Abstract
Objective: There are no comprehensive studies on survival outcomes and optimal treatment protocols for cervical esophageal cancer (CEC), due to its rare clinical prevalence. Our objective was to determine the relationship between pathological characteristics, treatment protocols, and survival outcomes in Chinese CEC patients.
Methods: A total of 500 Chinese CEC patients were selected from our 500,000 esophageal and gastric cardia carcinoma database (1973–2018). There were two main groups: patients treated with surgery, and patients receiving non-surgical treatments (radiotherapy, radiochemotherapy, and chemotherapy). The Chi-square test and Kaplan–Meier method were used to compare the continuous variables and survival.
Results: Among the 500 CEC patients, 278 (55.6%) were male, and the median age was 60.9 ± 9.4 years. A total of 496 patients (99.2%) were diagnosed with squamous cell carcinoma. In 171 (34.2%) patients who received surgery, 22 (12.9%) had undergone laryngectomy. In 322 (64.4%) patients who received non-surgical treatments, 245 (76.1%) received radiotherapy. Stratified survival analysis showed that only T stage was related with survival outcomes for CEC patients in the surgical group, and the outcomes between laryngectomy and non-laryngectomy patients were similar. It was noteworthy that the 5-year survival rate was similar in CEC patients among the different groups treated with surgery, radiotherapy, chemotherapy, or radiochemotherapy (P = 0.244).
Conclusions: The CEC patients had similar survival outcomes after curative esophagectomy and radiotherapy, including those with or without total laryngectomy. These findings suggest that radiotherapy could be the initial choice for treatment of Chinese CEC patients.
Keywords: Cervical esophageal cancer, survival, esophagectomy, radiochemotherapy
Introduction
Cervical esophagus is defined as the short part of the esophagus between the lower border of the cricoid cartilage and the thoracic inlet (suprasternal notch), ~18 cm from the incisor teeth1. Studies from Western countries have indicated that cervical esophageal cancer (CEC) is rare in clinic prevalence, with a ratio of 2%–10% for all esophageal cancers2–6. Prior to this report, the study with the largest sample size was from Italy5. It included 363 CEC (10.5%) patients, out of 3,445 esophageal cancer patients5, but only 148 CEC patients were eligible for the final analysis. A study from Japan indicated that there were only 64 CEC patients reported within 22 years (1960–1982)7. In China, an accurate incidence for CEC is unknown, and there have been no reports with a larger sample size for CEC. The clinicopathological features and treatment outcomes of CEC patients in China are also largely unknown. After almost 30 years of effort, we have established a database of esophageal and gastric cardia cancers in China, including 410,000 esophageal cancers (97% esophageal squamous cell carcinomas and 1.5% primary esophageal adenocarcinomas) and 90,000 gastric cardia adenocarcinomas8. Among these patients, more than 200,000 cases have been successfully followed-up since 1973. The present study selected 500 CEC patients from this database. Our aim was to characterize the clinicopathological features and treatment outcomes for Chinese CEC patients. To the best of our knowledge, this Chinese study has the largest sample size of any CEC study.
Materials and methods
Patients and follow-up
All patients were from the 500,000 esophageal and gastric cardia carcinoma database (1973–2018), established by Henan Key Laboratory for Esophageal Cancer Research of The First Affiliated Hospital, Zhengzhou University8. To select the present study cohort, the database was reviewed. All patients with accurate tumor location records and treatments for esophageal cancer were considered for inclusion in this study (1973–2018). CEC was defined as the epicenter of the esophageal tumor found between the esophageal orifice and the sternal notch. All medical records were reviewed for consistency and completeness.
The follow-up was performed with patients with accurate addresses through either yearly telephone or home interviews, until the death of patients. The last follow-up was completed in December 2018. Of the 500 CEC patients, 466 patients (93.2%) were followed-up successfully.
The collection of clinicopathological information and tumor staging
All clinical information for each patient was collected and digitalized based on in-patient medical records, including gender, age at diagnosis, family history (2 or more esophageal cancer patients in the same family within consecutive 3 generations), cigarette smoking, alcohol consumption, histopathology and treatment procedures. Pathological diagnosis was based on the medical record for each patient. All patients with esophagectomy were staged according to the 2002 American Joint Committee on Cancer (AJCC) tumor node metastasis staging system for esophageal cancer9.
Treatments
In this study, based on the medical records, the treatments for all patients were classified as esophagectomy, radiotherapy, chemotherapy, and radiochemotherapy. Based on the procedure and approach, esophagectomy was classified as esophagectomy with or without laryngectomy, left or right thoracotomy, and transhiatal esophagectomy. The total radiation dose was generally 40–60 Gy with 2 Gy per fraction per day. The chemotherapy was usually performed using cisplatin with 5-fluorouracil or taxol. Of the 500 CEC cases, there were 171 cases treated with surgery, 245 cases treated with radiotherapy, 11 cases treated with chemotherapy, 66 cases treated with radiochemotherapy, and 7 cases without any medical treatment.
Statistical analysis
Statistical analysis was performed using SPSS statistical software for Windows, version 21.0 (SPSS, Chicago. IL, USA). The t-test and Chi-square test were used to compare the differences of categorical and continuous variables between different CEC groups, respectively. The survival outcome was estimated by the Kaplan-Meier method and the multivariate Cox proportional hazards regression model. A value of P < 0.05 was considered statistically significant.
Results
Distribution of CEC patients by clinicopathological changes
Table 1 shows the distribution of all CEC patients by gender, age, family history, cigarette smoking, alcohol consumption, histopathology and treatment procedures from 1973–2018. There were 278 males with a mean age of 60.6 ± 9.3 years and 222 females with a mean age of 61.3 ± 9.5 years. Family aggregation for CEC patients was evident with a family history in 29.0% of the patients. In addition, almost all female CEC patients had no history of cigarette smoking and alcohol consumption. In contrast, half of the male patients had a history of cigarette smoking (52.2%) and less than one-third of the male patients had alcohol consumption (24.6%). Nearly all of the CEC patients were diagnosed with squamous cell carcinoma (99.2%). There were only 3 CEC patients with primary esophageal adenocarcinoma (0.6%), and 1 case was mucoepidermoid carcinoma. Of the CEC patients, more than one-third received esophagectomy (34.2%), including 22 cases with laryngectomy (12.9%) and 90 cases with left thoracotomy (52.6%). Two-thirds of the CEC patients received non-surgical treatments (64.4%), in which 76.1% received radiotherapy.
Table 1.
The distribution of 500 cases with CEC by clinicopathological information
| Characteristics | CEC, n (%) |
|---|---|
| Gender | |
| Male | 278 (55.6) |
| Female | 222 (44.4) |
| Male/female | 1/0.8 |
| Mean age, years (SD) | 60.9 (9.4) |
| Histological type | |
| Squamous cell carcinoma | 496 (99.2) |
| Adenocarcinoma | 3 (0.6) |
| Others | 1 (0.2) |
| Family history† | |
| Positive | 144 (29.0) |
| Negative | 352 (71.0) |
| Cigarette smoking‡ | |
| Yes | 141 (28.8) |
| No | 349 (71.2) |
| Alcohol consumption§ | |
| Yes | 66 (13.5) |
| No | 424 (86.5) |
| Type of treatment | |
| Surgery | 171 (34.2) |
| Radiotherapy | 245 (49.0) |
| Radiochemotherapy | 66 (13.2) |
| Chemotherapy | 11 (2.2) |
| UR* | 7 (1.4) |
*CEC, cervical esophageal cancer; SD, standard deviation; UR, unrecorded patients for treatment procedures or out-patients.
†Four cases with missing family histories.
‡Ten cases with missing smoking information.
§Ten cases with missing alcohol consumption.
Pathological features of CEC patients with esophagectomy
The pathological features of 171 CEC patients with esophagectomy from 1973–2018 are summarized in Table 2, including differentiation, T and N status, pathological stages, and incisal edge residue. Most patients were at advanced stage (IIa + IIb + III vs. 0 + I, 87.7% vs. 12.3%) and better degeneration (G1+G2, 82.2%), and the percentage of positive incisal edge residue was much higher (14.0%).
Table 2.
The distributions of 171 CEC cases through esophagectomy according to clinicopathological information
| Characteristics | CEC, n (%) |
|---|---|
| Gender | |
| Male | 110 (64.3) |
| Female | 61 (35.7) |
| Mean age, years, (SD) | 58.9 ± 8.0 |
| Histological type | |
| Squamous cell carcinoma | 168 (98.2) |
| Adenocarcinoma | 3 (1.8) |
| Others | 0 |
| T status | |
| Tis | 3 (1.8) |
| T1 | 22 (12.9) |
| T2 | 59 (34.5) |
| T3 | 75 (43.8) |
| T4 | 12 (7.0) |
| N status | |
| pN (−) | 125 (73.1) |
| pN (+) | 46 (26.9) |
| Differentiation† | |
| High (G1) | 27 (18.5) |
| Moderate (G2) | 93 (63.7) |
| Low (G3) | 26 (17.8) |
| Pathological stage | |
| 0 | 3 (1.8) |
| I | 18 (10.5) |
| IIa | 101 (59.1) |
| IIb | 11 (6.4) |
| III | 38 (22.2) |
| IV | 0 |
| Preoperative treatment | |
| None | 144 (84.2) |
| Chemotherapy | 5 (2.9) |
| Radio ± C* | 22 (12.9) |
| Surgical procedure | |
| Laryngectomy | 22 (12.9) |
| Non-laryngectomy | 149 (87.1) |
| Surgical approach | |
| Left thoracotomy | 90 (52.6) |
| Right thoracotomy | 24 (14.1) |
| Transhiatal esophagectomy | 57 (33.3) |
| Incisal edge residue | |
| Negative | 147 (86.0) |
| Positive | 24 (14.0) |
*CEC, cervical esophageal cancer; SD, standard deviation; Radio ± C, radiotherapy with or without chemotherapy.
†Twenty-five cases missing differentiation information.
Comparison of perioperative parameters of CEC patients with esophagectomy between laryngectomy and non-laryngectomy procedures
Table 3 shows the comparisons between laryngectomy and non-laryngectomy procedures by gender, age, differentiation, T and N status, pathological stage, incisal edge residue, and anastomotic leakage. It showed that the laryngectomy group had a significantly higher proportion of T3–T4 (90.9% vs. 45.0%, P = 0.000) and higher proportion of stage III (59.1% vs. 16.8%, P = 0.000), in other words, non-laryngectomy had lower percentage of earlier stage (0–II, 83.2% vs. 40.9%, P = 0.000). However, there were no difference between these 2 groups in the percentage of incisal edge residue (P = 0.699), and anastomotic leakage (P = 0.063).
Table 3.
The comparison of 171 CEC cases through esophagectomy with or without laryngectomy according to clinicopathological information
| Characteristics | Non-laryngectomy, n (%) | Laryngectomy, n (%) | P |
|---|---|---|---|
| Gender | 0.005 | ||
| Male | 90 (60.4) | 20 (90.9) | |
| Female | 59 (39.6) | 2 (9.1) | |
| Age | 0.200 | ||
| ≥ 60 | 73 (49) | 14 (63.6) | |
| < 60 | 76 (51) | 8 (36.4) | |
| T status | 0.000 | ||
| Tis-T2 | 82 (55) | 2 (9.1) | |
| T3-T4 | 67 (45) | 20 (90.9) | |
| N status | 0.036 | ||
| N0 | 113 (75.8) | 12 (54.5) | |
| N1 | 36 (24.2) | 10 (45.5) | |
| G status† | 0.093 | ||
| G1-2 | 107 (84.3) | 13 (68.4) | |
| G3 | 20 (15.7) | 6 (31.6) | |
| Pathological stage | 0.000 | ||
| 0–II | 124 (83.2) | 9 (40.9) | |
| III–IV | 25 (16.8) | 13 (59.1) | |
| Incisal edge residue | 0.699 | ||
| Negative | 127 (85.2) | 20 (90.9) | |
| Positive | 22 (14.8) | 2 (9.1) | |
| Anastomotic Leakage | 0.063 | ||
| Negative | 122 (81.9) | 22 (100) | |
| Positive | 27 (18.1) | 0 |
†Twenty-five cases missing differentiation information.
Treatment outcome analysis
In patients who had been followed-up successfully until December, 2018, 13.9% patients survived more than 10 years, with the longest survival time of 26.9 years. It was noteworthy that the 5-year survival rates were similar in CEC patients among the different groups treated with surgery, radiotherapy, chemotherapy, or radiochemotherapy (P = 0.244). In patients with or without radiotherapy, or radiochemotherapy before esophagectomy, the 5-year survival rates were similar (37.0% vs. 52.1%, P = 0.106).
Stratification analysis of survival-related factors
For CEC patients, the 5-year survival rates were similar (P > 0.05) in terms of gender, age, family history, cigarette smoking, alcohol consumption, histological type (Supplementary Figure S1A–F), LNM (lymph node metastasis), and types of differentiation (Supplementary Figure S2B, S2C), either in CEC patients with esophagectomy with radiotherapy, chemotherapy, or radiochemotherapy (Figure 1A, 1B). In CEC patients with esophagectomy, although it seemed that patients with laryngectomy had a poorer 5-year survival rate, there was no significant difference (44.8% vs. 50.3%, P = 0.532) (Figure 2). In CEC patients, the tumor invasion depth was correlated with survival outcome. The 5-year survival rate in patients with T3 and T4 was lower than those with Tis (tumor in situ), T1, and T2 (37.7% vs. 61.7%, P = 0.026) (Supplementary Figure S2A). Although the CEC patients with negative LNM had a higher 5-year survival rate than those with positive LNM, the difference was not statistically significant (55.8% vs. 33.9%, P = 0.056) (Supplementary Figure S2B). There was no significant difference in the 5-year survival rate among different grades of differentiation for the CEC patients (P = 0.545) (Supplementary Figure S2C). Regarding pathological stage, CEC patients with early stage (0 and I) had significantly better survival rates than stage III patients (80.0% vs. 34.2%, P = 0.016) (Supplementary Figure S2D).
Figure 1.
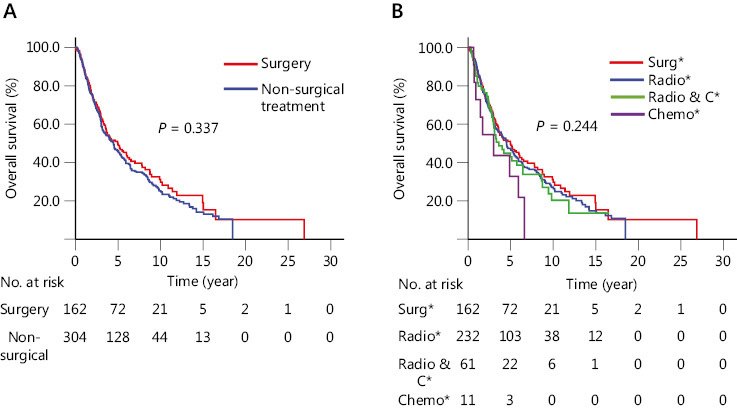
Kaplan–Meier curves comparing different treatment types of CEC patients. (A) Surgical and non-surgical treatments (i.e., radiotherapy, radiochemotherapy, and chemotherapy). (B) Different treatment types. *Surg, curable surgery; Radio, radiotherapy; Radio & C, radiochemotherapy; Chemo, chemotherapy.
Figure 2.
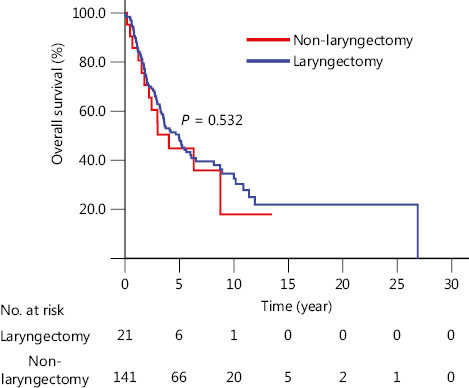
Kaplan-Meier curves comparing cervical esophageal cancer patients with or without laryngectomy.
Esophagectomy approach and survival analysis
In CEC patients, the 5-year survival rate was similar in patients with esophagectomy through left or right thoracotomy, and transhiatal approaches (49.8% vs. 47.3% vs. 50.1%, respectively, P = 0.696) (Supplementary Figure S3B).
Treatment period analysis
We divided all CEC patients into 3 groups (1973–1997, 1998–2007, and 2008–2018) (Supplementary Table S1). The survival rates were similar between the surgical and non-surgical treatment groups in different time periods (64.3 vs. 68.8%, P = 0.912) (Supplementary Figure S4A); (48.1% vs. 51.3%, P = 0.922) (Supplementary Figure S4B); and (48.5% vs. 34.4%, P = 0.111) (Supplementary Figure S4C). For patients receiving radiotherapy, we combined the groups of 1973–1997 and 1998–2017 into 1 group. The ratio of CEC patients treated before 1997 was less than those in the other 2 time groups, and there was no significant difference in the 5-year survival rates (50.4% vs. 40.3%, P = 0.156) (Supplementary Figure S5).
Discussion
To the best of our knowledge, this is the first report of Chinese CEC patients, with the largest sample size in terms of clinicopathological features and treatment outcomes. The present study showed a low CEC prevalence in the Chinese population. Moreover, CEC in China seemed to be more prevalent in females, predominantly involving squamous cell carcinomas.
In the present study, it was noteworthy that 3 CEC cases were identified as adenocarcinomas. Although adenocarcinoma is the major pathological type of esophageal cancer in Western countries, this phenomenon is difficult to explain using the Western model of esophageal adenocarcinoma formation, i.e., from reflux esophagitis to Barrett’s esophagus to dysplasia, and finally to an adenocarcinoma. Accumulated evidence has shown that primary esophageal adenocarcinomas in the Chinese population mostly originate from the esophageal propria gland or duct epithelium10. Endoscopic screening for high risk subjects and symptom-free early esophageal cancer patients in high incidence areas in Linxian, Henan showed a very low detection rate of Barrett’s esophagus and reflux esophagitis (as low as 0.7% and 4.5%, respectively)11. These findings suggest that different mechanisms in Chinese patients might be involved in the oncogenesis of CEC adenocarcinoma.
In our study, 29.0% of CEC patients had a positive family history. In China, family clustering has been observed for esophageal cancer in both low incidence and high incidence areas12,13. It has been reported that positive family history and genetic changes may increase the susceptibility to esophageal cancer,14–16 and esophageal cancer survival17. However, further studies are needed to further characterize the mechanism of family histories and the survival of CEC patients.
Regarding CEC survival outcomes, the present study showed that curative esophagectomy and non-surgical treatment as initial treatments for CEC had comparable survival outcomes (P = 0.337). For Chinese CEC patients, radiotherapy is usually the initial choice of most patient relatives or clinicians, because of the possible risk of laryngectomy or incisal edge residue due to complicated surgery. Reviewing recent reports3,5,6,18,19 on the comparisons between surgery and non-surgical treatments, we found there was no advantage of surgery (Supplementary Table S2).
The present study also showed that early CEC (Tis-1N0M0) with curative surgical treatment did not show any survival benefit when compared with advanced or progressed CEC, or with non-surgical treatment of CEC. Because of its rare prevalence, large series studies to compare different treatments of CEC in China are very limited. A few studies of CEC have shown that the survival outcomes for CEC are comparable for curative surgery and non-surgical treatment3,5. With improvements in radiotherapy and chemotherapy, the gold standard for CEC treatment has been changed from histologically pharyngo–laryngo–esophagectomy to adjuvant radiotherapy and/or chemotherapy, followed with or without surgery. Chen et al.20 reported that 63 CEC patients with concurrent radiochemotherapy had satisfactory outcomes.
Another interesting result in the present study was that in CEC patients, the 5-year survival rate was similar even in groups with esophagectomy plus laryngectomy or larynx preservation. There was no significant difference between the two different surgical procedures, although in the early 5-years or even longer times after the patients received esophagectomy with larynx preservation, they seemed to have better survival. Other group studies have recently reported that larynx-preserving esophagectomy for CEC is feasible and oncologically acceptable21,22. Our results also showed that patients receiving laryngectony did not have a lower rate of incisal edge residue. Considering the low quality of life following total laryngectomy and similar survival outcomes for esophagectomy and radiotherapy, or radiochemotherapy, the present study strongly suggested the advantage of radiotherapy as the initial choice in CEC treatment strategies. Our study showed that there was no difference in the survival rate of CEC patients who received radiotherapy between different decades. This might be because most cases enrolled in this study were from villages, so they were treated in local county hospitals in Henan province, which is an economically depressed area. It might be difficult to introduce new technology involving non-surgical treatments in these areas. Otherwise, in different decades, there was no advantage for surgical treatment, which suggests that CEC patients select radiotherapy or radiochemotherapy as the initial treatments.
The present results showed that the percentage of female patients with CEC was high, which was different from thoracic esophageal cancer. Saeki et al.2 also reported a higher percentage of female patients in the CEC group, and suggested that the higher percentage of female CEC patients might be due to cigarette smoking. Hoeben et al.1 reported that cigarette smoking and alcohol consumption were risk factors to CEC. Although consumptions of tobacco and alcohol have been recognized as risk factors for esophageal cancer, especially for esophageal squamous cell carcinoma23, in China, the historical and current consumptions of tobacco and alcohol in males are more common than in females. We therefore suggest that there might be other mechanisms involved in gender distributions in CEC patients, which needs to be further elucidated. A multidimensional analysis of molecular differences between male and female cancer patients has classified cancer types into two groups showing distinct incidence and mortality profiles. Extensive gender-biased gene expression signatures have been identified in some cancer types, which may be helpful in developing gender-specific therapeutic strategies and for elucidating the mechanisms involved in cancer related clinical controversy by gender24.
Conclusions
The present study determined the clinicopathological characteristics of CEC patients in terms of gender, alcohol consumption, cigarette smoking, family history, LNM, anastomotic leakage, and incisal edge residues. In CEC patients, the survival outcomes with curative esophagectomy (with or without total laryngectomy) and radiotherapy were similar. Considering the low quality of life following total laryngectomy and anastomotic leakage, radiotherapy should be the initial choice for treatment of CEC in Chinese patients.
Supporting Information
Acknowledgments
This work was supported by the National Key R&D Program “Precision Medicine” of China (Grant No. 2016YFC0901403), the Major Science and Technology Projects of Henan Province (Grant No. 16110031 1300), the Doctoral Team Foundation of the First Affiliated Hospital of Zhengzhou University (Grant No. 2016-BSTDJJ-03), the National Natural Science Foundation of China (Grant No. 81872032, U1804262), and the State Key Laboratory of Esophageal Cancer Prevention and Treatment (Grant No. Z2020-0010).
We thank Dr. Junyan Hong (Rutgers, The State University of New Jersey, USA) for reviewing and editing this manuscript; Dr. Haizhou Guo, (Department of Thoracic Surgery, The First Affiliated Hospital of Zhengzhou University) for helpful discussions during the manuscript preparation; and Dr. Zhiguang Ping, (Department of Health Statistics, College of Public Health in Zhengzhou University) for help in statistical analysis.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol. 2016;27:1664–74. doi: 10.1093/annonc/mdw183. [DOI] [PubMed] [Google Scholar]
- 2.Saeki H, Tsutsumi S, Yukaya T, Tajiri H, Tsutsumi R, Nishimura S, et al. Clinicopathological features of cervical esophageal cancer: retrospective analysis of 63 consecutive patients who underwent surgical resection. Ann Surg. 2017;265:130–6. doi: 10.1097/SLA.0000000000001599. [DOI] [PubMed] [Google Scholar]
- 3.Takebayashi K, Tsubosa Y, Matsuda S, Kawamorita K, Niihara M, Tsushima T, et al. Comparison of curative surgery and definitive chemoradiotherapy as initial treatment for patients with cervical esophageal cancer. Dis Esophagus. 2017;30:1–5. doi: 10.1111/dote.12502. [DOI] [PubMed] [Google Scholar]
- 4.Li HX, Liu J, Cheng Y, Liu MN, Fang WT, Lv CX. Concurrent chemoradiotherapy for cervical esophageal squamous cell carcinoma: treatment results from a prospective observational study. Dis Esophagus. 2018;31:1–6. doi: 10.1093/dote/dox144. [DOI] [PubMed] [Google Scholar]
- 5.Valmasoni M, Pierobon ES, Zanchettin G, Briscolini D, Moletta L, Ruol A, et al. Cervical esophageal cancer treatment strategies: a cohort study appraising the debated role of surgery. Ann Surg Oncol. 2018;25:2747–55. doi: 10.1245/s10434-018-6648-6. [DOI] [PubMed] [Google Scholar]
- 6.Grass GD, Cooper SL, Armeson K, Garrett-Mayer E, Sharma A. Cervical esophageal cancer: a population-based study. Head Neck. 2015;37:808–14. doi: 10.1002/hed.23678. [DOI] [PubMed] [Google Scholar]
- 7.Kakegawa T, Yamana H, Ando N. Analysis of surgical treatment for carcinoma situated in the cervical esophagus. Surgery. 1985;97:150–7. [PubMed] [Google Scholar]
- 8.Wang XM, Gao HJ. Exploration and practice in clinical biobanks. Shanghai: Shanghai Jiao Tong University Press; 2017. pp. 476–517. [in Chinese]. [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual (6th Edition) Berlin, Heidelberg: Springer; 2002. pp. 91–8. [Google Scholar]
- 10.Zhang YX, Qiao SJ, Shen Q, Qiu SL, Song MQ, Yan AH, et al. Study on histology and pathology of primary esophageal adenocarcinoma in high incidence region of esophageal cancer in China. Henan Med Res. 1994;3:295–300. [in Chinese] [Google Scholar]
- 11.Wang LD, Zheng S, Zheng ZY, Casson AG. Primary adenocarcinomas of lower esophagus, esophagogastric junction and gastric cardia: in special reference to China. World J Gastroenterol. 2003;9:1156–64. doi: 10.3748/wjg.v9.i6.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–44. [PubMed] [Google Scholar]
- 13.Gao Y, Hu N, Han X, Giffen C, Ding T, Goldstein A, et al. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC Cancer. 2009;9:269. doi: 10.1186/1471-2407-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu N, Wang Z, Song X, Wei L, Kim BS, Freedman ND, et al. Genome-wide association study of gastric adenocarcinoma in Asia: a comparison of associations between cardia and non-cardia tumours. Gut. 2016;65:1611–8. doi: 10.1136/gutjnl-2015-309340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, et al. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet. 2010;42:759–63. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 16.Wu C, Wang Z, Song X, Feng XS, Abnet CC, He J, et al. Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet. 2014;46:1001–6. doi: 10.1038/ng.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Li D, Jia W, Hu Z, Zhou Y, Yu D, et al. Genome-wide association study identifies common variants in SLC39A6 associated with length of survival in esophageal squamous-cell carcinoma. Nat Genet. 2013;45:632–8. doi: 10.1038/ng.2638. [DOI] [PubMed] [Google Scholar]
- 18.Tong DK, Law S, Kwong DL, Wei W I, Ng RW, Wong KH. Current management of cervical esophageal cancer. World J Surg. 2011;35:600–7. doi: 10.1007/s00268-010-0876-7. [DOI] [PubMed] [Google Scholar]
- 19.Cao CN, Luo JW, Gao L, Xu GZ, Yi JL, Huang XD, et al. Primary radiotherapy compared with primary surgery in cervical esophageal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140:918–26. doi: 10.1001/jamaoto.2014.2013. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Lu HI, Lo CM, Wang YM, Chou SY, Hsiao CC, et al. The clinical outcomes of locally advanced cervical esophageal squamous cell carcinoma patients receiving curative concurrent chemoradiotherapy: a population-based propensity score-matched analysis. Cancers (Basel) 2019:11. doi: 10.3390/cancers11040451. pii: E451. doi: 10.3390/cancers11040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun F, Li X, Lei D, Jin T, Liu D, Zhao H, et al. Surgical management of cervical esophageal carcinoma with larynx preservation and reconstruction. Int J Clin Exp Med. 2014;7:2771–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Makino T, Yamasaki M, Miyazaki Y, Takahashi T, Kurokawa Y, Takiguchi S, et al. Short- and long-term outcomes of larynx-preserving surgery for cervical esophageal cancer: analysis of 100 consecutive cases. Ann Surg Oncol. 2016;23:858–65. doi: 10.1245/s10434-016-5511-x. [DOI] [PubMed] [Google Scholar]
- 23.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y, Liu L, Chen H, Wang Y, Xu Y, Mao H, et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell. 2016;29:711–22. doi: 10.1016/j.ccell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


