Abstract
Simple Summary
Necrotic enteritis is one of the most important economic issues in the poultry industry, associated with sudden death rates of up to 50%. However, there is limited information on the role of probiotics and/or phytobiotic compounds on the treatment and prevention of Clostridium perfringens infections in broiler chicks. This study aimed to assess the effects of probiotic compounds (Maxus, CloStat, Sangrovit Extra, CloStat + Sangrovit Extra and Gallipro Tech) on the growth performance, blood biochemistry and intestinal health of broiler chicks in vivo. The results demonstrated that the inclusion of probiotic and/or phytobiotic compounds has a positive effect on performance, blood constituents, liver histopathology, intestinal morphology and histopathology. Furthermore, a notable reduction in both lesion scores was observed when probiotics and phytobiotics alone or in combination were included in the diets.
Abstract
This study evaluated the effects of feed additives on the growth, blood biochemistry and intestinal health of broiler chicks. A total of 378 of broiler chicks (Ross 308) were randomly allotted to seven groups. Chicks were fed a basal diet with 0.0 (control negative), 0.0 (control positive), 0.1, 0.5, 0.12, 0.5 + 0.12 and 0.2 g Kg−1 of Maxus, CloStat, Sangrovit Extra, CloStat + Sangrovit Extra and Gallipro Tech, respectively for 35 days. After 15 days, the chicks were inoculated with Clostridium perfringens. All feed additives were found to enhance growth performance and feed efficiency. The best feed conversion ratio was found in the Negative Control, CloStat + Sangrovit Extra and Gallipro Tect groups, respectively. A notable increase in villus length, total villus area, small intestine weight, ilium weight and total lesion score was found in chicks supplemented with Bacillus subtilis. Besides, the dietary inclusion of phytobiotic compounds showed potential in reducing the serum Alanine aminotransferase (ALT) concentration and increasing the glucose levels. All intestine and liver histopathological signs were reduced in chicks fed a probiotic-supplemented diet. Our findings indicate that supplementation with probiotics and phytobiotics alone or in combined form can be used to enhance performance, intestine health and blood constituents against C. perfringens infection in broiler chicks.
Keywords: probiotic, phytobiotic, broiler, C. perfringens, histopathological, intestinal health
1. Introduction
Clostridium perfringens (C. perfringens) is a Gram-positive, anaerobic, germ-growing bacteria found within the gastrointestinal (GI) tract of several animals and in the environment [1,2] and poses an important threat to animals [3]. C. perfringens strains can produce up to 17 different toxins, where each set of toxins is responsible for a specific disease [4]. C. perfringens bacteria are responsible for several known infections in animals, including enterotoxaemia, gangrenous dermatitis and necrotic enteritis (NE), especially in poultry [5,6]. The induction of NE by C. perfringens in chicks can result in sudden death, with mortality rates of up to 50% [7,8]. C. perfringens bacteria are also responsible for subclinical infections, related to the chronic intestinal mucosa damage [9], which can have serious consequences, including decreased growth performance and weight gain and economic losses [10,11]. The cost of NE not only includes the direct loss due to the sudden death of broilers but also veterinary and scrubbing costs [12]. C. perfringens is generally always found in healthy chicks, even if at low levels (<105 CFU/g) in the intestinal tract [9]. Several factors can create a favorable environment in the intestine for the proliferation of the bacterium and the subsequent development of disease [9]. The coccidiosis incidence is one of the most important of these factors [13,14]. However, there are also several factors related to diet that are conducive to disease, including the epithelial thickness, the incidence of non-digestible polysaccharides and the intestinal pH value [15,16,17]. Bacterial cells or spores infection can be induced in animals through the type of feed, contaminated litter or by cross-contamination with infected early-stage animals [18]. Young animals are primarily at risk due to their immature immune systems and underdeveloped intestinal flora [19]. Infected animals manifest lesions of the jejunum and ileum, while the small intestine shows a degenerated mucosa and can be swollen by gases produced by C. perfringens [20]. Some visible symptoms of infection include changes in behavior, less movement and diarrhea [21].
Several strategies have been suggested for the prevention and control of NE infection in poultry [22]. The use of feed additives for the prevention of gut diseases via enhancing the intestinal microbiota has drawn the attention of nutrition scientists in an animal in vivo studies [23]. The supplementation of diets with antibiotics is a well-known strategy against NE in broiler chicks [24]. Avilamycin, an oligosaccharides antibiotic, is effective against many pathogenic and Gram-positive bacteria [25]. Furthermore, it has wide in vitro bactericidal effects, especially against C. perfringens [25]. Paradis et al. [22] and Mwangi et al. [26] found a strong relationship between the inclusion rate of avilamycin in the diet and a reduction in the mortality rate, lesion scores and C. perfringens counts induced by NE diseases.
Using probiotics as a food supplement improves the intestinal microbial balance of the host [27]. Probiotics cooperate with the host to enhance intestinal immunity and morphology but can also induce metabolism function, thus decreasing the risk of infection by opportunistic pathogenic bacteria [9]. Moreover, probiotic bacteria can play an antimicrobial role by producing molecules with antibacterial activities, such as bacteriocins, which can target certain pathogens and prevent the adhesion of pathogens or the excretion of pathogenic toxins [28,29]. Furthermore, beneficial bacteria can protect the host against pathogenic strains growing within the digestive tract [30]. Several studies have previously reported on the beneficial role played by certain Bacillus and Lactobacillus strains against C. perfringens activity in vitro [31,32].
The supplementation of broiler diets with Bacillus spores (B. licheniformis) has also been reported to result in a beneficial role played by probiotics when supplemented at high doses for extended periods compared to antibiotics [33]. After the inoculation of 20-day-old chicks with a low dosage of C. perfringens, the supplementation of their diet with a single dose of 1 × 109 Bacillus subtilis mitigated the colonization and insistence of C. perfringens, even though Bacillus subtilis did not affect C. perfringens in vitro [34]. Similarly, Sokale et al. [35] found that the supplementation of broiler chicks with Bacillus subtilis alone resulted in improved production, growth performance and reduced mortality after a C. perfringens challenge. Therefore, supplementation with Bacillus subtilis can not only be used to control NE diseases but also enhances gut health in broiler chicks [36].
Despite these findings, there are no studies on the ameliorative role of other probiotics (Maxus, CloStat, Sangrovit Extra, Gallipro Tech) as feed additives alone or in combined form in terms of growth, blood biochemical parameters and gut health of C. perfringens-challenged broiler chicks. As such, this study investigated the beneficial effects of dietary supplementation with feed additives on the growth parameters, feed utilization, hematological profile, liver histopathology, intestine morphometrics and histopathology of broiler chicks, as well as their health and performance under disease stress.
2. Materials and Methods
Experiments were performed at the Animal Production Department, College of Food Science and Agriculture Science, King Saud University. All of the protocols were performed and approved according to the experimentation guidelines of the Ethics of Animal Use in Research Committee of King Saud University (Ethical reference No: SE-19-150).
2.1. Feeding Trial and Regimen
For the experimental trials, 378 1-day-old broiler chicks (Ross 308) were selected and randomly allocated into seven groups (Table 1). Each group contained nine replicates, with six chicks per replicate. Upon arrival, all chicks were verified for the absence of Salmonella and C. perfringens. The broiler chicks were raised in cage pens (Dimensions of each pen were 5 × 3 × 2.5 feet) under similar managerial and hygienic conditions in an environmentally controlled poultry unit. Shed temperature was set for 1 week to 35 °C and then every week it was decreased gradually until it reached 24 °C. The lighting program followed standard ROSS TECH Lighting for Broilers [37]. Standard starter (0–15) and finisher (16–33 days) diets comprised of isocaloric and isonitrogenous ingredients were provided in mash form based on corn-soybean meal (SBM) and formulated to meet the requirements of Ross 308 broiler chicks (Table 2).
Table 1.
Experimental groups and inclusion rates of feed additives used during the feeding trial period (day 0–35).
| Item | Inclusion rate (g/kg) | Product resource |
|---|---|---|
| NC 1 | ‒ | ‒ |
| PC 2 | ‒ | ‒ |
| M 3 | 0.1 | Maxus: 100 g of avilamycin (BIOFERM CZ, spol. sro.) per 1000 g. |
| CL4 | 0.5 | CloStat: Bacillus subtilis (2 × 107 CFU/g) (KEMIN Ind., Valley Center, CA, USA) per 1 g. |
| S 5 | 0.12 | Sangrovit Extra: Photobiotic compound (benzophenanthridine alkaloids, sanguinarine and protopine) (Albitalia s.r.L., Co., Milano, Italy) |
| CL + S 6 | 0.5 CL+ 0.12 S | CloStat + Sangrovit Extra |
| G 7 | 0.2 | Gallipro Tech: A highly-selected strain (DSM17299) of Bacillus subtilis (4 × 109 CFU/g DSM 17299) (Boege Alle Co., Hoersholm, Denmark) |
1 Negative control = control with no additive or challenge; 2 PC = positive control; 3 M = 1 kg of the product contains 100 g avilamycin; 4 Clostat = Bacillus subtilis (2 × 107 CFU/g); 5 Sangrovit Extra = 0.12 g/kg after Clostridium challenge; 6 CL + S = 0.5 g/kg CloStat + 0.12 g/kg Sangrovit Extra; 7 G = Bacillus licheniformis (1.5 × 1011 CFU/g) after Clostridium challenge.
Table 2.
Composition of starter and finisher diets.
| Ingredient | Treatment Period (0‒35) days | |
|---|---|---|
| Starter (0–15) | Finisher (15–35) | |
| Yellow corn | 57.39 | 61.33 |
| Soybean meal | 27.00 | 22.80 |
| Palm oil | 2.20 | 2.80 |
| Corn gluten meal | 8.80 | 6.0 |
| Wheat bran | 0.00 | 3.0 |
| DCP | 2.30 | 2.09 |
| Ground limestone | 0.70 | 0.62 |
| Choline chloride | 0.05 | 0.05 |
| DL-methionine | 0.105 | 0.075 |
| L-lysine | 0.39 | 0.36 |
| Salt | 0.40 | 0.20 |
| Threonine | 0.17 | 0.17 |
| V-M premix 1 | 0.50 | 0.50 |
| Total | 100 | 100 |
| Analysis | ||
| ME (kcal/kg) | 3000 | 3050 |
| Crude protein (%) | 23.0 | 20.5 |
| Non-phytate P (%) | 0.48 | 0.44 |
| Calcium (%) | 0.96 | 0.88 |
| Digestible lysine (%) | 1.28 | 1.15 |
| Digestible methionine (%) | 0.60 | 0.54 |
| Digestible sulfur amino acids (%) | 0.95 | 0.86 |
| Digestible threonine (%) | 0.86 | 0.77 |
1 V-M premix; vitamin-mineral premix contains in the following per kg: vitamin A, 2,400,000 IU; vitamin D, 1,000,000 IU; vitamin E, 16,000 IU; vitamin K, 800 mg; vitamin B1, 600 mg; vitamin B2, 1600 mg; vitamin B6, 1000 mg; vitamin B12, 6 mg; niacin, 8000 mg; folic acid, 400 mg; pantothenic acid, 3000 mg; biotin 40 mg; antioxidant, 3000 mg; cobalt, 80 mg; copper, 2000 mg; iodine, 400; iron, 1200 mg; manganese, 18,000 mg; selenium, 60 mg; zinc, 14.000 mg.
2.2. Challenge Inoculum
On day 15, all of the group except for the negative control group were challenged with C. perfringens (Micro Biologics, Cloud, MN, USA) at a rate of 4 × 108 CFU/g via oral gavages [38]. Necropsies were carried out from the first day of infection to determine the cause of mortality. Confluent necrosis and sloughing of the intestinal epithelium were considered as signs of NE. C. perfringens was isolated from chicks were died because of NE.
2.3. Performance Measurements
During the feeding trial period (days 0‒35), the growth performance of the broiler chicks was evaluated by recording their feed intake daily. To this end, the amount of feed rejected was subtracted from the feed offered to determine the feed intake. Also, live body weight was recorded at weekly intervals, while the final body weight and total feed consumption were recorded at the end of the feeding trial period. Body weight gain was calculated as the difference between the live body weight and the final body weight. The feed conversion ratio (FCR) was computed for each group Abudabos et al. [39] using the following formula—FCR = feed intake/weight gain. The production efficiency factor (PEF) was calculated Griffin [40] using the following formula:
| PEF = (livability × live weight (kg))/(age in days × FCR) × 100. | (1) |
During the feeding trial, the number of deaths was counted to calculate the survival rate as the percentage of the surviving to the initial number of broilers
2.4. Intestine Morphometric Analysis and Lesions Score
In order to evaluate the intestinal characteristic (small and large intestines, including the ceca) and the lesions score of necrotic enteritis were randomly collected from nine broiler chicks in each group and weighed immediately after slaughtering according to Jensen et al. [41]. Before slaughter, the feed was withdrawn for six hours to ensure that the digestive tract was empty and the live body weight was determined. After slaughtering, the birds were defeathered and eviscerated. After removal, the duodenum, jejunum, ileum, caeca and small intestine were weighed and measured. The small intestine was measured between the site from which the duodenum emerges from the gizzard and the beginning of the ceca. The relative organ weight was calculated according to Arif et al. [42] formula:
| the relative organ weight = (weight of organ/live weight of bird) × 100. | (2) |
All of the chicks were weighed before sacrifice. The gross intestinal lesions characteristic of necrotic enteritis were determined according to Long et al. [43] procedure. The lesion scores were annotated as follows: 0 = none, 1 = mild, 2 = moderate and 3 = marked (severe) [44].
The length, width and total surface area of the villus in the intestine were used as the intestinal morphometric variables [45]. 2-cm segments were dissected from the midpoint and the distal end of the small intestine of the broiler chicks. These segments were flushed with phosphate-buffered saline (PBS) (pH 7), fixed in Clark fixative for 45 min and stored in ethyl alcohol (50%). Each segment was then divided into two sections along its length (sections A and B). Section A was placed in periodic acid Schiff (PAS) reagent for 2–3 min and observed under a dissecting microscope at 100× in five randomly chosen fields of view (using a zigzag line). The types and number of different villi were recorded. Section B of each sample was allowed to stand in PAS for staining. The muscle layers were then separated from the mucosa and the rows of villi were cut in sagittal sections before being transferred onto glass slides and covered with a cover slip. These samples were examined using a microscope in the direction of the rows with an eye piece graticule at 100× [46]. The villus height (µm) and width (μm) were measured from the top of the villus to the top of the lamina propria using image capture and analysis system (Image-Pro Plus version 4.5, Media Cybernetics, Silver Spring, MD, USA). The surface area was calculated using the following formula:
| (3) |
where VW is the villus width and VL is the villus length [47]. This was repeated for 8 chicks/treatment/age in triplicate. For each chick, two segments from the medium and distal intestine were examined.
2.5. Blood Biochemical Measurements
At the end of the feeding trial, 3 ml of blood were obtained from the wing vein of six chicks per treatment. The blood samples were centrifuged at 3000× g for 10 min and the resulting serum was stored at −20 °C until further analysis. The serum indices, total protein (TP) and albumin values were determined using the method described by Reinhold [48]. The globulin levels were calculated as the difference between the total protein and albumin levels. Cholesterol [49], glucose, triglyceride [50] and serum enzyme (ALT and AST) activities were determined spectrophotometrically [51,52] using an enzymatic kit (Witte kamp 30. D-30163; MDI Europa GmbH, Hannover, Germany).
2.6. Histopathological Examination
For histopathological examination, the tow broiler chicks from each replicate were sacrificed at the end of the feeding trial. The Eighteen longitudinal sections of the intestines (from three parts, anterior, mid and posterior) and liver from each treatment were cut and fixed overnight in 10% buffered neutral formalin solution. The fixed tissues were processed and stained with hematoxylin and eosin (HE), as described by Naiel et al. [53]. Then, five μm stained sections were observed and analyzed histopathologically, as reported by Bancroft and Gamble [54].
2.7. Statistical Analysis
The collected data were statistically analyzed using one-way analysis of variance (ANOVA). Significant differences were determined using Duncan’s Multiple Range [55]. All analyses were performed using SPSS software (version 14) (Chicago, IL, USA). The results are presented as the mean ± standard error (SE).
3. Results
3.1. Growth Performance and Feed Efficiency
The effects of diet supplementation on growth performance parameters, such as feed intake (FI), body weight gain (BWG), feed conversion ratio (FCR) and production efficiency factor (PEF) in 0–35-day-old broiler chicks challenged with C. perfringens are presented in Table 3. The Final Body Weight (FBW) and Body weight gain (BWG) were found to increase significantly (p < 0.01) in the entire supplemented group compared with the positive control group. Concerning FI, none of the probiotic bacteria were found to affect this parameter after the feeding trial. Conversely, the FCR was found to decrease significantly (p < 0.001) in the treated groups compared to the positive control group. The lowest values were recorded in the NC, CL + S and G groups (1.60, 1.72 and 1.78, respectively). Concerning PEF, all experimental additives demonstrated highly significant (p < 0.05) PEF values compared to the positive control. Also, the percentage (%) of survival rate (SR) was increased in all of the experimental groups (Table 3).
Table 3.
Effect of probiotic diet supplementation on growth performance and feed efficiency in broiler chicks challenged with C. perfringens.
| Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | NC | PC | M | CL | S | CL + S | G | SEM | Sig. |
| IBW (g) | 36.8 | 36.7 | 36.9 | 36.8 | 36.8 | 36.9 | 36.8 | 0.04 | NS |
| FBW (g) | 1829.3 a | 1593.3 b | 1807.4 a | 1827.0 a | 1824.3 a | 1823.1 a | 1798.9 a | 2.121 | *** |
| FI (g) | 2284.7 | 2325.6 | 2366.7 | 2353.2 | 2382.6 | 2379.9 | 2404.4 | 2.865 | NS |
| BWG (g) | 1424.6 a | 1134.0 b | 1384.3 a | 1379.0 a | 1418.8 a | 1386.5 a | 1350.8 a | 1.961 | *** |
| FCR (g: g) | 1.60 c | 2.05 a | 1.71 b,c | 1.71 b,c | 1.68 b,c | 1.72 b | 1.78 b | 0.03 | *** |
| PEF | 326.2 a | 215.6 c | 301.5 a,b | 305.0 a,b | 310.0 a,b | 307.6 a,b | 288.5 b | 2.76 | *** |
| SR (%) | 100.0 a | 96.3 b | 98.1 a | 98.1 a | 98.1 a | 98.1 a | 98.1 a | 0.30 | *** |
NC, negative control group; PC, positive control group; M, Maxus supplemented group; CL, CloStat supplemented group; S, Sangrovit Extra supplemented group; CL+S, CloStat+Sangrovit Extra supplemented group; G, Gallipro Tech supplemented group. IBW, initial body weight; FBW, final body weight; FI, feed intake; BWG, body weight gain; FCR, feed conversion ratio; PEF, protein efficiency ratio; SR, survival rate. SEM, mean values of standard error. Mean values of three replicates with deferent letter (a, b, c) in the same column are significantly deferent (p < 0.05). Sig., significance; N.S, non-significance; ***, significant at 0.001.
3.2. Intestinal Histomorphometric Measurements
The effects of feed supplementation on the intestine histomorphometric measurements (villus length (VL), villus width (VW), total villus area (TVA), small intestine length (SIL), small intestine weight (SIW), duodenum length (DL), duodenum weight (DW), jejunum length (JL), jejunum weight (JW), ileum length (IL), ileum weight (IW), ceca length (CL) and ceca weight (CW)) in the broiler chicks challenged with C. perfringens are presented in Table 4. The villus length and total villus area were significantly (p < 0.01) enhancement by feed supplementation and in the negative control group compared to the positive control group. By contrast, villus length did not show any significant differences between any of the groups.
Table 4.
Effect of probiotic diet supplementation on histomorphometric measurements in the intestine of broiler chicks challenged with C. perfringens.
| Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | NC | PC | M | CL | S | CL + S | G | SEM | Sig. |
| Live body weight | 1815.9 a | 1593.3 b | 1807.4 a | 1827.0 a | 1824.3 a | 1823.1 a | 1798.9 a | 1.121 | *** |
| Histomorphometry measurements | |||||||||
| VL (μm) | 629.98 a | 514.14 b | 576.82 a,b | 642.83 a | 632.40 a | 622.70 a | 627.28 a | 2.053 | *** |
| VW (μm) | 71.433 | 60.858 | 71.433 | 69.426 | 72.323 | 73.565 | 73.141 | 3.759 | NS |
| VTA (mm2) | 0.142 a | 0.100 b | 0.135 a,b | 0.141 a | 0.144 a | 0.149 a | 0.150 a | 0.01 | ** |
| Morphological measurements | |||||||||
| SIL (cm) | 211.3 | 191.6 | 202.9 | 206.3 | 194.6 | 201.7 | 205.8 | 2.14 | NS |
| DL (cm) | 15.66 | 18.29 | 17.81 | 17.00 | 17.51 | 16.75 | 15.97 | 0.89 | NS |
| CL (cm) | 19.03 | 17.89 | 17.76 | 17.36 | 23.37 | 17.61 | 17.45 | 2.17 | NS |
| JL (cm) | 42.13 | 39.41 | 39.72 | 41.51 | 41.14 | 41.69 | 41.41 | 0.88 | NS |
| ILL (cm) | 42.20 | 42.29 | 42.47 | 41.48 | 41.35 | 41.56 | 42.62 | 0.68 | NS |
| Relative SIW (%) | 0.63 a | 0.54 b | 0.54 b | 0.61 a,b | 0.56 a,b | 0.59 a,b | 0.54 b | 0.61 | * |
| Relative DW (%) | 0.051 | 0.066 | 0.055 | 0.053 | 0.068 | 0.057 | 0.052 | 0.10 | NS |
| Relative JW (%) | 0.202 | 0.161 | 0.151 | 0.175 | 0.169 | 0.169 | 0.156 | 0.24 | NS |
| Relative ILW (%) | 0.186 a | 0.144 b | 0.116 b | 0.136 b | 0.137 a,b | 0.123 b | 0.121 b | 0.09 | ** |
| Relative CW (%) | 0.056 | 0.042 | 0.040 | 0.085 | 0.035 | 0.043 | 0.037 | 0.35 | NS |
| Lesion score | 0.00b | 2.50 a | 0.67 b | 0.67 b | 0.33 b | 0.50 b | 0.67 b | 0.26 | *** |
NC, negative control group; PC, positive control group; M, Maxus supplemented group; CL, CloStat supplemented group; S, Sangrovit Extra supplemented group; CL+ S, CloStat+Sangrovit Extra supplemented group; G, Gallipro Tech supplemented group. VL, villus length; VW, villus width; VTA, villus total area; SIL, small intestine length; SIW, small intestine weight; DL, duodenum length; DW, duodenum weight; JL, jejunum length; JW, jejunum weight; IL, ileum length; ILW, ileum weight; CL, caeca length; CW, caeca weight. (a,b,c) Significant differences (p < 0.001). Values within each column, means showing different letter of superscript were significantly different; N.S, non-significance; **, significant at 0.01; ***, significant at 0.001.
The majority of the intestinal morphological factors were not affected by dietary supplementation with probiotics, apart from the small intestine weight (SIW), jejunum weight (JW), ileum weight (ILW) and intestine score lesion. Small intestine weight (SIW) was not significantly (p < 0.05) altered in the broiler chicks supplemented with probiotics compared to the control groups. However, supplementation with probiotic bacteria was found to enhance SIW and JW. The CL, S and CL + S mixed diets were found to improve JW compared to the M, G and PC groups. The highest values of JW were found in the negative control group. In terms of ileum weight (ILW), none of the additives were found to affect the ILW. Moreover, the highest values of ILW were observed in the negative control. Highly significant (p < 0.001) decreases in the lesion score were detected in all experimental groups compared to the positive control group.
3.3. Serum Profile
The blood serum profiles in terms of the composition (total protein, TP; albumin, ALB; globulin, GLB; cholesterol, CHO; total glyceride, TG; glucose, Glu) and enzymatic activity (alanine amine transferase, ALT; alanine amine transferase, AST) are presented in Table 5. No significant differences (p > 0.05) were observed in blood composition or enzyme profiles between the different treatments in broiler chicks challenged with C. perfringens. However, the ALT and glucose levels were found to be significantly (p < 0.05) affected by diet supplementation. The levels of ALT were found to decrease significantly (p < 0.05) in the M, S, G and Nc groups, while the glucose concentration increased compared to the positive control and other treatments (Table 5).
Table 5.
Effect of probiotic diets supplementation on blood profile of broiler chicks challenged with C. perfringens.
| Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | NC | PC | M | CL | S | CL + S | G | SEM | Sig. |
| TP (g/dL) | 2.3 | 2.0 | 2.2 | 2.3 | 2.2 | 2.2 | 2.4 | 0.081 | NS |
| ALB (g/dL) | 1.4 | 1.2 | 1.1 | 1.4 | 1.3 | 1.4 | 1.3 | 0.062 | NS |
| GLB (g/dL) | 0.9 | 0.8 | 1.0 | 0.9 | 0.9 | 0.8 | 1.1 | 0.088 | NS |
| Glu (mg/dL) | 134.5 a | 85.3 b | 124.9 a | 121.8 a,b | 134.8 a | 115.9 a,b | 130.0 a | 2.816 | ** |
| ALT (IU/L) | 19.3 b | 41.8 a | 23.7 a,b | 21.2 b | 21.5 a,b | 27.0 a,b | 17.4 b | 1.433 | * |
| AST (IU/L) | 293.2 | 312.8 | 282.1 | 254.0 | 279.4 | 251.4 | 271.7 | 1.954 | NS |
| CHO | 76.2 | 76.5 | 71.6 | 76.7 | 72.5 | 83.9 | 77.3 | 3.268 | NS |
| TG | 34.9 | 46.4 | 44.4 | 47.2 | 39.9 | 46.9 | 50.9 | 2.043 | NS |
NC, negative control group; PC, positive control group; M, Maxus supplemented group; CL, CloStat supplemented group; S, Sangrovit Extra supplemented group; CL+ S, CloStat+Sangrovit Extra supplemented group; G, Gallipro Tech supplemented group. TP, total protein; ALB, albumin; GLB, globulin; Glu, glucose; ALT, alanine amine transferase; AST, alanine amine transferase; CHO, cholesterol; TG, total glyceride. a,b,c Significant differences p < 0.001). Values within each column, means showing different letter of superscript were significantly different; N.S, non-significance; *, significant at 0.05; **, significant at 0.01.
3.4. Histopathological Examination of Intestine and Liver
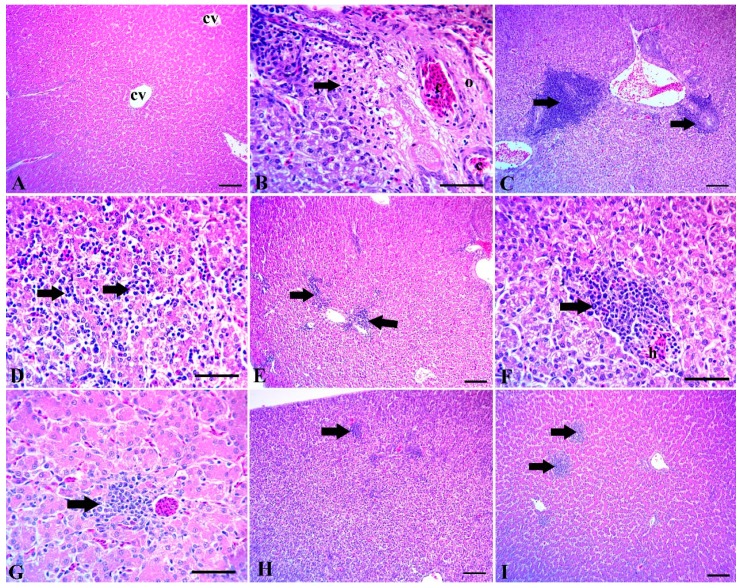
The examined liver sections of the control group showed normal tissue architecture with normal hepatic lobules, a normal central vein and normal hepatic sinusoids (Figure 1A). The liver of the challenged broiler chicks showed several histopathological signs such as portal blood vessel congestion with perivascular edema and coagulative necrosis of the surrounding hepatocytes (Figure 1B). Also, numerous lymphocytic aggregations around the congested portal blood vessels were detected (Figure 1C). Moreover, Extensive lymphocytic aggregations were detected among the hepatocytes in the Clostridium-challenged groups (Figure 1D). The liver of the Clostridium-challenged broiler chicks supplemented with Maxus reduced lymphocytic aggregations among the hepatocytes (Figure 1E). Similarly, the liver of Clostridium-challenge chicks supplemented with Clostat showed mild hemorrhaging and focal aggregation of lymphocytes (Figure 1F), while the liver of Clostridium-challenged chicks supplemented with Sangrovit showed mild perivascular lymphocytic aggregation (Figure 1G). The liver of Clostridium-challenged chicks supplemented with Clostat + Sangrovit showed mostly normal hepatic tissue, except for minute focal lymphocytic aggregation (Figure 1H). Lastly, the liver of Clostridium-challenged chicks supplemented with Gallipro showed moderate focal lymphocytic aggregations (Figure 1I).
Figure 1.
(A) Liver of control group without Clostridium challenge, showing normal tissue architecture, normal hepatic lobules, a normal central vein (cv) and hepatic sinusoids. (B–D) The intestine of chicks after the Clostridium challenge. (B) Congestion of portal blood vessels (c) with perivascular edema (o) and coagulative necrosis of the surrounding hepatocytes (arrow). (C) Numerous lymphocytic aggregations around the congested portal blood vessels. (D) Extensive lymphocytic aggregations among the hepatocytes (arrows). (E–I) Liver of chicks challenged with Clostridium and supplemented with: (E) Maxus, showing moderate scattered lymphocytic aggregations (arrows); (F) Clostat, showing mild hemorrhage and (H) focal aggregation of lymphocytes (arrow); (G) Sangrovit, showing mild perivascular lymphocytic aggregation (arrow); (H) Clostat + Sangrovit, showing mostly normal hepatic tissue, except for minute focal lymphocytic aggregations (arrow); (I) Gallipro, showing moderate focal lymphocytic aggregations. Scale bar = 100 µm (A,C,E,H,I) and 50 µm (B,D,F,G).
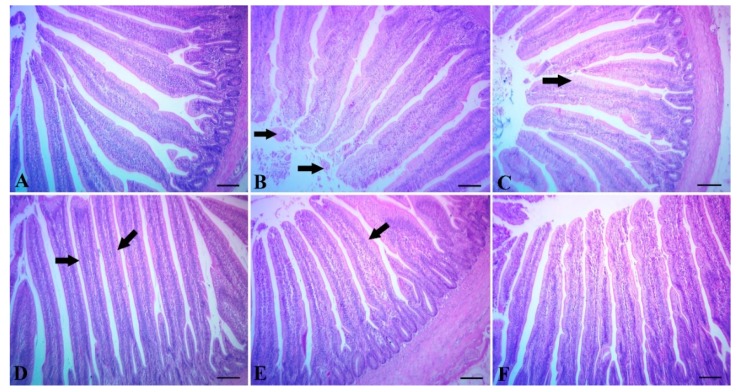
The intestine of the control group showed normal tissue architecture with normal intestinal villi (Figure 2A). Chicks supplemented with Maxus showed mild desquamation of the villous epithelium (Figure 2B), while chicks supplemented with Clostat or Sangrovit showed moderate metaplasia of the columnar epithelium lining the villi into goblet cells (Figure 2C,D). Chicks supplemented with Clostat + Sangrovit showed mostly normal villi, except for mild metaplasia of the columnar epithelium lining the villi into goblet cells (Figure 2E). Lastly, chicks supplemented with Gallipro showed normal tissue architecture with normal intestinal villi (Figure 2F).
Figure 2.
(A) Intestine of the control group without Clostridium challenge, showing normal tissue architecture. (B) The intestine of chicks supplemented with Maxus showing mild desquamation of villous epithelium (arrow). (C) The intestine of chicks supplemented with Clostat, the columnar epithelium lining the villi into goblet cells (arrow). (D) The intestine of chicks supplemented with Sangrovit, showing moderate metaplasia of the columnar epithelium lining the villi into goblet cells (arrow). (E) The intestine of chicks supplemented with Clostat + Sangrovit, showing mostly normal villi, except for mild metaplasia of the columnar epithelium lining the villi into goblet cells (arrow). (F) The intestine of chicks supplemented with Gallipro, showing normal tissue architecture with normal intestinal villi. Scale bar = 100 µm.
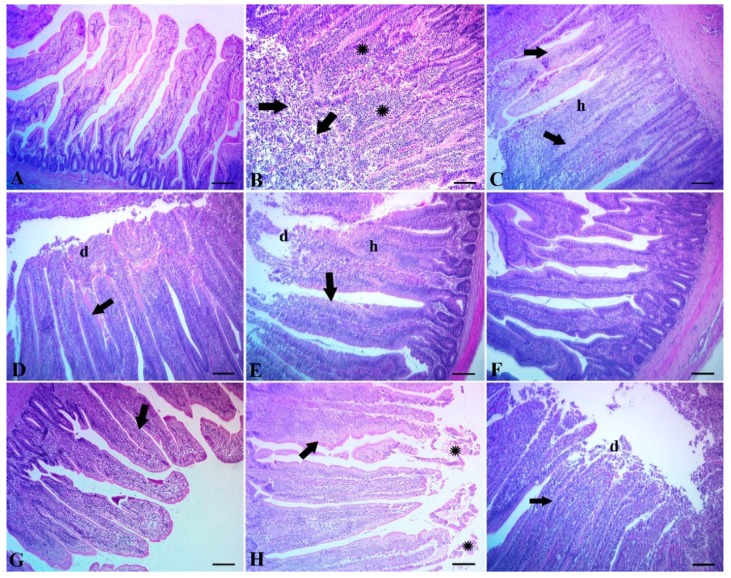
The intestine of the control group without the Clostridium challenge showed normal tissue architecture with normal intestinal villi (Figure 3A). Broiler chicks subjected to Clostridium challenge showed extensive degeneration and necrosis of the intestinal villi with extensive round cell aggregations (Figure 3B). The same groups showed extensive hyperplasia of the villous epithelium with thickening and shortening of villi, together with metaplasia into goblet cells (Figure 3C). Extensive degeneration of the villous epithelium, with metaplasia of the columnar epithelium into goblet cells, was also detected (Figure 3D). The intestine of chicks challenged with Clostridium and supplemented with Maxus showed extensive hyperplasia of the intestinal epithelium, metaplasia into goblet cells and moderate desquamation of the superficial epithelium (Figure 3E). The intestine of chicks challenged with Clostridium and supplemented with Clostat showed regeneration of the intestinal epithelium with a normal tissue architecture (Figure 3F), while the intestine of chicks supplemented with Sangrovit showed a normal tissue architecture with moderate metaplasia of the intestinal epithelium into goblet cells (Figure 3G). Lastly, the intestine of chicks supplemented with Clostat + Sangrovit showed sloughing of the superficial epithelium with moderate metaplasia into goblet cells (Figure 3H), while chicks with supplemented with Gallipro showed severe desquamation of the superficial epithelium and metaplasia into goblet cells (Figure 3I).
Figure 3.
(A) Intestine of the control group without Clostridium challenge, showing normal tissue architecture with normal intestinal villi. (B) The intestine of chicks subjected to Clostridium challenge, showing an extensive degeneration and necrosis of the intestinal villi (arrows) with extensive round cell aggregations (∗). (C) The intestine of Clostridium-challenged chicks showing extensive hyperplasia of the villous epithelium (h) with thickening and shortening of villi, together with metaplasia into goblet cells (arrows). (D) The intestine of Clostridium-challenged chicks, showing an extensive degeneration of the villous epithelium (d) with metaplasia of the columnar epithelium into goblet cells (arrow). (E–I) Intestine of chicks challenged with Clostridium) and supplemented with: (E Maxus, showing extensive hyperplasia of intestinal epithelium (h), metaplasia into goblet cells (arrow) and moderate desquamation of superficial epithelium (d); (F) Clostat, showing a regeneration of the intestinal epithelium with normal tissue architecture; (G) Sangrovit, showing a normal tissue architecture with moderate metaplasia of the intestinal epithelium into goblet cells (arrow); (H) Clostat + Sangrovit, showing a sloughing of the superficial epithelium (∗) with moderate metaplasia into goblet cells (arrow); (I) Gallipro, showing a severe desquamation of the superficial epithelium (d) and metaplasia into goblet cells (arrow). Scale bar = 100 µm.
4. Discussion
In the poultry industry, one of the most critical problems is necrotic enteritis (NE) [56]. Recently, antibiotics, antibacterial agents and probiotic agents have been becoming more common place as feed additives to enhance the health of animal, speed up growth and improve feed efficiency [57]. As a result, the supplementation of the diet with probiotic bacteria has increased [58]. Several studies have reported on the positive effects of the use of antibiotics and probiotics on the growth rate, feed utilization, feed efficiency and survival rate in broiler chickens challenged with Clostridium spp. [59,60]. However, strain selection, gene manipulation, strain combinations and probiotics combinations affect the degree of the positive effect attributed to the use of probiotics against the development of necrotic enteritis (NE) [61]. The best way of enhancing the action of probiotics seems to be by using multi-strain probiotics, which has a beneficial effect on the host by enhancing growth-promoting bacteria, combined with the viable antibiosis of pathogenic bacteria in the intestinal tract [62].
4.1. Growth Performance
In terms of growth, diet supplementation significant improved (p ≤ 0.05) the body weight gain (BWG), final body weight, production efficiency ratio (PER), feed conversion ratio (FCR) and survival rate of broiler chicks (days 0–35) during the overall experimental period improved groups compared to the chicks in the positive control group (Table 3).
These results correlated with those reported by Khaksefidi and Ghoorchi [63], who supplemented the diets of chickens with 50 mg/kg of Bacillus subtilis and found that the body weight gain of the chicks increased significantly during the finisher period (days 22–42) compared to chickens fed control diets. Moreover, the supplementation of diets with probiotics significantly reduced the feed conversion ratio of broiler chickens (days 22–42) compared to the control diet group. Similarly, Patel et al. [61] found that the body weight gain and feed conversion ratio were significantly enhanced by the inclusion of probiotics (Protexin) at 100 g/ton in the diet, without any adverse effects on feed intake, mortality or carcass characteristics. Likewise, Anjum et al. [64] and Singh et al. [65] obtained similar results. The improvement of all the performance parameters may be due to the biological role of probiotics in the modification of intestinal pH, which benefits the bacterial population, improves nutrient absorption and increases the efficiency of feed utilization [66].
Diet supplementation was found to reduce the mortality rate of the broiler chicks, confirming the positive effects that supplementation with antibiotics, probiotics and phytobiotics have on mortality (Table 3). Our findings were similar to those reported by Abdel-Hafeez et al. [67]. Similarly, Riad et al. [68] found that the addition of probiotics in feed decreased the mortality rate in broiler chicks. An increased percentage of survival rate in broiler chicks has been previously ascribed to the inhibitory effects of these additives towards enteric pathogenic microorganisms via adjusting the intestinal pH [67].
4.2. Intestinal Histomorphometric Parameters
The mucosal architecture and villus structure are closely related to the absorption function of the small intestine [69]. However, few studies have compared the effect of types of probiotic on the intestine morphology of broiler chickens. Some researchers have indicated that the addition of probiotics resulted in an increased height of intestinal villus [70]. The effects of probiotic-supplemented diets on the histomorphometric parameters of the small intestine are listed in Table 4. In our study, the inclusion of probiotics in the diet significantly (p < 0.01) increased villus length (VL) and villus total area (VTA). We found a tendency for the villi width in the probiotics groups to increase, however, these differences were not statistically significant (p > 0.05).
In the present study, the morphometric traits of the digestive tract were not significantly affected by diet supplementation with probiotics, apart from the small intestine weight (SIW), jejunum weight (JW), ileum weight (ILW) and lesion scores. Remarkably, we found a strong relationship between the small intestine weight and body weight [71]. In the present study, broiler chicks fed a diet supplemented with probiotics showed slight increases in SIW and ILW, which may be indicative of histological changes. We associated an increased villus height with an improved digestive and absorptive function of the intestine, due to an enlarged absorptive surface area, increased enzyme secretion and enhanced nutrient transport systems [72]. There is a strong correlation between increased villus height and the activation of intestinal villi function [73]. This suggests that the role of the villi is enhanced after the inclusion of probiotics in the diet. Moreover, improved inactive absorption of glucose and proline was previously reported in broiler chicks fed diets supplemented with Lactobacilli, B. thermophilum and E. faecium [70].
4.3. Blood Biochemical Parameters
Glucose is an important cellular source of energy and serves as a metabolic substrate [74]. The chicks that were administered probiotic-supplemented diets showed a significant increase (p ≤ 0.05) in their glucose levels and decreased ALT concentrations compared to the chicks in the positive control group. All other serum biochemical parameters did not show any significant differences between the different experimental groups. These findings correlate with those reported by Shareef and Al-Dabbagh [75], wherein serum glucose was found to be highest when probiotics were used against Clostridium perfringens infection in broiler chickens. Moreover, in a previous study, the concentration of certain serum biochemical parameters (total protein, lipids and albumin) in broiler chicks was not affected by probiotic supplementation [76]. Additionally, our results correlated with those reported by Al-Kassie et al. [77] and Ta et al. [78], since we did not find any significant differences between the chicks supplemented with probiotics and the control groups with regards to the blood composition (total protein, albumin and globulin). Moreover, Santoso et al. [79] found that the level of AST and ALT enzymes in the blood serum of broiler chickens was decreased by diet supplementation with probiotics. However, Hussein [80] found that probiotics (Saccharomyces cerevisiae) did not have any significant effect on serum AST and ALT activities in broiler chicks fed a supplemented diet compared to the control group. Diet supplementation with Bacillus subtilis and E. faecium results in normal liver function as a result of a significant decrease in the ALT and AST activities in the blood [81]. On the other hand, a significant increase in AST and ALT in the negative and positive control groups fed a basal diet could be used as an indicator of hepatocellular damage [82].
4.4. Liver and Intestinal Histopathological Signs
Supplementing diets with probiotics could help to prevent some of the harmful modifications caused by Salmonella enterica in the hepatocellular parenchyma [83]. NE can potentially lead to the degeneration and vacuolation of hepatocytes [84]. We found that the liver sections of chicks challenged with Clostridium were associated with several hepatocellular damages, including the congestion of portal blood vessels (Figure 1B) with numerous lymphocytic aggregations (Figure 1C), as well as edema and coagulative necrosis (Figure 1D). In contrast, the liver specimens of chicks fed supplemented diets showed an enhanced liver tissue structure against Clostridium challenge. The Sangrovit, Clostat + Sangrovit and Gallipro groups (Figure 1G, H and I) showed a normal hepatocellular structure with mild perivascular lymphocytic aggregation. The bacterial infection is the most important cause of lobular localization and may be a preamble to hepatocyte necrosis [85]. Moreover, the role of probiotics on the transfer of immune cells in the liver was reported by a previous study [86], which found that probiotic bacteria reduced the induction of monocytes and macrophages in the intestine and spleen of animals fed a supplemented compared with the controls. Finally, the supplementation of the diet with probiotics may enhance the enrollment of pro-inflammatory immune cells to systemic lymphoid tissues, including the liver and other organs [85].
Eeckhaut et al. [87] reported that probiotic Butyricicoccus pullicaecorum can prevent NE in the digestive tract of broiler chickens by reducing the number of potentially important pathogens in the caeca and ileum. In our recent study, the intestine samples from Gallipro- or Clostat-supplemented diets were examined and found to show a normal tissue architecture with normal intestinal villi (Figure 2F) and moderate metaplasia of the intestinal epithelium into goblet cells (Figure 3G), in addition to a negative control group (Figure 2A). Meanwhile, chicks challenged with Clostridium showed signs of injury to intestine tissue, including the degeneration and necrosis of intestinal villi, with extensive round cell aggregations (Figure 3B), wide hyperplasia of the villus epithelium, with a thickening and shortening of villi, together with metaplasia into goblet cells (Figure 3C), as well as a degeneration of the villous epithelium with metaplasia of the columnar epithelium into goblet cells (Figure 3D). These findings were in agreement with Wang et al. [56], who found that supplementing the diet of broiler chicks with Lactobacillus plantarum protected the intestinal structure from enterotoxigenic Clostridium infection by reducing irritation and preserving the integrity of the intestinal epithelial layer [88]. It is thus widely accepted that probiotics can prevent gut diseases by improving the immunity of the gut [89], promoting the development of gut histomorphology [90] and modifying the gut microbiota [91].
5. Conclusions
Using probiotic and phytobiotic compounds could be a useful strategy to preventing harmful effects of C. perfringens bacterium on the broiler performance, intestine, liver histopathological signs and some blood parameters alternative for using antibiotics. We suggest according to our study results that supplemented broiler diets with 0.5, 0.12 g Kg−1 of CloStat and Sangrovit Extra respectively, alone or in combined form promote growth and reduced the N.E. mortality rats.
Acknowledgments
Authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through Research Group Project No. RGP-273.
Author Contributions
Conceptualization, E.O.S.H. and S.H.A.; methodology, A.M.A.; data curation, M.A.E.N. and M.R.A.; writing—original draft preparation, M.A.E.N., M.M.A. and M.A.N.; writing—review and editing, G.M.S. and A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Saud University for its funding of this research through Research Group Project No. RGP-273.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Popoff M.C. In: Sécurité Sanitaire Des Aliments: Epidémiologie et Lutte Contre Les Contaminants Zoonotiques. Drider D., Salvat G., editors. Economica; Paris, France: 2013. pp. 165–189. [Google Scholar]
- 2.Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Grass J.E., Gould L.H., Mahon B.E. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013;10:131–136. doi: 10.1089/fpd.2012.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uzal F., Vidal J., McClane B., Gurjar A. Clostridium perfringens toxins involved in mammalian veterinary diseases. Open Toxinol. J. 2010;2:24. doi: 10.2174/1875414701003020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Timbermont L., De Smet L., Van Nieuwerburgh F., Parreira V.R., Van Driessche G., Haesebrouck F., Ducatelle R., Prescott J., Deforce D., Devreese B. Perfrin, a novel bacteriocin associated with netB positive Clostridium perfringens strains from broilers with necrotic enteritis. Vet. Res. 2014;45:40. doi: 10.1186/1297-9716-45-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Shall N.A., Awad A.M., El-Hack M.E.A., Naiel M.A., Othman S.I., Allam A.A., Sedeik M.E. The Simultaneous Administration of a Probiotic or Prebiotic with Live Salmonella Vaccine Improves Growth Performance and Reduces Fecal Shedding of the Bacterium in Salmonella-Challenged Broilers. Animals. 2020;10:70. doi: 10.3390/ani10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K., Lillehoj H., Jeong W., Jeoung H., An D. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors and vaccine development. Poult. Sci. 2011;90:1381–1390. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- 8.McDevitt R., Brooker J., Acamovic T., Sparks N. Necrotic enteritis; A continuing challenge for the poultry industry. World’s Poult. Sci. J. 2006;62:221–247. doi: 10.1079/WPS200593. [DOI] [Google Scholar]
- 9.Caly D.L., D’Inca R., Auclair E., Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: A microbiologist’s perspective. Front. Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaldhusdal M., Schneitz C., Hofshagen M., Skjerve E. Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl. Avian Dis. 2001;45:149–156. doi: 10.2307/1593022. [DOI] [PubMed] [Google Scholar]
- 11.Skinner J.T., Bauer S., Young V., Pauling G., Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010;54:1237–1240. doi: 10.1637/9399-052110-Reg.1. [DOI] [PubMed] [Google Scholar]
- 12.Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 13.Craven S., Stern N., Bailey J., Cox N. Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing. Avian Dis. 2001;45:887–896. doi: 10.2307/1592868. [DOI] [PubMed] [Google Scholar]
- 14.Williams R. Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- 15.Annett C., Viste J., Chirino-Trejo M., Classen H., Middleton D., Simko E. Necrotic enteritis: Effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- 16.Moran E.T., Jr. Intestinal events and nutritional dynamics predispose Clostridium perfringens virulence in broilers. Poult. Sci. 2014;93:3028–3036. doi: 10.3382/ps.2014-04313. [DOI] [PubMed] [Google Scholar]
- 17.Al-Sagheer A.A., Abd El-Hack M.E., Alagawany M., Naiel M.A., Mahgoub S.A., Badr M.M., Hussein E.O., Alowaimer A.N., Swelum A.A. Paulownia leaves as a new feed resource: Chemical composition and effects on growth, carcasses, digestibility, blood biochemistry and intestinal bacterial populations of growing rabbits. Animals. 2019;9:95. doi: 10.3390/ani9030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobsey M., Khatib L., Hill V., Alocilja E., Pillai S. In: Pathogens in Animal Wastes and the Impacts of Waste Management Practices on Their Survival, Transport and Fate. Rice J.M., Caldwell D.F., Humenik F.J., editors. ASABE; St. Joseph, MI, USA: 2006. Pp. 609–666 in Ani. Agri. and the Environ.: National Center for Manure and Animal Waste Management White Papers. [Google Scholar]
- 19.Bauer E., Williams B.A., Smidt H., Verstegen M.W., Mosenthin R. Influence of the gastrointestinal microbiota on development of the immune system in young animals. Curr. Issues Intest. Microbiol. 2006;7:35–52. [PubMed] [Google Scholar]
- 20.Paiva D., McElroy A. Necrotic enteritis: Applications for the poultry industry. J. Appl. Poult. Res. 2014;23:557–566. doi: 10.3382/japr.2013-00925. [DOI] [Google Scholar]
- 21.Fenichel G.M. Clinical Pediatric Neurology: A Signs and Symptoms Approach. 6th ed. Saunders; Philadelphia, PA, USA: 2009. [Google Scholar]
- 22.Paradis M.A., McMillan E., Bagg R., Vessie G., Zocche A., Thompson M. Efficacy of avilamycin for the prevention of necrotic enteritis caused by a pathogenic strain of Clostridium perfringens in broiler chickens. Avian Pathol. 2016;45:365–369. doi: 10.1080/03079457.2016.1165793. [DOI] [PubMed] [Google Scholar]
- 23.El-Naby F.S.A., Naiel M.A., Al-Sagheer A.A., Negm S.S. Dietary chitosan nanoparticles enhance the growth, production performance and immunity in Oreochromis niloticus. Aquaculture. 2019;501:82–89. doi: 10.1016/j.aquaculture.2018.11.014. [DOI] [Google Scholar]
- 24.Brennan J., Bagg R., Barnum D., Wilson J., Dick P. Efficacy of narasin in the prevention of necrotic enteritis in broiler chickens. Avian Dis. 2001;45:210–214. doi: 10.2307/1593030. [DOI] [PubMed] [Google Scholar]
- 25.Butaye P., Devriese L.A., Haesebrouck F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwangi S., Timmons J., Fitz-Coy S., Parveen S. Characterization of Clostridium perfringens recovered from broiler chicken affected by necrotic enteritis. Poult. Sci. 2018;98:128–135. doi: 10.3382/ps/pey332. [DOI] [PubMed] [Google Scholar]
- 27.Fooks L.J., Fuller R., Gibson G.R. Prebiotics, probiotics and human gut microbiology. Int. Dairy J. 1999;9:53–61. doi: 10.1016/S0958-6946(99)00044-8. [DOI] [Google Scholar]
- 28.Joerger R. Alternatives to antibiotics: Bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 2003;82:640–647. doi: 10.1093/ps/82.4.640. [DOI] [PubMed] [Google Scholar]
- 29.Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sassone-Corsi M., Raffatellu M. No vacancy: How beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 2015;194:4081–4087. doi: 10.4049/jimmunol.1403169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbosa T.M., Serra C.R., La Ragione R.M., Woodward M.J., Henriques A.O. Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2005;71:968–978. doi: 10.1128/AEM.71.2.968-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gérard P., Brézillon C., Quéré F., Salmon A., Rabot S. Characterization of cecal microbiota and response to an orally administered lactobacillus probiotic strain in the broiler chicken. J. Mol. Microbiol. Biotechnol. 2008;14:115–122. doi: 10.1159/000106090. [DOI] [PubMed] [Google Scholar]
- 33.Knap I., Lund B., Kehlet A., Hofacre C., Mathis G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010;54:931–935. doi: 10.1637/9106-101509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 34.La Ragione R.M., Woodward M.J. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003;94:245–256. doi: 10.1016/S0378-1135(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 35.Sokale A., Menconi A., Mathis G., Lumpkins B., Sims M., Whelan R., Doranalli K. Effect of Bacillus subtilis DSM 32315 on the intestinal structural integrity and growth performance of broiler chickens under necrotic enteritis challenge. Poult. Sci. 2019;98:5392–5400. doi: 10.3382/ps/pez368. [DOI] [PubMed] [Google Scholar]
- 36.Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- 37.Aviagen . Parent Stock Management Handbook: Ross. Aviagen, Ltd.; Huntsville, AL, USA: 2013. [Google Scholar]
- 38.Ao Z., Kocher A., Choct M. Effects of dietary additives and early feeding on performance, gut development and immune status of broiler chickens challenged with Clostridium perfringens. Asian Australas. J. Anim. Sci. 2012;25:541. doi: 10.5713/ajas.2011.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. J. Appl. Anim. Res. 2018;46:691–695. doi: 10.1080/09712119.2017.1383258. [DOI] [Google Scholar]
- 40.Griffin R. The response of cage-reared broiler cockerels to dietary supplements of nitrovin, zinc bacitracin or penicillin used singly or in paired combinations. Br. Poult. Sci. 1979;20:281–287. doi: 10.1080/00071667908416580. [DOI] [Google Scholar]
- 41.Jensen L., Johnson J., Ruff M. Selenium status and response of broiler chicks to coccidial infection. Proc. Poult. Sci. 2003;57:1147–1148. [Google Scholar]
- 42.Arif M., Iram A., Bhutta M.A., Naiel M.A., Abd El-Hack M.E., Othman S.I., Allam A.A., Amer M.S., Taha A.E. The Biodegradation Role of Saccharomyces cerevisiae against Harmful Effects of Mycotoxin Contaminated Diets on Broiler Performance, Immunity Status and Carcass characteristics. Animals. 2020;10:238. doi: 10.3390/ani10020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long J., Barnum D., Pettit J. Necrotic enteritis in broiler chickens II. Pathology and proposed pathogenesis. Can. J. Comp. Med. 1974;38:467. [PMC free article] [PubMed] [Google Scholar]
- 44.Hofacre C., Froyman R., Gautrias B., George B., Goodwin M., Brown J. Use of Aviguard and other intestinal bioproducts in experimental Clostridium perfringens-associated necrotizing enteritis in broiler chickens. Avian Dis. 1998;42:579–584. doi: 10.2307/1592685. [DOI] [PubMed] [Google Scholar]
- 45.Langhout D.J. Ph.D. Thesis. Wageningen University & Research; Wageningen, The Netherlands: 1998. The Role of the Intestinal Flora as Affected by Non-Starch Polysaccharides in Broiler Chicks. [Google Scholar]
- 46.Teshfam M., Nodeh H., Hassanzadeh M. Alterations in the intestinal mucosal structure following oral administration of triiodothyronine (T3) in broiler chickens. J. Appl. Anim. Res. 2005;27:105–108. doi: 10.1080/09712119.2005.9706550. [DOI] [Google Scholar]
- 47.Sakamoto K., Hirose H., Onizuka A., Hayashi M., Futamura N., Kawamura Y., Ezaki T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- 48.Reinhold J. Total protein, albumin and globulin. Stand. Methods Clin. Chem. 1953;1:88–94. [Google Scholar]
- 49.Ogunwole O., Abu O., Adedeji B., Jemiseye F., Ojelade A., Tewe O. Haematology and Serum Indices of Finisher Broiler Chickens Fed Acidified Blood Meal-based Diets. JABB. 2017;11:1–7. doi: 10.9734/JABB/2017/30227. [DOI] [Google Scholar]
- 50.Kim Y., Han I.K., Choi Y., Shin I., Chae B., Kang T. Effects of dietary levels of chromium picolinate on growth performance, carcass quality and serum traits in broiler chicks. Asian Australas. J. Anim. Sci. 1996;9:341–347. doi: 10.5713/ajas.1996.341. [DOI] [Google Scholar]
- 51.Holder M., Rej R. In: Alanine Transaminase in Methods of Enzymatic Analysis. 3rd ed. Bergmeyer H.U., Bergemeyer J., Grassl M., editors. Weinhein Velagehemie; Hoboken, NJ, USA: 1983. pp. 380–401. [Google Scholar]
- 52.Kornblatf M.J., Klugerman A., Nagy F. Characterization and localization of alkaline phosphatase activity in rat testes. Biol. Reprod. 1983;29:157–164. doi: 10.1095/biolreprod29.1.157. [DOI] [PubMed] [Google Scholar]
- 53.Naiel M.A., Ismael N.E., Shehata S.A. Ameliorative effect of diets supplemented with rosemary (Rosmarinus officinalis) on aflatoxin B1 toxicity in terms of the performance, liver histopathology, immunity and antioxidant activity of Nile Tilapia (Oreochromis niloticus) Aquaculture. 2019;511:734264. doi: 10.1016/j.aquaculture.2019.734264. [DOI] [Google Scholar]
- 54.Bancroft J.D., Gamble M. Theory and Practice of Histological Techniques. 6th ed. Churchill Livingstone Elsevier; Philadelphia, PA, USA: 2008. [Google Scholar]
- 55.Duncan D.B. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 56.Wang H., Ni X., Qing X., Liu L., Lai J., Khalique A., Li G., Pan K., Jing B., Zeng D. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front. Immunol. 2017;8:1592. doi: 10.3389/fimmu.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kefali S., Kaygisiz F., Toker N. Effect of probiotics on feed consumption, live weight gain and production cost in broilers. Indian Vet. J. 2007;84:267–269. [Google Scholar]
- 58.De Jesus R., Waitzberg D., Campos F. More Channels Showcase Channel Catalog. Rev. Assoc. Med. Bras. 2000;46 doi: 10.1590/S0104-42302000000300010. [DOI] [PubMed] [Google Scholar]
- 59.El-Naga M. Effect of dietary yeast supplementation on broiler performance. Egypt. Poult. Sci. J. 2012;32:95–106. [Google Scholar]
- 60.Sen S., Ingale S., Kim Y., Kim J., Kim K., Lohakare J., Kim E., Kim H., Ryu M., Kwon I. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Patel S.G., Raval A.P., Bhagwat S.R., Sadrasaniya D.A., Patel A.P., Joshi S.S. Effects of probiotics supplementation on growth performance, feed conversion ratio and economics of broilers. J. Anim. Res. 2015;5:155. doi: 10.5958/2277-940X.2015.00026.1. [DOI] [Google Scholar]
- 62.Lukic J., Chen V., Strahinic I., Begovic J., Lev-Tov H., Davis S.C., Tomic-Canic M., Pastar I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017;25:912–922. doi: 10.1111/wrr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khaksefidi A., Ghoorchi T. Effect of probiotic on performance and immunocompetence in broiler chicks. J. Poult. Sci. 2006;43:296–300. doi: 10.2141/jpsa.43.296. [DOI] [Google Scholar]
- 64.Anjum M., Khan A., Azim A., Afzal M. Effect of dietary supplementation of multi-strain probiotic on broiler growth performance. Pak. Vet. J. 2005;25:25–29. [Google Scholar]
- 65.Singh S., Niranjan P., Singh U., Koley S., Verma D. Effects of dietary supplementation of probiotics on broiler chicken. Anim. Nutr. Feed Technol. 2009;9:85–90. [Google Scholar]
- 66.Dunne C. Adaptation of bacteria to the intestinal niche: Probiotics and gut disorder. Inflamm. Bowel Dis. 2001;7:136–145. doi: 10.1097/00054725-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Abdel-Hafeez H.M., Saleh E.S., Tawfeek S.S., Youssef I.M., Abdel-Daim A.S. Effects of probiotic, prebiotic and synbiotic with and without feed restriction on performance, hematological indices and carcass characteristics of broiler chickens. Asian Australas. J. Anim. Sci. 2017;30:672. doi: 10.5713/ajas.16.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riad S., Safaa H., Mohamed F. Influence of probiotic, prebiotic and/or yeast supplementation in broiler diets on the productivity, immune response and slaughter traits. J. Anim. Poult. Prod. 2010;1:45–60. [Google Scholar]
- 69.Liu T., She R., Wang K., Bao H., Zhang Y., Luo D., Hu Y., Ding Y., Wang D., Peng K. Effects of rabbit sacculus rotundus antimicrobial peptides on the intestinal mucosal immunity in chickens. Poult. Sci. 2008;87:250–254. doi: 10.3382/ps.2007-00353. [DOI] [PubMed] [Google Scholar]
- 70.Chichlowski M., Croom W., Edens F., McBride B., Qiu R., Chiang C., Daniel L., Havenstein G., Koci M. Microarchitecture and spatial relationship between bacteria and ileal, cecal and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac and salinomycin. Poult. Sci. 2007;86:1121–1132. doi: 10.1093/ps/86.6.1121. [DOI] [PubMed] [Google Scholar]
- 71.Awad W., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- 72.Pluske J.R., Thompson M.J., Atwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of villus height and crypt depth and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996;76:409–422. doi: 10.1079/BJN19960046. [DOI] [PubMed] [Google Scholar]
- 73.Shamoto K., Yamauchi K. Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult. Sci. 2000;79:718–723. doi: 10.1093/ps/79.5.718. [DOI] [PubMed] [Google Scholar]
- 74.Nahavandinejad M., Seidavi A., Asadpour L., Payan-Carreira R. Blood biochemical parameters of broilers fed differently thermal processed soybean meal. Rev. MVZ Córdoba. 2014;19:4301–4315. doi: 10.21897/rmvz.92. [DOI] [Google Scholar]
- 75.Shareef A., Al-Dabbagh A. Effect of probiotic (Saccharomyces cerevisiae) on performance of broiler chicks. Iraqi J. Vet. Sci. 2009;23:23–29. [Google Scholar]
- 76.Alkhalf A., Alhaj M., Al-Homidan I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J. Biol. Sci. 2010;17:219–225. doi: 10.1016/j.sjbs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Kassie G., Al-Jumaa Y., Jameel Y. Effect of probiotic (Aspergillus niger) and prebiotic (Taraxacum officinale) on blood picture and biochemical properties of broiler chicks. Int. J. Poult. Sci. 2008;7:1182–1184. doi: 10.3923/ijps.2008.1182.1184. [DOI] [Google Scholar]
- 78.Ta A., Raji M., Hassan B., Kawu M., Kobo P., Ayo J. Effect of Different Levels of Supplemental Yeast on Performance Indices and Serum Biochemistry of Broiler Chickens. Open Conf. Proc. J. 2012;3:41–45. [Google Scholar]
- 79.Santoso U., Tanaka K., Ohtani S. Effect of dried Bacillus subtilis culture on growth, body composition and hepatic lipogenic enzyme activity in female broiler chicks. Br. J. Nutr. 1995;74:523–529. doi: 10.1079/BJN19950155. [DOI] [PubMed] [Google Scholar]
- 80.Hussein A. Effect of biological additives on growth indices and physiological responses of weaned Najdi ram lambs. J. Exp. Biol. Agric. Sci. 2014;2:6. [Google Scholar]
- 81.Hatab M., Elsayed M., Ibrahim N. Effect of some biological supplementation on productive performance, physiological and immunological response of layer chicks. J. Radiat. Res. Appl. Sci. 2016;9:185–192. doi: 10.1016/j.jrras.2015.12.008. [DOI] [Google Scholar]
- 82.Yalçın S., Uzunoğlu K., Duyum H., Eltan Ö. Effects of dietary yeast autolysate (Saccharomyces cerevisiae) and black cumin seed (Nigella sativa L.) on performance, egg traits, some blood characteristics and antibody production of laying hens. Livest. Sci. 2012;145:13–20. doi: 10.1016/j.livsci.2011.12.013. [DOI] [Google Scholar]
- 83.Hayashi R.M., Lourenço M.C., Kraieski A.L., Araujo R.B., Gonzalez-Esquerra R., Leonardecz E., da Cunha A.F., Carazzolle M.F., Monzani P.S., Santin E. Effect of Feeding Bacillus subtilis Spores to Broilers Challenged with Salmonella enterica serovar Heidelberg Brazilian Strain UFPR1 on Performance, Immune Response and Gut Health. Front. Vet. Sci. 2018;5:13. doi: 10.3389/fvets.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swaggerty C.L., Genovese K.J., He H., Byrd J.A., Kogut M.H. Mechanisms of persistence, survival and transmission of bacterial foodborne pathogens in production animals. Front. Vet. Sci. 2018;5:139. doi: 10.3389/fvets.2018.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gkretsi V., Mars W.M., Bowen W.C., Barua L., Yang Y., Guo L., St-Arnaud R., Dedhar S., Wu C., Michalopoulos G.K. Loss of Integrin Linked Kinase from Mouse HepatocytesIn VitroandIn VivoResults in Apoptosis and Hepatitis. Hepatology. 2007;45:1025–1034. doi: 10.1002/hep.21540. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W., Wen K., Azevedo M.S., Gonzalez A., Saif L.J., Li G., Yousef A.E., Yuan L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet. Immunol. Immunopathol. 2008;121:222–231. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eeckhaut V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Van Immerseel F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Q., Ni X., Wang Q., Peng Z., Niu L., Wang H., Zhou Y., Sun H., Pan K., Jing B. Lactobacillus plantarum BSGP201683 isolated from giant panda feces attenuated inflammation and improved gut microflora in mice challenged with enterotoxigenic Escherichia coli. Front. Microbiol. 2017;8:1885. doi: 10.3389/fmicb.2017.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vieira A.T., Teixeira M.M., Martins F.D.S. The role of probiotics and prebiotics in inducing gut immunity. Front. Immunol. 2013;4:445. doi: 10.3389/fimmu.2013.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olnood C.G., Beski S.S., Choct M., Iji P.A. Novel probiotics: Their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 2015;1:184–191. doi: 10.1016/j.aninu.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ubeda C., Pamer E.G. Antibiotics, microbiota and immune defense. Trends Immunol. 2012;33:459–466. doi: 10.1016/j.it.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]