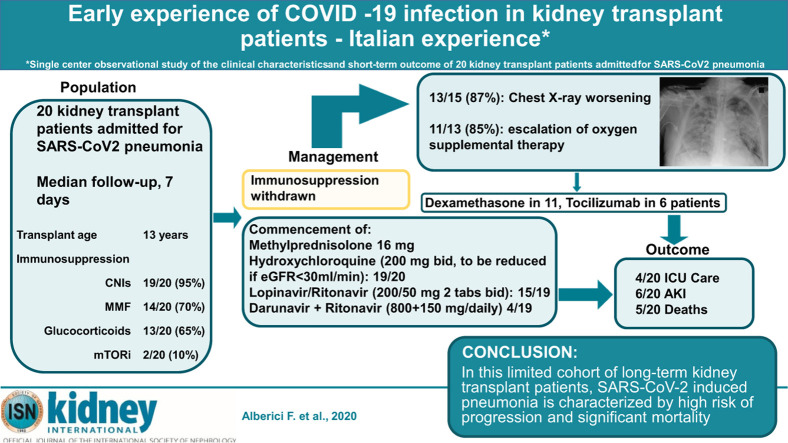
Abstract
The outcome of SARS-CoV2 infection in patients who have received a kidney allograft and are being treated with immunosuppression is unclear. We describe 20 kidney transplant recipients (median age 59 years [inter quartile range 51-64 years], median age of transplant 13 years [9-20 years], baseline eGFR 36.5 [23-47.5]) with SARS-CoV2 induced pneumonia. At admission, all had immunosuppression withdrawn and were started on methylprednisolone 16 mg/day, all but one was commenced on antiviral therapy and hydroxychloroquine with doses adjusted for kidney function. At baseline, all patients presented fever but only one complained of difficulty in breathing. Half of patients showed chest radiographic evidence of bilateral infiltrates while the other half showed unilateral changes or no infiltrates. During a median follow-up of seven days, 87% experienced a radiological progression and among those 73% required escalation of oxygen therapy. Six patients developed acute kidney injury with one requiring hemodialysis. Six of 12 patients were treated with tocilizumab, a humanized monoclonal antibody to the IL-6 receptor. Overall, five kidney transplant recipients died after a median period of 15 days [15-19] from symptom onset. These preliminary findings describe a rapid clinical deterioration associated with chest radiographic deterioration and escalating oxygen requirement in renal transplant recipients with SARS-Cov2 pneumonia. Thus, in this limited cohort of long-term kidney transplant patients, SARS-CoV-2 induced pneumonia is characterized by high risk of progression and significant mortality.
Keywords: acute kidney injury, inflammation, tocilizumab, transplantation
Graphical abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) infection is posing challenges to all the health systems in the world. The ideal therapeutic approach is still debated, and data on subgroups of patients at high risk are still scarce.1 We have developed an internal treatment protocol for the management of patients with SARS-CoV2 pneumonia who had undergone renal transplantation.2 Since the first days of March 2020, we reorganized our ward to admit patients with SARS-CoV2 pneumonia who had undergone kidney transplantation; we felt this rearrangement was necessary because our center acts as referral for a population of 1200 patients who have had kidney transplantation. Due to the onset of the coronavirus disease 2019 epidemic, acute transplantation surgery was suspended in our center from February 20, 2020. The first admission of a patient with SARS-CoV2 pneumonia who had undergone transplantation occurred on February 27, 2020, the second 6 days later, and subsequently up to March 24, 2020 (when this analysis was performed and patients’ follow-up was censored), an average rate of 1.2 patients who had undergone kidney transplantation were admitted per day. We describe here the clinical course and renal outcomes of the first 20 patients who had undergone kidney transplantation admitted and followed in our unit with pneumonia secondary to SARS-CoV2 infection.
Results
In this report, we describe the progress of all patients with SARS-CoV2 pneumonia who had undergone kidney transplantation admitted up to March 24, 2020. These patients had a median inpatient stay of 7 days (interquartile range [IQR], 4–15), and the main baseline clinical characteristics are shown in Tables 1 and 2 . Briefly, all patients presented with fever; however, only 1 of the 20 complained of dyspnea; 50% of the patients who had undergone transplantation had radiographic changes of bilateral infiltrates on admission, while the remaining 50% showed unilateral changes or no infiltrates; 7 did not require supplemental oxygen at admission.
Table 1.
Baseline clinical characteristics of 20 patients affected by SARS-CoV2 infection followed in our unit who had undergone kidney transplantation
| Characteristics | |
|---|---|
| Male/female (n) | 16/4 |
| Age (yr) | 59 (51–64) |
| Comorbidities (%) | |
| Hypertension | 85 |
| Ischemic cardiac disease | 15 |
| Diabetes | 15 |
| HCV infection | 10 |
| Kidney transplantation age (yr) | 13 (9–20) |
| Time from symptoms onset to admission (d) | 5.5 (3.3–8) |
| Baseline serum creatinine (mg/dl) | 1.95 (1.5–2.8) |
| Baseline eGFR (ml/min)a | 36.5 (23–47.5) |
| Baseline immunosuppression (n) | |
| CNIs | 19/20 |
| MMF | 14/20 |
| Low-dose glucocorticoidb | 13/20 |
| mTORi | 2/20 |
| SARS-CoV2 infection symptoms onset (%) | |
| Temperature >37.5 °C | 100 |
| Cough | 50 |
| Gastrointestinal symptoms | 15 |
| Pharyngitis | 10 |
| Shortness of breath | 5 |
| Myalgia | 5 |
| Chest X-ray at hospital admission (%) | |
| No infiltrates | 15 |
| Unilateral infiltrates | 35 |
| Bilateral infiltrates | 50 |
| Blood tests at hospital admission | |
| WBCs (NV: 4.00–10.80 × 103/μl) | 5470 (4115–6193) |
| Neutrophils (NV: 1.50–8.00 × 103/μl) | 3700 (2280–4500) |
| Lymphocytes (NV: 0.90–4.00 × 103/μl) | 1170 (620–1305) |
| Platelets (NV: 130–400 × 103/μl) | 196,000 (119,000–202,000) |
| LDH (NV: 135–225 U/l) | 231 (190–260) |
| CPK (NV: 39–308 U/l) | 69 (44–121) |
| AST (NV: 18–54 U/l) | 37 (26–35) |
| ALT (NV: 10–50 U/l) | 23 (16–30) |
| Bilirubin (NV: <1.20 mg/dl) | 0.8 (0.4–0.9) |
| CRP (NV: <5.0 mg/l) | 49 (19–62) |
| Procalcitonin (NV: <0.5 ng/ml) | 0.22 (0.1–0.35) |
| Ferritin (NV: 30–400 μg/l) | 831 (284–882) |
| Fibrinogen (NV: 170–410 mg/d) | 461 (343–614) |
| D-dimer (NV: <232 ng/ml) | 279 (277–563) |
| Urea (NV: 17–49 mg/dl) | 46 (48–106) |
| Creatinine (NV: 0.70–1.20 mg/dl) | 1.8 (1.7–3.5) |
| Antiviral therapy (n of 19 patients) | |
| Lopinavir/ritonavir | 15 |
| Darunavir + ritonavir | 4 |
| Ventilation requirement at hospital admission | |
| No oxygen | 7 |
| LOR | 8 |
| HOR | 5 |
| NIV | 0 |
| MV | 0 |
ALT, alanine transaminase; AST, aspartate transaminase; CNI, calcineurin inhibitor; CPK, creatine phosphokinase; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; HOR, high oxygen requirement; LDH, lactate dehydrogenase; LOR, low oxygen requirement; MMF, mofetil mycophenolate; mTORi, mammalian target of rapamycin inhibitor; MV, mechanical ventilation; NIV, non-invasive ventilation; NV, normal value; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cell.
Data are reported as percentages or median (interquartile range) unless otherwise indicated. Unless specified, counts are from the total cohort (N = 20).
Determined with the CKD Epidemiology Collaboration’s CKD-EPI equation.
Prednisone 5 mg/d or methylprednisolone 4 mg/d.
Table 2.
Clinical characteristics and outcome of 20 patients with COVID-19 infection who had undergone kidney transplantation
| Patient | Age, yr/sex | Tx date | Comorbidities | Respiratory and renal involvement | Baseline creatinine, μmol/l (eGFR, ml/min per 1.73 m2) | Baseline immunosuppression and treatment (± tocilizumab) | ACEi or ARB | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 70/F | 12/2002 | Hypertension | NIV | 185 (23) | CNI/mTORi COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone |
ACEi | Discharged |
| 2 | 47/F | 3/2011 | None | ICU, AKI, ARDS | 282 (16) | MMF/CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone Tocilizumab |
ACEi | Inpatient |
| 3 | 71/M | 1/2007 | Ischemic cardiac disease | NIV, ARDS | 159 (37) | MMF/CNI/low-dose steroids COVID treatment: no antivirals or hydroxychloroquine Dexamethasone |
ARB | Death |
| 4 | 57/M | 8/2018 | HCV infection | ICU, ARDS | 141 (47) | MMF/CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone Tocilizumab |
– | Death |
| 5 | 51/M | 3/1997 | Hypertension HCV infection |
NIV | 221 (29) | MMF/CNI COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone Tocilizumab |
– | Discharged |
| 6 | 46/M | 9/2017 | Hypertension | NIV | 132 (55) | MMF/CNI COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone |
– | Discharged |
| 7 | 59/M | 2/2015 | Hypertension | ICU, ARDS | 256 (23) | MMF/CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone |
ACEi | Death |
| 8 | 70/F | 7/2004 | Hypertension | ICU, AKI, ARDS | 300 (13) | CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone |
ACEi | Death |
| 9 | 60/M | 10/2011 | Hypertension | Room air | 150 (43) | MMF/CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine |
ACEi | Inpatient |
| 10 | 73/M | 9/2013 | Hypertension Diabetes |
NIV, ARDS | 132 (46) | MMF/CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine |
ACEi | Inpatient |
| 11 | 59/M | 3/2010 | Hypertension Ischemic cardiac disease Diabetes |
NIV, AKI, ARDS | 238 (25) | MMF/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone Tocilizumab |
ARB | Inpatient |
| 12 | 63/M | 8/2004 | Hypertension | NIV, ARDS | 203 (29) | MMF/CNI COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone Tocilizumab |
– | Death |
| 13 | 49/M | 6/2018 | Hypertension | NIV, AKI, ARDS | 185 (36) | MMF/CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine Dexamethasone Tocilizumab |
– | Inpatient |
| 14 | 60/F | 6/2018 | Hypertension | NIV, ARDS | 106 (49) | MMF/CNI/low-dose steroids COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine |
– | Inpatient |
| 15 | 57/M | 6/2009 | Hypertension | Room air | 106 (67) | MMF/CNI COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine |
– | Inpatient |
| 16 | 54/M | 10/2002 | Hypertension | NIV, AKI, ARDS | 344 (16) | CNI/low-dose steroids COVID treatment: darunavir, ritonavir, and hydroxychloroquine |
ARB | Inpatient |
| 17 | 60/M | 4/2007 | Hypertension Ischemic cardiac disease |
Room air | 141 (46) | CNI COVID treatment: combination of lopinavir and ritonavir, hydroxychloroquine |
– | Inpatient |
| 18 | 50/M | 11/2010 | Hypertension | Room air | 123 (58) | MMF/CNI/low-dose steroids COVID treatment: darunavir, ritonavir, and hydroxychloroquine |
– | Inpatient |
| 19 | 69/M | 7/1998 | Hypertension Diabetes |
AKI | 309 (17) | CNI/low-dose steroids COVID treatment: darunavir, ritonavir, and hydroxychloroquine |
– | Inpatient |
| 20 | 44/M | 7/2006 | Hypertension | Room air | 114 (66) | CNI mTORi COVID treatment: darunavir, ritonavir, and hydroxychloroquine |
– | Inpatient |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; ARDS, acute respiratory distress syndrome; AKI, acute kidney injury; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; F, female; HCV, hepatitis C virus; ICU, intensive care unit; M, male, MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitor; NIV, non-invasive ventilation; Tx, transplant.
All patients had their usual transplant immunosuppression withdrawn and were started on methylprednisolone 16 mg or equivalent dose of prednisone, and 19 of the 20 received antiviral therapy and hydroxychloroquine as per our protocol.2 As antiviral therapy is known to interfere with calcineurin inhibitor metabolism, in 4 patients, tacrolimus levels were monitored after these therapeutic changes were instituted. The median trough values before antiviral therapy were 7.05 ng/ml (IQR, 5.5–8.6): 1 patient had the level rechecked after 3 days with no change compared with baseline; 1 patient had the level rechecked 4 and 5 days after admission (−17% and −18% compared with baseline); 1 was rechecked 6 days after admission (−12% compared with baseline); and 1 was rechecked 8 days after admission (−21% compared with baseline). The median times from symptom onset and admission to these therapeutic changes were, respectively, 5 days (IQR, 3–8.25) for antiviral therapy and 0 days (IQR, 0–0) for hydroxychloroquine. During the follow-up, 1 patient had hydroxychloroquine withdrawn due to toxicity (nausea, vomiting); among the treated patients no prolongation of the cardiac QTc interval compared with baseline or cardiac arrhythmias were observed. Antibiotics were administered to 11 of the 20 patients (55%): cephalosporins in 64%; beta-lactams in 36%; fluoroquinolones in 25%; carbapenems in 10%; and glycopeptides in 5%. During hospitalization, chest radiographs were repeated in 15 patients and radiological findings worsened in 13 of those 15 (87%). In the 13 individuals with worsening radiological findings, 11 (85%) required an escalation of the oxygen supplemental therapy, including 1 patient switched from regular breathing to low oxygen requirement, 3 from regular breathing to high oxygen requirement, 3 from low to high oxygen requirement, 2 from high oxygen requirement to non-invasive ventilation, and 2 from high oxygen requirement to mechanical ventilation. The changes of the main blood tests compared with baseline are shown in Supplementary Figure S1.
Additional anti-inflammatory therapy with dexamethasone or tocilizumab, respectively, was given to 11 (55%) and 6 of the 20 patients (30%) (see Supplementary Methods for protocol details); in these patients, respectively, 4 (36%) and 2 (33%) subsequently died.
The characteristics of the patients treated with tocilizumab are shown in Table 3 ; among these 6 patients, 3 (50%) experienced a reduction of the oxygen requirements and 2 (33%) showed amelioration of the radiological findings. Two patients who were treated with tocilizumab eventually died and 1 was discharged from hospital 9 days after the administration of tocilizumab.
Table 3.
Characteristics of 6 patients with SARS-CoV2 pneumonia who had undergone kidney transplantation treated with tocilizumab and outcome after treatment
| Patient | Day of tocilizumab administration | Oxygen requirement at tocilizumab | Follow-up after tocilizumab (d) | Oxygen requirement after tocilizumab | Chest X-ray improvement | Outcome |
|---|---|---|---|---|---|---|
| 1 | 6 | NIV | 11 | HOR | No | Inpatient |
| 2 | 6 | HOR | 10 | MV | No | Death |
| 3 | 7 | LOR | 9 | Room air | No | Discharged |
| 4 | 3 | HOR | 4 | HOR | Yes | Inpatient |
| 5 | 5 | NIV | 3 | NIV | N/A | Death |
| 6 | 4 | HOR | 3 | LOR | Yes | Inpatient |
HOR, high-flux oxygen; LOR, low-flux oxygen; MV, mechanical ventilation; N/A, not available; NIV, non-invasive ventilation; SARS-CoV2, severe acute respiratory syndrome coronavirus 2.
In terms of kidney function, the medium creatinine level at admission was +17% (IQR, 12%–26%; range, 0%–143%) compared with baseline, and the highest creatinine level observed during the follow-up was +33% (IQR, 13%–59%; range, 0%–157%) compared with baseline; 6 of 20 patients developed acute kidney injury and 1 of those 6 required hemodialysis.
During follow-up of the 20 patients, 4 (20%) required intensive care and 3 of these individuals subsequently died. Overall, 5 patients died after a median of 11 days from admission (IQR, 11–14 days) and 15 days (IQR, 15–19 days) from symptom onset; of these 5 patients, 4 died from complications of the respiratory failure secondary to SARS-CoV2 infection and 1 died of probable bacterial sepsis (fever, rise of C-reactive protein, and procalcitonin) despite a satisfactory recovery from SARS-CoV2 pneumonia–induced respiratory failure, need for intensive care unit care, and treatment with dexamethasone and tocilizumab.
Three patients were discharged, after 7 days in 1 case and after 16 in the 2 remaining cases; at discharge, creatinine levels compared with baseline were 3.6 versus 2.1, 2.3 versus 2.5, and 2.1 versus 1.5 mg/dl. In terms of immunosuppressive therapy, 2 patients were discharged receiving methylprednisolone 16 mg/d and 1 with methylprednisolone 12 mg/d.
Discussion
SARS-CoV2 infection is challenging health care systems around the world. The mortality of the disease has been proposed to be around the 2.3% with age and comorbidities such as cardiovascular diseases, diabetes, chronic respiratory diseases, hypertension, and cancer being associated with worse prognoses.3 , 4 Immunosuppression and chronic kidney disease may represent additional risk factors, although specific data are not available at the moment. Here we reported the clinical characteristics and the outcomes of the first 20 patients affected by SARS-CoV2 pneumonia at our center who had received kidney transplantation. Despite on average a relatively benign onset of the disease, a large proportion of the patients displayed worsening chest radiographs and required an escalation of the supplemental oxygen. Of note, 25% of the patients died despite an aggressive approach to immunosuppression withdrawal and early commencement of antiviral therapy.
The role of lopinavir/ritonavir in SARS-CoV2 management is debated, with some data supporting a greater benefit with early start compared with a delayed commencement;1 our cohort was started on antiviral therapy a median of 5.5 days after symptom onset. Lopinavir/ritonavir may interact with calcineurin inhibitors impacting on their level: the 4 patients of our cohort with serial calcineurin inhibitor monitoring confirmed that; of note none of these patients died and 3 have been discharged.
Reports suggest a role for hydroxychloroquine treatment in reducing the viral load.5 In our cohort, 19 patients received this drug although toxicity led to treatment withdrawal in 1 case.
Hydroxychloroquine and lopinavir/ritonavir may interact causing a prolongation of the cardiac QTc interval; however, none of the patients in this series experienced this complication. Preliminary data and understanding of the pathogenesis of the pneumonia secondary to SARS-CoV2 infection suggest a central role of inflammatory cytokines in inducing the rapid clinical deterioration in association with worsening chest radiographs and escalating oxygen requirement, observed in an average of 7 to 10 days from the symptom onset.6 In this context, glucocorticoids with or without tocilizumab have been suggested to be a therapeutic strategy.7 Our subgroup of patients treated by this approach experienced a poor outcome, although encouraging signals in terms of potential beneficial effects were observed in the patients treated with tocilizumab: 50% reduced oxygen therapy requirement and 33% experiencing improvement of radiological changes. Despite that, 2 patients died. Our results are too preliminary and the sample size too small to draw firm conclusions.
The high mortality rate of this population suggests aggressive management is needed for patients with SARS-CoV2 infection who had received kidney transplantation, in particular early hospitalization should be considered in case of pneumonia. Furthermore, more effective treatment protocols need to be identified.
Our study has limitations: the sample size is small and the median follow-up time is short. Therefore, our findings are preliminary and will need to be confirmed in larger cohorts with longer follow-up times. Some strengths may be acknowledged as well; in particular, the monocentric approach and the homogeneity of the clinical treatment employed.
In conclusion, patients with SARS-CoV2 pneumonia who had undergone kidney transplantation may present with an unfavorable disease course and a poor outcome; hospitalization is required and repeat chest X-rays are advisable. Clinical management needs to be improved to have an impact on the prognosis of these patients.
Methods
All the patients with SARS-CoV2 infection admitted in the Nephrology Unit of the Spedali Civili Hospital of Brescia who had undergone kidney transplantation have been included. The therapeutic approach followed our already-published protocol.2 In brief, all admitted patients had immunosuppresion withdrawn and were commenced on methylprednisolone 16 mg/d. Antiviral therapy with lopinavir/ritonavir plus hydroxychloroquine (dose adjusted for kidney function) was considered in all patients if not contraindicated. In case of shortage of lopinavir/ritonavir, darunavir and ritonavir have been employed (see the Supplementary Methods). Patients experiencing clinical deterioration after at least 7 days from symptom onset or no temperature for >72 hours but with escalating oxygen requirements or progression of the chest radiography and no signs of bacterial infection were considered for dexamethasone (20 mg/d for 5 days, then 10 mg/d for 5 days) and up to 2 tocilizumab infusions at intervals of 12 to 24 hours (8 mg/kg of body weight, maximum dose per infusion 800 mg). Details on the indications for dexamethasone and tocilizumab have been provided in the Supplementary Methods.
Oxygen requirements have been categorized as follow: no oxygen needs; low oxygen requirement, from nasal cannula up to Venturi mask with FiO2 of 0.5, high oxygen requirement, including Venturi mask with FiO2 of 0.6, reservoir mask with oxygen at 15 l/min, and high-flow nasal ventilation; non-invasive ventilation; and mechanical ventilation. Acute kidney injury was defined as per previous publications.8
Considering the well-documented effects of lopinavir/ritonavir and hydroxychloroquine on increasing the cardiac QTc interval, electrocardiograms were performed every 2 to 3 days. In case of prolongation compared with baseline, dose reduction was performed.
Due to the small sample size, only descriptive statistics have been performed, results are expressed as count (percentage) for categorical variables and median and IQR for continuous variables.
According to the Italian regulations, ethical approval for the study has been obtained.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary Methods.
Figure S1. Changes of WBC, LDH, AST, ALT, CRP, and creatinine compared with baseline.
Supplementary Material
References
- 1.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 [e-pub ahead of print]. N Engl J Med. https://doi.org/10.1056/NEJMoa2001282. Accessed March 22, 2020. [DOI] [PMC free article] [PubMed]
- 2.Alberici F., Delbarba E., Manenti C. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5:580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Novel Coronavirus Pneumoniae Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVID-19)—China. Available at: http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. Accessed March 22, 2020. [PMC free article] [PubMed]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [e-pub ahead of print]. Int J Antimicrob Agents.https://doi.org/10.1016/j.ijantimicag.2020.105949. Accessed March 26, 2020. [DOI] [PMC free article] [PubMed] [Retracted]
- 6.Frey N., Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2019;25:e123–e127. doi: 10.1016/j.bbmt.2018.12.756. [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [e-pub ahead of print]. JAMA Intern Med. 10.1001/jamainternmed.2020.0994. Accessed March 22, 2020. [DOI] [PMC free article] [PubMed]
- 8.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.