Abstract
This study investigated the protective effect of a Vitis vinifera L. grape skin extract (ACH09) on blood pressure, lipid profile, and oxidative status in spontaneously hypertensive rats (SHR). Systolic blood pressure (SBP), total cholesterol, triglyceride, and glucose levels, as well as oxidative damage and antioxidant activity in the plasma and kidney, were evaluated in four experimental groups: control Wistar rats (W-C) and SHR-C that received water, and Wistar rats and SHR treated with ACH09 (200 mg/kg/d) in drinking water for 12 weeks (W-ACH09 and SHR-ACH09, respectively). SBP increased in the SHR group compared with the W groups and the treatment with ACH09 prevented the development of hypertension. Plasma triglyceride and total cholesterol levels increased in SHR compared with W-C rats; these changes prevented by treatment with ACH09. Glucose levels did not differ between the groups. The SHR group had increased oxidative damage in plasma, as expressed by 2-thiobarbituric acid reactive substances (TBARS) levels, and this prevented by ACH09. Levels of TBARS in the kidneys were lower in the SHR-ACH09 group than in the SHR-C group. Further, ACH09 increased the superoxide dismutase activity in both the plasma and kidneys of both SHR and Wistar rats. These results suggest that ACH09 is protective against disruption of blood pressures, oxidant status, and lipid profile in SHR, and provide important evidence on the benefits of ACH09 on hypertension and associated cardiovascular complications.
Keywords: grape skin extract, hypertension, oxidative stress, spontaneously hypertensive rats
INTRODUCTION
Hypertension is an independent risk factor for cardiovascular disease. Generally, hypertension demands high costs due to its complications as cerebrovascular disease, coronary artery disease, heart failure, chronic renal failure, and peripheral vascular disease (James et al., 2014).
Hypertension is often associated with metabolic abnormalities such as dyslipidemia, impaired glucose tolerance, insulin resistance, and obesity (Ceriello, 2008). Moreover, there is increasing evidence that oxidative stress and associated oxidative damage are mediators of vascular injury and therefore may be involved in the pathogenesis of hypertension (Grossman, 2008; Rodrigo et al., 2011; Singh et al., 2015).
Reactive oxygen species (ROS) are constantly formed in aerobic organisms and play important roles in normal regulation of many physiological processes. However, at high levels, ROS are harmful and cause widespread damage to cells and tissues, and disrupt many physiological processes (Hamza and Dyck, 2014). Overproduction of ROS or inefficient antioxidant defenses characterize oxidative stress. ROS may react with cellular components, especially lipids and proteins, causing tissue damage (Pisoschi and Pop, 2015), which is thought to be involved in the development and progression of hypertension (Siti et al., 2015). In addition, under pathological conditions, superoxide decreases the bioavailability of nitric oxide (NO) contributing to vascular damage and renal dysfunction (Savoia et al., 2011).
Grapes are a rich dietary source of polyphenolic compounds, which have beneficial effects for human health and experimental models of chronic diseases (Soares de Moura et al., 2002; Zang et al., 2006; Yadav et al., 2009; Soares de Moura et al., 2012). Consumption of polyphenols in large amounts reduces the incidence of cardiovascular diseases (Stoclet et al., 2004; Khurana et al., 2013; Hügel et al., 2016). These benefits of polyphenols may be related to their antioxidant, vasodilator (Rocha et al., 2007; Khurana et al., 2013), and antihypertensive effects (Soares de Moura et al., 2002; Khurana et al., 2013), and their roles in reducing serum cholesterol levels (de Oliveira et al., 2010) and protecting against atherosclerotic plaque (Waddington et al., 2004; Khurana et al., 2013).
Previously, we have demonstrated that Vitis vinifera L. grape skin extract (ACH09) is predominantly composed of anthocyanins (Resende et al., 2013), a large family of polyphenols. These natural compounds may be responsible for the antihypertensive effect of the extract in desoxycorticosterone acetate-salt and Nw-nitro-L-arginine methyl ester (L-NAME) experimental models (Soares de Moura et al., 2002; Soares de Moura et al., 2007). In addition, we have demonstrated that this extract induces a vasodilator response (Soares de Moura et al., 2002), has an antioxidant effect (Emiliano et al., 2011; Soares de Moura et al., 2012; Resende et al., 2013) and has a protective effect on the metabolism of adult offspring whose mothers were fed a high fat diet (Resende et al., 2013).
However, effects of ACH09 on spontaneously hypertensive rats (SHR), an experimental model that mimics the main abnormalities of human essential hypertension, such as high peripheral vascular resistance, have not been investigated.
MATERIALS AND METHODS
Preparation of the grape skin extract (ACH09)
V. vinifera L. (Vitaceae) was obtained from the South of Brazil. The dried and powdered skin were extracted in an aqueous solution at 100℃ with occasional shaking for 120 min. Aqueous extract was concentrated under a vacuum, introduced into an ion-exchange resin column (cationic), and washed sequentially with ethanol, ethanol/H2O (1:1), and H2O. The ethanolic and hydroalcoholic fractions were placed together and evaporated under vacuum at 60℃, followed by spray drying of the concentrated solution (inlet temperature 190℃ and outlet temperature 85℃). The fine powder (ACH09) obtained in the process was soluble in H2O, and contained about 30% of total polyphenols, according to the Folin-Ciocalteau procedure (Singleton and Rossi, 1965).
Polyphenol analyses
Analysis of the composition of ACH09 was performed by high-performance liquid chromatography (HPLC) as previously reported (Resende et al., 2013). Briefly, HPLC analysis of the dried hydroalcoholic ACH09 involved dissolving 10 mg of the extract in 1 mL methanol:H2O (1:1) with 0.5% formic acid (HPLC quality). All compounds showed the same ultraviolet (UV) spectra in the LC/UV/diode array detector analysis that is characteristic of anthocyanins. The compounds were identified as peonidin-3-O-glucoside, petunidin-3-O-glucoside, malvidin-3-O-glucoside, and malvidin-3-(6-O-trans-p-coumaryl)-5-O-diglicoside. The composition of ACH09 was confirmed by comparison of retention time with UV and mass spectrometry (MS) data from the LC/UV/MS, using commercially available standards (Resende et al., 2013).
Animals
All experiments were reviewed and approved by the Ethics Committee for Experimental Animals Use and Care (CEUA) of the Institute of Biology Roberto Alcântara Gomes/Rio de Janeiro State University (protocol: CEUA/022/2010). Male Wistar (W) and spontaneously hypertensive rats (SHR) were obtained from the facilities at the Rio de Janeiro State University at 3 weeks of age. The animals were maintained under standard conditions (12-h light/dark cycles, 23±2℃, humidity 60±10%, and 15 min/h air exhaustion cycle) and were divided into 4 groups (n=7 for each group): Wistar and SHR that received ACH09 (200 mg/kg/d in drinking water) for 12 weeks (W-ACH09 and SHR-ACH09, respectively), and corresponding controls (W-C and SHR-C) that received only water during the experimental protocol. Fluid intake was recorded every two days.
Systolic blood pressure measurement
Systolic blood pressure (SBP) was measured by the tail-cuff method using Letica LE 5000 devices (Letica Scientific Instruments, Póvoa de Varzim, Portugal). Rats were trained before starting the experimental protocol so that blood pressure could be recorded consistently with minimal restraint and stress to the animals. SBP measurements were recorded at the end of the experimental period; just before the measurement of SBP, rats were kept at 30∼32℃ for 15 min to ensure the pulsations in the tail artery were detectable.
Plasma assays
After 12 weeks of treatment and when rats were 15 weeks of age, rats were fasted for 12 h and then anesthetized with thiopental (70 mg/kg i.p.). Blood samples were collected by aorta puncture and centrifuged at 3,000 rpm for 10 min at 4℃. Plasma samples were separated and then stored at −80℃. Plasma glucose, triglyceride, and total cholesterol levels were measured by a colorimetric assay (Gold Analisa Diagnóstica, Belo Horizonte, Brazil).
Determination of oxidative damage
Lipid membrane damage was determined by analyzing the formation of products of lipid peroxidation [2-thiobarbituric acid reactive substances (TBARS)] in kidney homogenates and plasma. Briefly, samples were mixed with 1 mL of trichloroacetic acid 10%, and 1 mL of 0.67% thiobarbituric acid, and heated in a boiling water bath for 30 min. TBARS was determined by measuring the absorbance at 532 nm and the results were expressed in nmol/mg protein (Draper et al., 1993).
Determination of antioxidant enzyme activity
Superoxide dismutase (SOD) and catalase (CAT) activities were determined in kidney homogenates and plasma. SOD activity was assayed by measuring the inhibition of adrenaline auto-oxidation at an absorbance of 480 nm (Bannister and Calabrese, 1987). CAT activity was measured by the rate of decrease in hydrogen peroxide at 240 nm (Aebi, 1984). The total protein content in each sample was determined using the Bradford method (Bradford, 1976).
Statistical analysis
Data are shown as mean±standard error of the mean (SEM). Differences among groups were analyzed by a one-way analysis of variance (one-way ANOVA), followed by the Bonferroni post hoc test using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA, USA). Statistical significance was calculated at P<0.05. Graphs were created using SigmaPlot version 12.0 (Systat Software, Inc., San Jose, CA, USA).
RESULTS
Effect of ACH09 on SBP
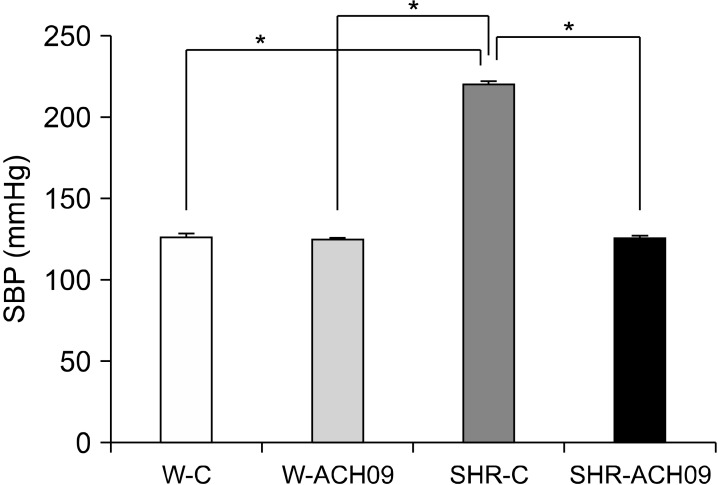
SBP (mm Hg) was significantly higher (P<0.05, n=7) in the SHR-C group than in the Wistar groups at the end of the experimental protocol (Fig. 1). Treatment with ACH09 prevented the increase in SBP (P<0.05) in the SHR group, and there was no difference between the SHR group and the Wistar groups at the end of treatment.
Fig. 1.
Effect of Vitis vinifera L. grape skin extract (ACH09) on systolic blood pressure (SBP) in Wistar and spontaneously hypertensive rats (SHR). W-C, control Wistar rats; SHR-C, control SHR rats; W-ACH09, Wistar rats received ACH09 (200 mg/kg/d) in drinking water; SHR-ACH09, SHR rats received ACH09 (200 mg/kg/d) in drinking water. Values are reported as mean±SEM (n=7). *P<0.05.
Effect of ACH09 on blood glucose, triglyceride, and total cholesterol levels
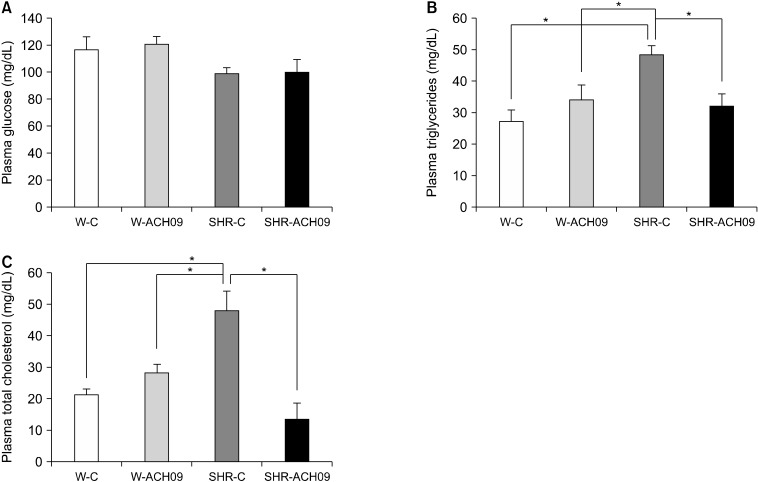
Fasting glucose did not differ between the experimental groups (n=7, Fig. 2A). Plasma triglyceride (n=7, Fig. 2B) and total cholesterol (n=7, Fig. 2C) levels were higher in the SHR-C group than in the Wistar group (P<0.05). Treatment with ACH09 restored both total cholesterol and triglyceride concentrations (P<0.05) to normal levels in the SHR group (Fig. 2B and 2C).
Fig. 2.
Effect of Vitis vinifera L. grape skin extract (ACH09) on plasma glucose (A), triglyceride (B), and total cholesterol (C) levels in Wistar and spontaneously hypertensive rats (SHR). W-C, control Wistar rats; SHR-C, control SHR rats; W-ACH09, Wistar rats received ACH09 (200 mg/kg/d) in drinking water; SHR-ACH09, SHR rats received ACH09 (200 mg/kg/d) in drinking water. Values are reported as mean±SEM (n=7). *P<0.05.
Effect of ACH09 on oxidative damage
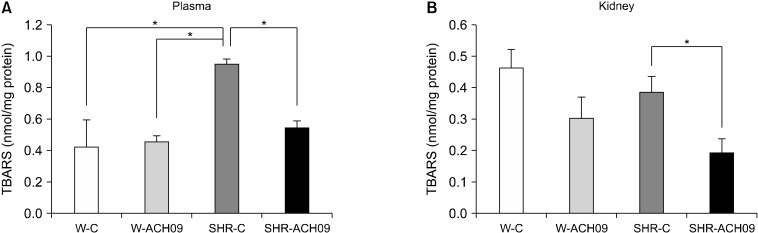
Oxidative damage, assessed by levels of TBARS, was evaluated in the plasma (n=7, Fig. 3A) and kidneys (n=7, Fig. 3B) from all groups. TBARS levels were significantly higher (P<0.05) in the plasma from the SHR-C group than the Wistar groups (Fig. 3A). Rats in the SHR-ACH09 showed lower levels of TBARS in plasma than the SHR-C group (P<0.05). The levels of TBARS in the kidneys were significantly lower (P<0.05) in the SHR-ACH09 group than in the SHR-C group, but there were no differences between the SHR-C and Wistar groups (Fig. 3B).
Fig. 3.
Effect of Vitis vinifera L. grape skin extract (ACH09) on 2-thiobarbituric acid reactive substances (TBARS) levels in plasma (A) and kidney (B) of Wistar and spontaneously hypertensive rats (SHR). W-C, control Wistar rats; SHR-C, control SHR rats; W-ACH09, Wistar rats received ACH09 (200 mg/kg/d) in drinking water; SHR-ACH09, SHR rats received ACH09 (200 mg/kg/d) in drinking water. Values are reported as mean±SEM (n=7). *P<0.05.
Effect of ACH09 on antioxidant activity
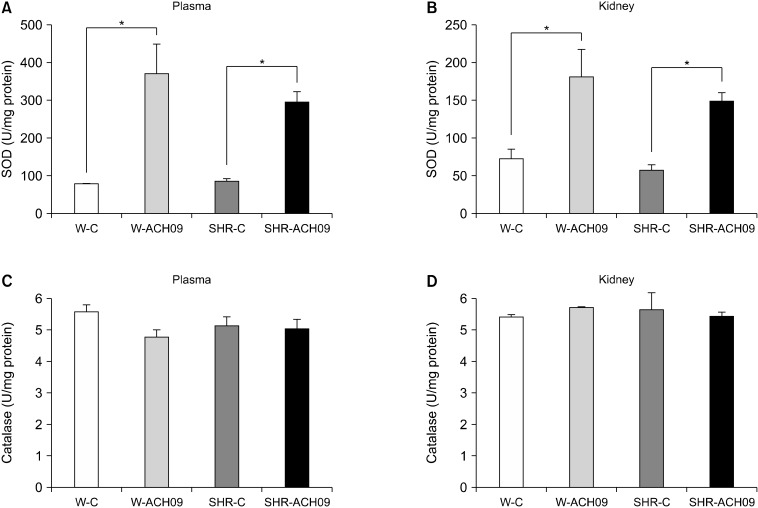
SOD and CAT activities were evaluated in the plasma and kidneys of all groups. Rats in both the SHR-ACH09 and W-ACH09 groups showed increased (P<0.05) SOD activity in both the plasma (n=7, Fig. 4A) and kidneys (n=7, Fig. 4B) compared with their respective controls. However, there was no difference in catalase activity in the plasma (n=7, Fig. 4C) and kidneys (n=7, Fig. 4D) between experimental groups.
Fig. 4.
Effect of Vitis vinifera L. grape skin extract (ACH09) on the antioxidant activities of superoxide dismutase (A and B) and catalase (C and D) in the plasma and kidneys of Wistar and spontaneously hypertensive rats (SHR). W-C, control Wistar rats; SHR-C, control SHR rats; W-ACH09, Wistar rats received ACH09 (200 mg/kg/d) in drinking water; SHR-ACH09, SHR rats received ACH09 (200 mg/kg/d) in drinking water. Values are reported as means mean±SEM (n=7). *P<0.05.
DISCUSSION
This study evaluated the effects of chronic treatment with a hydroalcoholic extract obtained from the V. vinifera grape skin (ACH09) on blood pressure, oxidative stress, and lipid profile in SHR. The main results demonstrate that ACH09 prevents development of hypertension, oxidative stress, and altered lipid profile in SHR, which demonstrates its potential beneficial use against cardiovascular diseases.
The most common form of human hypertension is primary or essential hypertension, which is best represented by genetic animal models of hypertension such as the SHR (Sarikonda et al., 2009). In SHR, blood pressure rises progressively from 4 weeks of age. Therefore, in this study, 3-week-old SHR rats were used as we could observe the protective effect of long-term intake of ACH09 on the development of hypertension.
These results indicate that systolic blood pressure is significantly elevated in adult SHR, as previously reported (Erejuwa et al., 2012; Booranasubkajorn et al., 2017). We demonstrate for the first time that chronic treatment with ACH09 prevents development of hypertension in SHR. Levels of systolic blood pressure in SHR-ACH09 were not different from those of the Wistar groups. The antihypertensive effect of ACH09 has been previously demonstrated by our group in the models of hypertension induced by administration of desoxycorticosterone acetate-salt and L-NAME (Soares de Moura et al., 2002; Soares de Moura et al., 2007). Moreover, ACH09 has been shown to protect normally fed offspring of high fat-fed dams during lactation from phenotypic and metabolic characteristics of metabolic syndrome, including hypertension (Emiliano et al., 2011).
The antihypertensive effect of ACH09 is likely mediated by endothelium-dependent vasodilation induced by the extract (Soares de Moura et al., 2002). Madeira et al. (2005) showed that ACH09 induces vasodilation of pre-constricted isolated mesenteric vascular beds, which is endothelium-dependent and mediated by the release of NO in combination with endothelium-derived hyperpolarizing factor.
Growing evidence suggests that oxidative stress is a crucial molecular mechanism involved in the pathogenesis of hypertension and hypertensive renal damage (Grossman, 2008; Rodrigo et al., 2011; Mennuni et al., 2014; Singh et al., 2015; Sun et al., 2015). In the present study, we showed that the oxidative damage, assessed by levels of the lipid peroxidation marker TBARS, was increased in plasma of SHR with established hypertension, which is in agreement with previously published data (Erejuwa et al., 2012; Shang et al., 2016). Interestingly, ACH09 extract significantly reduced TBARS plasma levels in SHR, thereby demonstrating an antioxidant effect. However, levels of TBARS in the kidneys of SHR did not differ from those of Wistar groups. In contrast, a previous study showed that SHR rats have increased oxidative damage in the kidney compared with control group (Erejuwa et al., 2012). The difference between our results and this previous may be explained by the animals’ age. Erejuwa et al. (2012) used 24-week-old SHR whereas 15-week-old rats were used in the present study. Although we did not observe increased lipid peroxidation in the kidneys of SHR compared with the controls, ACH09 reduced lipid peroxidation, which is associated with increased activity of SOD, the major antioxidant enzyme; these results suggest ACH09 may also show renoprotective effect. The antioxidant capacity of ACH09 has been reported in experimental models of obesity (da Costa et al., 2017; Santos et al., 2017), diabetes (Soares de Moura et al., 2012), and metabolic programming (Resende et al., 2013), possibly attributable to anthocyanins, particularly peonidin, petunidin, and malvidins in ACH09 (Resende et al., 2013). Therefore, in the present study, the antioxidant effect of ACH09 in SHR may be related to induction of SOD, which is the first line of defense against oxidative stress. We speculate that the reduced levels of superoxide may increase bioavailability of nitric oxide, an important vasodilator, thus contributing to the antihypertensive effect of ACH09.
Dyslipidemia is strongly associated with increased cardiovascular risk (Sánchez-Íñigo et al., 2016). Therefore, the development of a new agent that can simultaneously lower blood pressure and improve lipid profile is important for treatment of cardiovascular disease and hypertension. In the present study, we observed high levels of plasma triglycerides and total cholesterol in SHR compared with Wistar groups. We also demonstrated that ACH09 has an important role in regulating lipid profile in SHR. The results showed that ACH09 decreased levels of triglycerides ant total cholesterol in SHR to levels similar to normotensive groups. In our previous studies, we observed a significant reduction in serum total cholesterol and triglycerides in mice fed a high-fat diet and treated with ACH09, suggesting ACH09 has lipid-lowering properties (da Costa et al., 2017; Santos et al., 2017), related to the increased cholesterol excretion through ATP-binding cassette sub-family G member (ABCG) 5 and ABCG8 transporters in the liver (Santos et al., 2017). Anthocyanins present in ACH09 (Resende et al., 2013) may be responsible for this improvement in lipid profile since polyphenols, such as procyanidins from grape seed extracts, improve the hyperlipidemic state in the fructose-fed rat model (Downing et al., 2015).
In summary, the findings from this study show that chronic treatment with ACH09 is protective against development of high blood pressure in spontaneously hypertensive rats, and that this effect may be related to antioxidant effect of superoxide dismutase. Moreover, the improvement in lipid profile may contribute to the protective cardiovascular effect. These preclinical results reveal the possibility for use of ACH09 in the prevention or treatment of hypertension in human.
ACKNOWLEDGEMENTS
Supported by grants from the National Council of Scientific and Technological Development (CNPq) and Rio de Janeiro State Research Agency (FAPERJ).
Footnotes
AUTHOR DISCLOSURE STATEMENT
Roberto Soares de Moura is the inventor of a patent (PCT/BR02/00038) that supported the development of a new patent application (PI0605693 A2-8). The other authors declare no conflict of interest.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bannister JV, Calabrese L. Assays for superoxide dismutase. Methods Biochem Anal. 1987;32:279–312. doi: 10.1002/9780470110539.ch5. [DOI] [PubMed] [Google Scholar]
- Booranasubkajorn S, Huabprasert S, Wattanarangsan J, Chotitham P, Jutasompakorn P, Laohapand T, et al. Vasculoprotective and vasodilatation effects of herbal formula (Sahatsatara) and piperine in spontaneously hypertensive rats. Phytomedicine. 2017;24:148–156. doi: 10.1016/j.phymed.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31:S181–S184. doi: 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- da Costa GF, Santos IB, de Bem GF, Cordeiro VSC, da Costa CA, de Carvalho LCRM, et al. The beneficial effect of anthocyanidin-rich Vitis vinifera L. grape skin extract on metabolic changes induced by high-fat diet in mice involves antiinflammatory and antioxidant actions. Phytother Res. 2017;31:1621–1632. doi: 10.1002/ptr.5898. [DOI] [PubMed] [Google Scholar]
- de Oliveira PR, da Costa CA, de Bem GF, de Cavalho LC, de Souza MA, de Lemos Neto M, et al. Effects of an extract obtained from fruits of Euterpe oleracea Mart. in the components of metabolic syndrome induced in C57BL/6J mice fed a high-fat diet. J Cardiovasc Pharmacol. 2010;56:619–626. doi: 10.1097/FJC.0b013e3181f78da4. [DOI] [PubMed] [Google Scholar]
- Downing LE, Heidker RM, Caiozzi GC, Wong BS, Rodriguez K, Del Rey F, et al. A grape seed procyanidin extract ameliorates fructose-induced hypertriglyceridemia in rats via enhanced fecal bile acid and cholesterol excretion and inhibition of hepatic lipogenesis. PLoS One. 2015;10:e0140267. doi: 10.1371/journal.pone.0140267. doi: 10.1371/journal.pone.0140267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med. 1993;15:353–363. doi: 10.1016/0891-5849(93)90035-S. [DOI] [PubMed] [Google Scholar]
- Emiliano AF, de Cavalho LC, da Silva Cristino Cordeiro V, da Costa CA, de Oliveira PB, Queiroz EF, et al. Metabolic disorders and oxidative stress programming in offspring of rats fed a high-fat diet during lactation: effects of a vinifera grape skin (ACH09) extract. J Cardiovasc Pharmacol. 2011;58:319–328. doi: 10.1097/FJC.0b013e3182244a51. [DOI] [PubMed] [Google Scholar]
- Erejuwa OO, Sulaiman SA, Ab Wahab MS, Sirajudeen KN, Salleh S, Gurtu S. Honey supplementation in spontaneously hypertensive rats elicits antihypertensive effect via amelioration of renal oxidative stress. Oxid Med Cell Longev. 2012;2012:374037. doi: 10.1155/2012/374037. doi: 10.1155/2012/374037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E. Does increased oxidative stress cause hypertension? Diabetes Care. 2008;31:S185–S189. doi: 10.2337/dc08-s246. [DOI] [PubMed] [Google Scholar]
- Hamza SM, Dyck JRB. Systemic and renal oxidative stress in the pathogenesis of hypertension: modulation of long-term control of arterial blood pressure by resveratrol. Front Physiol. 2014;5:292. doi: 10.3389/fphys.2014.00292. doi: 10.3389/fphys.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügel HM, Jackson N, May B, Zhang AL, Xue CC. Polyphenol protection and treatment of hypertension. Phytomedicine. 2016;23:220–231. doi: 10.1016/j.phymed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai TC. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5:3779–3827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira SV, de Castro Resende A, Ognibene DT, de Sousa MA, Soares de Moura R. Mechanism of the endothelium-dependent vasodilator effect of an alcohol-free extract obtained from a vinifera grape skin. Pharmacol Res. 2005;52:321–327. doi: 10.1016/j.phrs.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014;28:74–79. doi: 10.1038/jhh.2013.55. [DOI] [PubMed] [Google Scholar]
- Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Resende AC, Emiliano AF, Cordeiro VS, de Bem GF, de Cavalho LC, de Oliveira PR, et al. Grape skin extract protects against programmed changes in the adult rat offspring caused by maternal high-fat diet during lactation. J Nutr Biochem. 2013;24:2119–2126. doi: 10.1016/j.jnutbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Rocha APM, Carvalho LCRM, Sousa MAV, Madeira SVF, Sousa PJC, Tano T, et al. Endothelium-dependent vasodilator effect of Euterpe oleracea Mart. (Açaí) extracts in mesenteric vascular bed of the rat. Vasc Pharmacol. 2007;46:97–104. doi: 10.1016/j.vph.2006.08.411. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- Sánchez-Íñigo L, Navarro-González D, Pastrana-Delgado J, Fernández-Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34:1257–1265. doi: 10.1097/HJH.0000000000000941. [DOI] [PubMed] [Google Scholar]
- Santos IB, de Bem GF, Cordeiro VSC, da Costa CA, de Carvalho LCRM, da Rocha APM, et al. Supplementation with Vitis vinifera L. skin extract improves insulin resistance and prevents hepatic lipid accumulation and steatosis in high-fat diet-fed mice. Nutr Res. 2017;43:69–81. doi: 10.1016/j.nutres.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Sarikonda KV, Watson RE, Opara OC, Dipette DJ. Experimental animal models of hypertension. J Am Soc Hypertens. 2009;3:158–165. doi: 10.1016/j.jash.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Savoia C, Burger D, Nishigaki N, Montezano A, Touyz RM. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med. 2011;13:e11. doi: 10.1017/S1462399411001815. doi: 10.1017/S1462399411001815. [DOI] [PubMed] [Google Scholar]
- Shang P, Liu W, Liu T, Zhang Y, Mu F, Zhu Z, et al. Acetyl-11-keto-β-boswellic acid attenuates prooxidant and profibrotic mechanisms involving transforming growth factor-β1, and improves vascular remodeling in spontaneously hypertensive rats. Sci Rep. 2016;6:39809. doi: 10.1038/srep39809. doi: 10.1038/srep39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev. 2015;31:113–126. doi: 10.1002/dmrr.2558. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Soares de Moura R, Costa Viana FS, Souza MA, Kovary K, Guedes DC, Oliveira EP, et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J Pharm Pharmacol. 2002;54:1515–1520. doi: 10.1211/002235702153. [DOI] [PubMed] [Google Scholar]
- Soares de Moura R, da Costa GF, Moreira AS, Queiroz EF, Moreira DD, Garcia-Souza EP, et al. Vitis vinifera L. grape skin extract activates the insulin-signalling cascade and reduces hyperglycaemia in alloxan-induced diabetic mice. J Pharm Pharmacol. 2012;64:268–276. doi: 10.1111/j.2042-7158.2011.01395.x. [DOI] [PubMed] [Google Scholar]
- Soares de Moura R, Resende AC, Moura AS, Maradei MF. Protective action of a hydroalcoholic extract of a vinifera grape skin on experimental preeclampsia in rats. Hypertens Pregnancy. 2007;26:89–100. doi: 10.1080/10641950601147960. [DOI] [PubMed] [Google Scholar]
- Stoclet JC, Chataigneau T, Ndiaye M, Oak MH, El Bedoui J, Chataigneau M, et al. Vascular protection by dietary polyphenols. Eur J Pharmacol. 2004;500:299–313. doi: 10.1016/j.ejphar.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang T, Li L, Wang J. Induction of apoptosis by hypertension via endoplasmic reticulum stress. Kidney Blood Press Res. 2015;40:41–51. doi: 10.1159/000368481. [DOI] [PubMed] [Google Scholar]
- Waddington E, Puddey IB, Croft KD. Red wine polyphenolic compounds inhibit atherosclerosis in apolipoprotein E-deficient mice independently of effects on lipid peroxidation. Am J Clin Nutr. 2004;79:54–61. doi: 10.1093/ajcn/79.1.54. [DOI] [PubMed] [Google Scholar]
- Yadav M, Jain S, Bhardwaj A, Nagpal R, Puniya M, Tomar R, et al. Biological and medicinal properties of grapes and their bioactive constituents: an update. J Med Food. 2009;12:473–484. doi: 10.1089/jmf.2008.0096. [DOI] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]