Abstract
Since triple-negative breast cancer (TNBC) was first defined over a decade ago, increasing studies have focused on its genetic and molecular characteristics. Patients diagnosed with TNBC, compared to those diagnosed with other breast cancer subtypes, have relatively poor outcomes due to high tumor aggressiveness and lack of targeted treatment. Metabolic reprogramming, an emerging hallmark of cancer, is hijacked by TNBC to fulfill bioenergetic and biosynthetic demands; maintain the redox balance; and further promote oncogenic signaling, cell proliferation, and metastasis. Understanding the mechanisms of metabolic remodeling may guide the design of metabolic strategies for the effective intervention of TNBC. Here, we review the metabolic reprogramming of glycolysis, oxidative phosphorylation, amino acid metabolism, lipid metabolism, and other branched pathways in TNBC and explore opportunities for new biomarkers, imaging modalities, and metabolically targeted therapies.
Keywords: Metabolic reprogramming, triple-negative breast cancer, aerobic glycolysis, Warburg effect, cancer stem cell, targeted therapy
Introduction
Breast cancer is a heterogeneous disease comprising four major molecular subtypes, namely, luminal A, luminal B, human epidermal growth factor receptor 2 (HER2), and triple-negative1. Triple-negative breast cancer (TNBC) is defined as a tumor that lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and HER2 (also known as ERBB2)2. Basal-like breast cancer (BLBC), which is a major subtype of breast cancer according to PAM50 subtyping, is generally categorized as the TNBC subtype. Patients with the TNBC subtype account for 15% of all breast cancer patients and have a relatively poor prognosis due to the high metastatic capacity of their tumors, especially to the brain and lungs3. The high risk of metastasis in these patients may be associated with epithelial-mesenchymal transition (EMT), which is induced by several transcriptional factors such as Snail1, Smad2, and Twist1/2. EMT confers TNBC with cancer stem cell (CSC)-like characteristics, including elevated metastatic potential and enhanced resistance to chemotherapy4. Nevertheless, chemotherapy currently remains the mainstay of systemic treatment due to the lack of effective targeted therapy such as anti-HER2 and anti-ER5. In addition, patients carrying a mutation in the breast cancer susceptibility gene (BRCA1) usually exhibit the triple-negative or basal-like phenotype and tend to be sensitive to the inhibitor of poly ADP-ribose polymerase (PARP)3. Although some patients with TNBC have a relatively better prognosis after chemotherapy or treatment with PARP inhibitors, many TNBC patients are still insensitive to these therapies. Hence, there is an urgent need to identify new druggable targets.
Cancer cells display significant metabolic reprogramming, which enables them to survive and rapidly proliferate in a nutrient-poor tumor microenvironment. For example, cancer cells preferentially utilize glycolysis despite the availability of adequate oxygen (known as aerobic glycolysis or the Warburg effect), thereby maintaining high levels of glycolytic intermediates for biosynthetic requirements, while decreasing the production of reactive oxygen species (ROS) from oxidative phosphorylation (OXPHOS)6. This rewired metabolic network depends on the dysregulation of many key enzymes, such as pyruvate kinase muscle isozyme M2 (PKM2), which redirects glycolytic intermediates into the anabolic pathway7. Appropriate intervention against the dysregulation of these pivotal enzymes may rectify cancer metabolism and hinder tumor growth, suggesting novel therapeutic strategies.
Glycolysis in TNBC
Aberrant expression of glycolysis-related enzymes
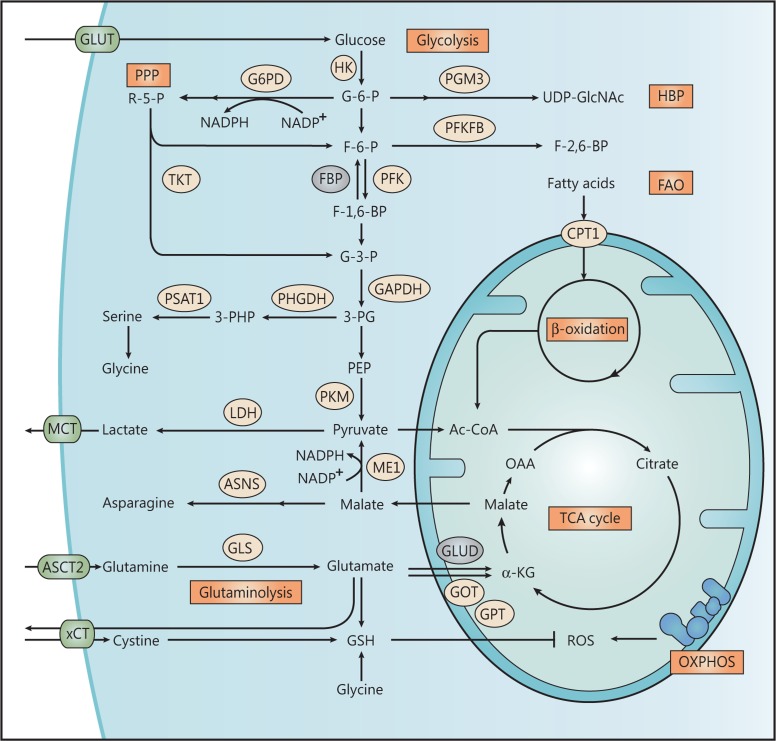
Compared with other breast cancer subtypes, TNBC is more dependent on glycolysis; it displays elevated glucose uptake and lactate secretion, with the upregulation of several key glycolytic enzymes and transporters, including lactate dehydrogenase (LDH), glucose transporter (GLUT), and monocarboxylate transporter (MCT) (Figure 1). Knocking down GLUT4 diminishes glucose uptake and lactate release, thus normalizing the metabolism of cancer by reallocating the glycolytic flux to OXPHOS, which leads to compromised cell proliferation and viability under hypoxia8. Either tumoral LDH-A or LDH-B is closely associated with poor clinical outcomes in patients with TNBC9,10. Two isoforms of MCT, MCT1 and MCT4, are also specifically upregulated in TNBC, where they mediate lactate extrusion and acidification of the tumor microenvironment9,11. Disruption of lactate transport impairs tumor growth in vitro and in vivo, accompanied by reduced tumor aggressiveness12.
Figure 1.
Metabolic reprogramming in triple-negative breast cancer (TNBC). TNBC upregulates several key glycolytic enzymes and transporters such as GLUT, HK, LDH, and MCT, therefore displaying high rate of glycolysis and glycolytic branched pathways, including serine synthesis and pentose phosphate pathway (PPP) for NADPH generation, and hexosamine biosynthetic pathway (HBP) for protein glycosylation. Other pathways such as fatty acid oxidation (FAO), glutaminolysis and cystine uptake are also induced in TNBC to meet its bioenergetic or biosynthetic demands, and mitigate reactive oxygen species (ROS) that is generated from oxidative phosphorylation (OXPHOS). Enzymes that are upregulated in TNBC are shown as light-yellow ovals, and enzymes downregulated in TNBC are shown as gray ovals.
Crosstalk between glycolysis and oncogenic signaling
The aberrant expression of glycolysis-related enzymes in TNBC can be attributed to dysregulated signaling, such as the epidermal growth factor receptor (EGFR), HIF-1α, and c-Myc pathways. The EGFR signaling, frequently active in TNBC, triggers the expression of hexokinase 2 (HK2) and stabilizes GLUT1 on the cell membrane13, whereas it reduces the activity of PKM214, thus causing an accumulation of the glycolytic intermediate, fructose-1,6-bisphosphate (F-1,6-BP). In turn, elevated F-1,6-BP directly binds to EGFR and enhances its activity, further promoting EGFR-mediated aerobic glycolysis. Combining the glycolytic inhibitor 2-deoxy-D-glucose with the EGFR inhibitor gefitinib effectively suppresses TNBC cell proliferation14. Under normoxic conditions, HIF-1α is hydroxylated by the proline hydroxylase domain (PHD2; also known as EglN1) in an oxygen- and α-ketoglutarate (α-KG)-dependent manner, which results in its ubiquitination and subsequent degradation15. However, a long non-coding RNA abundant in TNBC, LINK-A, can activate normoxic HIF-1α signaling. Mechanistically, LINK-A induces BRK-mediated phosphorylation of HIF-1α at Tyr 565, which interferes with the hydroxylation of the adjacent Pro 564 by PHD2, leading to normoxic HIF-1α stabilization16. Once activated, HIF-1α transcriptionally promotes the expression of most glycolytic enzymes and transporters (e.g., GLUT1, HK2, LDH-A, and MCT4) to enhance the ability of cancer cells to perform glycolysis. HIF-1α also inactivates the pyruvate dehydrogenase complex and attenuates the entry of pyruvate into the tricarboxylic acid (TCA) cycle, thereby reinforcing the glycolytic phenotype and decreasing OXPHOS15,17. BLBC with c-Myc activation overexpresses many c-Myc-targeted genes, including LDH-A, pyruvate dehydrogenase kinase (PDK), and glutaminase (GLS)18 (Figure 2). c-Myc also represses the level of thioredoxin-interacting protein, a potent negative regulator of glucose uptake and glycolytic gene expression, to further activate aerobic glycolysis19.
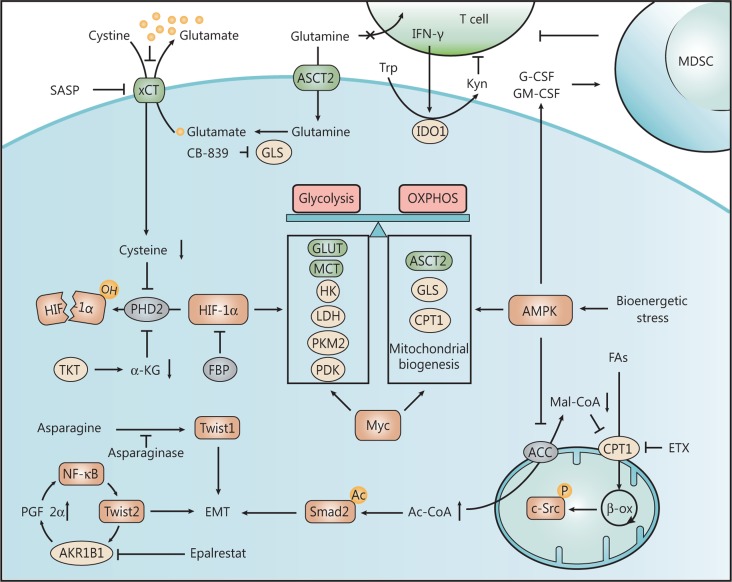
Figure 2.
Switchable metabolic dependence on glycolysis and oxidative phosphorylation (OXPHOS) regulated by HIF-1α and AMPK. Under normoxic condition, HIF-1α is hydroxylated and subsequently degraded by PHD2 in an α-KG- and cysteine-dependent manner. In TNBC, decreased levels of α-KG and cysteine due to altered activity of transketolase (TKT) and xCT cystine/glutamate antiporter, respectively, contribute to normoxic activation of HIF-1α signaling, which triggers aerobic glycolysis with the upregulation of glycolysis-related enzymes and transporters. Although TNBC cells rely on glycolysis for rapid proliferation, OXPHOS which generate ATP more efficiently is also required, especially under bioenergetic stress. Activated AMPK and Myc pathways induce mitochondrial biogenesis and enzymes involved in glutaminolysis and fatty acid oxidation (FAO) to facilitate OXPHOS, thus endowing TNBC with metabolic plasticity to switch between glycolysis and OXPHOS. In addition, AMPK signaling also promotes epithelial-mesenchymal transition (EMT) through regulating lipid metabolism and increases the secretion of cytokines to recruit immunosuppressive myeloid-derived suppressor cells (MDSCs). Many drugs have been developed to target these key processes in the metabolic reprogramming of TNBC.
The metabolic rewiring of glycolysis due to dysregulated signaling can, in turn, promote oncogenic pathways through altered metabolites or energy status. Overexpression of GLUT3 in nonmalignant human breast cells contributes to loss of polarity and activates several known oncogenic pathways, including the EGFR, β1-integrin, MEK, and AKT pathways. In contrast, decreasing glucose uptake in breast cancer cells suppresses these oncogenic pathways and promotes the formation of organized structures20. In TNBC, glutathione S-transferase Pi 1 (GSTP1) interacts with and activates glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to facilitate glycolytic flux, whereas the inhibition of GSTP1 by LAS17 impairs glycolysis and lowers the generation of ATP and macromolecular building blocks, such as lipids and nucleotides. This energy reduction ultimately blocks oncogenic signaling through the activation of AMPK and the inhibition of mTOR signaling21. Unexpectedly, the bifunctional enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4), a glycolytic stimulator responsible for the phosphorylation of F-6-P to F-2,6-BP, can also act as a protein kinase. PFKFB4 phosphorylates oncogenic steroid receptor coactivator-3 (Src-3) and enhances its transcriptional activity, which leads to the upregulation of transketolase (TKT), a major enzyme that mediates the non-oxidative pentose phosphate pathway (PPP), thus shunting glycolytic flux into the PPP for purine synthesis22. Overexpression of TKT in TNBC is also required for the activation of HIF-1α signaling. TKT depletion upregulates the expression of succinate dehydrogenase and fumarate hydratase, thereby decreasing the levels of oncometabolites, succinate and fumarate, both of which can suppress PHD2 activity and stabilize HIF-1α. Suppressing TKT by oxythiamine leads to the disruption of HIF-1α-induced aerobic glycolysis and breast cancer metastasis23. In addition, a high rate of aerobic glycolysis in TNBC increases the secretion of G-CSF and GM-CSF through the AMPK-ULK1-autophagy pathway, which promotes myeloid-derived suppressor cells (MDSCs) development and an immunosuppressive microenvironment24 (Figure 2).
Amino acid metabolism
Glutamine addiction and dysregulated glutaminolysis
Circulating glutamine is the most abundant amino acid in the blood. Although it is a non-essential amino acid, many cancers, including TNBC, are dependent on glutamine and hijack glutaminolysis, the process by which glutamine is catabolized for entry into the TCA cycle, to support biosynthesis, energy generation, and glutathione (GSH) production25,26. Under physiologic conditions, glutamine is transported into cells via many transporters, such as alanine, serine, cysteine-preferring transporter 2 (ASCT2; also known as SLC1A5), and L-type amino acid transporter 1 (LAT1; also known as SLC7A5). Intracellular glutamine is deaminated by GLS to glutamate, which can then be converted to α-KG by either glutamate dehydrogenase (GLUD) or several aminotransferases, such as glutamate-oxaloacetate transaminase (GOT), glutamate-pyruvate transaminase (GPT), and phosphoserine aminotransferase (PSAT), following which α-KG enters the TCA cycle26 (Figure 1).
In TNBC, both ASCT2 and LAT1 are overexpressed27,28. High expression of ASCT2 is critical for the uptake of glutamine and subsequent glutaminolysis, leading to the activation of the mTORC1 nutrient-sensing pathway27. Metabolomics analysis also reveals a low level of glutamine and a high level of glutamate in TNBC, indicating enhanced glutaminolysis29. Compared with other breast cancer subtypes, TNBC is more glutamine dependent and susceptible to glutaminolysis-targeting therapeutics because of the overexpression of GLS30,31, which is associated with high-grade metastatic breast cancer32. Several small-molecule inhibitors of GLS, such as CB-839, BPTES, and compound 96833, have been developed to target dysregulated glutaminolysis. In addition, GLS expression in TNBC is significantly correlated with a low level of tumor-infiltrating lymphocytes (TILs), suggesting a metabolic competition between cancer cells and TILs in the tumor microenvironment, where active consumption of intercellular glutamine by GLS-overexpressing TNBC cells deprives TILs of glutamine and hinders their proliferation34 (Figure 2). As explained above, glutamate is converted to α-KG through two mechanisms, via transaminases or GLUD. Compared with quiescent cells, highly proliferative cells prefer to catabolize glutamate via transaminases to synthesize non-essential amino acids (aspartate and alanine) and downregulate GLUD to reduce ammonia production. Consistently, among the four major breast cancer subtypes, the most proliferative basal breast tumors express high levels of GPT2 and PSAT1, whereas they express relatively low levels of GLUD1/235. In contrast, ER-positive breast cancers exhibit increased GLUD expression, which accounts for their glutamine independence. Mechanistically, GLUD reversibly catalyzes the reductive amination of α-KG to glutamate under glutamine deprivation. Through this metabolic recycling of ammonia, elevated glutamate levels enable the synthesis of other amino acids, such as aspartate and proline36. Another reason for the glutamine independence of luminal-type breast cancer is the high expression of glutamine synthetase (GS), which is directly induced by a key luminal transcription factor, GATA3. Luminal cells can rescue basal cells in co-culture without glutamine, indicating possible glutamine symbiosis within breast ducts37. In addition to GPT2 and PSAT1, another transaminase, GOT2, is also overexpressed in TNBC; it facilitates cell proliferation by increasing aspartate and α-KG production. BRCA1 protein transcriptionally represses GOT2 expression, but this repression mechanism is impaired due to the frequently observed BRCA1 deficiency in TNBC3,38. Intriguingly, as the product of glutamate, gamma-aminobutyric acid (GABA), is a major neurotransmitter in mammals, the catabolic pathway of GABA is remodeled. GABA is catabolized to succinic semialdehyde by gamma-aminobutyrate aminotransferase (ABAT). We discovered that, compared with other subtypes, ABAT is considerably decreased in BLBC due to Snail-mediated transcriptional repression, thus causing the accumulation of GABA; the elevated GABA then activates GABA-A receptor (GABAA) and subsequently triggers the activation of Ca2+-NFAT1 signaling to promote the aggressive behavior of BLBC. In breast tumor patients, loss of ABAT is strongly correlated with large tumor size, high tumor grade, and metastatic tendency39.
Cystine uptake by xCT is required for the CSC phenotype
High levels of glutaminolytic flux and glutamate indirectly support environmental cystine acquisition via the xCT cystine/glutamate antiporter (SLC7A11), a major transporter for the uptake of cystine in exchange for intracellular glutamate. The xCT antiporter is overexpressed in one-third of TNBCs and is essential for GSH synthesis and the maintenance of CSCs31. Silencing of xCT impairs tumorsphere formation and the redox balance in breast cancer stem cells (BCSCs)31,40. In turn, chemotherapy induces the enrichment of BCSCs in TNBC by upregulating xCT in a HIF-1-dependent manner to facilitate the synthesis of GSH and activate the gene encoding the pluripotency factor, Nanog41. The CD44 variant (CD44v), a marker of CSCs, interacts with and stabilizes xCT at the cell membrane42. Meanwhile, mucin 1 (MUC1), a transmembrane glycoprotein that is aberrantly overexpressed in TNBC, binds directly to the intracellular domain of CD44v and further promotes the stability of xCT43. Nevertheless, the accumulation of extracellular glutamate secreted by the xCT antiporter in turn inhibits the xCT antiporter and cystine uptake. Subsequently, the depletion of intracellular cysteine disables PHD2, which hydroxylates HIF-1α for degradation, thus leading to the induction of HIF-1α signaling and triple-negative breast carcinogenesis44 (Figure 2). The secreted glutamate can also induce metabotropic glutamate receptors (mGluR) on the membrane of TNBC and endothelial cells, promoting tumor growth and angiogenesis and inhibiting inflammation through the mGluR1 signaling. These tumor-promoting effects can be blocked by riluzole, a Food and Drug Administration (FDA)-approved drug for the treatment of amyotrophic lateral sclerosis45–47. Moreover, cystine starvation induces mitochondrial fragmentation and ROS production, leading to necroptosis and ferroptosis in BLBC cells via the tumor necrosis factor alpha (TNFα) and MEKK4-p38-Noxa pathways, while luminal-type breast cancer, without activation of these pathways, is cystine independent48,49.
Serine synthesis promotes NADPH generation and anaplerosis
The serine synthetic pathway diverts glycolytic carbon fluxes from 3-phosphoglycerate into de novo serine and glycine biosynthesis, conferring several metabolic advantages. These advantages include limiting ATP production, synthesis of serine for one-carbon metabolism and NADPH formation, and the generation of α-KG from glutamate. Phosphoglycerate dehydrogenase (PHGDH), as a key enzyme in the first step of serine synthesis, is overexpressed in both TNBC and BLBC, in part, because of copy number amplification50,51. Ectopic expression of PHGDH in breast epithelial cells disrupts acinar morphogenesis and induces phenotypic alterations that may contribute to oncogenesis52. Under hypoxia or doxorubicin treatment, the expression of PHGDH and downstream enzymes in the serine synthesis or one-carbon cycle is upregulated. This metabolic reprogramming maintains the level of NADPH to counter hypoxia- or chemotherapy-induced ROS stress and plays a role in BCSC enrichment and lung metastasis53,54. Suppression of PHGDH leads to markedly decreased cell proliferation and serine synthesis; although it does not affect intracellular serine concentration, it lowers the level of α-KG, an intermediate in the TCA cycle that is produced by PSAT1, which is downstream of PHGDH. In cells with high PHGDH expression, the serine synthesis pathway shunts 8%–9% of the glycolytic flux towards serine production and simultaneously contributes approximately 50% of the total anaplerotic flux of glutamine into the TCA cycle as α-KG50. The overexpression of PHGDH and PSAT1 is significantly associated with poor clinical outcome and malignant phenotypic features of breast cancer55.
Catabolism of tryptophan, arginine, and other amino acids
Most tryptophan catabolism occurs via the kynurenine pathway, which is catalyzed in the first step by indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO)56. Tryptophan can also be catabolized to 5-hydroxytryptamine (5-HT; also known as serotonin) by tryptophan hydroxylase 1 (TPH1). The expression of these enzymes is aberrant in TNBC, and their products, kynurenine and 5-HT, may modulate immune surveillance or oncogenic signaling. For example, in response to T cell-derived interferon (IFN)-γ, TNBC exhibits highly inducible expression of IDO1 to counteract immune cells57 (Figure 2). Overexpression of TDO2 is also found in TNBC cells in suspension in a nuclear factor kappa B (NF-κB)-dependent manner, rendering TNBC more resistant to anoikis, a programmed cell death process triggered by substratum detachment during metastasis. Mechanistically, the increased kynurenine generated by TDO2 activates the aryl hydrocarbon receptor (AhR), an endogenous kynurenine receptor, thus facilitating the proliferation, invasion, and metastatic capacity of TNBC58,59. In addition, 5-HT promotes the invasion and proliferation of TNBC cells via 5-HT7 receptor and increases the expression of TPH1 and vascular endothelial growth factor (VEGF)56.
Arginine is required for the growth of TNBC because of its two products, ornithine and nitric oxide (NO). Arginine uptake and ornithine synthesis are induced during the S/G2/M phases in transformed cells but not in normal cells. Cancer cells exclusively use arginase 2 (ARG2) to synthesize ornithine, whereas normal epithelial cells depend on ornithine aminotransferase (OAT). Knockdown of ARG2 in BLBC markedly reduces cancer cell growth and causes G2/M arrest but does not prompt compensation via OAT60. Rosuvastatin may impede breast cancer development because it inhibits arginase enzymatic activity and reduces the levels of ornithine and polyamine61. Silencing of argininosuccinate lyase (ASL), an enzyme responsible for the production of arginine, also delays the G2/M transition of TNBC cell line62. Another use of arginine is to synthesize NO via nitric oxide synthase (NOS). A high activity of inducible NOS (iNOS) is associated with poor survival of TNBC patients. Increased generation of NO induces the EGFR pathway via S-nitrosylation and subsequently activates several oncogenic signal transduction pathways (including c-Myc, Akt, and β-catenin). The NO signaling also triggers upregulation of the stem cell marker CD44 and other proteins that are characteristic of BLBC, potentiating EMT, chemoresistance, and invasion capacity63–66. In addition, NO can directly inhibit the catalytic activity of the demethylase KDM3A; thereby, it alters cellular histone methylation patterns67, leading to changes in the expression levels of numerous oncogenes such as Ets-168.
The branched-chain amino acid transaminase 1 (BCAT1), which is involved in the breakdown of branched-chain amino acids, is overexpressed in TNBC because of the hypomethylation on its promoter. BCAT1 indirectly controls cell cycle and enhances invasion capacity69. Asparagine, methionine, and glutamine metabolism are also essential for modulating the metastasis, stemness, and apoptosis of TNBC. Dietary restriction or otherwise inhibiting the synthesis of these amino acids has tumor-suppressing effects, which is discussed later in the section of clinical practice70–73.
Lipid metabolism
Increased oxidation and decreased synthesis of fatty acid
In addition to glucose and amino acids, cancer cells also utilize fatty acids (acquired from the extracellular matrix or from de novo synthesis) as an alternative energy source through fatty acid oxidation (FAO), which is a very efficient way to produce energy. FAO begins with the acylation of fatty acid to form acyl-CoA, and then, acyl-CoA is transported into the mitochondria through carnitine palmitoyltransferase (CPT) as the first committed step of the FAO. Myc-overexpressing TNBC shows upregulated CPT activity and increased bioenergetic reliance on FAO through targeted metabolomics analysis74. Upregulation of CPT1C promotes FAO and ATP generation, contributing to cell resistance against metabolic stress such as hypoxia, glucose deprivation, or mTOR inhibition75. Increased levels of ATP through mitochondrial FAO also activate the oncoprotein Src via autophosphorylation, whereas interfering with CPT1 abolishes Src activation and reverses the Src-regulated gene pattern, leading to decreased tumor growth and metastasis in vivo76. Elevated FAO in TNBC is associated with the upregulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α), the master regulator of mitochondria biogenesis and respiration74, which activates FAO to promote energy homeostasis and cell viability, especially under attachment loss or metabolic stress, thus promoting the metastasis of TNBC77. In contrast, fatty acid synthesis (FAS) and lipogenic enzymes are downregulated in TNBC compared with the HER2 subtype [upregulating ATP citrate lyase (ACLY) and fatty acid synthase (FASN) to accelerate FAS]25,74. Acetyl-CoA carboxylase (ACC) is the key lipogenic enzyme that irreversibly catalyzes acetyl-CoA to malonyl-CoA, which is an inhibitor of CPT1 and FAO. TNBC downregulates ACC activity via post-translational modification to decrease malonyl-CoA generation and boost FAO flux. Mechanistically, PHD3 hydroxylates ACC2 to promote its activity, whereas loss of PHD3 in BCSCs inactivates ACC2 and enables tumor metabolic reliance on FAO78,79. In addition, in response to TGF-β or leptin, ACC1 is phosphorylated and inactivated through the AMPK signaling, resulting in the elevation of cellular acetyl-CoA, which promotes the acetylation of EMT-inducing Smad2 and, ultimately, EMT programs and metastasis80 (Figure 2). Nevertheless, blocking FASN and lipogenesis by metformin or EGCG also induces antitumor effects on TNBC81,82, suggesting an intricate role of FAS in cancer cells.
Other lipid metabolic pathways
Triglycerides in circulating lipoprotein particles can provide an additional, exogenous source of fatty acid. TNBC exhibits enhanced lipid uptake by secreting lipoprotein lipase (LPL, which hydrolyzes the triglycerides in lipoproteins into fatty acids) and expressing CD36 (the channel for cellular fatty acid uptake)83. Several fatty acid-binding proteins, including FABP5 and FABP7, have also been identified as prognostic markers for poor outcomes in TNBC. They promote cell proliferation by enhancing fatty acid uptake and activating the retinoic acid pathway84,85. Additionally, cholesterol biosynthesis is enhanced through the upregulation of HMG-CoA reductase (HMGCR) and HMG-CoA synthase 1 (HMGCS1), key enzymes in the mevalonate pathway86. Meanwhile, elevated activity of low-density lipoprotein (LDL), caveolin-1, and cholesterol acyltransferase 1 (ACAT1) in TNBC is associated with greater uptake and utilization of cholesterol87. We have reported that the aldo-keto reductase 1 member B1 (AKR1B1) is activated in TNBC by Twist2, an inducer of EMT, and the accumulation of its major metabolite PGF2α, in turn, activates NF-κB signaling to upregulate Twist2 expression, which eventually forms a positive feedback loop to enhance the CSC properties in TNBC88 (Figure 2). Lipin-1 is a phosphatidic acid phosphatase that controls the synthesis of phospholipid for membrane biogenesis. In TNBC, lipin-1 is dramatically upregulated, whereas knockdown of lipin-1 blocks phospholipid synthesis and changes the membrane lipid composition, leading to apoptosis and growth arrest89.
OXPHOS, ROS, and the anti-oxidant pathway
OXPHOS is downregulated to reduce ROS production
Although OXPHOS generates more ATP than glycolysis does, when oxygen availability is compromised, electrons can escape from the electron transport chain, and they can be captured by O2, causing excessive ROS (such as superoxide and hydrogen peroxide) and ROS-mediated DNA damage90,91. Hence, most tumors, including TNBC, tend to utilize glycolysis to meet bioenergetic demands and rigidly control the level of ROS by downregulating OXPHOS. Consistently, metabolomics analysis of TNBC cell lines cultured under hypoxia shows that these cells have increased glucose uptake and higher glycolytic rate, while the conversion rate of glucose into the TCA cycle for OXPHOS is decreased92. We have discovered that a key gluconeogenic enzyme, fructose-1,6-bisphosphatase (FBPase), is a key regulator that triggers the metabolic switch from glycolysis to OXPHOS. Ubiquitous loss of FBP1 in TNBC is required for the maintenance of aerobic glycolysis and the CSC phenotype91,93. Mechanistically, FBP1 suppresses HIF-1α activity by directly binding to its inhibitory domain, thus acting as a transcriptional corepressor in the nucleus to repress the transcription of HIF-1α target glycolysis-related genes, including GLUT1, LDHA, and PDK194. FBP1 also enhances OXPHOS by activating mitochondrial electron transporter complex I through the upregulation of the mitochondrial transcription factor B1M (TFB1M), which is essential for complex I protein translation93. Nevertheless, it has been reported that Myc and MCL1 cooperatively promote OXPHOS and ROS generation to activate HIF-1α signaling, which confers chemoresistance by expanding BCSCs95. A similar mechanism is observed in TNBC cells with mitophagy defect96. The roles of ROS and OXPHOS in TNBC remain controversial and require further exploration.
Powerful anti-oxidant system
As described, through the high GLS1 activity and enhanced uptake of cystine, TNBC cells can quickly synthesize abundant GSH to antagonize transitory ROS elevation. To accelerate the regeneration of reduced GSH, some metabolic pathways relevant to NADPH formation are also upregulated in TNBC. We found that malic enzyme 1 (ME1), a cytosolic NADP-dependent enzyme that decarboxylates malate to pyruvate with NADPH generation, is dramatically upregulated in BLBC, where it serves as part of an important NADPH-supplying pathway97 (Figure 1). Nicotinamide phosphoribosyltransferase (NAMPT) is a rate-limiting enzyme in the synthesis of NAD+. TNBC cells utilize NAMPT to increase the intracellular NAD+ pool, which is converted to NADP+ and finally reduced to NADPH via the PPP98. In addition, ferritin is a family of proteins associated with iron storage, oxidation, and deposition. Some TNBC cell lines show aberrantly increased heavy subunits of ferritin in the nucleus, where it can act as a GSH-independent anti-oxidative system, protecting the DNA from damage by oxidizing Fe2+ to Fe3+ to mitigate ROS stress after exposure to UV99,100.
Switchable dependence on glycolysis or OXPHOS
Although TNBC relies on metabolic reprogramming from OXPHOS to glycolysis, and glycolytic inhibitor can indeed significantly suppress the aggressiveness and CSC phenotype of TNBC cells101, it is noteworthy that inhibition of glycolysis also promotes the transition of BCSC in TNBC from a quiescent, mesenchymal-like state [characterized by high expression of aldehyde dehydrogenase (ALDH)] to a proliferative, epithelial-like state (characterized by CD24-CD44+ expression) through ROS-induced AMPK-HIF-1α pathway, in a process that resembles the EMT process. Epithelial-like BCSCs exhibit elevated OXPHOS coupled with the upregulation of GSH metabolism and anti-oxidant defense102, which is also observed in “energetic” cancer stem cells (e-CSCs) and TNBC brain or lung metastases103–106. Similarly, after chemotherapy, the residual tumors are highly sensitive to the inhibitor of OXPHOS, suggesting increased dependence on OXPHOS107. In addition, loss of the tumor-suppressor retinoblastoma gene (RB1) in TNBC induces mitochondrial protein translation, OXPHOS, and anabolic metabolism, thus equipping cancer cells to utilize scant oxygen levels and migrate away from the hypoxic area108,109. In fact, TNBC cells stably maintain a hybrid metabolic phenotype that is characterized by both high activity of glycolysis/OXPHOS and high levels of HIF-1/AMPK. Cells with this metabolic phenotype display maximum proliferation and clonogenicity relative to cells exhibiting a more glycolytic or more OXPHOS phenotype. The hybrid metabolic phenotype endows TNBC with the metabolic plasticity to switch between glycolysis and OXPHOS as a compensatory strategy in response to metabolic targeting drugs or an altered tumor environment110 (Figure 2). Therefore, dual targeting of glycolysis and mitochondrial bioenergetics or antioxidant pathways decreases cellular bioenergetics and increases the death of breast cancer cells102,111. Understanding this switchable metabolic dependence mechanism during tumor development and clinical process will facilitate the development of precise treatments.
Other metabolic pathways
Recently, we reported that increased expression of urine diphosphate–galactose ceramide galactosyltransferase (UGT8) in BLBC facilitates tumor aggressiveness through the sulfatide-αVβ5 axis and predicts poor prognosis. Mechanistically, UGT8 is transcriptionally upregulated by Sox10 to induce the biosynthesis of sulfatide, which then activates integrin αVβ5-mediated signaling, including TGF-β and NF-κB, to potentiate tumor viability and metastasis112. Dysregulation of protein glycosylation, resulting from an increased flux of the hexosamine biosynthetic pathway (HBP), contributes to breast tumor progression or metastasis113. N-acetylglucosamine-phosphate mutase (PGM3), which is responsible for the conversion of N-acetylglucosamine-6-P (GlcNAc-6-P) into N-acetylglucosamine-1-P (GlcNAc-1-P), is the key enzyme in the synthesis of UDP-GlcNAc, the donor substrate of protein glycosylation. In TNBC, the inhibition of PGM3 blocks the HBP and alters glycosylation levels and the stability of proteins, leading to unfolded protein response and arrested cell proliferation114. The de novo synthesis of pyrimidine from glutamine is induced in TNBC subjected to chemotherapy. Metabolic flux through this pathway is controlled by the multifunctional enzyme carbamoyl-phosphate synthetase 2, aspartate transcarbamoylase, dihydroorotase (CAD), and dihydroorotate dehydrogenase (DHODH). Exposure to doxorubicin likely stimulates pyrimidine synthesis by regulating the post-translational modification of CAD and its activity, whereas inhibiting DHODH sensitizes TNBC cells to doxorubicin and significantly induces the regression of TNBC xenografts115. In addition, targeting DHODH causes synthetic lethality in phosphatase and tensin homolog (PTEN)-mutant TNBC due to inherent defects in DNA repair, leading to accumulated DNA damage and impaired cell replication116.
Metabolic reprogramming of TNBC in the clinic
Functional imaging
Neo-adjuvant chemotherapy based on anthracyclines and taxanes is increasingly used prior to surgery to reduce the size of unresectable TNBC. Traditionally, the response to chemotherapy is assessed by tumor volume, which represents a post-treatment event117. Because of the significantly higher uptake of glucose and the glucose analogue 18F-fluorodeoxyglucose (18F-FDG), 18F-FDG PET/CT is used for early determination of TNBC sensitivity to neo-adjuvant chemotherapy and for prediction of recurrence after surgery118,119. Additionally, the thymidine analogue 3’-[18F]fluoro-3’-deoxythymidine (18F-FLT), which reflects the activity of thymidine kinase and pyrimidine salvage, is also a potential marker of chemotherapy efficacy in TNBC117. By using high-resolution 1H magnetic resonance spectroscopy (MRS), TNBC with high GLS activity exhibits low cellular glutamine pool size (glutamine concentration), which significantly increases after the inhibition of GLS120. Recently, novel imaging techniques have been developed, such as [18F](2S,4R)4-fluoroglutamine (18F-FGln) PET and glutamate-weighted chemical exchange saturation transfer MR imaging (GluCEST MRI), to study glutamine transport and kinetics. Similar to MRS, these methods enable the observation of altered levels of glutamine or glutamate upon GLS inhibition, indicating their feasibility to non-invasively detect the early therapeutic response of TNBC to GLS inhibitors120,121.
Targeting glutaminolysis and cystine uptake
Several classes of compounds have been developed to target GLS and block glutaminolysis in TNBC, and among these compounds, the most promising is CB-839, a potent, selective, and orally bioavailable inhibitor of GLS. CB-839 has an antiproliferative effect on TNBC cells both in vitro and in xenograft models, accompanied by marked decreases in glutaminolytic flux, oxygen consumption, and levels of GSH and TCA cycle intermediates122. In clinical trials, CB-839 (Telaglenastat) has been shown to be safe, and it exhibits a disease control rate of 55% in combination with paclitaxel in TNBC patients who are refractory to taxane therapy. CB-839 also suppresses mTOR activity in TNBC and synergizes with mTOR inhibition30. In combination with the mTOR inhibitor everolimus, it shows a disease control rate of 92% in renal cell carcinoma patients; the FDA has granted fast track designation for CB-839 in combination with everolimus or cabozantinib for the treatment of renal cell carcinoma123. Other combination therapies, such as the combination of GLS inhibition and bevacizumab (an anti-angiogenesis monoclonal antibody that targets VEGF), also exhibit antitumor effects on TNBC124. In addition, increased generation of glutamate by GLS can support the uptake of exogenous cystine through the cystine/glutamate antiporter xCT to maintain the redox balance. Sulfasalazine (SASP), as a clinically approved anti-inflammatory drug, is found to inhibit xCT activity and retards TNBC growth31 (Figure 2). By immunizing mice with a DNA-based vaccine expressing xCT protein, immunotargeting of the xCT antigen on the cell surface efficiently attenuates tumor growth and pulmonary metastasis and increases BCSC chemosensitivity to doxorubicin40. However, because the antibody titers achieved upon using the DNA vaccine are low, a virus-like-particle (VLP; AX09-0M6) immunotherapy has been developed, which elicits a stronger antibody response against xCT to decrease BCSC growth and prevent self-renewal125.
Dietary restriction of amino acids
Besides glutamine and cysteine, TNBC cells are also dependent, to some extent, on the availability of certain other amino acids such as methionine, asparagine, and arginine, which suggests that limiting the supply of these amino acids may confer therapeutic benefits. The depletion of either methionine or glutamine can increase the cell surface expression of pro-apoptotic TNF-related apoptosis-inducing ligand receptor-2 (TRAIL-R2) and sensitize TNBC cells to TRAIL-induced apoptosis71,72. Dietary methionine deprivation enhances cell susceptibility to lexatumumab, an agonistic monoclonal antibody targeting TRAIL-R2, and reduces the lung metastasis rate71,73. Additionally, many tumor and stem cells depend on the biosynthesis of the universal methyl-donor S-adenosylmethionine (SAM) from exogenous methionine by methionine adenosyltransferase 2α (MAT2A) to maintain their epigenome126,127. Methionine restriction is sufficient to cripple the tumor-initiating capability of TNBC partly because of impaired SAM generation. Limiting dietary methionine induces MAT2A expression in TNBC as an adaptive response; therefore, the combination of methionine restriction and the MAT2A inhibitor cycloleucine has a synergistic antitumor effect128. Under normal physiological conditions, the asparagine levels in the serum are lower than those in the mammary gland, thus making asparagine bioavailability a key regulator of circulating tumor cells and the metastatic potential of breast cancer. Limiting asparagine by knocking down asparagine synthetase (ASNS), treatment with l-asparaginase, or dietary asparagine restriction suppresses metastasis and decreases the level of asparagine-enriched proteins, especially proteins associated with EMT, which, at least in part, accounts for the inhibition of metastasis progression70 (Figure 2). The depletion of arginine by recombinant human arginase (rhArg) leads to TNBC cell apoptosis via ROS and induces adaptive autophagy, whereas blocking autophagic flux by autophagy-targeting drugs potentiates the cytotoxicity of rhArg129. Other dietary modifications, such as caloric restriction, also cause tumor regression in combination with radiation130.
Blocking other metabolic pathways
Overexpression of Myc induces a bioenergetic reliance on FAO in TNBC cells, especially under glucose deprivation or matrix detachment. Etomoxir (ETX), as a clinically tested, specific inhibitor of the enzyme CPT1 involved in FAO, causes ATP depletion and energetic stress, retarding tumor growth in a xenograft model74. As FAO activates oncogenic Src and its target genes, as discussed previously, ETX also reverses the Src-regulated gene pattern and abolishes the metastasis potential of TNBC76. We have identified that zoledronic acid (ZA), a marketed drug for the management of osteoporosis or bone metastasis, is the direct inhibitor of UGT8 in the sulfatide biosynthetic pathway. Significantly, ZA impedes sulfatide-induced oncogenic signaling, suppressing tumor aggressiveness and pulmonary metastasis of TNBC112. In addition, our early work also showed that epalrestat, which is used in the targeted treatment of diabetic complications, inhibits the activity of AKR1B1 and controls the NF-κB pathway, thus attenuating the BCSC phenotype and tumor metastasis88 (Figure 2). In response to genotoxic chemotherapy exposure, TNBC cells adaptively upregulate pyrimidine synthesis to increase the production of nucleotides necessary for DNA repair, whereas using leflunomide, a clinically approved inhibitor of de novo pyrimidine synthesis for the management of rheumatoid arthritis, sensitizes TNBC cells to chemotherapy and causes significant tumor regression when used in combination with doxorubicin115. Moreover, leflunomide induces the depletion of nucleotides and the accumulation of DNA damage during replication, leading to synthetic lethality in TNBC cells with the loss of PTEN, which is vital for DNA repair activity116. A novel inhibitor of the HBP enzyme PGM3, FR054, reduces both N- and O-glycosylation levels to regulate cell adhesion and migration, impairing tumor growth in vivo114.
Concluding remarks and future perspectives
In this review, we outline the metabolic rewiring of glycolysis, OXPHOS, amino acid metabolism, lipid metabolism, and other important branched pathways in TNBC. Cancer cells flexibly regulate key enzymes in these pathways to meet their bioenergetic and biosynthetic demands; subsequent alternations in intracellular energy, biomass precursors, and ROS levels further influence oncogenic signaling, cell viability, proliferation, and CSC phenotype91. Understanding the metabolic remodeling mechanisms should help in discovering new metabolic strategies and repurposing existing drugs for the effective intervention of TNBC.
However, metabolic heterogeneity and the adaptive response of TNBC pose considerable challenges to the transition of metabolically targeted therapy to the clinic. Although TNBC is characterized by the metabolic rewiring from OXPHOS to glycolysis, in a specific malignant mass, not all TNBC cells depend on aerobic glycolysis. Indeed, they can also transit to an epithelial-like state to rely on OXPHOS102 or stably maintain a hybrid metabolic phenotype with a high activity of both glycolysis and OXPHOS110. This metabolic heterogeneity and plasticity of cancer cells arise from inherent gene dysregulation or from extrinsic cues in the tumor microenvironment, such as hypoxia, acidification, nutrient availability, and antitumor drugs. The tumor microenvironment and the different cells in this microenvironment are often modified by the dysregulated metabolism of tumor cells to further facilitate tumor growth in a symbiotic manner131–133. Dysbiosis of the microbiome and microbial metabolome also plays a role in breast cancer metabolism and progression134. In addition, TNBC itself is a heterogeneous disease that can be classified into four distinct molecular subtypes according to gene expression profiling, with each subtype displaying a differential response to chemotherapy135. Therefore, in the future, systemic metabolic therapeutics in combination with radiation, chemotherapy, targeted therapy, or immunotherapy may be used to combat tumor heterogeneity and induce synthetic lethality, ultimately improving outcomes for patients with TNBC.
Acknowledgments
This work was supported by grants from the Key Program of Zhejiang Provincial Natural Science Foundation (Grant No. LZ17H160002), National Natural Science Foundation of China (Grant No. 81972456 and 81772801), the National Key R&D Program of China (Grant No. 2016YFC1303200), the Fundamental Research Funds for Central Universities of China (to C.D.), and the Thousand Young Talents Plan of China (to C.D.).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–60. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrido P, Osorio FG, Moran J, Cabello E, Alonso A, Freije JM, et al. Loss of GLUT4 induces metabolic reprogramming and impairs viability of breast cancer cells. J Cell Physiol. 2015;230:191–8. doi: 10.1002/jcp.24698. [DOI] [PubMed] [Google Scholar]
- 9.McCleland ML, Adler AS, Shang Y, Hunsaker T, Truong T, Peterson D, et al. An integrated genomic screen identifies LDHB as an essential gene for triple-negative breast cancer. Cancer Res. 2012;72:5812–23. doi: 10.1158/0008-5472.CAN-12-1098. [DOI] [PubMed] [Google Scholar]
- 10.Dong T, Liu Z, Xuan Q, Wang Z, Ma W, Zhang Q. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci Rep. 2017;7:6069. doi: 10.1038/s41598-017-06378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyen J, Trastour C, Ettore F, Peyrottes I, Toussant N, Gal J, et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun. 2014;451:54–61. doi: 10.1016/j.bbrc.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Morais-Santos F, Granja S, Miranda-Goncalves V, Moreira AH, Queiros S, Vilaca JL, et al. Targeting lactate transport suppresses in vivo breast tumour growth. Oncotarget. 2015;6:19177–89. doi: 10.18632/oncotarget.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avanzato D, Pupo E, Ducano N, Isella C, Bertalot G, Luise C, et al. High USP6NL Levels in Breast Cancer Sustain Chronic AKT Phosphorylation and GLUT1 Stability fueling aerobic glycolysis. Cancer Res. 2018;78:3432–44. doi: 10.1158/0008-5472.CAN-17-3018. [DOI] [PubMed] [Google Scholar]
- 14.Lim SO, Li CW, Xia W, Lee HH, Chang SS, Shen J, et al. EGFR signaling enhances aerobic glycolysis in triple-negative breast cancer cells to promote tumor growth and immune escape. Cancer Res. 2016;76:1284–96. doi: 10.1158/0008-5472.CAN-15-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 16.Lin A, Li C, Xing Z, Hu Q, Liang K, Han L, et al. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18:213–24. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–6. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palaskas N, Larson SM, Schultz N, Komisopoulou E, Wong J, Rohle D, et al. 18F-fluorodeoxy-glucose positron emission tomography marks MYC-overexpressing human basal-like breast cancers. Cancer Res. 2011;71:5164–74. doi: 10.1158/0008-5472.CAN-10-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen L, O’Shea JM, Kaadige MR, Cunha S, Wilde BR, Cohen AL, et al. Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proc Natl Acad Sci U S A. 2015;112:5425–30. doi: 10.1073/pnas.1501555112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onodera Y, Nam JM, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest. 2014;124:367–84. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie SM, Grossman EA, Crawford LA, Ding L, Camarda R, Huffman TR, et al. GSTP1 is a driver of triple-negative breast cancer cell metabolism and pathogenicity. Cell Chem Biol. 2016;23:567–78. doi: 10.1016/j.chembiol.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasgupta S, Rajapakshe K, Zhu B, Nikolai BC, Yi P, Putluri N, et al. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature. 2018;556:249–54. doi: 10.1038/s41586-018-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng CW, Kuo WH, Chan SH, Chan HL, Chang KJ, Wang LH. Transketolase regulates the metabolic switch to control breast cancer cell metastasis via the alpha-ketoglutarate signaling pathway. Cancer Res. 2018;78:2799–812. doi: 10.1158/0008-5472.CAN-17-2906. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific cebpb isoform in triple-negative breast cancer. Cell Metab. 2018;28:87–103 e6. doi: 10.1016/j.cmet.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogrodzinski MP, Bernard JJ, Lunt SY. Deciphering metabolic rewiring in breast cancer subtypes. Transl Res. 2017;189:105–22. doi: 10.1016/j.trsl.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–34. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201–8. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Ansari R, Craze ML, Miligy I, Diez-Rodriguez M, Nolan CC, Ellis IO, et al. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 2018;20:21. doi: 10.1186/s13058-018-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao MD, Lamichhane S, Lundgren S, Bofin A, Fjosne H, Giskeodegard GF, et al. Metabolic characterization of triple negative breast cancer. BMC Cancer. 2014;14:941. doi: 10.1186/1471-2407-14-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lampa M, Arlt H, He T, Ospina B, Reeves J, Zhang B, et al. Glutaminase is essential for the growth of triple-negative breast cancer cells with a deregulated glutamine metabolism pathway and its suppression synergizes with mTOR inhibition. PLoS One. 2017;12:e0185092. doi: 10.1371/journal.pone.0185092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmerman LA, Holton T, Yuneva M, Louie RJ, Padro M, Daemen A, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24:450–65. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassago A, Ferreira AP, Ferreira IM, Fornezari C, Gomes ER, Greene KS, et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci U S A. 2012;109:1092–7. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mates JM, Campos-Sandoval JA, Marquez J. Glutaminase isoenzymes in the metabolic therapy of cancer. Biochim Biophys Acta Rev Cancer. 2018;1870:158–64. doi: 10.1016/j.bbcan.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Kim JY, Heo SH, Choi SK, Song IH, Park IA, Kim YA, et al. Glutaminase expression is a poor prognostic factor in node-positive triple-negative breast cancer patients with a high level of tumor-infiltrating lymphocytes. Virchows Arch. 2017;470:381–89. doi: 10.1007/s00428-017-2083-5. [DOI] [PubMed] [Google Scholar]
- 35.Coloff JL, Murphy JP, Braun CR, Harris IS, Shelton LM, Kami K, et al. Differential glutamate metabolism in proliferating and quiescent mammary epithelial cells. Cell Metab. 2016;23:867–80. doi: 10.1016/j.cmet.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Spinelli JB, Yoon H, Ringel AE, Jeanfavre S, Clish CB, Haigis MC. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science. 2017;358:941–46. doi: 10.1126/science.aam9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kung HN, Marks JR, Chi JT. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 2011;7:e1002229. doi: 10.1371/journal.pgen.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong R, Zhang W, Xia X, Zhang K, Wang Y, Wu M, et al. Preventing BRCA1/ZBRK1 repressor complex binding to the GOT2 promoter results in accelerated aspartate biosynthesis and promotion of cell proliferation. Mol Oncol. 2019;13:959–77. doi: 10.1002/1878-0261.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Cao Q, Liao R, Wu X, Xun S, Huang J, et al. Loss of ABAT-mediated GABAergic system promotes basal-like breast cancer progression by activating Ca(2+)-NFAT1 axis. Theranostics. 2019;9:34–47. doi: 10.7150/thno.29407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzardo S, Conti L, Rooke R, Ruiu R, Accart N, Bolli E, et al. Immunotargeting of antigen xCT attenuates stem-like cell behavior and metastatic progression in breast cancer. Cancer Res. 2016;76:62–72. doi: 10.1158/0008-5472.CAN-15-1208. [DOI] [PubMed] [Google Scholar]
- 41.Lu H, Samanta D, Xiang L, Zhang H, Hu H, Chen I, et al. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc Natl Acad Sci U S A. 2015;112:E4600–9. doi: 10.1073/pnas.1513433112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa M, Takahashi H, Rajabi H, Alam M, Suzuki Y, Yin L, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7:11756–69. doi: 10.18632/oncotarget.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briggs KJ, Koivunen P, Cao S, Backus KM, Olenchock BA, Patel H, et al. Paracrine induction of HIF by glutamate in breast cancer: EglN1 senses cysteine. Cell. 2016;166:126–39. doi: 10.1016/j.cell.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sexton RE, Hachem AH, Assi AA, Bukhsh MA, Gorski DH, Speyer CL. Metabotropic glutamate receptor-1 regulates inflammation in triple negative breast cancer. Sci Rep. 2018;8:16008. doi: 10.1038/s41598-018-34502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speyer CL, Smith JS, Banda M, DeVries JA, Mekani T, Gorski DH. Metabotropic glutamate receptor-1: a potential therapeutic target for the treatment of breast cancer. Breast Cancer Res Treat. 2012;132:565–73. doi: 10.1007/s10549-011-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speyer CL, Hachem AH, Assi AA, Johnson JS, DeVries JA, Gorski DH. Metabotropic glutamate receptor-1 as a novel target for the antiangiogenic treatment of breast cancer. PLoS One. 2014;9:e88830. doi: 10.1371/journal.pone.0088830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang X, Ding CK, Wu J, Sjol J, Wardell S, Spasojevic I, et al. Cystine addiction of triple-negative breast cancer associated with EMT augmented death signaling. Oncogene. 2017;36:4235–42. doi: 10.1038/onc.2016.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen MS, Wang SF, Hsu CY, Yin PH, Yeh TS, Lee HC, et al. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget. 2017;8:114588–602. doi: 10.18632/oncotarget.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–50. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samanta D, Semenza GL. Serine synthesis helps hypoxic cancer stem cells regulate redox. Cancer Res. 2016;76:6458–62. doi: 10.1158/0008-5472.CAN-16-1730. [DOI] [PubMed] [Google Scholar]
- 52.Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–74. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samanta D, Park Y, Andrabi SA, Shelton LM, Gilkes DM, Semenza GL. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 2016;76:4430–42. doi: 10.1158/0008-5472.CAN-16-0530. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Bai W. Repression of phosphoglycerate dehydrogenase sensitizes triple-negative breast cancer to doxorubicin. Cancer Chemother Pharmacol. 2016;78:655–9. doi: 10.1007/s00280-016-3117-4. [DOI] [PubMed] [Google Scholar]
- 55.Pollari S, Kakonen SM, Edgren H, Wolf M, Kohonen P, Sara H, et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat. 2011;125:421–30. doi: 10.1007/s10549-010-0848-5. [DOI] [PubMed] [Google Scholar]
- 56.Gautam J, Banskota S, Regmi SC, Ahn S, Jeon YH, Jeong H, et al. Tryptophan hydroxylase 1 and 5-HT7 receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol Cancer. 2016;15:75. doi: 10.1186/s12943-016-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noonepalle SK, Gu F, Lee EJ, Choi JH, Han Q, Kim J, et al. Promoter methylation modulates indoleamine 2,3-dioxygenase 1 induction by activated T cells in human breast cancers. Cancer Immunol Res. 2017;5:330–44. doi: 10.1158/2326-6066.CIR-16-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75:4651–64. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers TJ, Christenson JL, Greene LI, O’Neill KI, Williams MM, Gordon MA, et al. Reversal of triple-negative breast cancer EMT by miR-200c decreases tryptophan catabolism and a program of immunosuppression. Mol Cancer Res. 2019;17:30–41. doi: 10.1158/1541-7786.MCR-18-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roci I, Watrous JD, Lagerborg KA, Lafranchi L, Lindqvist A, Jain M, et al. Mapping metabolic events in the cancer cell cycle reveals arginine catabolism in the committed SG2M phase. Cell Rep. 2019;26:1691–700 e5. doi: 10.1016/j.celrep.2019.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erbas H, Bal O, Cakir E. Effect of rosuvastatin on arginase enzyme activity and polyamine production in experimental breast cancer. Balkan Med J. 2015;32:89–95. doi: 10.5152/balkanmedj.2015.15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang HL, Chen WC, Hsu HP, Cho CY, Hung YH, Wang CY, et al. Argininosuccinate lyase is a potential therapeutic target in breast cancer. Oncol Rep. 2015;34:3131–9. doi: 10.3892/or.2015.4280. [DOI] [PubMed] [Google Scholar]
- 63.Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest. 2010;120:3843–54. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garrido P, Shalaby A, Walsh EM, Keane N, Webber M, Keane MM, et al. Impact of inducible nitric oxide synthase (iNOS) expression on triple negative breast cancer outcome and activation of EGFR and ERK signaling pathways. Oncotarget. 2017;8:80568–88. doi: 10.18632/oncotarget.19631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Switzer CH, Glynn SA, Cheng RY, Ridnour LA, Green JE, Ambs S, et al. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol Cancer Res. 2012;10:1203–15. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walsh EM, Keane MM, Wink DA, Callagy G, Glynn SA. Review of Triple negative breast cancer and the impact of inducible nitric oxide synthase on tumor biology and patient outcomes. Crit Rev Oncog. 2016;21:333–51. doi: 10.1615/CritRevOncog.2017021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickok JR, Vasudevan D, Antholine WE, Thomas DD. Nitric oxide modifies global histone methylation by inhibiting Jumonji C domain-containing demethylases. J Biol Chem. 2013;288:16004–15. doi: 10.1074/jbc.M112.432294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vasudevan D, Hickok JR, Bovee RC, Pham V, Mantell LL, Bahroos N, et al. Nitric oxide regulates gene expression in cancers by controlling histone posttranslational modifications. Cancer Res. 2015;75:5299–308. doi: 10.1158/0008-5472.CAN-15-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thewes V, Simon R, Hlevnjak M, Schlotter M, Schroeter P, Schmidt K, et al. The branched-chain amino acid transaminase 1 sustains growth of antiestrogen-resistant and ERalpha-negative breast cancer. Oncogene. 2017;36:4124–34. doi: 10.1038/onc.2017.32. [DOI] [PubMed] [Google Scholar]
- 70.Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554:378–81. doi: 10.1038/nature25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strekalova E, Malin D, Good DM, Cryns VL. Methionine deprivation induces a targetable vulnerability in triple-negative breast cancer cells by enhancing TRAIL receptor-2 expression. Clin Cancer Res. 2015;21:2780–91. doi: 10.1158/1078-0432.CCR-14-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mauro-Lizcano M, Lopez-Rivas A. Glutamine metabolism regulates FLIP expression and sensitivity to TRAIL in triple-negative breast cancer cells. Cell Death Dis. 2018;9:205. doi: 10.1038/s41419-018-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeon H, Kim JH, Lee E, Jang YJ, Son JE, Kwon JY, et al. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Oncotarget. 2016;7:67223–34. doi: 10.18632/oncotarget.11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22:427–32. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–51. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park JH, Vithayathil S, Kumar S, Sung PL, Dobrolecki LE, Putluri V, et al. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 2016;14:2154–65. doi: 10.1016/j.celrep.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carracedo A, Weiss D, Leliaert AK, Bhasin M, de Boer VC, Laurent G, et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. 2012;122:3088–100. doi: 10.1172/JCI62129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.German NJ, Yoon H, Yusuf RZ, Murphy JP, Finley LW, Laurent G, et al. PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2. Mol Cell. 2016;63:1006–20. doi: 10.1016/j.molcel.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iriondo O, Rábano M, Domenici G, Carlevaris1 O, López-Ruiz JA, Zabalza I, et al. Distinct breast cancer stem/progenitor cell populations require either HIF1α or loss of PHD3 to expand under hypoxic conditions. Oncotarget. 2015;06:31721–39. doi: 10.18632/oncotarget.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rios Garcia M, Steinbauer B, Srivastava K, Singhal M, Mattijssen F, Maida A, et al. Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 2017;26:842–55 e5. doi: 10.1016/j.cmet.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Giro-Perafita A, Palomeras S, Lum DH, Blancafort A, Vinas G, Oliveras G, et al. Preclinical evaluation of fatty acid synthase and EGFR inhibition in triple-negative breast cancer. Clin Cancer Res. 2016;22:4687–97. doi: 10.1158/1078-0432.CCR-15-3133. [DOI] [PubMed] [Google Scholar]
- 82.Wahdan-Alaswad RS, Cochrane DR, Spoelstra NS, Howe EN, Edgerton SM, Anderson SM, et al. Metformin-induced killing of triple-negative breast cancer cells is mediated by reduction in fatty acid synthase via miRNA-193b. Horm Cancer. 2014;5:374–89. doi: 10.1007/s12672-014-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–36. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu RZ, Graham K, Glubrecht DD, Lai R, Mackey JR, Godbout R. A fatty acid-binding protein 7/RXRbeta pathway enhances survival and proliferation in triple-negative breast cancer. J Pathol. 2012;228:310–21. doi: 10.1002/path.4001. [DOI] [PubMed] [Google Scholar]
- 85.Liu RZ, Graham K, Glubrecht DD, Germain DR, Mackey JR, Godbout R. Association of FABP5 expression with poor survival in triple-negative breast cancer: implication for retinoic acid therapy. Am J Pathol. 2011;178:997–1008. doi: 10.1016/j.ajpath.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhardwaj A, Singh H, Trinidad CM, Albarracin CT, Hunt KK, Bedrosian I. The isomiR-140-3p-regulated mevalonic acid pathway as a potential target for prevention of triple negative breast cancer. Breast Cancer Res. 2018;20:150. doi: 10.1186/s13058-018-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antalis CJ, Arnold T, Rasool T, Lee B, Buhman KK, Siddiqui RA. High ACAT1 expression in estrogen receptor negative basal-like breast cancer cells is associated with LDL-induced proliferation. Breast Cancer Res Treat. 2010;122:661–70. doi: 10.1007/s10549-009-0594-8. [DOI] [PubMed] [Google Scholar]
- 88.Wu X, Li X, Fu Q, Cao Q, Chen X, Wang M, et al. AKR1B1 promotes basal-like breast cancer progression by a positive feedback loop that activates the EMT program. J Exp Med. 2017;214:1065–79. doi: 10.1084/jem.20160903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He J, Zhang F, Tay LWR, Boroda S, Nian W, Levental KR, et al. Lipin-1 regulation of phospholipid synthesis maintains endoplasmic reticulum homeostasis and is critical for triple-negative breast cancer cell survival. FASEB J. 2017;31:2893–904. doi: 10.1096/fj.201601353R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Z, Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends in Cancer. 2019;5:30–45. doi: 10.1016/j.trecan.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Yang J, Cheng J, Sun B, Li H, Wu S, Dong F, et al. Untargeted and stable isotope-assisted metabolomic analysis of MDA-MB-231 cells under hypoxia. Metabolomics. 2018;14:40. doi: 10.1007/s11306-018-1338-8. [DOI] [PubMed] [Google Scholar]
- 93.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–31. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–5. doi: 10.1038/nature13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee KM, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL, Hutchinson KE, et al. MYC and MCL1 Cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab. 2017;26:633–47 e7. doi: 10.1016/j.cmet.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chourasia AH, Tracy K, Frankenberger C, Boland ML, Sharifi MN, Drake LE, et al. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep. 2015;16:1145–63. doi: 10.15252/embr.201540759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liao R, Ren G, Liu H, Chen X, Cao Q, Wu X, et al. ME1 promotes basal-like breast cancer progression and associates with poor prognosis. Sci Rep. 2018;8:16743. doi: 10.1038/s41598-018-35106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong SM, Park CW, Kim SW, Nam YJ, Yu JH, Shin JH, et al. NAMPT suppresses glucose deprivation-induced oxidative stress by increasing NADPH levels in breast cancer. Oncogene. 2016;35:3544–54. doi: 10.1038/onc.2015.415. [DOI] [PubMed] [Google Scholar]
- 99.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–99. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Shpyleva SI, Tryndyak VP, Kovalchuk O, Starlard-Davenport A, Chekhun VF, Beland FA, et al. Role of ferritin alterations in human breast cancer cells. Breast Cancer Res Treat. 2011;126:63–71. doi: 10.1007/s10549-010-0849-4. [DOI] [PubMed] [Google Scholar]
- 101.O’Neill S, Porter RK, McNamee N, Martinez VG, O’Driscoll L. 2-Deoxy-D-Glucose inhibits aggressive triple-negative breast cancer cells by targeting glycolysis and the cancer stem cell phenotype. Sci Rep. 2019;9:3788. doi: 10.1038/s41598-019-39789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo M, Shang L, Brooks MD, Jiagge E, Zhu Y, Buschhaus JM, et al. Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 2018;28:69–86 e6. doi: 10.1016/j.cmet.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67:1472–86. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- 104.Wright HJ, Hou J, Xu B, Cortez M, Potma EO, Tromberg BJ, et al. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc Natl Acad Sci U S A. 2017;114:E6556–E65. doi: 10.1073/pnas.1703791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fiorillo M, Sotgia F, Lisanti MP. “Energetic” cancer stem cells (e-CSCs): A new hyper-metabolic and proliferative tumor cell phenotype, driven by mitochondrial energy. Front Oncol. 2018;8:677. doi: 10.3389/fonc.2018.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sotgia F, Fiorillo M, Lisanti MP. Hallmarks of the cancer cell of origin: Comparisons with “energetic” cancer stem cells (e-CSCs). Aging (Albany NY). 2019;11:1065–68. doi: 10.18632/aging.101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Echeverria GV, Ge Z, Seth S, Zhang X, Jeter-Jones S, Zhou X, et al. Resistance to neoadjuvant chemotherapy in triple-negative breast cancer mediated by a reversible drug-tolerant state. Sci Translational Med. 2019;11:eaav0936. doi: 10.1126/scitranslmed.aav0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones RA, Robinson TJ, Liu JC, Shrestha M, Voisin V, Ju Y, et al. RB1 deficiency in triple-negative breast cancer induces mitochondrial protein translation. J Clin Invest. 2016;126:3739–57. doi: 10.1172/JCI81568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zacksenhaus E, Shrestha M, Liu JC, Vorobieva I, Chung PED, Ju Y, et al. Mitochondrial OXPHOS induced by RB1 deficiency in breast cancer: Implications for anabolic metabolism, stemness, and metastasis. Trends Cancer. 2017;3:768–79. doi: 10.1016/j.trecan.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Jia D, Lu M, Jung KH, Park JH, Yu L, Onuchic JN, et al. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci U S A. 2019;116:3909–18. doi: 10.1073/pnas.1816391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lucantoni F, Dussmann H, Prehn JHM. Metabolic targeting of breast cancer cells with the 2-deoxy-d-glucose and the mitochondrial bioenergetics inhibitor MDIVI-1. Front Cell Dev Biol. 2018;6:113. doi: 10.3389/fcell.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao Q, Chen X, Wu X, Liao R, Huang P, Tan Y, et al. Inhibition of UGT8 suppresses basal-like breast cancer progression by attenuating sulfatide-alphaVbeta5 axis. J Exp Med. 2018;215:1679–92. doi: 10.1084/jem.20172048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Milde-Langosch K, Karn T, Schmidt M, zu Eulenburg C, Oliveira-Ferrer L, Wirtz RM, et al. Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res Treat. 2014;145:295–305. doi: 10.1007/s10549-014-2949-z. [DOI] [PubMed] [Google Scholar]
- 114.Ricciardiello F, Votta G, Palorini R, Raccagni I, Brunelli L, Paiotta A, et al. Inhibition of the hexosamine biosynthetic pathway by targeting PGM3 causes breast cancer growth arrest and apoptosis. Cell Death Dis. 2018;9:377. doi: 10.1038/s41419-018-0405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown KK, Spinelli JB, Asara JM, Toker A. Adaptive reprogramming of de novo pyrimidine synthesis is a metabolic vulnerability in triple-negative breast cancer. Cancer Discov. 2017;7:391–99. doi: 10.1158/2159-8290.CD-16-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mathur D, Stratikopoulos E, Ozturk S, Steinbach N, Pegno S, Schoenfeld S, et al. PTEN regulates glutamine flux to pyrimidine synthesis and sensitivity to dihydroorotate dehydrogenase inhibition. Cancer Discov. 2017;7:380–90. doi: 10.1158/2159-8290.CD-16-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raccagni I, Belloli S, Valtorta S, Stefano A, Presotto L, Pascali C, et al. [18F]FDG and [18F]FLT PET for the evaluation of response to neo-adjuvant chemotherapy in a model of triple negative breast cancer. PLoS One. 2018;13:e0197754. doi: 10.1371/journal.pone.0197754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Humbert O, Riedinger JM, Charon-Barra C, Berriolo-Riedinger A, Desmoulins I, Lorgis V, et al. Identification of biomarkers including 18FDG-PET/CT for early prediction of response to neoadjuvant chemotherapy in triple-negative breast cancer. Clin Cancer Res. 2015;21:5460–8. doi: 10.1158/1078-0432.CCR-15-0384. [DOI] [PubMed] [Google Scholar]
- 119.Kim YI, Kim YJ, Paeng JC, Cheon GJ, Lee DS, Chung JK, et al. Prediction of breast cancer recurrence using lymph node metabolic and volumetric parameters from (18)F-FDG PET/CT in operable triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1787–95. doi: 10.1007/s00259-017-3748-7. [DOI] [PubMed] [Google Scholar]
- 120.Zhou R, Pantel AR, Li S, Lieberman BP, Ploessl K, Choi H, et al. [(18)F](2S,4R)4-Fluoroglutamine PET detects glutamine pool size changes in triple-negative breast cancer in response to glutaminase inhibition. Cancer Res. 2017;77:1476–84. doi: 10.1158/0008-5472.CAN-16-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou R, Bagga P, Nath K, Hariharan H, Mankoff DA, Reddy R. Glutamate-weighted chemical exchange saturation transfer magnetic resonance imaging detects glutaminase inhibition in a mouse model of triple-negative breast cancer. Cancer Res. 2018;78:5521–26. doi: 10.1158/0008-5472.CAN-17-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 123.Gras J. Telaglenastat glutaminase inhibitor treatment of advanced solid tumors. Drugs of the Future. 2018;43:881–89. [Google Scholar]
- 124.Huang Q, Stalnecker C, Zhang C, McDermott LA, Iyer P, O’Neill J, et al. Characterization of the interactions of potent allosteric inhibitors with glutaminase C, a key enzyme in cancer cell glutamine metabolism. J Biol Chem. 2018;293:3535–45. doi: 10.1074/jbc.M117.810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bolli E, O’Rourke JP, Conti L, Lanzardo S, Rolih V, Christen JM, et al. A virus-like-particle immunotherapy targeting epitope-specific anti-xCT expressed on cancer stem cell inhibits the progression of metastatic cancer in vivo. Oncoimmunology. 2018;7:e1408746. doi: 10.1080/2162402X.2017.1408746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borrego SL, Fahrmann J, Datta R, Stringari C, Grapov D, Zeller M, et al. Metabolic changes associated with methionine stress sensitivity in MDA-MB-468 breast cancer cells. Cancer Metab. 2016;4:9. doi: 10.1186/s40170-016-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Z, Yip LY, Lee JHJ, Wu Z, Chew HY, Chong PKW, et al. Methionine is a metabolic dependency of tumor-initiating cells. Nat Med. 2019;25:825–37. doi: 10.1038/s41591-019-0423-5. [DOI] [PubMed] [Google Scholar]
- 128.Strekalova E, Malin D, Weisenhorn EMM, Russell JD, Hoelper D, Jain A, et al. S-adenosylmethionine biosynthesis is a targetable metabolic vulnerability of cancer stem cells. Breast Cancer Res Treat. 2019;175:39–50. doi: 10.1007/s10549-019-05146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z, Shi X, Li Y, Fan J, Zeng X, Xian Z, et al. Blocking autophagy enhanced cytotoxicity induced by recombinant human arginase in triple-negative breast cancer cells. Cell Death & Disease. 2014;5:e1563–e63. doi: 10.1038/cddis.2014.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saleh AD, Simone BA, Palazzo J, Savage JE, Sano Y, Dan T, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. 2013;12:1955–63. doi: 10.4161/cc.25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gandhi N, Das GM. Metabolic reprogramming in breast cancer and its therapeutic implications. Cells. 2019;8:pii: E89. doi: 10.3390/cells8020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–17. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, et al. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–43. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miko E, Kovacs T, Sebo E, Toth J, Csonka T, Ujlaki G, et al. Microbiome-microbial metabolome-cancer cell interactions in breast cancer-familiar, but unexplored. Cells. 2019;8:pii: E293. doi: 10.3390/cells8040293. doi: 10.3390/cells8040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]