Abstract
As health systems worldwide grapple with the coronavirus disease-2019 (COVID-19) pandemic, patients with durable LVAD support represent a unique population at risk for the disease. This paper outlines the case of such a patient who developed COVID-19 complicated by a “cytokine storm” with severe acute respiratory distress syndrome and myocardial injury and describes the challenges that arose during management.
Key Words: ARDS, COVID-19, cytokine storm, LVAD
Abbreviations and Acronyms: ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease-2019; LVAD, left ventricular assist device; MODS, multiorgan dysfunction syndrome; PEA, pulseless electrical activity; RV, right ventricle; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Central Illustration
As health systems worldwide grapple with the coronavirus disease 2019 (COVID-19) pandemic, patients with durable LVAD support represent a unique…
History of Presentation
The patient was a 70-year-old male with a destination therapy HeartMate 3 (Abbott Laboratory, Lake Bluff, Illinois) left ventricular assist device (LVAD) implanted in 2016 who developed fever, flank pain, and hematuria 3 days after attending a party. He was evaluated for nephrolithiasis at a local emergency department by abdomen and pelvis computed tomography imaging, which incidentally found possible atypical or viral pneumonia. He was tested for coronavirus disease-2019 (COVID-19), but he left against medical advice. In the ensuing days, he continued to have fever, new onset myalgia, diarrhea, and dyspnea. He returned to the emergency department and was in acute hypoxic respiratory failure requiring supplemental oxygen to maintain peripheral oxygen saturation ≥94%.
Learning Objectives
-
•
To describe high-risk clinical features in a patient on durable LVAD support who developed COVID-19.
-
•
To illustrate potential complications and clinical dilemmas in managing COVID-19 in a patient supported with a durable LVAD.
Medical History
His medical history included ischemic cardiomyopathy, stage 3 chronic kidney disease, and obesity. His post-LVAD complications include gastrointestinal bleeding, ventricular tachycardia, and right ventricular (RV) dysfunction but no infectious complications. He did not have diabetes or use tobacco. His blood group was type A positive.
Differential Diagnosis
Pretest probability for COVID-19 infection was moderate to high due to fever, dyspnea, and hypoxia as well as prior imaging showing peripheral ground-glass opacities.
Investigations
Reverse transcription polymerase chain reaction results for severe acute respiratory syndrome-coronavirus-2 (SAR-CoV-2) was positive at the initial emergency department visit and at the authors’ institution. Serial laboratory and imaging tests are detailed in Table 1. Several markers of disease severity were abnormal including absolute lymphocyte count, C-reactive protein level, and cardiac enzymes. Chest radiographs showed bilateral infiltrates concerning for atypical pneumonia (Figure 1).
Table 1.
Vitals and Laboratory Values of the Patient With LVAD While Receiving Inpatient Treatment for COVID-Related Cytokine Storm With Severe ARDS and Multiorgan Dysfunction
| Reference Values | Last Visit∗ |
HoD 0 |
HoD 3† |
HoD 6‡ |
||||
|---|---|---|---|---|---|---|---|---|
| Absolute Value | Absolute Value | Relative Change‖ | Absolute Value | Relative Change‖ | Absolute Value | Relative Change‖ | ||
| Maximum temperature, °C | 36–37.9 | 36.6 | 38.5 | 5% | 37.5 | 2% | 37.8 | 3% |
| MAP mm Hg | 70–85 | 90 | 75 | −17% | 74 | −18% | 74 | −18% |
| Pulse, beats/min | 60–100 | 80 | 80 | 0% | 80 | 80 | 0% | |
| CVP, mm Hg | 5–10 | 10 | 14 | 40% | 10 | 0% | ||
| LVAD speed, rpm | 5,600 | 5,600 | 5,600 | 5,600 | ||||
| LVAD flow, lpm | 4.8 | 4.6 | −4% | 4.5 | −6% | 3.0 | −38% | |
| O2 saturation, % | 92–100 | 98 | 90 | 98 | 100 | |||
| Pao2, mm Hg | 80–105 | 68 | 143 | |||||
| Fio2, % | 21 | 21 | 80 | 80 | ||||
| Pao2 to Fio2 ratio | >300 | 85 | 178 | |||||
| White blood cell, ×103 cells | 4.5–11 | 5.1 | 3.0 | −41% | 6.1 | 20% | 4.9 | −4% |
| Absolute neutrophil, ×103 cells | 1.9–8.0 | 3.7 | 2.0 | −46% | 4.9 | 32% | 3.7 | 0% |
| Absolute lymphocyte, ×103 cells | 1.0–4.5 | 1.4 | 0.7 | −50% | 0.8 | −43% | 0.7 | −50% |
| eGFR, ml/min/1.73 m3 | >90 | 51 | 44 | −14% | 58 | 14% | 55 | 8.0% |
| Urobilinogen | Negative | Negative | Negative | |||||
| AST, U/l | 1–35 | 19 | 69 | 263% | 86 | 353% | 121 | 537% |
| ALT, U/l | 1–45 | 8 | 11 | 38% | 15 | 88% | 24 | 200% |
| Total bilirubin, mg/dl | 0.1–1.2 | 1.0 | 1.0 | 0% | 1.8 | 80% | 1.6 | 60% |
| Direct bilirubin, mg/dl | 0.0–0.8 | 0.5 | 1.2 | 1.0 | ||||
| Interleukin-1 | <5 | <5 | ||||||
| Interleukin-6 | 0.0–15.5 | 135 | 260 | >3,000 | ||||
| C-reactive protein, mg/l | 0–5.0 | 75 | 158 | 63 | ||||
| LDH, U/l | 100–220 | 247 | 485 | 96% | 802 | 225% | 647 | 162% |
| D-dimer, μg/ml) | 0.00–0.50 | 1.32 | 1.1 | |||||
| Ferritin, ng/ml | 30–400 | 23 | 376 | 1,534% | 719 | 3,026% | ||
| Procalcitonin | <0.49 | 0.43 | 0.61 | 0.33 | ||||
| Troponin, ng/ml | 0.00–0.03 | 0.02 | 0.1 | 400% | 0.09 | 350% | 0.33 | 1,550% |
| CK-MB, ng/ml | 0.60–6.30 | 2.60 | 1.8 | |||||
| Creatine kinase, U/l | 30–200 | 1,183 | 863 | 188 | ||||
| BNP, pg/ml | 0–100 | 281 | 580 | 106% | 721 | 157% | 404 | 44% |
| Pro-BNP, pg/ml | 300–899 | 6,075 | 4,709 | 9,820 | ||||
| Lactate, mmol/l | 0.50–1.99 | 1.8 | 1.5 | 2.0 | ||||
Values in bold are in-hospital values that were consistent with baseline values.
ALT = alanine aminotransferase; ARDS = acute respiratory distress syndrome; AST = aspartate aminotransferase; BNP = B-type natriuretic peptide; CK-MB = creatine kinase MB; CVP = central venous pressure (obtained from right heart catherization at baseline, and from central venous line in the hospital); eGFR = estimated glomerular filtration rate; Fio2 = fraction of inspired oxygen; HoD = hospital day; LDH = lactate dehydrogenase; LVAD = left ventricular assist device; MAP = mean arterial pressure (obtained from Doppler or arterial line); Pao2 = arterial partial pressure of oxygen; WBC = white blood cell.
Last visit values were the latest values obtained within the previous 6 months. Baseline LDH, WBC, platelet, absolute polymorphonuclear leukocytes, and absolute lymphocytes were recorded as an average of the previous 3 values measured within 1 year.
This patient was placed on ventilator support on the night of HoD 2 and was given tocilizumab on the evening of HoD 3.
This patient experienced pulseless electrical activity arrest after the return of spontaneous circulation.
Relative change is the percentage of increase or decrease from baseline value.
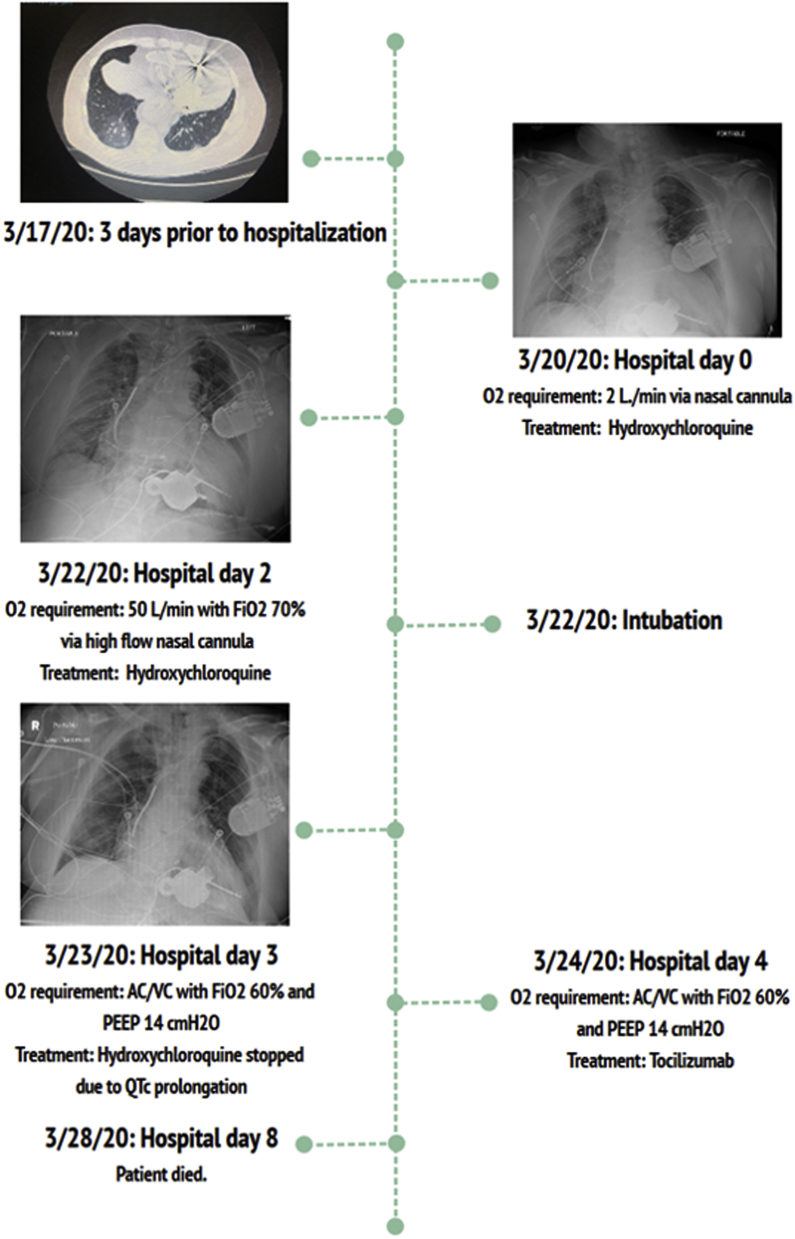
Figure 1.
Clinical Course Timeline of Severe COVID-19 Infection in This Patient on LVAD Support
Management
The patient was quarantined in a negative-pressure intensive care room. Figure 1 details his clinical course. Based on the Mount Sinai protocol (Central Illustration), the patient was started on hydroxychloroquine therapy for COVID-19 pneumonia while his QTc complex was monitored. He experienced severe acute respiratory distress syndrome (ARDS) necessitating endotracheal intubation and ventilator support with low tidal volume, according to the ARDSNet protocol (1). Despite aggressive supportive care, a “cytokine storm” ensued consisting of multiple organ dysfunction syndrome (MODS) with evidence of: 1) elevated inflammatory markers; 2) severe ARDS; 3) myocardial injury; and 4) vasodilatory shock requiring vasopressors. Two successive doses of intravenous tocilizumab (interleukin-6 receptor antagonist) at 8 mg/kg (maximum dose of 800 mg) were administered. Although clinical improvement was observed after the initial tocilizumab therapy, the patient developed worsening shock, refractory hypoxemia, and pulseless electrical activity (PEA) arrest with successful return of spontaneous circulation.
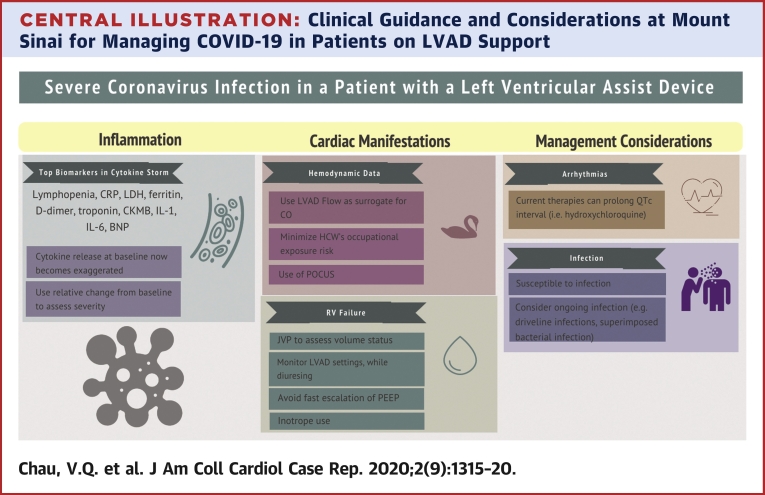
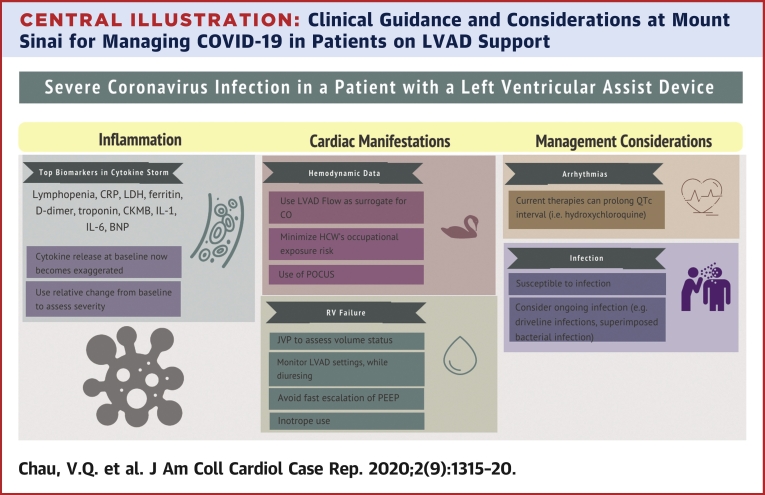
Central Illustration.
Clinical Guidance and Considerations at Mount Sinai for Managing COVID-19 in Patients on LVAD Support
Discussion
COVID-19 is a pandemic caused by the novel coronavirus SARS-CoV-2 and has taken a stronghold in New York State (2). Patients with heart failure on LVAD support are a unique population at risk for COVID-19 infection. This report presents the case of such a patient who developed COVID-19 complicated by a “cytokine storm” with severe ARDS and myocardial injury and illustrates clinical considerations that arose during his clinical course.
Inflammation and COVID-19 in LVAD support
The host response to COVID-19 infection is often localized in the lung parenchyma, but a surge in proinflammatory cytokines can occur (3,4). Known as a “cytokine storm,” this phenomenon is described in graft-versus-host disease and viral illnesses that include influenza and COVID-19 (3). One complication is MODS, which includes ARDS and cardiac manifestation. Biomarkers of a COVID-19-related cytokine storm include lymphopenia, C-reactive protein, lactate dehydrogenase, ferritin, D-dimer, and troponin (3, 4, 5). Siddiqi and Mehra (5) proposed a schema to assess the severity of “systemic hyperinflammation” in COVID-19 disease to guide therapies. Serial evaluation of inflammatory markers should be done to stratify the risk of critical illness in patients with milder disease.
Inflammation and myocardial injury from COVID-19 must be differentiated from baseline inflammation often encountered during LVAD support (6). In the present LVAD patient, several biomarkers including lactate dehydrogenase, absolute lymphocyte count, brain natriuretic peptide, and troponin had been obtained previously. In lieu of absolute values, the relative changes in these biomarkers may be more pertinent in grading COVID-19 severity in patients on LVAD support. The present patient had increased inflammatory and cardiac biomarker profiles from baseline that worsened with disease progression. This inflammatory biomarker profile improved after he received tocilizumab therapy. Changes in the pattern of these laboratory markers may be a reliable tool to follow LVAD patients with COVID-19 infection.
Cardiac manifestations
Experience in China has shown that COVID-19 could adversely affect the cardiovascular system (4). The present patient had evidence of myocardial injury as part of this COVID-19 presentation. The myocardial effect is likely a multifactorial process due to “bystander effect” from MODS, viral myocarditis, and activation of adverse remodeling mechanisms (4,5). Furthermore, the PEA arrest in this patient may be a sequela of such cardiac damage, and as such, LVAD support may have been instrumental in his resuscitation. In this context, management of patients on LVAD support with COVID-19 disease is difficult as there is a complex interplay between volume status and biventricular dynamics. We should closely monitor for: 1) RV failure and need for inotropic support; 2) drops in LVAD speed or suction events, low flow, or pulsatility index events due to vasoplegia associated with infection.
Management Considerations
To limit health care workers’ exposure to COVID-19, nonessential testing such as echocardiograms, radiographs, computed tomography, and pulmonary artery catheters were deferred. Physical examination of jugular venous pressure, pulmonary crackles, and hepatojugular reflux are essential to making clinical decisions. Also, LVAD monitor displays of flow parameters may be useful as a surrogate for cardiac output if the display flow correlates with prior invasive hemodynamic data.
Although there is no definitive therapy for COVID-19 disease, there are multiple ongoing randomized trials evaluating different treatments (3). The antimalarial medication hydroxychloroquine, which was chosen as the initial treatment agent for our patient, was shown to reduce in vitro SAR-CoV-2 cell entry, and a retrospective study suggested its clinical benefit in COVID-19 (3,4). A major side effect is QTc prolongation, so the present protocol provides monitoring guidance of this complication. Immunomodulatory biological agents such as tocilizumab are reserved for severe COVID-19, defined by the presence of both worsening respiratory failure and a cytokine storm as shown by increasing inflammatory markers. Still, caution is warranted as major adverse effects of tocilizumab include infection, infusion reactions, dyslipidemia, neutropenia, and potential malignancy (7). Patients on LVAD support are particularly vulnerable to infectious complications due to the inherent presence of hardware and driveline exposure as well as the fact that prolonged support has been associated with immune dysregulation (6).
Finally, prone ventilation is beneficial in cases of severe ARDS. The maneuver has been effective in improving lung mechanics and gas exchanges, and in some cases, it may prevent the need to escalate to venous-venous extracorporeal membrane oxygenation (8,9). Although there are no published outcomes, early experience in Wuhan, China indicates that prone position was widely used in patients with COVID-19-related severe ARDS with possible benefits (9). Nonetheless, it may be prohibitive in heart failure patients on LVAD support. Prone positioning could result in complications such as compression of outflow graft and driveline, impaired venous return from increased thoracic pressure, hardware malpositioning, and worsening RV hemodynamics (10,11). There may be additional anxiety for staff caring for COVID-19 patients who are not otherwise familiar with LVAD management. Due to these considerations, prone ventilation was not performed in this patient. Its potential for benefit in LVAD supported patients with ARDS warrants further study.
Follow-Up
Although this patient’s inflammatory profile improved after tocilizumab therapy, he remained in critical condition on maximal ventilatory support and on vasopressors for shock. Given his multiple complications, early discussion of palliative care was initiated. His MODS continued to worsen, and methylene blue was administered without improvement in hemodynamics. The patient ultimately expired on hospital day 8.
Conclusions
This case highlights a systematic approach and various considerations for a patient with an LVAD who developed life-threatening manifestations of COVID-19 infection.
Footnotes
Dr. Pinney is a consultant for Abbott Laboratories, CareDx, Medtronic, and Procyrion. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.ARDSnet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.New York State Department of Health. NYSDOH (New York State Department of Health) COVID-19 Tracker. Persons Tested Positive by County. Albany, NY: NYSDOH Available at: https://coronavirus.health.ny.gov/county-county-breakdown-positive-cases. Accessed April 9, 2020.
- 3.Tisoncik J.R., Korth M.J., Simmon C.P. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerkin K.J., Fried J.A., Raikhelkar J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqi H.K., Mehra M.R. COVID-19 Illness in native and immunosuppressed states: A clinical therapeutic staging proposal. J Heart Lung Transpl. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radley G., Pieper I.L., Ali S. The inflammatory response to ventricular assist devices. Front Immunol. 2018;9:2651. doi: 10.3389/fimmu.2018.02651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanmarti R., Ruiz-Esquide V., Bastida C., Soy D. Tocilizumab in the treatment of adult rheumatoid arthritis. Immunotherapy. 2018;10:447–464. doi: 10.2217/imt-2017-0173. [DOI] [PubMed] [Google Scholar]
- 8.Aoyama H., Uchida K., Aoyama K. Assessment of therapeutic interventions and lung protective ventilation in patients with moderate to severe acute respiratory distress syndrome: a systematic review and network meta-analysis. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng L., Qiu H., Wan L. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan's Experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollmar J.P., Colquhoun D.A., Huffmyer J.L. Anesthetic challenges for posterior spine surgery in a patient with left ventricular assist device: a case report. A A Case Rep. 2017;9:77–80. doi: 10.1213/XAA.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 11.Zochios V., Parhar K., Tunnicliffe W. The right ventricle in ARDS. Chest. 2017;152:181–193. doi: 10.1016/j.chest.2017.02.019. [DOI] [PubMed] [Google Scholar]