W. anomalus DMKU-RE13. In this study, examination of yeast population on rice sheath surface during controlling rice sheath blight revealed a decrease of population from 7.29–8.74 × 104 CFU/cm2 to 2.51–2.58 × 10 CFU/cm2 after 5 days of spraying yeast cell suspension. However, the disease lesion development when inoculating R. solani with yeast was lower when compared to inoculating R. solani alone. This indicated that a yeast population of 102–105 CFU/cm2 was enough to reduce sheath blight disease. However, Fokkema et al. [68] suggested that at least 104 CFU/cm2 of yeast was necessary to control necrotrophic fungal pathogens on rye and wheat leaves and the higher values of antagonist populations might be required to obtain a better control of decay. The decreasing of yeast population on leaf surface was already found on bean leaves when applied with R. glutinis or Cry. albidus [69] and on wheat leaves applied with Sp. roseus or Cry. laurentii [70].
Sheath blight is the second most important rice disease worldwide after blast [17,18]. In this study, strains of T. indica (two strains) and W. anomalus (one strain) were evaluated for their ability to control rice sheath blight disease in rice plants in the greenhouse. Although the suppression of rice sheath blight by these antagonistic yeast strains was high, it did not reach the level of efficacy of the chemical fungicide, validamycin. However, using antagonistic yeasts as biocontrol agents in agricultural crops is an environmentally friendly alternative method. Some bacteria, actinomycetes and yeast strains have been found to be capable of controlling rice sheath blight disease in the greenhouse. A strain of Streptomyces philanthi was found to be effective in the control of rice sheath blight disease in the greenhouse when either spores or a cell suspension was applied [14]. Sporobolomyces sp. LR951565 was reported to control rice sheath blight disease in the greenhouse [71]; however, the efficacy of this yeast strain seems to be less than the strains in this study. W. anomalus DMKU-CE53 and W. anomalus DMKU-RE13 were reported to control rice sheath blight disease in the greenhouse and the biocontrol efficiency were 55.2–65.1%. This is similar to the efficiency of W. anomalus DMKU-RP25, the strain in this study (66.4%.) [65]. To our knowledge, this is the first report of using T. indica for the biocontrol of rice sheath blight disease caused by R. solani.
Acknowledgments
The authors would like to thank Phatthalung Rice Research Center, Phatthalung, Thailand for providing Pyricularia oryzae, the cause of blast disease in rice plant, and Pathum Thani Rice Research Center, Pathum Thani, Thailand for providing Chai Nat 1 rice cultivar seeds.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/3/362/s1, Table S1: Sequence similarity analysis of the D1/D2 domain of rice phylloplane yeasts and their closely related species, Table S2: Sequence similarity analysis of the ITS region of corn phylloplane isolates showing ≥2 nucleotide substitutions in the D1/D2 region, Figure S1: Phylogenetic relationship of the sequences of the D1/D2 region of the LSU rRNA gene of yeast strains isolated from the phylloplane of rice belonging to the phylum Ascomycota, subphylum Saccharomycotina and their closely related yeast, Figure S2: Phylogenetic relationship of the sequences of the D1/D2 region of the LSU rRNA gene of yeast strains isolated from the phylloplane of rice belonging to the phylum Basidiomycota and their closely related yeast.
Author Contributions
P.I. performed the data curation, formal analysis, investigation, methodology and writing—original draft preparation. P.K., S.J. and S.A.-i. performed investigation and methodology. S.L. and W.I.; provided conceptualization and supervision, S.L. provided funding acquisition, project administration, resources, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Thailand Research Fund through the TRF Research-Team Promotion Grant (RTA5480009 and RTA6080004) and the Royal Golden Jubilee PhD programme grant no. PHD/0025/2556, Thailand.
Conflicts of Interest
The authors declare that they have no conflicts of interest in the publication.
References
- 1.Phaff H.J., Starmer W.T. Yeasts associated with plants, insects and soil. In: Rose A.H., Harrison J.S., editors. The Yeasts. 2nd ed. Academic Press; London, UK: 1987. pp. 123–180. [Google Scholar]
- 2.Fiala V., Glad C., Martin M., Jolivet E., Derridj S. Occurrence of soluble carbohydrates on thephylloplane of maize (Zea mays L.): Variations in relation to leaf heterogeneity and position on the plant. New Phytol. 1990;115:609–615. doi: 10.1111/j.1469-8137.1990.tb00492.x. [DOI] [Google Scholar]
- 3.Xin G., Glawe D., Doty S.L. Characterization of three endophytic, indole-3-acetic acidproducing yeasts occurring in Populus trees. Mycol. Res. 2009;113:973–980. doi: 10.1016/j.mycres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 4.de Azeredo L.A.I., Gomes E.A.T., Mendonca-Hagler L.C., Hagler A.N. Yeast communities associated with sugarcane in Campos, Rio de Janeiro, Brazil. Int. Microbiol. 1998;1:205–208. [PubMed] [Google Scholar]
- 5.Inácio J., Portugal L., Spencer-Martins I., Fonseca Á. Phylloplane yeasts from Portugal: Seven novel anamorphic species in the Tremellales lineage of the Hymenomycetes (Basidiomycota) producing orange-coloured colonies. FEMS Yeast Res. 2005;5:1167–1183. doi: 10.1016/j.femsyr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Nasanit R., Jaibangyang S., Tantirungkij M., Limtong S. Yeast diversity and novel yeast D1/D2 sequences from corn phylloplane obtained by a culture-independent approach. Antonie Leeuwenhoek. 2016;109:1615–1634. doi: 10.1007/s10482-016-0762-x. [DOI] [PubMed] [Google Scholar]
- 7.Nasanit R., Krataithong K., Tantirungkij M., Limtong S. Assessment of epiphytic yeast diversity in rice (Oryza sativa) phyllosphere in Thailand by a culture-independent approach. Antonie Leeuwenhoek. 2015;107:1475–1490. doi: 10.1007/s10482-015-0442-2. [DOI] [PubMed] [Google Scholar]
- 8.Nasanit R., Tangwong-o-thai A., Tantirungkij M., Limtong S. The assessment of epiphytic yeast diversity in sugarcane phyllosphere in Thailand by culture-independent method. Fungal Biol. 2015;119:1145–1157. doi: 10.1016/j.funbio.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Plodpai P., Chuenchitt S., Petcharat V., Chakthong S., Voravuthikunchai S.P. Anti-Rhizoctonia solani activity by Desmos chinensis extracts and its mechanism of action. Crop Prot. 2012;43:65–71. doi: 10.1016/j.cropro.2012.09.004. [DOI] [Google Scholar]
- 10.Boukaew S., Prasertsan P. Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot. 2014;61:1–10. doi: 10.1016/j.cropro.2014.02.012. [DOI] [Google Scholar]
- 11.Groth D.E. Azoxystrobin rate and timing effects on rice sheath blight incidence and severity and rice grain and milling yields. Plant Dis. 2005;89:1171–1174. doi: 10.1094/PD-89-1171. [DOI] [PubMed] [Google Scholar]
- 12.Nandakumar R., Babu S., Viswanathan R., Raguchander T., Samiyappan R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol. Biochem. 2001;33:603–612. doi: 10.1016/S0038-0717(00)00202-9. [DOI] [Google Scholar]
- 13.Groth D.E., Bond J.A. Effects of cultivars and fungicides on rice sheath blight, yield, and quality. Plant Dis. 2007;91:1647–1650. doi: 10.1094/PDIS-91-12-1647. [DOI] [PubMed] [Google Scholar]
- 14.Boukaew S., Klinmanee C., Prasertsan P. Potential for the integration of biological and chemical control of sheath blight disease caused by Rhizoctonia solani on rice. World J. Microbiol. Biotechnol. 2013;29:1885–1893. doi: 10.1007/s11274-013-1353-x. [DOI] [PubMed] [Google Scholar]
- 15.Williamson M.A., Fokkema N.J. Phyllosphere yeasts antagonize penetration from appressoria and subsequent infection of maize leaves by Colletotrichum graminicola. Neth. J. Plant Pathol. 1985;91:265–276. doi: 10.1007/BF02000012. [DOI] [Google Scholar]
- 16.Ziedan E.S.H.E., Farrag E.S. Application of yeasts as biocontrol agents for controlling foliar diseases on sugar beet plants. J. Agric. Technol. 2011;7:1789–1799. [Google Scholar]
- 17.Curtis F.D., Cicco V.D., Lima G. Efficacy of biocontrol yeasts combined with calcium silicate or Sulphur for controling durum wheat powdery mildew and increasing grain yield components. Field Crops Res. 2012;134:36–46. doi: 10.1016/j.fcr.2012.04.014. [DOI] [Google Scholar]
- 18.Suzzi G., Romano P., Ponti I., Montuschi C. Natural wine yeasts as biocontrol agents. J. Appl. Bacteriol. 1995;78:304–308. doi: 10.1111/j.1365-2672.1995.tb05030.x. [DOI] [Google Scholar]
- 19.Bar-Shimon M., Yehuda H., Cohen L., Weiss B., Kobeshnikov A., Duas A., Goldway M., Wisniewski M., Droby S. Characterization of extracellular lytic enzymes produced by the yeast biological agent Candida oleophila. Curr. Genet. 2004;45:140–148. doi: 10.1007/s00294-003-0471-7. [DOI] [PubMed] [Google Scholar]
- 20.Droby S., Wisniewski M., Macarisin D., Wilson C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009;52:137–145. doi: 10.1016/j.postharvbio.2008.11.009. [DOI] [Google Scholar]
- 21.Rosa M.M., Tauk-Tornisielo S.M., Rampazzo P.E., Ceccato-Antonini S.R. Evaluation of the biological control by the yeast Torulaspora globosa against Colletotrichum sublineolum in sorghum. World J. Microbiol. Biotechnol. 2010;26:1491–1502. doi: 10.1007/s11274-010-0324-8. [DOI] [Google Scholar]
- 22.Schisler D.A., Janisiewicz W.J., Boekhout T., Kurtzman C.P. Agriculturally important yeasts: Biological control of field and postharvest diseases using yeast antagonists, and yeasts as pathogens of plants. In: Kurtzman C.P., Fell J.W., Boekhout T., editors. The Yeasts, A Taxonomic Study. 5th ed. Elsevier; New York, NY, USA: 2011. pp. 45–52. [Google Scholar]
- 23.Bautista-Rosales P.U., Calderon-Santoyo M., Servín-Villegas R., Ochoa-Álvarez N.A., Ragazzo-Sánchez J.A. Action mechanisms of the yeast Meyerozyma caribbica for the control of the phytopathogen Colletotrichum gloeosporioides in mangoes. Biol. Control. 2013;65:293–301. doi: 10.1016/j.biocontrol.2013.03.010. [DOI] [Google Scholar]
- 24.Spadaro D., Droby S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016;47:39–49. doi: 10.1016/j.tifs.2015.11.003. [DOI] [Google Scholar]
- 25.Cordero-Bueso G., Mangieri N., Maghradez D., Foschino R., Valdetara F., Cantoral J.M., Vigentini I. Wild grape-associated yeasts as promising biological agents against Vitis vinifera fungal pathogen. Front. Microbiol. 2017;8:2025. doi: 10.3389/fmicb.2017.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vespermann A., Kai M., Piechulla B. Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl. Environ. Microbiol. 2007;73:5639–5641. doi: 10.1128/AEM.01078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang R., Li G.Q., Zhang J., Yang L., Che H.J., Jiang D.H., Huang H.C. Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology. 2011;101:859–869. doi: 10.1094/PHYTO-09-10-0255. [DOI] [PubMed] [Google Scholar]
- 28.Ando H., Hatanaka K., Ohata I., Yamashita-Kitaguchi Y., Kurata A., Kishimoto N. Antifungal activities of volatile substances generated by yeast isolated from Iranian commercial cheese. Food Control. 2012;26:472–478. doi: 10.1016/j.foodcont.2012.02.017. [DOI] [Google Scholar]
- 29.Huang R., Che H.J., Zhang J., Yang L., Jiang D.H., Li G.Q. Evaluation of Sporidiobolus pararoseus strain YCXT3 as biocontrol agent of Botrytis cinerea on post-harvest strawberry fruits. Biol. Control. 2012;62:53–63. doi: 10.1016/j.biocontrol.2012.02.010. [DOI] [Google Scholar]
- 30.Hua S.S.T., Beck J.J., Sarreal S.B.L., Gee W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014;30:71–78. doi: 10.1007/s12550-014-0189-z. [DOI] [PubMed] [Google Scholar]
- 31.Chanchaichaovivat A., Panijpan B., Ruenwongsa P. Putative modes of action of Pichia guilliermondii strain R13 in controlling chilli anthracnose after harvest. Biol. Control. 2008;47:207–215. doi: 10.1016/j.biocontrol.2008.07.018. [DOI] [Google Scholar]
- 32.Masih E.I., Paul B. Secretion of β-1,3-glucanases by the yeast Pichia membranifaciens and its possible role in the biocontrol of Botrytis cinerea causing grey mold disease of the grapevine. Curr. Microbiol. 2002;44:391–395. doi: 10.1007/s00284-001-0011-y. [DOI] [PubMed] [Google Scholar]
- 33.Dikin A., Sijam K., Kadir J., Seman I.A. Mode of Action of Antimicrobial Substances from Burkholderia multivorans and Microbacterium testaceum Against Scizophyllum commune. J. Agric. Biol. 2007;9:311–314. [Google Scholar]
- 34.Calvente V., De Orellano M.E., Sansone G., Benuzzi D., De Tosetti M.S. Effect of nitrogen source and pH on siderophore production by Rhodotorula strains and their application to biocontrol of phytopathogenic moulds. J. Ind. Microbiol. Biot. 2001;26:226–229. doi: 10.1038/sj.jim.7000117. [DOI] [PubMed] [Google Scholar]
- 35.Sansone G., Rezza I., Calvente V., Benuzzi D., de Tosetti M.I.S. Control of Botrytis cinerea strains resistant to iprodione in apple with rhodotorulic acid and yeasts. Postharvest Biol. Technol. 2005;35:245–251. doi: 10.1016/j.postharvbio.2004.09.005. [DOI] [Google Scholar]
- 36.Sharma R.R., Singh D., Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009;50:205–221. doi: 10.1016/j.biocontrol.2009.05.001. [DOI] [Google Scholar]
- 37.Zhang D., Spadaro D., Garibaldi A., Gullino M.L. Efficacy of the antagonist Aureobasidium pullulans PL5 against postharvest pathogens of peach, apple and plum and its modes of action. Biol. Control. 2010;54:172–180. doi: 10.1016/j.biocontrol.2010.05.003. [DOI] [Google Scholar]
- 38.Platania C., Restuccia C., Muccilli S., Cirvilleri G. Efficacy of killer yeasts in the biological control of Penicillium digitatum on Tarocco orange fruits (Citrus sinensis) Food Microbiol. 2012;30:219–225. doi: 10.1016/j.fm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Tian Y.Q., Li W., Jiang Z.T., Jing M.M., Shao Y.Z. The preservation effect of Metschnikowia pulcherrima yeast on anthracnose of postharvest mango fruits and the possible mechanism. Food Sci. Biotechnol. 2018;27:95–105. doi: 10.1007/s10068-017-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surussawadee J., Jindamorakot S., Nakase T., Lee C.F., Limtong S. Hannaella phyllophila sp. nov., a novel basidiomycetous yeast species associated with plants in Thailand and Taiwan. Int. J. Syst. Evolut. Microbiol. 2015;65:2135–2140. doi: 10.1099/ijs.0.000231. [DOI] [PubMed] [Google Scholar]
- 41.Limtong S., Koowadjanakul N., Jindamorakot S., Yongmanitchai W., Nakase T. Candida sirachaensis sp. nov. and Candida sakaeoensis sp. nov. two anamorphic yeast species from phylloplane in Thailand. Antonie Leeuwenhoek. 2012;102:221–229. doi: 10.1007/s10482-012-9728-9. [DOI] [PubMed] [Google Scholar]
- 42.Kurtzman C.P., Robnett C.J. Identification and phylogeny of ascomycete yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek. 1998;73:331–371. doi: 10.1023/A:1001761008817. [DOI] [PubMed] [Google Scholar]
- 43.Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fell J.W., Boekhout T., Fonseca A., Scorzetti G., Statzell-Tallman A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evolut. Microbiol. 2000;50:1351–1371. doi: 10.1099/00207713-50-3-1351. [DOI] [PubMed] [Google Scholar]
- 45.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evolut. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Francesco A., Ugolini L., Lazzeri L., Mari M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control. 2015;81:8–14. doi: 10.1016/j.biocontrol.2014.10.004. [DOI] [Google Scholar]
- 48.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 49.Khan M.A., Hamid R., Ahmad M., Abdin M.Z., Javed S. Optimization of culture media for enhanced chitinase production from a novel strain of Stenotrophomonas maltophilia using response surface methodology. J. Microbiol. Biotechnol. 2010;20:1597–1602. doi: 10.4014/jmb.0909.09040. [DOI] [PubMed] [Google Scholar]
- 50.Zaidi S., Usmani S., Singh B.R., Musarrat J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991–997. doi: 10.1016/j.chemosphere.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 51.Rokhbakhsh-Zamin F., Sachdev D., Kazemi-Pour N., Engineer A., Pardesi K.R., Zinjarde S., Dhakephalka P.K., Chopade B.A. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011;21:556–566. [PubMed] [Google Scholar]
- 52.Schwyn B., Neilands J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 53.Růžička F., Holá V., Votava M., Tekkalov R. Importance of biofilm in Candida parapsilosis and evaluation of its susceptibility to antifungal agents by colorimetric method. Folia Microbiol. 2007;52:209–214. doi: 10.1007/BF02931300. [DOI] [PubMed] [Google Scholar]
- 54.Spadaro D., Ciavorella A., Zhang D., Garibaldi A., Gullino M.L. Effect of culture media and pH on the biomass production and biocontrol efficacy of a Metschnikowia pulcherrima strain to be used as a biofungicide for postharvest disease control. Can. J. Microbiol. 2010;56:128–137. doi: 10.1139/W09-117. [DOI] [PubMed] [Google Scholar]
- 55.Park D., Sayler R., Hong Y., Nam M., Yang Y. A method for inoculation and evaluation of rice sheath blight disease. Plant Dis. 2008;92:25–29. doi: 10.1094/PDIS-92-1-0025. [DOI] [PubMed] [Google Scholar]
- 56.Jayaprakashvel M., Selvakumar M., Srinivasan K., Ramesh S., Mathivanan N. Control of sheath blight disease in rice by thermostable secondary metabolites of Trichoderma roseum MML003. Eur. J. Plant Pathol. 2010;126:229–239. doi: 10.1007/s10658-009-9535-y. [DOI] [Google Scholar]
- 57.Nix S., Burpee L.L., Buck J.W. Responses of 2 epiphytic yeasts to foliar infection by Rhizoctonia solani or mechanical wounding on the phylloplane of tall fescue. Can. J. Microbiol. 2009;55:1160–1165. doi: 10.1139/W09-072. [DOI] [PubMed] [Google Scholar]
- 58.Jindamorakot S., Am-In S., Kaewwichian R., Limtong S. Yamadazyma insecticola fa, sp. nov. and Yamadazyma epiphylla fa, sp. nov., two novel yeast species. Int. J. Syst. Evolut. Microbiol. 2015;65:1290–1296. doi: 10.1099/ijs.0.000100. [DOI] [PubMed] [Google Scholar]
- 59.Kaewwichian R., Jindamorakot S., Am-In S., Sipiczki M., Limtong S. Hannaella siamensis sp. nov. and Hannaella phetchabunensis sp. nov., two new anamorphic basidiomycetous yeast species isolated from plants. Int. J. Syst. Evolut. Microbiol. 2015;65:1297–1303. doi: 10.1099/ijs.0.000101. [DOI] [PubMed] [Google Scholar]
- 60.Srisuk N., Nutaratat P., Surussawadee J., Limtong S. Yeast communities in sugarcane phylloplane. Microbiology. 2019;88:353–369. doi: 10.1134/S0026261719030135. [DOI] [Google Scholar]
- 61.Limtong S., Kaewwichian R. The diversity of culturable yeasts in the phylloplane of rice in Thailand. Ann. Microbiol. 2015;65:667–675. doi: 10.1007/s13213-014-0905-0. [DOI] [PubMed] [Google Scholar]
- 62.Coelho A.R., Celli M.G., Ono E.Y.S., Wosiacki G., Hoffmann F.L., Pagnocca F.C., Hirooka Y.E. Penicillium expansum versus antagonist yeasts and patulin degradation in vitro. Braz. Arch. Biol. Technol. 2007;50:725–733. doi: 10.1590/S1516-89132007000400019. [DOI] [Google Scholar]
- 63.Sundh I., Melin P. Safety and regulation of yeasts used for biocontrol or biopreservation in the food or feed chain. Antonie Leeuwenhoek. 2011;99:113–119. doi: 10.1007/s10482-010-9528-z. [DOI] [PubMed] [Google Scholar]
- 64.Freimoser F.M., Rueda-Mejia M.P., Tilocca B., Migheli Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019;35:154. doi: 10.1007/s11274-019-2728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khunnamwong P., Lertwattanasakul N., Jindamorakot S., Suwannarach N., Matsui K., Limtong S. Evaluation of antagonistic activity and mechanisms of endophytic yeasts against pathogenic fungi causing economic crop diseases. Folia Microbiol. 2019:1–18. doi: 10.1007/s12223-019-00764-6. [DOI] [PubMed] [Google Scholar]
- 66.Oro L., Feliziani E., Ciania M., Romanazzib G., Comitinia F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018;265:18–22. doi: 10.1016/j.ijfoodmicro.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 67.Sperandio E.M., do Vale H.M.M., Moreira G.A.M. Yeasts from native Brazilian Cerrado plants: Occurrence, diversity and use in the biocontrol of citrus green mould. Fungal Boil. 2015;119:984–993. doi: 10.1016/j.funbio.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Fokkema N.J., den Houter J.G., Kosterman Y.J.C., Nelis A.L. Manipulation of yeasts on field-grown wheat leaves and their antagonistic effect on Cochliobolus sativus and Septoria nodorum. Trans. Br. Mycol. Soc. 1979;72:19–29. doi: 10.1016/S0007-1536(79)80003-0. [DOI] [Google Scholar]
- 69.Elead Y., Köhl J., Fokkema N.J. Control of infection and sporulation of Botrytis cinerea on bean and tomato by saprophytic bacteria and fungi. Eur. J. Plant Pathol. 1994;100:315–336. doi: 10.1007/BF01876443. [DOI] [Google Scholar]
- 70.Dik A.J., Fokkema N.J., van Pelt J.A. Influence of climatic and nutritional factors on yeast population dynamics in the phyllosphere of wheat. Microb. Ecol. 1992;23:41–52. doi: 10.1007/BF00165906. [DOI] [PubMed] [Google Scholar]
- 71.Shahjahan A.K.M., Rush M.C., Groth D.E. Phylloplane yeasts as potential biocontrol agents for rice sheath blight disease. In: Sreenivasaprasad S., Johnson R., editors. Major Fungal Diseases of Rice. Kluwer Academic; New York, NY, USA: 2001. pp. 235–252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.