Abstract
Soil-borne pathogens, including phytopathogenic fungi and root-knot nematodes, could synergistically invade vegetable roots and result in serious economic losses. The genus of Trichoderma has been proven to be a promising reservoir of biocontrol agents in agriculture. In this study, the search for antagonistic metabolites from a marine-derived fungus, Trichoderma longibrachiatum, obtained two structural series of sesquiterpenes 1–6 and cyclodepsipeptides 7–9. Notably, the novel 1 was a rare norsesquiterpene characterized by an unprecedented tricyclic-6/5/5-[4.3.1.01,6]-decane skeleton. Their structures were elucidated by extensive spectroscopic analyses, while the absolute configuration of novel 1 was determined by the comparison of experimental and calculated ECD spectra. The novel 1 and known 2 and 3 showed significant antifungal activities against Colletotrichum lagrnarium with MIC values of 8, 16, and 16 μg/mL respectively, even better than those of the commonly used synthetic fungicide carbendazim with 32 μg/mL. They also exhibited antifungal potential against carbendazim-resistant Botrytis cinerea. Cyclodepsipeptides 7–9 showed moderate nematicidal activities against the southern root-knot nematode (Meloidogyne incognita). This study constitutes the first report on the antagonistic effects of metabolites from T. Longibrachiatum against soil-borne pathogens, also highlighting the integrated antagonistic potential of marine-derived T. Longibrachiatum as a biocontrol agent.
Keywords: marine-derived fungus, sesquiterpenes, cyclodepsipeptides, antagonistic activity
1. Introduction
Colletotrichum spp., Botrytis cinerea, and Fusarium oxysporum are three well-known and seriously damaging soil-borne phytopathogenic fungi worldwide, which cause anthracnose, gray mold, and wilt diseases of vegetables, respectively [1]. The southern root-knot nematode (Meloidogyne incognita) is also a typical soil-borne pathogen which invades vegetable roots [2,3]. More seriously, soil-borne fungi could synergistically interact with southern root-knot nematodes and therefore result in an even greater threat to vegetable cultivation [4,5]. Hence, the development of integrated control agents is always in demand.
Currently, the main strategy for controlling soil-borne pathogens is highly dependent on synthetic agrochemicals [6]. However, their ongoing overuse has resulted in a series of negative consequences, such as residual toxicity, the developing resistance of targeted pathogens, and many other environmental issues [6,7]. Therefore, the search for environmentally friendly integrated control alternatives has attracted more and more attention. Notably, the genus of Trichoderma has been successfully applied to control soil-borne pathogens, such as Trichoderma harzianum and T. viride [8,9,10]. Recently, the species of T. longibrachiatum has also been suggested as a biocontrol agent against phytopathogens due to its potent antagonistic effect [11,12,13]. Research on its mechanism has mainly focused on its mycoparasitic and enzyme-producing abilities, with few reports on its antagonistic metabolites [12,13,14]. T. longibrachiatum is generally isolated from terrestrial soil and plants [11,12,13,14], being a rare marine resource [15]. Ji and co-workers isolated various Trichoderma species from marine alga which could produce structurally unique and biologically active metabolites [16,17,18,19], also indicating the research potential of marine-derived Trichoderma species.
During our ongoing search for biocontrol agents in agriculture [20,21,22], a marine-derived fungus T. longibrachiatum attracted our attention due to its potent antagonistic ability to destroy soil-borne pathogens. Furthermore, the search for its antagonistic metabolites obtained two structural series of sesquiterpenes 1–6 and cyclodepsipeptides 7–9 (Figure 1), especially including a new rare norsesquiterpene 1. The isolation, structural elucidation, and antagonistic evaluation of the isolated metabolites (1–9) are discussed herein.
Figure 1.
Structures of sesquiterpenes 1–6 and cyclodepsipeptides 7–9.
2. Results and Discussion
2.1. Structural Elucidation
The molecular formula of compound 1 was obtained as C14H22O6 by HRESIMS (Figure S1 in materials, SI), implying four degrees of unsaturation. The one-dimensional NMR and HSQC data (Table 1 and Figure S5) exhibited one carbonyl carbon (δC 214.7), one oxygenated (δC 73.6) and one aliphatic (δC 43.4) quaternary carbons, four CH (δC 66.1, 48.0, 37.5, and 37.1), four CH2 (δC 35.9, 33.0, 20.6, and 20.3) and three CH3 groups (δC 28.0, 25.6, and 20.6). Therefore, in addition to one degree of unsaturation from the carbonyl group, three remaining ones could indicate the presence of a tricyclic system in 1.
Table 1.
1H and 13C NMR data of compound 1 (δ: ppm).
| Compound 1 a | Compound 1 b | |||
|---|---|---|---|---|
| No. | δC (type) | δH (mult., J in Hz) | δC (type) | δH (mult., J in Hz) |
| 1 | 66.1, CH | 2.24, br. s | 68.4, CH | 2.15, d (5.6) |
| 2 | 43.4, C | 45.6, C | ||
| 3 | 33.0, CH2 | 2.56, m; 2.37, ov | 34.8, CH2 | 2.61, ddd (18.2, 11.4, 6.6) 2.34, ddd (18.2, 9.5, 1.9) |
| 4a 4b |
20.1, CH2 | 2.13, m; 2.02, m |
21.73, CH2 | 2.19, ov; 2.03, dddd (13.8, 9.5, 6.6, 2.1) |
| 5 | 214.7, CO | 219.0, CO | ||
| 6 | 37.1, CH | 2.39, ov | 39.2, CH | 2.40, dt (5.6, 2.1) |
| 7 | 37.5, CH | 2.40, s | 39.6, CH | 2.46, s |
| 8 | 48.0, CH | 1.83, ov | 50.4, CH | 1.80, ov |
| 9 | 20.3, CH2 | 1.73, ov | 22.2, CH2 | 1.77, ov |
| 10 | 35.9, CH2 | 1.78, ov | 38.0, CH2 | 1.83, ov; 1.72, ov |
| 11 | 20.6, CH3 | 0.87, s | 21.71, CH3 | 0.84, s |
| 12 | 73.6, C | 75.0, C | ||
| 13 | 28.0, CH3 | 1.23, s | 28.5, CH3 | 1.18, s |
| 14 | 25.6, CH3 | 1.19, s | 25.7, CH3 | 1.16, s |
a,b Compound 1 was determined using CDCl3 a (1H of 500 MHz and 13C of 125 MHz) and CD3OD b (1H of 600 MHz and 13C of 150 MHz), respectively; ov: overlapped 1H NMR signals.
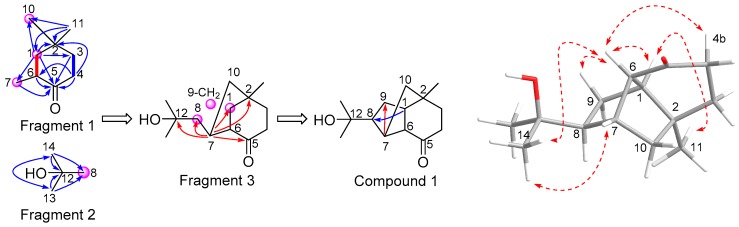
Firstly, the obvious COSY correlations between H-3a and H2-4 could confirm a structural fragment of CH2(3)-CH2(4) (Figure 2 and Figure S6). Further, due to the partially overlapped 1H NMR signals of H-6 and H-3b, another CH(1)-CH(6) or CH2(3) could be deduced by the COSY cross-peak from H-1 to H-6 or 3b (Figure S6). In order to clearly distinguish H-6 and 3b in the NMR spectra, the novel 1 was further determined using CD3OD with a higher sensitivity of 600 MHz. As shown in Figure 2 and Figure S11, the fragment could be unambiguously confirmed as CH(1)-CH(6) or CH(1)-CH2(3). However, the NMR signals of H-8, H2-10 and 9 were still seriously overlapped using CDCl3 (Figures S2 and S6) or CD3OD (Figures S8 and S11), which were therefore difficult to clearly distinguish for the structure elucidation.
Figure 2.
Key COSY (bond lines), HMBC (arrows), and NOE (dashed lines) correlations of 1 a. (a blue and red lines represented NMR signals determining CDCl3 and CD3OD, respectively).
Secondly, the presence of a cyclohexanone residue (fragment 1) could be deduced by two groups of key HMBC correlations (Figure 2 and Figure S7), one including HMBC signals from H-1/6 and H2-3/4 to CO-5 (Figure 2 and Figure S7A), while another containing HMBC correlations from H-1, H2-4, H3-11 to C-2, from H3-11 to CH-1, from H-1 to CH2-3, and from H2-4 to CH-6 (Figure 2 and Figure S7B). The connection between the methylene residue (δC 35.9, CH2-10) and the quaternary carbon (C-2) could be confirmed by the HMBC cross-peaks from H-1 and H3-11 to CH2-10. Fragment 2 could also be deduced by the HMBC correlations shown in Figure 2 and Figure S7B.
Thirdly, as shown in Figure 2 and Figure S12, the obvious HMBC correlations from H-7 to CH-1 and CO-5 could indicate that the CH-7 group was connected to CH-6 of the cyclohexanone residue. The significant HMBC cross-peaks from H-7 to C-2, as well as from H-7 to CH-8 and C-12, could join fragments 1 and 2 to obtain fragment 3, through a CH(7)-CH2(10)-C2 bridge and a CH7-CH8 bond, respectively (Figure 2 and Figure S12B).
Finally, the last cyclopentane residue could be formed through the CH1-CH2(9)-CH8 linkage as evidenced by the key HMBC correlations from H-1 to CH-8 and from H-7 to CH2-9 (Figure 2 and Figure S12B). Therefore, based on the analyses of MS and NMR data, the novel 1 could be determined as a norsesquiterpene with tricyclic-6/5/5-[4.3.1.01,6]decane skeleton.
H-7 of novel 1 was nearby to H-6, 8 and H2-10, respectively, but only showed a clear singlet peak in 1H NMR spectrum, no matter which deuterated reagents of CDCl3 (500 MHz, Figure S2), CD3OD (600 MHz, Figure S8) and DMSO-d6 (500 MHz, Figure S14) had been applied. Therefore, based on the Karplus-type equation showing the relationship between the coupling constant and dihedral angles [23,24], the singlet peak of H-7 should be related to the nearly perpendicular angles of nearby hydrogens of H-6, 8 and H2-10 (Figure 2). Similar results have been reported in many terpenoids, such as CH-8 (δH 2.15, dd, 4.8, 3.9) in sesquiterpene of penicibilaene A [25] and CH-7 (δH 3.81, d, 4.9) in diterpene of conidiogenone G [26].
As shown in Table 1 and Figure S8B, the large 5.6 Hz of H-6 was the vicinal coupling between H-6 (a bond) and H-1 (e bond), while the small 2.1 Hz of H-6 should be a long-range coupling between H-6 and H-4b, which usually appeared in bridged-ring or unsaturated compounds. The H-1 could be observed as a doublet peak with a coupling constant of 5.6 Hz, which was identical to that of H-6 (Figure S8B). This “d” peak of H-1 indicated that there was no vicinal coupling between H-1 and H2-9, probably due to their mutual nearly perpendicular angles. The lack of some COSY correlations of H-7 and H2-9 (Figures S6 and S11) should also be related to their perpendicular positions to nearby hydrogens [25].
The 13C NMR shift of CH-1 was assigned as unusually large, 66.1, which should be related to the anisotropic deshielding effects of nearby C-C bonds, while the shift of CH-6 (δC 37.1) to a higher field might be connected with the anisotropic shielding effect of the C=O bond, which also resulted in the higher-field-shifted CH2-4 (δC 20.1) compared with CH2-3 (δC 33.0). Similar results were usually found in bridgehead carbons of polycyclic terpenoids, such as CH-5 (δC 61.5) in penicibilaene A [25], as well as CH-6 (δC 66.0) and CH-15 (δC 70.1) in conidiogenone G [26].
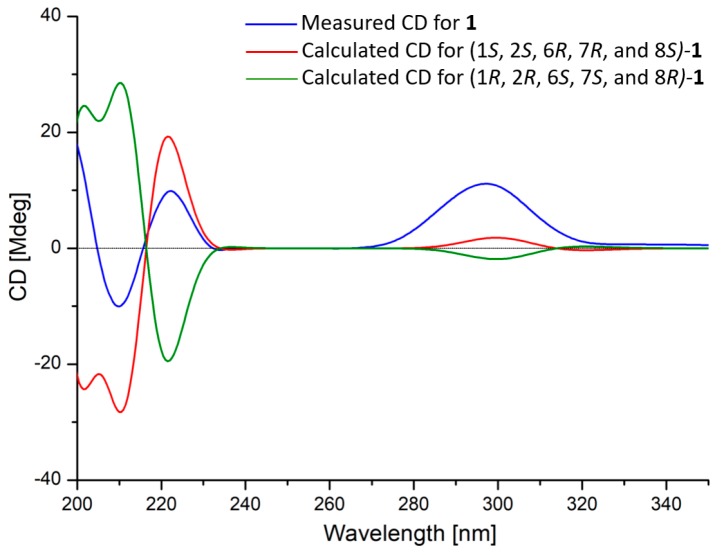
The relative configuration of compound 1 was deduced through the NOESY experiment (Figure 2 and Figure S13). The consecutive NOE correlations from H3-11 to H-1, from H-1 to H-6, from H-6 to H-4b, Haxial-9b and H3-14, as well as from H3-14 to H-7, suggested the co-face orientation of these protons. The absolute configuration of 1 was determined by the comparison of its experimental and calculated ECD (Electronic Circular Dichroism) spectra. As shown in Figure 3, the calculated ECD data of (1S, 2S, 6R, 7R, and 8S)-1 showed positive cotton effects (CEs) near 220 and 295 nm, as well as negative CE around 210 nm, the same as the experimental ones, while the calculated ECD spectra of (1R, 2R, 6S, 7S, and 8R)-1 exhibited opposite corresponding CEs.
Figure 3.
Comparisons of calculated ECD spectra for (1S, 2S, 6R, 7R, and 8S) and (1R, 2R, 6S, 7S, and 8R) with the experimental one of compound 1 in CH3OH.
The molecular formula of compound 1 only showed fourteen carbons, one less than the fifteen of the general sesquiterpenes, and therefore belongs to the rare norsesquiterpene. Compound 1 also possessed an unprecedented tricyclic-6/5/5-[4.3.1.01,6]decane skeleton, especially with a C2-C10-C7 bridge across two carbon rings. The ring system of 1 was similar to that of the pupukeanan skeleton (tricyclic-6/5/6-[4.3.1.03,7]decane) with its carbon bridge located in the contiguous carbon of the cyclohexanone residue in 1, which also suggested that they should be biosynthesized with similar pathways [27,28,29,30]. To our best knowledge, the carbon skeleton of novel 1 was first reported in the norsesquiterpene series and could be named as norpupukeanane skeleton. Therefore, the novel 1 could be named as norpupukeanane A, which was also first reported from marine-derived fungus. Its proposed biosynthesis via mevalonic acid pathway might be similar to that of previous norsesquiterpenes [31,32].
The isolation of antagonistic fractions from the culture extract also resulted in other known metabolites, including sesquiterpenes (2–6) and cyclodepsipeptides (7–9). Their structures were determined by detailed analyses of their spectroscopic data and comparisons with previously published reports as follows: trichothecinol A (2) [33], 8-deoxy-trichothecin (3) [34], trichothecinol B (4) [33], 10-cycloneren-3,5,7-triol (5) [17], 10(E)-cyclonerotriol (6) [35], homodestcardin (7) [11], trichomide B (8) [11], and homodestruxin B (9) [11]. Compounds 2–4 and 5 and 6 belong to sesquiterpenes of the trichothecene and cyclonerodiol series, respectively, while 7–9 are attributed to cyclohexadepsipeptides of the trichomide series. All the known metabolites (2–9) were first reported to be isolated from the species of T. longibrachiatum.
2.2. Antagonistic Evaluation
The isolated metabolites of sesquiterpenes 1–6 and cyclodepsipeptides 7–9 were evaluated for their antagonistic potential (Table 2), including antifungal activities against three groups of representative soil-borne phytopathogenic fungi—Colletotrichum lagrnarium, Colletotrichum fragariae, carbendazim-resistant strains of Botrytis cinerea from grape (PTQ1) and strawberry (CMQ1), Fusarium oxysporum f. sp. cucumerinum, and Fusarium oxysporum f. sp. Lycopersici—as well as nematicidal effects against the southern root-knot nematode (M. incognita).
Table 2.
Antifungal (MIC: μg/mL) and nematicidal (IC50: μg/mL) activities of the isolated metabolites 1–9.
| Compounds | C. lagrnarium | C. fragariae | PTQ1 | CMQ1 |
F. oxysporum f. sp. cucumerinum |
F. oxysporum f. sp. lycopersici |
M. incognita |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 16 | 64 | 32 | 128 | 256 | — |
| 2 | 16 | 64 | 32 | 32 | 64 | 32 | — |
| 3 | 16 | 64 | 64 | 32 | 32 | 32 | — |
| 4 | 64 | 128 | 256 | 128 | 128 | 256 | — |
| 5 | — | — | — | — | — | — | 38.2% c |
| 6 | — | — | — | — | — | — | 42.7% c |
| 7 | — | — | 256 | — | — | — | 149.2 |
| 8 | — | — | — | — | — | — | 140.6 |
| 9 | — | — | — | 256 | — | — | 198.7 |
| Carbendazim a | 32 | 16 | 256 | 256 | 4 | 8 | |
| Abamectin b | 24.9 |
“—”: no activity. a,b carbendazim and abamectin for antifungal and nematicidal bioassays, respectively. c J2s lethal rate of M. incognita at concentration of 200 μg/mL.
The novel norsesquiterpene 1 showed significant antifungal activities against two Colletotrichum species and two carbendazim-resistant strains of B. cinerea with MIC values ranging from 8 to 64 μg/mL. These results are better than those from the commonly used carbendazim, a benzimidazole fungicide which binds to the β-tubulin proteins and then inhibits cell division [36]. Therefore, although there are almost no reports concerning antifungal mechanisms of norsesquiterpenes, the novel 1 could show multiple-target potential.
The known trichothecene sesquiterpenes 2 and 3 exhibited broad-spectrum antifungal activities against all tested soil-borne phytopathogenic fungi, while the trichothecene congener 4 only showed far weaker effects compared to those of 2 and 3, suggesting that the 4-OH substituted in the cyclohexane ring of 4 might negatively modulate its antifungal activity. Trichothecenes are known as mycotoxins, which show antifungal, phytotoxic and cytotoxic activities [37,38,39]. Antifungal SAR research of trichothecene congeners showed that the 12-epoxide was essential to its activity [37], while the substituted groups in C-4 and C-8 could also modulate the effect [38], which was identical to the SAR of isolated 2–4. Trichoderma trichothecenes have been reported to be able to induce the expression of plant defense-related genes [39].
Trichomide cyclodepsipeptides 7–9 exhibited moderate nematicidal activities against M. incognita. Cyclonerodiol sesquiterpenes 5 and 6 could also partially kill the second-stage juveniles (J2s) of M. incognita at concentration of 200 μg/mL. Although the metabolites 5–9 showed weaker nematicidal potential compared to the positive control of abamectin, they could also exert synergistic effects with the antifungal 1–4 for the control of soil-borne diseases. The antifungal and nematicidal potential of known metabolites (2–9) was also first reported.
3. Experimental Section
3.1. General Procedures
One- and two-dimensional NMR spectra were recorded at 500 and 125 MHz for 1H and 13C, respectively, using a Bruker Avance III spectrometer (Bruker Biospin Group, Karlsruhe, Germany) with TMS as internal standard. HRESIMS data were determined on a mass spectrometer, the Thermo Scientific Orbitrap Fusion Lumos Tribrid (Thermo Scientific, Waltham, MA, USA). Optical rotation was obtained using a Jasco P-1020 digital polarimeter (Jasco Corporation, Tokyo, Japan). CD spectrum was acquired on a Chirascan spectropolarimeter (Applied Photophysics Ltd., Surrey, UK). Column chromatography (CC) was performed with Si gel (200–300 mesh; Qingdao Haiyang Chemical Co., Qingdao, China), Lobar LiChroprep RP-18 (40–63 μm; Merck, Kenilworth, NJ, USA), and Sephadex LH–20 (18–110 μm; Merck). Semi-preparative HPLC (semi-pHPLC) was performed using a Dionex HPLC system equipped with a P680 pump (flow rate: 3 mL/min), an ASI-100 automated sample injector, and a UVD340U multiple wavelength detector (Detection wavelength: 220 nm) controlled using Chromeleon software, version 6.80 (Dionex Corporation, Sunnyvale, CA, USA).
3.2. Fungal Material
The fungal strain T. longibrachiatum was isolated from the root of Suaeda glauca, a highly halophile plant collected from the intertidal zone of Jiaozhou Bay, Qingdao, China in October 2015. The fungus was identified on the basis of morphological characteristics and molecular analyses of ITS (internal transcribed spacer)-5.8S Rdna region sequence [20]. The strain was preserved in the Natural Products Laboratory, College of Chemistry and Pharmacy, Qingdao Agricultural University.
3.3. Fermentation, Extraction and Isolation
Fresh mycelia of the fungus were statically fermented at 28 °C for 30 days on liquid Potato Dextrose Broth (PDB) media. The liquid culture was conducted in 50 × 1 L conical flasks containing 300 mL of PDB medium (2% glucose and 20% potato juice in natural seawater).
The PDB culture was exhaustively extracted using EtOAc to obtain a crude extract, which was further fractionated via silica gel vacuum liquid chromatography (VLC) with petroleum ether/acetone (20:1, 10:1, 5:1, and 1:1) and chloroform/MeOH gradients (20:1, 10:1, 5:1, and 1:1) to yield eight fractions (Frs. 1–8). The potential antagonistic Fr. 4 was purified via CC over RP-C18, eluting with a MeOH-H2O gradient (from 1:9 to 1:0) to obtain five subfractions (Fr. 3-1 to 3-5). Fr. 3-3 was first separated using Sephadex LH-20 (MeOH), and then isolated via CC over silica gel with a chloroform/MeOH gradient (from 40:1 to 10:1) to yield compounds 5 (5.6 mg) and 6 (11.3 mg). Fr. 3-4 was purified via CC over Sephadex LH-20 (MeOH) and further separated using semi-pHPLC (45% MeCN-H2O) to obtain compounds 2 (6.5 mg, tR 13.4 min), 3 (9.6 mg, tR 18.1 min) and 4 (7.7 mg, tR 15.8 min). Fr. 3-5 was isolated using Sephadex LH-20 (Acetone) to yield compound 1 (3.1 mg) and a subfraction, which was further purified via semi-pHPLC (70% MeOH-H2O) to obtain compounds 7 (8.5 mg, tR 16.7 min), 8 (3.4 mg, tR 18.1 min) and 9 (10.2 mg, tR 21.4 min).
Trichodermene A (1): Colorless oil. [α= +7.4, c 1.20, CH3OH; UV (CH3OH) λmax (log ε) 205 (2.28), 284 (1.05) nm; IR (CH3OH) νmax 3431, 3257, 2945, 2832, 1652, 1043 cm−1; ECD (CH3OH) λmax ([θ]) 210 (–10.05), 222 (+9.90), 297 (+11.13) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 245.1489 [M + Na]+ (calcd for NaC14H22O6, 245.1484).
3.4. Antagonistic Evaluation
In order to evaluate the antagonistic potential of T. longibrachiatum metabolites against soil-borne pathogens, its antifungal activities were tested against three groups of representative soil-borne phytopathogens, including Colletotrichum spp. (C. fragariae and C. lagenarium), carbendazim-resistant strains of B. cinerea from grape (PTQ1) and strawberry (CMQ1), and Fusarium oxysporum, using a broth microdilution method in 96-well plates [21,40].
Another synergetic soil-borne pathogen, the southern root-knot nematode (M. incognita), was also selected for nematicidal bioassay in 24-well plates. Briefly, J2s of M. incognita were collected to prepare the nematode suspension based on the protocol reported previously [21]. The isolated metabolites (1–9) were dissolved and diluted in DMSO to obtain sample solvents with a series of different concentrations. The sample solvents (5 μL) were added to each well containing the nematode suspension (495 μL) with about 60 J2s, while the same amount of DMSO (5 μL) was added for the negative control. The plates were maintained at 25 °C for 48 h and then observed using a stereomicroscope to evaluate the nematode mortalities. Nematodes were defined to be dead if their bodies became straight and did not react to mechanical touches. The experiment was repeated three times under the same conditions.
4. Conclusions
The investigation of antagonistic metabolites from marine-derived fungus T. longibrachiatum obtained two structural series of sesquiterpenes 1–6 and cyclodepsipeptides 7–9. Notably, the novel 1 was a rare norsesquiterpene possessing an unprecedented tricyclic-6/5/5-[4.3.1.01,6]decane skeleton. Its absolute configuration was determined by the comparison of experimental and calculated ECD spectra. The novel 1 and known 2 and 3 showed significant antifungal activities against two Colletotrichum species and two carbendazim-resistant strains of B. cinerea with MIC values ranging from 8 to 64 μg/mL, even better than those of the commonly used fungicide carbendazim. Cyclodepsipeptides 7–9 showed moderate nematicidal activities against the southern root-knot nematode (M. incognita). The antifungal activities of 1–4 and nematicidal effects of 5–9 were reported for the first time and further revealed the synergistically antagonistic potential of marine-derived T. longibrachiatum against soil-borne pathogens.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/18/3/165/s1, Figure S1: HRESIMS spectrum of compound 1; Figures S2–S14: annotated 1D NMR and selected 2D NMR spectra of compound 1; Experimental Section: The method of calculated ECD spectrum of compound 1.
Author Contributions
F.-Y.D. and G.-L.J. both performed the experiments for the isolation and bioactivity evaluation of the isolated compounds; F.-Y.D. also prepared the manuscript; L.X. contributed to the antagonistic evaluation; Y.-M.Z. determined the structures of the isolated compounds; X.W. supervised the research and also prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Key Research and Development Program of China (SQ2018YFD020082), Natural Science Foundation of China (No. 31401795), Shandong Province Key Research and Development Project (Nos. 2016GSF121007 and 2018GSF121036), and High-level Talents Foundation of Qingdao Agricultural University (No. 631419 and No. 631431).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ralph D., Jan A.L.V.K., Zacharias A.P., Ki E.H., Antonio D.P., Pietro D.S., Jason J.R., Marty D., Regine K., Jeff E., et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu T.T., Wu H.B., Jiang H.Y., Zhang L., Zhang Y.N., Mao L.G. Thiophenes from Echinops grijsii as a preliminary approach to control disease complex of root-knot nematodes and soil-borne fungi: Isolation, activities, and structure−nonphototoxic activity relationship analysis. J. Agric. Food Chem. 2019;67:6160–6168. doi: 10.1021/acs.jafc.9b01306. [DOI] [PubMed] [Google Scholar]
- 3.Muhammad Z.K., Tariq M., Muhammad A.H. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop. Prot. 2017;92:207–212. [Google Scholar]
- 4.Huang S.Z., Huang H.N., Ma Q.Y., Mo M.H., Zhu M.L., Dai H.F., Ji Y.P., Wang Q.H., Zhao Y.X. The phytochemicals with antagonistic activities toward pathogens of a disease complex caused by Meloidogyne incogni and Ralstonia solanacearumta. J. Pure Appl. Microbiol. 2015;9:209–213. [Google Scholar]
- 5.Van der Putten W.H., Dijk C.V., Peters B.A.M. Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature. 1993;362:53–56. doi: 10.1038/362053a0. [DOI] [Google Scholar]
- 6.Russell P.E. The development of commercial disease control. Plant Pathol. 2006;55:585–594. doi: 10.1111/j.1365-3059.2006.01440.x. [DOI] [Google Scholar]
- 7.Charles L.C., Franck E.D., Stephen O.D. Natural products as sources for new pesticides. J. Nat. Prod. 2012;75:1231–1242. doi: 10.1021/np300024u. [DOI] [PubMed] [Google Scholar]
- 8.Vincenzo M., Aurélie S., Enrico B., Catia P., Cindy C., Patricia T.A., Christophe C., Laura M., Florence F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018;102:1189–1217. doi: 10.1094/PDIS-08-17-1181-FE. [DOI] [PubMed] [Google Scholar]
- 9.Rosa H., Ada V., Ilan C., Enrique M. Plant-beneficial effects of Trichoderma and of its genes. Microbiology. 2012;158:17–25. doi: 10.1099/mic.0.052274-0. [DOI] [PubMed] [Google Scholar]
- 10.Morton C.O., Penny R.H., Brian R.K. Infection of plant-parasitic nematodes by nematophagous fungi—A review of the application of molecular biology to understand infection processes and to improve biological control. Nematology. 2004;6:161–170. doi: 10.1163/1568541041218004. [DOI] [Google Scholar]
- 11.Nashwa M.A.S., Amal M.I.E., Ahmed S. Effect of Trichoderma spp. On fusarium wilt disease of tomato. Mol. Biol. Rep. 2019;46:4463–4470. doi: 10.1007/s11033-019-04901-9. [DOI] [PubMed] [Google Scholar]
- 12.Kamal A.M.A., Sobhy I.I.A., Ismail R.A. Isolation of Trichoderma and evaluation of their antagonistic potential against Alternaria porri. J. Phytopathol. 2014;162:567–574. [Google Scholar]
- 13.AL-Shammari T.A., Bahkali A.H., Elgorban A.M., El-Kahky M.T., Al-Sum B.A. The use of Trichoderma longibrachiatum and Mortierella alpina against root-knot nematode, Meloidogyne javanica on tomato. J. Pure Appl. Microbiol. 2013;7:199–207. [Google Scholar]
- 14.Cesar G.O., Víctor G.P., Stefania L., Matteo L. Enzyme activity of extracellular protein induced in Trichoderma asperellum and T. longibrachiatum by substrates based on Agaricus bisporus and Phymatotrichopsis omnivora. Fungal Biol. 2014;118:211–221. doi: 10.1016/j.funbio.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Song Y.P., Miao F.P., Liu X.H., Ji N.Y. Responses of marine-derived Trichoderma fungi to seawater and their potential antagonistic behavior. J. Oceanol. Limnol. 2019;37:525–534. doi: 10.1007/s00343-019-8059-3. [DOI] [Google Scholar]
- 16.Song Y.P., Shi Z.Z., Miao F.P., Fang S.T., Yin X.L., Ji N.Y. Tricholumin A, a highly transformed ergosterol derivative from the alga-endophytic fungus Trichoderma asperellum. Org. Lett. 2018;20:6306–6309. doi: 10.1021/acs.orglett.8b02821. [DOI] [PubMed] [Google Scholar]
- 17.Song Y.P., Fang S.T., Miao F.P., Yin X.L., Ji N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018;81:2553–2559. doi: 10.1021/acs.jnatprod.8b00714. [DOI] [PubMed] [Google Scholar]
- 18.Shi Z.Z., Fang S.T., Miao F.P., Yin X.L., Ji N.Y. Trichocarotins A–H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg. Chem. 2018;81:319–325. doi: 10.1016/j.bioorg.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Liang X.R., Miao F.P., Song Y.P., Liu X.H., Ji N.Y. Citrinovirin with a new norditerpene skeleton from the marine algicolous fungus Trichoderma citrinoviride. Bioorg. Med. Chem. Lett. 2016;26:5029–5031. doi: 10.1016/j.bmcl.2016.08.093. [DOI] [PubMed] [Google Scholar]
- 20.Du F.Y., Li X., Li X.M., Zhu L.W., Wang B.G. Indolediketopiperazine alkaloids from Eurotium cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar. Drugs. 2017;15:24. doi: 10.3390/md15020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y.M., Ju G.L., Xiao L., Zhang X.F., Du F.Y. Cyclodepsipeptides and sesquiterpenes from marine-derived fungus Trichothecium roseum and their biological functions. Mar. Drugs. 2018;16:519. doi: 10.3390/md16120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L., Zhou Y.M., Zhang X.F., Du F.Y. Antifungal activity against apple fruit pathogens of Notopterygium incisum extract and bioactive secondary metabolites. Pestic. Biochem. Phys. 2018;150:59–65. doi: 10.1016/j.pestbp.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Yuan X.R., Duan E.H., Zhang Y., Hu S., Liu Y.H. Determining the configuration of trans-4-propyl-cyclohexylcarboxylic acid by NMR method. Mol. Cryst. Liq. Cryst. 2010;528:147–155. doi: 10.1080/15421406.2010.504639. [DOI] [Google Scholar]
- 24.Abraham R.J., Leonard P., Tormena C.F. 1H NMR spectra. Part 28: Proton chemical shifts and couplings in three-membered rings. A ring current model for cyclopropane and a novel dihedral angle dependence for 3JHH couplings involving the epoxy proton. Magn. Reson. Chem. 2012;50:305–313. doi: 10.1002/mrc.3808. [DOI] [PubMed] [Google Scholar]
- 25.Meng L.H., Li X.M., Liu Y., Wang B.G. Penicibilaenes A and B, sesquiterpenes with a tricyclo[6.3.1.01,5]dodecane skeleton from the marine isolate of Penicillium bilaiae MA-267. Org. Lett. 2014;16:6052−6055. doi: 10.1021/ol503046u. [DOI] [PubMed] [Google Scholar]
- 26.Du L., Li D.H., Zhu T.J., Cai S.X., Wang F.P., Xiao X., Gu Q.Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron. 2009;65:1033–1039. doi: 10.1016/j.tet.2008.11.078. [DOI] [Google Scholar]
- 27.Chang N.C., Chang C.K. Total synthesis of (±)-2-pupukeanone. J. Org. Chem. 1996;61:4967–4970. doi: 10.1021/jo9603338. [DOI] [Google Scholar]
- 28.Liu L., Liu S.C., Jiang L.H., Chen C.L., Guo L.D., Che Y.S. Chloropupukeananin, the first chlorinated pupukeanane derivative, and its precursors from Pestalotiopsis fici. Org. Lett. 2008;10:1397–1400. doi: 10.1021/ol800136t. [DOI] [PubMed] [Google Scholar]
- 29.Liu L., Niu S.B., Lu X.H., Chen X.L., Zhang H., Guo L.D., Che Y.S. Unique metabolites of Pestalotiopsis fici suggest a biosynthetic hypothesis involving a Diels–Alder reaction and then mechanistic diversification. Chem. Commun. 2010;46:460–462. doi: 10.1039/B918330B. [DOI] [PubMed] [Google Scholar]
- 30.Liu L., Bruhn T., Guo L.D., Gçtz D.C.G., Brun R., Stich A., Che Y.S., Bringmann G. Chloropupukeanolides C–E: Cytotoxic pupukeanane chlorides with a spiroketal skeleton from Pestalotiopsis fici. Chem. Eur. J. 2011;17:2604–2613. doi: 10.1002/chem.201003129. [DOI] [PubMed] [Google Scholar]
- 31.Shen T., Qian H., He Y.L., Li L.M., Wang Y.D. Longifodiol, a novel rearranged triquinane norsesquiterpene from the root of Leontopodium longifolium. Chem. Lett. 2018;47:461–463. doi: 10.1246/cl.171046. [DOI] [Google Scholar]
- 32.Li B., Li L., Peng Z.G., Liu D., Si L.L., Wang J., Yuan B.C., Huang J., Proksch P., Lin W.H. Harzianoic acids A and B, new natural scaffolds with inhibitory effects against hepatitis C virus. Bioorg. Med. Chem. 2019;27:560–567. doi: 10.1016/j.bmc.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 33.Akira I., Kazuhide K., Hiroki K., Kiyoshi T., Harukuni T., Hoyoku N. Trichothecinols A, B and C, potent anti-tumor promoting sesquiterpenoids from the fungus Trichothecium roseum. Tetrahedron Lett. 1996;37:9219–9220. [Google Scholar]
- 34.Kenji T., Ronald D.P., Reiko Y., Masatoshi M., Masaru M., Shoichi K., Manfred G., Gen O. 8-deoxy-trichothecin production by Spicellum roseum isolated from a cultivated mushroom in Japan. Mycotoxins. 2001;51:71–77. [Google Scholar]
- 35.Pang X.Y., Lin X.P., Tian Y.Q., Liang R., Wang J.F., Yang B., Zhou X.F., Kumaravel K., Luo X.W., Tu Z.C., et al. Three new polyketides from the marine sponge derived fungus Trichoderma sp. SCSIO41004. Nat. Prod. Res. 2018;32:105–111. doi: 10.1080/14786419.2017.1338286. [DOI] [PubMed] [Google Scholar]
- 36.Yin W.X., Adnan M., Shang Y., Lin Y., Luo C.X. Sensitivity of Botrytis cinerea from nectarine/cherry in China to six fungicides and characterization of resistant isolates. Plant Dis. 2018;102:2578–2585. doi: 10.1094/PDIS-02-18-0244-RE. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto M., Nishiyama M., Maeda H., Tonouchi A., Konno K., Hashimoto M. Structure-activity relationships of trichothecenes against COLO201 cells and Cochliobolus miyabeanus: The role of 12-epoxide and macrocyclic moieties. Bioorg. Med. Chem. Lett. 2019;29:982–985. doi: 10.1016/j.bmcl.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Cheng J.L., Zheng M., Yao T.T., Li X.L., Zhao J.H., Xia M., Zhu G.N. Synthesis, antifungal activity, and QSAR study of novel trichodermin derivatives. J. Asian Nat. Prod. Res. 2015;17:47–56. doi: 10.1080/10286020.2014.962522. [DOI] [PubMed] [Google Scholar]
- 39.Malmierca M.G., Cardoza R.E., Alexander N.J., McCormick S.P., Hermosa R., Monte E., Gutiérrez S. Involvement of Trichoderma trichothecenes in the biocontrol activity and induction of plant defense-related genes. Appl. Environ. Microbiol. 2012;78:4856–4868. doi: 10.1128/AEM.00385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang P., Yuan X.L., Du Y.M., Zhang H.B., Shen G.M., Zhang Z.F., Liang Y.J., Zhao D.L., Xu K. Angularly prenylated indole alkaloids with antimicrobial and insecticidal activities from an endophytic fungus Fusarium sambucinum TE-6L. J. Agric. Food Chem. 2019;67:11994−12001. doi: 10.1021/acs.jafc.9b05827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.