Abstract
In recent decades, the prevalence of diabetes has rapidly increased worldwide. Medical nutrition therapy has been identified as a major therapeutic support for diabetic patients, while preventive strategies in prediabetic or high-risk individuals have mainly focused on supplementation with bioactive compounds. Recently, meal-based interventions have been investigated as novel and safe long-term strategies for improving glucose regulation. However, evaluation of meal-based interventions is difficult since it requires analysis of sensitive markers. Biomarkers can also be used to identify individuals at risk for diabetes, which is important for disease prevention. In this review, we summarize current evidence from meal-based intervention studies conducted with the aim of improving glucose homeostasis in individuals at risk of diabetes using clinical biomarkers currently used to assess diabetic risk. Very low-calorie diets have significantly improved glucose regulation in obese adults and in adults with type 2 diabetes mellitus. In particular, changing the ratios of macronutrients through calorie restriction reduces fasting glucose level and hemoglobin A1c levels in patients with diabetes mellitus. However, this effect is limited in both obese and healthy adults. To date, multiple glucose-related markers have been identified as clinical biomarkers of diabetes. Additional clinical biomarkers include cholesterol levels, hematological markers, and inflammatory markers. Taken together, the evidence presented in this review may help for selection of clinical biomarkers for meal-based preventive approaches for non- or pre-diabetic individuals to prevent onset of diabetes.
Keywords: biomarker, clinical, diabetes mellitus, glucose, meals
INTRODUCTION
Diabetes is a metabolic diseases caused by defects in insulin secretion and/or insulin action (American Diabetes Association, 2010). The global prevalence of diabetes has risen from 4.7% to 8.5% over the past 30 years, and affects a higher number of overweight and obese individuals (WHO, 2016). In Korean adults 30 years of age or older, the prevalence of diabetes was 14.4% in 2016 (Korean Diabetes Association, 2018). Diabetes and the associated complications are major causes of mortality (Cowie et al., 2006). Depending on insulin dependency, diabetes can be categorized as type 1 or type 2 (American Diabetes Association, 2013). Type 2 diabetes (T2D) accounts for more than 90% of diabetic cases. In individuals with T2D, insulin is regularly secreted in the body, however target cells exhibit insulin resistance (IR) (American Diabetes Association, 2013). If left untreated, T2D can cause serious damage to nerves and blood vessels due to prolonged hyperglycemia (Kwon and Chung, 2013). Even in nondiabetic individuals, high blood glucose concentrations are considered a risk factor for mortality in middle-aged adults (Balkau et al., 1998). Thus, regulating glucose concentrations is important for maintaining good health.
Multiple genetic and environmental factors are major risk factors for T2D. Of these, obesity is one of the most significant determinants (Hu et al., 2001). Lack of exercise, high stress, and high fat and sugar intake are well-known factors that contribute to onset of T2D, and are characteristics of lifestyles that often lead to obesity. Therefore, interventions aimed at modulating these lifestyles, such as via diet and exercise, are critical for preventing T2D in populations at high risk of diabetes. Dietary intervention has been extensively studied in the context of prevention and treatment of T2D (Ben-Avraham et al., 2009; Schwingshackl et al., 2018). One of the most well-known strategies is modulating glycemic control (Stolar, 2010). High levels of fasting glucose can increase the risk of complications in diabetes patients. Thus, improving glycemic control is both a major goal of T2D therapy and a preventive strategy.
The effects of individual nutrients and functional compounds on regulating blood glucose levels for management of T2D have been previously reported (Heer and Egert, 2015; Russell et al., 2016; Alkhatib et al., 2017). For example, high fiber diets and soluble fiber supplements have been reported to significantly improve blood glucose levels in patients with T2D (Silva et al., 2013; Thompson et al., 2017). Recently, meal-based interventions has also emerged as a potential strategy for prevention of and treatment of T2D. Meal-based interventions can be used for long-term management of individuals at risk by modulating meal patterns, and as a short-term approach through supplementing meals with nutrients and bioactive compound. It is recognized that meal-based interventions have great potential for disease prevention, but are limited by practical obstacles associated with providing individuals with whole meals. Consequently, only a few studies have evaluated the efficacy of meal-based interventions on glucose regulation. Moreover, most the sensitivities of most clinical biomarkers for diabetes have not been fully validated in the context of assessing glucose changes in non-diabetic risk groups.
There are multiple clinical biomarkers currently available for the diagnosis and classification of diabetes (American Diabetes Association, 2013). Fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) levels are the most commonly used glycemic parameters. FPG levels are a key factor for determining threshold glucose levels, whereas HbA1c levels reflect average blood glucose levels over a period of two to three months. Currently, HbA1c is widely used as a marker of chronic glycemia (Kwon and Chung, 2013), and is used as a readout of glycemic control in the management of diabetes (American Diabetes Association, 2013). Further, hyperglycemic indicators, anthropometric measurements, insulin indexes, and lipid profiles are used to diagnose, classify, and monitor diabetes. While these markers are commonly used, it is not clear whether these markers are sufficiently sensitive to analyze changes in glucose homeostasis in nondiabetic individuals, particularly in response to dietary intervention.
In this review, we examined recent evidence regarding meal-based interventions and clinical biomarkers for intervention in both healthy individuals and individuals at risk of diabetes. This information should facilitate selection of appropriate clinical biomarkers and will help establish long-term prevention strategies for patients with T2D and individuals in target risk populations participating in meal-based interventions.
MATERIALS AND METHODS
Search strategy
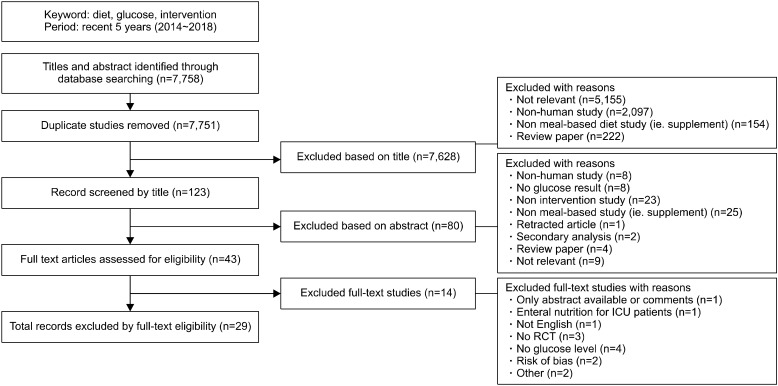
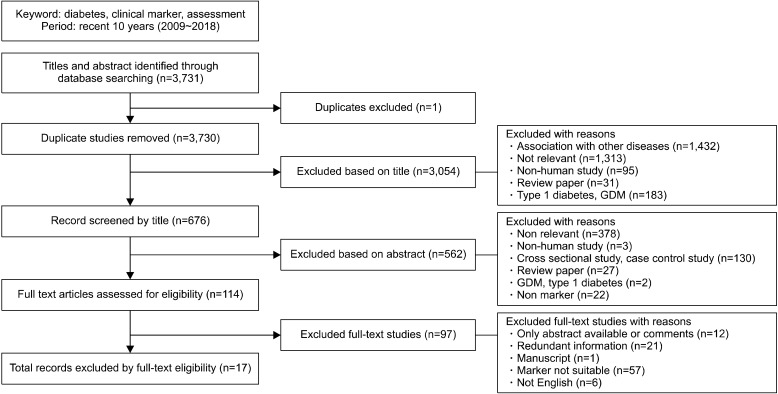
Two search strategies were used to review publications reporting improvements in glucose regulation. First, we performed a search for meal-based dietary interventions in nondiabetic individuals through searching the PubMed database. The terms, ‘diet, glucose, and intervention’, was used to search publications published between 2014 and 2018. We then performed a second comprehensive literature search for clinical markers used to assess glucose regulation. The terms ‘clinical marker, assessment, and diabetes’ were used to search publications in MEDLINE through PubMed published between 2009 and 2018. Only articles published in English were considered. Relevant literature was extracted based on the title and abstract. Full text articles were subsequently checked according to the criteria listed in Fig. 1 and 2. A total of 29 articles reporting meal-based diet interventions and 17 articles reporting clinical biochemical markers were selected.
Fig. 1.
Flow diagram of studies included in the review of meal-based dietary intervention on blood glucose regulation. ICU, intensive care units; RCT, randomized controlled trial.
Fig. 2.
Flow diagram of studies included in the review of clinical markers for type 2 diabetes. GDM, gestational diabetes mellitus.
Selection criteria
For analysis of meal-based dietary interventions, all included studies were intervention studies that involved human subjects and used glucose regulation markers, such as FPG or HbA1c (Fig. 1). Following identification of relevant papers according to the titles, we excluded non-human studies, review papers, and non-meal-based studies. Next, the abstracts of the remaining papers were reviewed. Papers were excluded if results of glucose control were not provided. Finally, a total of 29 articles were selected for inclusion in this review.
Criteria for selecting articles involving clinical biomarkers of diabetes mellitus were included data derived from T2D patients and cohort studies that included adult with and without diabetes (Fig. 2). We excluded studies which 1) were derived from cross-sectional studies and case control studies, due to insufficient explanation for cause- and-effect relationships and difficulty in obtaining valid indicators to assess disease in time-specific investigations, 2) were identified in individuals with type 1 diabetes, gestational diabetes mellitus, and other diseases, and 3) were based on insufficient information regarding clinical markers. By screening titles and abstracts, articles were excluded based on unrelated diseases, non-human studies, review papers, and studies of individuals with other types of diabetes. Finally, a total of 17 articles were selected for this review.
RESULTS
Meal-based dietary interventions for improving glucose regulation
Initially, we categorized the 29 studies of meal-based dietary interventions for improving glucose regulation according to meal type: low caloric meals (n=5), macronutrient ratio-based meals (n=14), and food pattern studies (n=10). The low caloric meal intervention studies are summarized in Table 1. We observed that changes in FPG in obese individuals were not consistent between the different studies (Lee et al., 2014a; Perichart-Perera et al., 2014). Moreover, in the studies reporting low-calorie diets, 12-week, but not 6-month interventions, were shown to decrease FPG levels. However, very low-calorie restriction diet (e.g. low fat diets with a limitation of 624∼700 kcal/d) significantly reduced FPG and HbA1c levels in both obese and T2D adults regardless of study duration (Norén and Forssell, 2014; Steven and Taylor, 2015; Steven et al., 2016). Interestingly, the effect of very low-calorie restriction on HbA1c levels was not significant in adults who had diabetes for more than 8 years (Steven and Taylor, 2015), indicating that the effect depends on the severity of glucose impairment.
Table 1.
Intervention studies of low caloric meals on blood glucose regulation
| Meal types of intervention | Subjects (country) | Duration of intervention | Results | Comments | Reference |
|---|---|---|---|---|---|
| Low-calorie diet | Overweight or obese postmenopausal women (Mexico) | 6 months | FPG: no change TC, HDL-C, LDL-C, and TG: no change |
1,300∼1,400 kcal/d | Perichart-Perera et al. (2014) |
| Obese adult women (Korea) | 12 weeks | FPG and AUC glucose: decrease TC, HDL-C, and LDL-C: decrease TG: no change |
1,200 kcal/d | Lee et al. (2014a) | |
| Very low-calorie diet (less than 800 kcal/d) | Obese adults (partially T2DM) | 4 weeks | FPG and HbA1c: decrease TG, cholesterol: decrease |
680 kcal/d | Norén and Forssell (2014) |
| T2DM adults | 8 weeks | FPG and HbA1c: decrease TG, TC, and non-HDL-C: decrease HDL-C: no change |
624∼700 kcal/d HbA1c no changed in long-duration disease (>8 years) group |
Steven and Taylor (2015) | |
| FPG and HbA1c: decrease TG and non-HDL-C: decrease |
624∼700 kcal/d | Steven et al. (2016) |
T2DM, type 2 diabetes mellitus; FPG, fasting plasma glucose; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; AUC glucose, area under the curve of glucose; HbA1c, hemoglobin A1c.
Recent evidence from multiple meal-based intervention studies examining various macronutrient ratios is summarized in Table 2. The ratio of the macronutrients, rather than calorie intake, determined cardiometabolic health and aging in ad libitum-fed mice (Solon-Biet et al., 2014). In addition, limited carbohydrate diets under calorie restriction, such as low carbohydrate and high protein diets, effectively lowered HbA1c and fasting glucose levels in both pre-diabetic and diabetic adults (Kempf et al., 2014; Tay et al., 2014; Goday et al., 2016); however, this result was not observed in nondiabetic overweight or obese adults (Rajaie et al., 2014). We also observed that these meal-based interventions with various macronutrient ratios affected both glucose markers and serum lipid profiles, including triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). However, the latter affects are less consistent (Kempf et al., 2014; Tay et al., 2014; Goday et al., 2016; Stentz et al., 2016). Intriguingly, the effects of a two-year intervention involving a low carbohydrate and low saturated fat diet on HbA1c and fasting glucose levels did not significantly differ from effects observed following a high carbohydrate and low fat diet (Tay et al., 2018). It is possible that different ratios of carbohydrate and fat may not be critical to long-term intake. Ratios of macronutrients, even in the absence of calorie restriction, have been found to influence glucose and lipid metabolism (Table 2). Furthermore, low carbohydrate diets have been effective for decreasing HbA1c level in adult diabetes patients, although their effects on fasting glucose levels and serum lipid profiles have been relatively limited (Yamada et al., 2014; Sato et al., 2017; Wang et al., 2018).
Table 2.
Intervention studies of various macronutrient ratio-based meals on blood glucose regulation
| Meal types of intervention (carbohydrate:fat:protein) | Subjects (country) | Duration of intervention | Results | Comments | Reference | |
|---|---|---|---|---|---|---|
| Calorie restriction | Moderately restricted carbohydrate diet (45:40:15) compared to high carbohydrate diet (60:20:20) | Overweight/obese adults | 6 weeks per diet (cross-over) | FPG: no change | 350∼700 kcal less than the energy requirement | Rajaie et al. (2014) |
| TG, HDL-C, LDL-C, and TC: no change | Only TG significantly decreased comparing baseline to post-intervention in moderately restricted carbohydrate diet group. | |||||
| Low carbohydrate diet (14:58:28) compared to high carbohydrate diet (53:<30:17) | T2DM adults | 6 months | FPG: no change | Low carbohydrate diet consisting of low-saturated fat | Tay et al. (2014) | |
| HbA1c and blood glucose range: decrease | Calorie restriction with 500∼1,000 kcal less than the energy requirement | |||||
| TC and LDL-C: no change | Greater increased in HDL-C occurred with the low carbohydrate diet for participants with a baseline HDL-C <1.3 mmol/L. | |||||
| HDL-C: increase | No diet effect in participants with initial HbA1c ≤ 62 mmol/mol | |||||
| TG: decrease | ||||||
| 24 months | FPG and HbA1c: no change | Tay et al. (2018) | ||||
| HDL-C: increase | ||||||
| TG: decrease | ||||||
| TC, LDL-C, and non-HDL-C: no change | ||||||
| High protein diet (40:30:30) | Pre-diabetes adults | 6 months | HbA1c, cholesterol, TG, and LDL-C: decrease | Stentz et al. (2016) | ||
| HDL-C: no change | ||||||
| High protein diet | T2DM adults | 3 months | FPG and HbA1c: decrease | A data after 1.5 years of follow-up was available | Kempf et al. (2014) | |
| HDL-C: increase | ||||||
| TG: decrease | ||||||
| TC and LDL-C: no change | ||||||
| Very low carbohydrate (ketogenic) diet compared to low calorie diet | T2DM adults | 4 months | FPG, HbA1c, and TG: decrease | Very low caloric diets with 600∼800 kcal/d | Goday et al. (2016) | |
| TC, HDL-C, LDL-C: no change | <50 g carbohydrate from vegetables daily | |||||
| Low calorie diet with 500∼1,000 kcal less than the energy requirement | ||||||
| T2DM duration <10 years | ||||||
| Non-calorie restriction | Low carbohydrate-high fat diet (20:65:15) | Healthy adults | 7 days | FPG: increase | 65% total energy from fat | Parry et al. (2017) |
| HDL-C: increase | ||||||
| TG: decrease | ||||||
| TC and LDL-C: no change | ||||||
| Low carbohydrate-high fat diet (20:70:10) compared to control diet (60:30:10) | 3 days per diet(cross-over) | TC, HDL-C, and LDL-C: no change | FPG and TG were significantly decreased comparing baseline to post-intervention. | Numao et al. (2016) | ||
| TG: decrease | ||||||
| Low carbohydrate-high fat diet (40:50:10) compared to control diet (60:30:10) | FPG: no change | |||||
| Low carbohydrate-high fat diet | Overweight/obese adults | 12 weeks | HDL-C: increase | Carbohydrate intake less than 45% of total energy | Zinn et al. (2017) | |
| Serum glucose, TG: decrease | Fat intake more than 33% of total energy | |||||
| HbA1c and LDL-C: no change | ||||||
| Low carbohydrate-high fat diet (15:67:18) compared to low fat diet (67:15:18) | 5 days per diet(cross-over) | Mean glucose and AUC: decrease | Parr et al. (2018) | |||
| FPG: no change | ||||||
| High complex-carbohydrate diet (60:25:15) | Gestational diabetes | 7 weeks | TG: increase | |||
| FPG: decrease | High carbohydrate was based on complex carbohydrate. | Hernandez et al. (2016) | ||||
| TC, HDL-C, and LDL-C: no change | ||||||
| Low carbohydrate diet | T2DM adults (Japan) | 6 months | HbA1c: decrease | Carbohydrate intake: 130 g/d. | Sato et al. (2017) | |
| TG, HDL-C, and LDL-C: no change | A data after a year of follow-up was available | |||||
| T2DM adults | 6 months | HbA1c and TG: decrease | Carbohydrate intake less than 130 g/d | Yamada et al. (2014) | ||
| FPG: no change | ||||||
| LDL-C and HDL-C: no change | ||||||
| Low carbohydrate diet (38:42:20) | T2DM adults (China) | 3 months | FPG and HbAlc: decrease | Wang et al. (2018) | ||
| TC: decrease | ||||||
T2DM, type 2 diabetes mellitus; FPG, fasting plasma glucose; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; HbA1c, hemoglobin A1c; AUC, area under the curve.
The types of carbohydrates consumed, as well as the proportion of energy they represent in a diet, are critical for achieving low fasting glucose levels in gestational diabetic patients. Meanwhile, a high complex carbohydrate diet has been found to reduce fasting glucose levels compared with a control diet containing a relatively low carbohydrate content (Hernandez et al., 2016). Interestingly, the positive effects of a low carbohydrate/high fat diet (50∼70% of total calories from fat) were not found to be significant in healthy and obese adults (Numao et al., 2016; Parry et al., 2017; Zinn et al., 2017; Parr et al., 2018).
Recently, intervention studies with various food patterns have been reported (Table 3). For example, in a four-year intervention study of adults with T2D adhering to a Mediterranean diet, HbA1c levels were markedly reduced (Esposito et al., 2014). Low glycemic index (GI) diets, conventionally recommended for diabetes patients, have been shown to significantly lower 24 h glucose incremental area under the curve data for both diabetes patients and healthy adults, as compared with high GI diets (Camps et al., 2017; Henry et al., 2017). However, these diets have exhibited different effects on glucose regulation in obese children and adolescents. The Mediterranean diet has been shown to decrease glucose levels and serum lipid profiles (Velázquez-López et al., 2014), whereas low GI diets have shown no effect on FPG levels and serum lipid profiles in obese children (Visuthranukul et al., 2015). In addition, a dietary approaches to stop hypertension diet did not change glycemic viability in type 1 diabetes adolescents (Peairs et al., 2017), and a Mexican diet did not affect FPG compared to a US diet in healthy adults (Santiago-Torres et al., 2016). Culturally modified diet patterns, such as the Okinawan-based Nordic diet, have exhibited potential for reducing FPG and HbA1c levels in adults with T2D (Darwiche et al., 2016). Meanwhile, a high-red meat diet and a high-dairy diet did not induce differences in fasting glucose level in obese adults (Turner et al., 2015). Finally, plant-based burgers have been shown to induce limited changes to postprandial plasma glucose levels in both healthy adults and adults with T2D (Belinova et al., 2014).
Table 3.
Intervention studies of various food patterns on blood glucose regulation
| Meal types of intervention | Subjects (country) | Duration of intervention | Change of phenotype | Comments | Reference |
|---|---|---|---|---|---|
| Mediterranean diet | T2DM adults | 48 months | HbA1c: decrease | 1,500 kcal/d for women | Esposito et al. (2014) |
| 1,800 kcal/d for men | |||||
| Obese children and adolescents | 4 months | FPG: decrease | Velázquez-López et al. (2014) | ||
| HDL-C: increase | |||||
| TC, TG, and LDL-C: decrease | |||||
| Low GI diet compared to low calorie diet | Obese children | 6 months | FPG: no change | No change within the group | Visuthranukul et al. (2015) |
| TC, TG, HDL-C, and LDL-C: no change | |||||
| Low GI diet compared to high GI diet | Healthy male adults (China) | 1 day per diet (cross-over) | 24 h glucose iAUC: decrease | Camps et al. (2017) and Henry et al. (2017) | |
| Mexican diet compared to US diet | Healthy female adults (Mexico) (partially overweight) | 24 days per diet (cross-over) | FPG: no change | Mexican diet: mixture of native Mesoamerican foods and Hispanic foods | Santiago-Torres et al. (2016) |
| US diet: based on 2003∼2004 NHANES | |||||
| Okinawan-based Nordic diet | T2DM adults (Scandinavia) | 3 months | HDL-C: increase in follow-up | A data after four months of follow-up was available. | Darwiche et al. (2016) |
| FPG and HbA1c: decrease | |||||
| TG, TC, and LDL-C: decrease | |||||
| DASH diet specifically modified for diabetes | T1DM adolescents | 3 days | Glycemic viability: no change | Peairs et al. (2017) | |
| High-red meat diet compared to high-dairy diet | Overweight/obese adults | 4 weeks per diet (cross-over) | FPG: no change | Turner et al. (2015) | |
| Plant-based burger compared to processed meat burger | T2DM and healthy adults | 1 day(several times) | Postprandial plasma glucose: increased | Increased only one time point | Belinova et al. (2014) |
GI, glycemic index; T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus; HbA1c, hemoglobin A1c; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; iAUC, incremental area under the curve; DASH, dietary approaches to stop hypertension; NHANES, National Health and Nutrition Examination Survey.
Taken together, calorie restriction and macronutrient ratio adjustments, such as low carbohydrate diets, have been shown to be effective for reducing fasting glucose and HbA1c levels in diabetic patients. However, these effects are limited in both obese and healthy adults. Specifically, HbA1c and fasting glucose levels are altered in obese and healthy subjects following short-term interventions, yet similar results are not shown following long-term interventions. Thus, it appears that clinical biomarkers for glucose regulation may need to be re-evaluated for interventions involving non-diabetic subjects.
Clinical biomarkers for improved glucose regulation
A total of 17 studies were reviewed to evaluate currently available clinical markers for improved glucose regulation. In these studies, diagnostic criteria for diabetes were mainly based on criteria published by the World Health Organization or by the American Diabetes Association. The former includes FPG ≥7.0 mmol/L (126 mg/dL) or a 2-hr plasma glucose ≥11.1 mmol/L (200 mg/dL) (WHO, 2006), and the latter includes FPG ≥126 mg/dL (7.0 mmol/L), an HbA1c ≥6.5%, a 2-hr plasma glucose level ≥200 mg/dL, or a random plasma glucose ≥200 mg/dL (11.1 mmol/L) with symptoms of hyperglycemia or hyperglycemic crisis (American Diabetes Association, 2013).
Clinical markers considered in this review are associated with diabetes-related phenotypes, including IR. In addition, conventionally used anthropometric markers were considered, such as height, weight, waist circumference, hip circumference, waist-hip circumference ratio, body mass index (BMI), and blood pressure. Clinical markers of serum parameters [e.g., fasting blood glucose, fasting insulin, homeostatic model assessment (HOMA)-IR, HDL-C, LDL-C, TC, and TG] were used in most of the human intervention studies that assessed glucose regulation. In addition, triglyceride glucose (TyG) indexes and insulinogenic indexes (IGI) were reported. TyG indexes are accepted as a surrogate marker for IR (Roth et al., 2017), whereas IGI represent insulin levels in response to changes in serum glucose (Singh and Saxena, 2010).
In diabetic adults, a more conserved set of clinical biomarkers have been used, including BMI as an anthropometric marker, and insulin, glucose, cholesterol, and HOMA as biochemical markers (Ren et al., 2016) (Table 4). In addition, IGI and insulin sensitivity indexes were used (Chung et al., 2012). Novel markers, including activin A, activin B, and follistatin, have also been reported as useful indicators for the severity of T2D and IR (Wu et al., 2012). Activin A is a member of the transforming growth factor β superfamily and enhanced activin A activity has been associated with T2D (Ueland et al., 2012). Follistatin is a glycosylated plasma protein that plays a role in IR (Hansen et al., 2013). Both glycoprotein acetylation and leptin have been associated with risk and incidence of T2D (Welsh et al., 2009; Connelly et al., 2016).
Table 4.
Clinical markers to access improvement of glucose regulation in diabetic adults
| Subjects (country) | Investigated markers | Major outcome markers | Diagnosis standard | Reference |
|---|---|---|---|---|
| T2DM (China) | Anthropometric markers | - TG/HDL-C ratio | World Health Organization | Ren et al. (2016) |
| Height, weight, WC, HC, WHR, BMI, and BP | - High TG/HDL-C ratio: ALT, HbA1c, HOMA-IR, HOMA-β , HDL-C, LDL-C, TC, TG, FPG, FINS, IGI, ISI increase | |||
| Clinical markers | ||||
| ALT, HbA1c, HOMA-IR, HOMA-β , HDL-C, LDL-C, TC, TG, FPG, 2hPG, FINS, 2hPINS, IGI, and ISI | - High TG/HDL-C ratio: HDL-C decrease | |||
| T2DM (Korea) | Anthropometric markers | - BMI | American Diabetes Association | Chung et al. (2012) |
| BMI and BP | - High BMI: TG, FPI, fasting insulin, 2-h insulin, HOMA-IR, HOMA-β , and IGI: increase | |||
| Clinical markers | ||||
| HbA1c, HOMA-IR, HOMA-β , HDL-C, TC, TG, fasting glucose, fasting insulin, 2-h glucose, 2-h insulin, DI, IGI, and ISIcomp | - High BMI: DI decrease | |||
| T2DM (Australia) | Anthropometric markers | - Crcl, HDL-C, LDL-C, TC, TG, hsCRP, fasting glucose, HbA1c, fasting insulin, and HOMA-IR | American Diabetes Association and World Health Organization | Wu et al. (2012) |
| WHR and BMI | ||||
| Clinical markers | ||||
| HbA1c, HOMA-IR, fasting glucose, fasting insulin, HDL-C, LDL-C, TC, TG, Crcl, hsCRP, activin A, activin B, and follistatin | - Markers of the severity of T2D and insulin resistance: activin A, activin B, or follistatin | |||
| Diabetes (Netherlands) | Clinical markers | - High GlycA and low hsCRP: incidence of T2D | - One or more of the following: 1) FPG ≥7.0 mmol/L (126 mg/dL) 2) Random sample FPG ≥11.1 mmol/L (200 mg/dL) 3) Self-report of a physician diagnosis of T2DM 4) Initiation of glucose-lowering medication use, retrieved from a central pharmacy registry |
Connelly et al. (2016) |
| GlycA and hsCRP | ||||
| Diabetes (Scotland, Ireland, or Netherlands) | Clinical markers | - Leptin | - Based on baseline glucose measurements (>95% fasting) | Welsh et al. (2009) |
| Leptin |
T2DM, type 2 diabetes mellitus; WC, waist circumference; HC, hip circumference; WHR, waist-hip ratio; BMI, body mass index; BP, blood pressure; ALT, alanine transaminase; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-β, homeostatic model assessment of β-cell function; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; FPG, fasting plasma glucose; 2hPG, 2-hour postprandial plasma glucose; FINS, fasting serum insulin; 2hPINS, 2-hour postprandial serum insulin; IGI, insulinogenic index; ISI, insulin sensitivity index; DI, disposition index; ISIcomp, insulin sensitivity index composite; Crcl, creatinine clearance; hsCRP, high sensitivity C-reactive protein; GlycA, glycoprotein acetylation.
Clinical indicators associated with diabetes-related phenotypes in nondiabetic and diabetic subjects was summarized in Table 5. Of the various anthropometric markers, BMI followed by waist circumference is the most widely used for both nondiabetic and diabetic subjects. In addition to conventional clinical markers (e.g., glucose, insulin, HDL-C, LDL-C, TC, TG, and HOMA index), alanine transaminase (ALT), and gamma-glutamyl transferase (GGT) have also been used as biomarkers to assess diabetes risk. Higher levels of both ALT and GGT are detected in diabetic compared with non-diabetic individuals (Nguyen et al., 2011; Kurosaki et al., 2014). In another study, levels of HbA1c, TC, LDL-C, high sensitivity C-reactive protein (hsCRP), HOMA-IR, interleukin (IL)-6, tumor necrosis factor (TNF), and IL-12 were higher in diabetic compared with non-diabetic individuals (Mishra et al., 2011). However, in another study, levels of adipsin and IL-1β were lower and higher, respectively, in diabetic compared with non-diabetic individuals (Zhou et al., 2018). Adipsin is one of the major proteins in adipose cells and is useful for identifying diabetic patients at high risk of beta cell failure. In contrast with GIs, such as HbA1c levels, which are well controlled, levels of adipsin have been proposed to provide more sensitive monitoring during the introduction of insulin therapy (Lo et al., 2014). Higher levels of ferritin and IL-2 receptor alpha have been observed in individuals with diabetes, whereas higher levels of adiponectin have been observed in individuals without diabetes (Lyssenko et al., 2012). A positive correlation between plasma ferritin and fasting insulin and glucose levels have also been reported (Fumeron et al., 2006), with elevated ferritin concentrations associated with increased risk of diabetes. Thus, serum ferritin is considered to be an independent predictor of diabetes (Forouhi et al., 2007).
Table 5.
Clinical markers to access improvement of glucose regulation in nondiabetic and diabetic adults
| Subjects (country) | Investigated markers | Major outcome markers | Diagnosis standard | Reference |
|---|---|---|---|---|
| Normoglycemic, prediabetic, and diabetic adults (USA) | Anthropometric markers | - Insulin, LDL-C, TG, and HOMA-IR: high (in diabetic) | American Diabetes Association | Nguyen et al. (2011) |
| BMI, BP, and MAP | ||||
| Clinical markers | - ALT and GGT: high (in diabetic) | |||
| HDL-C, LDL-C, TG, glucose, insulin, HOMA-IR, ALT, and GGT | ||||
| Nondiabetic and diabetic subjects (Japan) | Anthropometric markers | - Fasting glucose, HbA1c, fasting insulin, HOMA-IR, TG, RLP-C, and VLDL/TG: high (in diabetic) | FPG >7.0 mmol/L and/or HbA1c >6.5% | Kurosaki et al. (2014) |
| BMI | ||||
| Clinical markers | ||||
| HbA1c, HOMA-IR, fasting insulin, fasting glucose, HDL-C, LDL-C, RLP-C, TC, TG, pre heparin LPL mass, and VLDL/TG | ||||
| Healthy and diabetic subjects (India) | Anthropometric markers | - HbA1c, FBS, PPBS, TC, LDL-C, hsCRP, HOMA-IR, IL-6, TNF, and IL-12: high (in diabetic) | American Diabetes Association | Mishra et al. (2011) |
| Height, weight, WC, HC, WHR, BMI, SBP, DBP | ||||
| Clinical markers | ||||
| FBS, PPBS, insulin, HbA1c, HOMA-IR, hsCRP, HDL-C, LDL-C, VLDL-C, TC, TG, NOx, ICAM, VCAM, IL-6, TNF, and IL-12 | - Endothelial dysfunction markers: NOx, VCAM, and ICAM high (in diabetic) | |||
| Healthy and diabetic subjects (China) | Anthropometric markers | - FFA, FPG, 2hPG, FINS, HbA1c, HOMA-IR, HOMA-β , TG, hsCRP, and IL‐1b: high (in diabetic) | World Health Organization | Zhou et al. (2018) |
| Height, weight, WC, HC, WHR, BMI, and BP | ||||
| Clinical markers | ||||
| FFA, FPG, FINS, HbA1c, hsCRP, HOMA-IR, 2hPG, HDL-C, LDL-C, TC, TG, Adipsin, and IL‐1b | - Adipsin: low (in diabetic) | |||
| Healthy and diabetic subjects (Denmark) | Anthropometric markers | - HbA1c, FPG, fasting insulin, and CRP: high (in diabetic) | American Diabetes Association | Lyssenko et al. (2012) |
| Height, weight, WC, HC, BMI, SBP, and DBP | ||||
| Clinical markers | - Adiponectin: low (in diabetic) | |||
| HbA1c, FPG, fasting insulin, adiponectin, CRP, ferritin, and IL2Ra | - Ferritin and IL2Ra: high (in diabetic) |
BMI, body mass index; BP, blood pressure; MAP, mean arterial pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; HOMA-IR, homeostatic model assessment of insulin resistance; ALT, alanine transaminase; GGT, gamma-glutamyl transferase; HbA1c, hemoglobin A1c; RLP-C, remnant-like particle cholesterol; TC, total cholesterol; LPL, lipoprotein lipase; VLDL, very low-density lipoprotein; WC, waist circumference; HC, hip circumference; WHR, waist-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; PPBS, post prandial blood sugar; hsCRP, high sensitivity C-reactive protein; NOx, nicotinamide adenine dinucleotide phosphate hydrate oxidase; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; IL, interluekin; TNF, tumor necrosis factor; FPG, fasting plasma glucose; FINS, fasting serum insulin; FFA, fasting fatty acid; 2hPG, 2-hour postprandial plasma glucose; IL2Ra, interleukin-2 recepter alpha.
We also reviewed clinical markers as indicators for confirming the incidence of diabetes in non-diabetic groups (Table 6). TyG indexes are a valuable surrogate marker for the degree of IR and for predicting diabetes incidence. Correspondingly, TyG levels are low in non-diabetic subjects (Lee et al., 2014b). Red cell distribution width (RDW) is also an easily measured marker and is positively associated with HbA1c. Thus, it has been proposed that RDW potentially represents a biomarker for risk of diabetes (Engström et al., 2014). High levels of biochemical parameters, such as blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR), apolipoprotein (Apo) A1, leukocyte, sCD163, and soluble plasminogen activator urokinase receptor (suPAR), have been associated with risk of diabetes (Haugaard et al., 2012; Møller et al., 2011; Xie et al., 2018), and are therefore useful for predicting diabetes incidence. In contrary, eGFR levels have been negatively associated with risk of diabetes (Xie et al., 2018).
Table 6.
Clinical markers to access improvement of glucose regulation in nondiabetic adults
| Subjects (country) | Investigated markers | Major outcome markers | Diagnosis standard | Reference |
|---|---|---|---|---|
| Nondiabetic (Canada) | Anthropometric markers | - Hematological variables | World Health Organization | Hanley et al. (2009) |
| Height, weight, WC, BMI, and BP | - High Hct, Hgb, RBC, and WBC: HOMA-IR increase: ISogtt, IGI/HOMA-IR, and ISSI-2 decrease | |||
| Clinical markers | ||||
| Hct, Hgb, RBC, WBC, HOMA-IR, fasting glucose, 2-h glucose, fasting insulin, IGI, ISogtt, IGI/HOMA-IR, ISSI-2, normal glucose tolerance, impaired glucose test, and impaired fasting glucose | ||||
| Adults without diabetes (USA) | Anthropometric markers | - BUN: high incidence of diabetes | diabetes medication prescription (including insulinsand oral hypoglycemic agents); or an HbA1c test result >6.4% | Xie et al. (2018) |
| BMI | - eGFR: low incidence of diabetes | |||
| Clinical markers | ||||
| BUN and eGFR | ||||
| Adults without diabetes (China) | Anthropometric markers | - ALT, fasting glucose, creatinine, and TG: decrease (in nondiabetic) | fasting glucose ≥7.0 mmol/L or if subjects were receiving active treatment for T2DM | Wu et al. (2017) |
| BMI, SBP, and DBP | ||||
| Clinical markers | - ApoA1: increase (in nondiabetic) | |||
| AST, ALT, creatinine, fasting glucose, ApoA1, HDL-C/ApoA1 ratio, ApoB, HDL-C, LDL-C, TC, and TG | ||||
| Nondiabetic subjects (Korea) | Anthropometric markers | - FPG, fasting insulin, HOMA-IR, TC, and TG: low (in nondiabetic) | American Diabetes Association | Lee et al. (2014b) |
| Height, weight, WC, HC, WHR, BMI, SBP, and DBP | ||||
| Clinical markers | ||||
| FPG, Fasting insulin, HOMA-IR, HOMA-β , HDL-C, LDL-C, TC, TG, and serum creatinine | - TyG index: low (in nondiabetic) | |||
| Nondiabetic subjects (Sweden) | Anthropometric markers | - High RDW: insulin, glucose, TG, and HOMA index decrease | FPG ≥7.0 mmol/L | Engström et al. (2014) |
| Height, weight, WC, and BMI | ||||
| Clinical markers | ||||
| Insulin, HOMA index, TG, RDW, MCV, RBC, and leukocyte count | - Low RDW: incidence of diabetes | |||
| Healthy adults (Denmark) | Anthropometric markers | - High sCD163 (in diabetes): predict increased risk of diabetes | World Health Organization | Møller et al. (2011) |
| BMI, SBP, DBP, WHR | - High RDW: Insulin, glucose, TG, and HOMA index decrease | |||
| Clinical markers | ||||
| Glucose, hsCRP, fibrinogen, α1-antitrypsin, orosomucoid, TC, HDL-C, LDL-C, ApoA1, ApoB, TG, and sCD163 | - High sCD163: HDL-C, ApoA1, and TG decrease | |||
| Healthy adults (Denmark) | Anthropometric markers | - Leucocytes, suPAR, and CRP: low (in nondiabetic) | International Classification of Disease or FPG ≥6.9 mmol/L or use of antidiabetic drugs | Haugaard et al. (2012) |
| WC, WHR, BMI, SBP, and DBP | ||||
| Clinical markers | - Plasma glucose, serum insulin, HOMA-IR, and TG: low (in nondiabetic) | |||
| Plasma glucose, serum insulin, HOMA-IR, suPAR, CRP, leukocytes, HDL-C, LDL-C, TC, and TG |
WC, waist circumference; BMI, body mass index; BP, blood pressure; Hct, hematocrit; Hgb, hemoglobin; RBC, red blood cell; WBC, white blood cell; HOMA-IR, homeostatic model assessment of insulin resistance; IGI, insulinogenic index; ISogtt, insulin sensitivity index for oral glucose tolerance tests; ISSI-2, insulin secretion sensitivity index-2; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; SBP, systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate aminotransferase; ALT, alanine transaminase; Apo, apolipoprotein; HC, hip circumference; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; T2DM, type 2 diabetes mellitus; WHR, waist-hip ratio; FPG, fasting plasma glucose; HOMA-β, homeostatic model assessment of β-cell function; TyG index, triglyceride and glucose index; MCV, mean corpuscular volume; RDW, red cell distribution width; suPAR, soluble urokinase-type plasminogen activator receptor.
In this review, clinical markers of T2D and interventions for preventing diabetes in non-diabetic groups were investigated. Currently, clinical markers of diabetes include well-known indicators of anthropometric measurements, blood samples, and indices. Of these, activin A, activin B, and follistatin are useful for determining the severity of diabetes. Hematological and biochemical markers used to identify the onset of diabetes in non-diabetic (healthy subjects) include RDW, hemoglobin (Hgb), hematocrit (Hct), BUN, eGFR, Apo A1, Apo B, sCD163, and suPAR. Finally, fasting glucose and HbA1c levels have been confirmed as appropriate markers for identifying development of diabetes in non-diabetic subjects.
DISCUSSION
Recent evidence from meal-based interventions supports the following results: 1) very low-calorie diets greatly improve glucose regulation in obese and adults with T2D, whereas low-calorie diets have no effect on obese adults; 2) calorie restriction and the ratio of macronutrients, such as low carbohydrate ratios, are effective for reducing fasting blood glucose and HbA1c levels in diabetes patients, although the effects are limited in obese and healthy adults; 3) glucose-related markers change in obese and healthy subjects following short-term interventions, yet these effects are not observed following long-term interventions; 4) Mediterranean and low GI diets are effective for lowering fasting blood glucose and/or HbA1c levels in healthy and T2D adults.
For meal-based interventions, it is necessary to consider the ratios used to define high or low carbohydrate and fat macronutrient compositions. An additional and important consideration for interpreting meal-based intervention data is the cultural aspect of the food. Variations in food patterns and cooking methods in different cultures may affect the observed results (Knowler et al., 2002). For example, conventional macronutrient ratios vary between Asian and Western cuisines. In Korea, meals with a relatively high carbohydrate content are common, and the Korean Dietary Reference Intake recommends that 55∼65% of total energy should be derived from carbohydrates and 15∼30% should be derived from fat (Korean Nutrition Society, 2015). However, North American meals include relatively high proportions of protein and fat, and the US Dietary Reference Intake recommends that 45∼65% of total energy should come from carbohydrates and 20∼35% of should come from fat (USDA, 2015). Therefore, results from meal-based interventions for risk groups should be carefully interpreted.
Of note, the observed effects of meal-based interventions on glucose regulation differed between diabetic patients and healthy or nondiabetic obese subjects. Specifically, HbA1c and fasting glucose levels were often only affected by short-term meal-based interventions in healthy or nondiabetic obese subjects. This limited effect may be due to sensitivities of the markers used. Because clinical markers for glucose regulation mostly target T2D, more sensitive and appropriate markers should be identified to evaluate prevention in healthy subjects.
FPG and HbA1c indicators are mainly used to identify changes in glycemic control in response to meal-based interventions. These indicators are used as diagnostic criteria for diabetes (American Diabetes Association, 2013) and as representative indicators of changes to blood sugar control. BMI is an anthropometric marker and a mandatory indicator of T2D. In particular, BMI ≥25 kg/m2 indicates an increased risk of T2D (Hsu et al., 2015). In general, fasting insulin, HOMA-IR, and HOMA of β-cell function are recognized as major clinical markers of diabetes, with high levels of these indicators present in diabetes patients (Haffner et al., 1996; Tangvarasittichai et al., 2010; Wang et al., 2011). In addition, lipid profiles of TC, TG, LDL-C, and HDL-C are used as basic markers for diabetes. For example, individuals with diabetes exhibit higher levels of triglycerides, LDL-C, and cholesterol, and lower levels of HDL-C, compared with healthy controls (Tangvarasittichai et al., 2010; Uttra et al., 2011); these markers are all significant indicators of diabetes. Other clinical indicators, such as white blood cell, red blood cell, Hgb, Hct, Apo A1, Apo B, sCD163, and suPAR, also exhibit associations with diabetic risk (Hanley et al., 2009; Møller et al., 2011; Haugaard et al., 2012; Wu et al., 2017).
Several markers are regarded as indicators for diagnosing the severity of diabetes and for predicting the incidence of diabetes. For example, activin A, activin B, and follistatin have been reported to be useful markers to assess the severity of T2D (Wu et al., 2012). In addition, TyG indexes have been reported as a novel marker for IR in healthy adults. As such, TyG has been proposed to serve as a surrogate marker for predicting further risk of diabetes (Lee et al., 2014b). It is important to appropriately select clinical indicators that can predict the onset and estimate the severity of diabetes. Recently, nontargeted metabolite profiling (Hanhineva et al., 2015) and continuous 24 h glucose measurements have been used to diagnose risk of diabetes in non-diabetic healthy subjects (Philippou et al., 2008). Such methods profile biological changes, rather than serving as biomarkers, to identify development of diabetes. Despite such limitations, these methods can monitor overall changes and provide strong data regarding dietary intake (Nansel et al., 2016). Thus, these methods are candidates for identifying onset of diabetes in non-diabetic subjects.
The effects of meal-based dietary interventions on glucose regulation can differ according to the characteristics of the subjects and factors such as the type of the intervention (e.g., meal type and duration). For example, FPG levels were not changed following a 6-month low-calorie diet intervention in obese adult women, but were decreased following a 4-weeks very low-calorie diets in obese adults (Norén et al., 2014; Perichart-Perera et al., 2014). However, general biomarkers for glucose regulation, such as HbA1c and HOMA-IR, are higher in diabetic individuals compared with healthy controls in most studies (Mishra et al., 2011; Kurosaki et al., 2014; Zhou et al., 2018). Therefore, more thorough investigation of appropriate clinical biomarkers is required.
This review has several limitations. First, it was not designed as a systematic review so did not produce comprehensive evidence. Second, studies examining meal-based interventions were based on articles published within the last five years to provide recent examples, whereas studies examining clinical biomarkers were screened from an extended period of time, i.e. the last decade. These screening cut offs may constrain the wider implications of this review. In future, a systematic and comprehensive review may be needed.
Overall, this review presents recent evidence from the effects of meal-based dietary interventions on glucose homeostasis in diabetic risk groups, and clinical biomarkers used to assess diabetic risk, and to predict the onset and severity of diabetes. It is anticipated that the insights presented in this review will guide further investigations of meal-based preventive approaches in both non-diabetic and prediabetic populations.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Alkhatib A, Tsang C, Tiss A, Bahorun T, Arefanian H, Barake R, et al. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients. 2017;9:E1310. doi: 10.3390/nu9121310. doi: 10.3390/nu9121310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36:S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkau B, Shipley M, Jarrett RJ, Pyörälä K, Pyörälä M, Forhan A, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21:360–367. doi: 10.2337/diacare.21.3.360. [DOI] [PubMed] [Google Scholar]
- Belinova L, Kahleova H, Malinska H, Topolcan O, Vrzalova J, Oliyarnyk O, et al. Differential acute postprandial effects of processed meat and isocaloric vegan meals on the gastrointestinal hormone response in subjects suffering from type 2 diabetes and healthy controls: a randomized crossover study. PLoS One. 2014;9:e107561. doi: 10.1371/journal.pone.0107561. doi: 10.1371/journal.pone.0107561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Avraham S, Harman-Boehm I, Schwarzfuchs D, Shai I. Dietary strategies for patients with type 2 diabetes in the era of multi-approaches; review and results from the Dietary Intervention Randomized Controlled Trial (DIRECT) Diabetes Res Clin Pract. 2009;86:S41–S48. doi: 10.1016/S0168-8227(09)70008-7. [DOI] [PubMed] [Google Scholar]
- Camps SG, Kaur B, Quek RYC, Henry CJ. Does the ingestion of a 24 hour low glycaemic index Asian mixed meal diet improve glycaemic response and promote fat oxidation? A controlled, randomized cross-over study. Nutr J. 2017;16:43. doi: 10.1186/s12937-017-0258-1. doi: 10.1186/s12937-017-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JO, Cho DH, Chung DJ, Chung MY. Associations among body mass index, insulin resistance, and pancreatic β-cell function in Korean patients with new-onset type 2 diabetes. Korean J Intern Med. 2012;27:66–71. doi: 10.3904/kjim.2012.27.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly MA, Gruppen EG, Wolak-Dinsmore J, Matyus SP, Riphagen IJ, Shalaurova I, et al. GlycA, a marker of acute phase glycoproteins, and the risk of incident type 2 diabetes mellitus: PREVEND study. Clin Chim Acta. 2016;452:10–17. doi: 10.1016/j.cca.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- Darwiche G, Höglund P, Roth B, Larsson E, Sjöberg T, Wohlfart B, et al. An Okinawan-based Nordic diet improves anthropometry, metabolic control, and health-related quality of life in Scandinavian patients with type 2 diabetes: a pilot trial. Food Nutr Res. 2016;60:32594. doi: 10.3402/fnr.v60.32594. doi: 10.3402/fnr.v60.32594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström G, Smith JG, Persson M, Nilsson PM, Melander O, Hedblad B. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med. 2014;276:174–183. doi: 10.1111/joim.12188. [DOI] [PubMed] [Google Scholar]
- Esposito K, Maiorino MI, Petrizzo M, Bellastella G, Giugliano D. The effects of a Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: follow-up of a randomized trial. Diabetes Care. 2014;37:1824–1830. doi: 10.2337/dc13-2899. [DOI] [PubMed] [Google Scholar]
- Forouhi NG, Harding AH, Allison M, Sandhu MS, Welch A, Luben R, et al. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia. 2007;50:949–956. doi: 10.1007/s00125-007-0604-5. [DOI] [PubMed] [Google Scholar]
- Fumeron F, Péan F, Driss F, Balkau B, Tichet J, Marre M, et al. Ferritin and transferrin are both predictive of the onset of hyperglycemia in men and women over 3 years: the Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) study. Diabetes Care. 2006;29:2090–2094. doi: 10.2337/dc06-0093. [DOI] [PubMed] [Google Scholar]
- Goday A, Bellido D, Sajoux I, Crujeiras AB, Burguera B, García-Luna PP, et al. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr Diabetes. 2016;6:e230. doi: 10.1038/nutd.2016.36. doi: 10.1038/nutd.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138–1141. doi: 10.2337/diacare.19.10.1138. [DOI] [PubMed] [Google Scholar]
- Hanhineva K, Lankinen MA, Pedret A, Schwab U, Kolehmainen M, Paananen J, et al. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J Nutr. 2015;145:7–17. doi: 10.3945/jn.114.196840. [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Retnakaran R, Qi Y, Gerstein HC, Perkins B, Raboud J, et al. Association of hematological parameters with insulin resistance and β-cell dysfunction in nondiabetic subjects. J Clin Endocrinol Metab. 2009;94:3824–3832. doi: 10.1210/jc.2009-0719. [DOI] [PubMed] [Google Scholar]
- Hansen J, Rinnov A, Krogh-Madsen R, Fischer CP, Andreasen AS, Berg RMG, et al. Plasma follistatin is elevated in patients with type 2 diabetes: relationship to hyperglycemia, hyperinsulinemia, and systemic low-grade inflammation. Diabetes Metab Res Rev. 2013;29:463–472. doi: 10.1002/dmrr.2415. [DOI] [PubMed] [Google Scholar]
- Haugaard SB, Andersen O, Hansen TW, Eugen-Olsen J, Linneberg A, Madsbad S, et al. The immune marker soluble urokinase plasminogen activator receptor is associated with new-onset diabetes in non-smoking women and men. Diabet Med. 2012;29:479–487. doi: 10.1111/j.1464-5491.2011.03513.x. [DOI] [PubMed] [Google Scholar]
- Heer M, Egert S. Nutrients other than carbohydrates: their effects on glucose homeostasis in humans. Diabetes Metab Res Rev. 2015;31:14–35. doi: 10.1002/dmrr.2533. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Kaur B, Quek RYC, Camps SG. A low glycaemic index diet incorporating isomaltulose is associated with lower glycaemic response and variability, and promotes fat oxidation in Asians. Nutrients. 2017;9:E473. doi: 10.3390/nu9050473. doi: 10.3390/nu9050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez TL, Van Pelt RE, Anderson MA, Reece MS, Reynolds RM, de la Houssaye BA, et al. Women with gestational diabetes mellitus randomized to a higher-complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study. Diabetes Care. 2016;39:39–42. doi: 10.2337/dc15-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care. 2015;38:150–158. doi: 10.2337/dc14-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- Kempf K, Schloot NC, Gärtner B, Keil R, Schadewaldt P, Martin S. Meal replacement reduces insulin requirement, HbA1c and weight long-term in type 2 diabetes patients with >100 U insulin per day. J Hum Nutr Diet. 2014;27:21–27. doi: 10.1111/jhn.12145. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korean Diabetes Association. [cited 2019 Dec 12];Diabetes Fact Sheet in Korea 2018. 2018 Available from: https://www.diabetes.or.kr/pro/news/admin.php?.category=A&code=admin&number=1546&mode=view.
- Korean Nutrition Society. Korean Dietary Reference Intakes (Ministry of Health & Welfare's research project, 2015 KDRIs) Seoul, Korea: 2015. pp. 62–101. [Google Scholar]
- Kurosaki Y, Tsukushi T, Munekata S, Kanoh Y, Moriya T, Nishinari M, et al. Is there a relation between triglyceride concentrations in very low density lipoprotein and the index of insulin resistance in nondiabetic subjects? J Clin Lab Anal. 2014;28:269–274. doi: 10.1002/jcla.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JY, Chung HY. Study on the correlation between the nutrient intakes and clinical indices of type 2 diabetes patients. Korean J Food Nutr. 2013;26:909–918. doi: 10.9799/ksfan.2013.26.4.909. [DOI] [Google Scholar]
- Lee HO, Yim JE, Kim YS, Choue R. Moderate diet-induced weight loss is associated with improved insulin sensitivity in middle-aged healthy obese Korean women. Nutr Res Pract. 2014a;8:469–475. doi: 10.4162/nrp.2014.8.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One. 2014b;9:e90430. doi: 10.1371/journal.pone.0090430. doi: 10.1371/journal.pone.0090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158:41–53. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Jørgensen T, Gerwien RW, Hansen T, Rowe MW, McKenna MP, et al. Validation of a multi-marker model for the prediction of incident type 2 diabetes mellitus: combined results of the Inter99 and Botnia studies. Diab Vasc Dis Res. 2012;9:59–67. doi: 10.1177/1479164111424762. [DOI] [PubMed] [Google Scholar]
- Mishra M, Kumar H, Bajpai S, Singh RK, Tripathi K. Level of serum IL-12 and its correlation with endothelial dysfunction, insulin resistance, proinflammatory cytokines and lipid profile in newly diagnosed type 2 diabetes. Diabetes Res Clin Pract. 2011;94:255–261. doi: 10.1016/j.diabres.2011.07.037. [DOI] [PubMed] [Google Scholar]
- Møller HJ, Frikke-Schmidt R, Moestrup SK, Nordestgaard BG, Tybjærg-Hansen A. Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin Chem. 2011;57:291–297. doi: 10.1373/clinchem.2010.154724. [DOI] [PubMed] [Google Scholar]
- Nansel TR, Lipsky LM, Liu A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am J Clin Nutr. 2016;104:81–87. doi: 10.3945/ajcn.115.126136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QM, Srinivasan SR, Xu JH, Chen W, Hassig S, Rice J, et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: the Bogalusa Heart Study. Diabetes Care. 2011;34:2603–2607. doi: 10.2337/dc11-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norén E, Forssell H. Very low calorie diet without aspartame in obese subjects: improved metabolic control after 4 weeks treatment. Nutr J. 2014;13:77. doi: 10.1186/1475-2891-13-77. doi: 10.1186/1475-2891-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numao S, Kawano H, Endo N, Yamada Y, Takahashi M, Konishi M, et al. Short-term high-fat diet alters postprandial glucose metabolism and circulating vascular cell adhesion molecule-1 in healthy males. Appl Physiol Nutr Metab. 2016;41:895–902. doi: 10.1139/apnm-2015-0702. [DOI] [PubMed] [Google Scholar]
- Parr EB, Devlin BL, Callahan MJ, Radford BE, Blankenship JM, Dunstan DW, et al. Effects of providing high-fat versus high-carbohydrate meals on daily and postprandial physical activity and glucose patterns: a randomised controlled trial. Nutrients. 2018;10:E557. doi: 10.3390/nu10050557. doi: 10.3390/nu10050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry SA, Smith JR, Corbett TR, Woods RM, Hulston CJ. Short-term, high-fat overfeeding impairs glycaemic control but does not alter gut hormone responses to a mixed meal tolerance test in healthy, normal-weight individuals. Br J Nutr. 2017;117:48–55. doi: 10.1017/S0007114516004475. [DOI] [PubMed] [Google Scholar]
- Peairs AD, Shah AS, Summer S, Hess M, Couch SC. Effects of the dietary approaches to stop hypertension (DASH) diet on glucose variability in youth with type 1 diabetes. Diabetes Manag. 2017;7:383–391. [PMC free article] [PubMed] [Google Scholar]
- Perichart-Perera O, Balas-Nakash M, Muñoz-Manrique C, Legorreta-Legorreta J, Rodríguez-Cano A, Mier-Cabrera J, et al. Structured hypocaloric diet is more effective than behavioral therapy in reducing metabolic syndrome in Mexican postmenopausal women: a randomized controlled trial. Menopause. 2014;21:711–720. doi: 10.1097/GME.0000000000000160. [DOI] [PubMed] [Google Scholar]
- Philippou E, McGowan BM, Brynes AE, Dornhorst A, Leeds AR, Frost GS. The effect of a 12-week low glycaemic index diet on heart disease risk factors and 24 h glycaemic response in healthy middle-aged volunteers at risk of heart disease: a pilot study. Eur J Clin Nutr. 2008;62:145–149. doi: 10.1038/sj.ejcn.1602688. [DOI] [PubMed] [Google Scholar]
- Rajaie S, Azadbakht L, Khazaei M, Sherbafchi M, Esmaillzadeh A. Moderate replacement of carbohydrates by dietary fats affects features of metabolic syndrome: a randomized crossover clinical trial. Nutrition. 2014;30:61–68. doi: 10.1016/j.nut.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Ren X, Chen ZA, Zheng S, Han T, Li Y, Liu W, et al. Association between triglyceride to HDL-c ratio (TG/HDL-C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PLoS One. 2016;11:e0154345. doi: 10.1371/journal.pone.0154345. doi: 10.1371/journal.pone.0154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth CL, Elfers C, Hampe CS. Assessment of disturbed glucose metabolism and surrogate measures of insulin sensitivity in obese children and adolescents. Nutr Diabetes. 2017;7:301. doi: 10.1038/s41387-017-0004-y. doi: 10.1038/s41387-017-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WR, Baka A, Björck I, Delzenne N, Gao D, Griffiths HR, et al. Impact of diet composition on blood glucose regulation. Crit Rev Food Sci Nutr. 2016;56:541–590. doi: 10.1080/10408398.2013.792772. [DOI] [PubMed] [Google Scholar]
- Santiago-Torres M, Kratz M, Lampe JW, Tapsoba Jde D, Breymeyer KL, Levy L, et al. Metabolic responses to a traditional Mexican diet compared with a commonly consumed US diet in women of Mexican descent: a randomized crossover feeding trial. Am J Clin Nutr. 2016;103:366–374. doi: 10.3945/ajcn.115.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J, Kanazawa A, Hatae C, Makita S, Komiya K, Shimizu T, et al. One year follow-up after a randomized controlled trial of a 130 g/day low-carbohydrate diet in patients with type 2 diabetes mellitus and poor glycemic control. PLoS One. 2017;12:e0188892. doi: 10.1371/journal.pone.0188892. doi: 10.1371/journal.pone.0188892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. 2018;33:157–170. doi: 10.1007/s10654-017-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev. 2013;71:790–801. doi: 10.1111/nure.12076. [DOI] [PubMed] [Google Scholar]
- Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stentz FB, Brewer A, Wan J, Garber C, Daniels B, Sands C, et al. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: randomized control trial. BMJ Open Diabetes Res Care. 2016;4:e000258. doi: 10.1136/bmjdrc-2016-000258. doi: 10.1136/bmjdrc-2016-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care. 2016;39:808–815. doi: 10.2337/dc15-1942. [DOI] [PubMed] [Google Scholar]
- Steven S, Taylor R. Restoring normoglycaemia by use of a very low calorie diet in long- and short-duration type 2 diabetes. Diabet Med. 2015;32:1149–1155. doi: 10.1111/dme.12722. [DOI] [PubMed] [Google Scholar]
- Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med. 2010;123:S3–S11. doi: 10.1016/j.amjmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Tangvarasittichai S, Poonsub P, Tangvarasittichai O. Association of serum lipoprotein ratios with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2010;131:641–648. [PubMed] [Google Scholar]
- Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al. A very low-carbohydrate, low-saturated fat diet for type 2 diabetes management: a randomized trial. Diabetes Care. 2014;37:2909–2918. doi: 10.2337/dc14-0845. [DOI] [PubMed] [Google Scholar]
- Tay J, Thompson CH, Luscombe-Marsh ND, Wycherley TP, Noakes M, Buckley JD, et al. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: a 2-year randomized clinical trial. Diabetes Obes Metab. 2018;20:858–871. doi: 10.1111/dom.13164. [DOI] [PubMed] [Google Scholar]
- Thompson SV, Hannon BA, An R, Holscher HD. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017;106:1514–1528. doi: 10.3945/ajcn.117.163246. [DOI] [PubMed] [Google Scholar]
- Turner KM, Keogh JB, Clifton PM. Red meat, dairy, and insulin sensitivity: a randomized crossover intervention study. Am J Clin Nutr. 2015;101:1173–1179. doi: 10.3945/ajcn.114.104976. [DOI] [PubMed] [Google Scholar]
- Ueland T, Aukrust P, Aakhus S, Smith C, Endresen K, Birkeland KI, et al. Activin A and cardiovascular disease in type 2 diabetes mellitus. Diab Vasc Dis Res. 2012;9:234–237. doi: 10.1177/1479164111431171. [DOI] [PubMed] [Google Scholar]
- USDA. 2015-2020 Dietary Guidelines for Americans. U.S. Department of Agriculture Publication; Washington, DC, USA: 2015. p. 97. [Google Scholar]
- Uttra KM, Devrajani BR, Shah SZA, Devrajani T, Das T, Raza S, et al. Lipid profile of patients with diabetes mellitus (a multidisciplinary study) World Appl Sci J. 2011;12:1382–1384. [Google Scholar]
- Velázquez-López L, Santiago-Díaz G, Nava-Hernández J, Muñoz-Torres AV, Medina-Bravo P, Torres-Tamayo M. Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity. BMC Pediatr. 2014;14:175. doi: 10.1186/1471-2431-14-175. doi: 10.1186/1471-2431-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visuthranukul C, Sirimongkol P, Prachansuwan A, Pruksananonda C, Chomtho S. Low-glycemic index diet may improve insulin sensitivity in obese children. Pediatr Res. 2015;78:567–573. doi: 10.1038/pr.2015.142. [DOI] [PubMed] [Google Scholar]
- Wang LL, Wang Q, Hong Y, Ojo O, Jiang Q, Hou YY. The effect of low-carbohydrate diet on glycemic control in patients with type 2 diabetes mellitus. Nutrients. 2018;10:E661. doi: 10.3390/nu10060661. doi: 10.3390/nu10060661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh P, Murray HM, Buckley BM, de Craen AJ, Ford I, Jukema JW, et al. Leptin predicts diabetes but not cardiovascular disease: results from a large prospective study in an elderly population. Diabetes Care. 2009;32:308–310. doi: 10.2337/dc08-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. World Health Organization; Geneva, Switzerland: 2006. pp. 1–50. [Google Scholar]
- WHO. Global report on diabetes. World Health Organization; Geneva, Switzerland: 2016. pp. 20–81. [Google Scholar]
- Wu H, Wu M, Chen Y, Allan CA, Phillips DJ, Hedger MP. Correlation between blood activin levels and clinical parameters of type 2 diabetes. Exp Diabetes Res. 2012;2012:410579. doi: 10.1155/2012/410579. doi: 10.1155/2012/410579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yu Z, Su W, Isquith DA, Neradilek MB, Lu N, et al. Low levels of ApoA1 improve risk prediction of type 2 diabetes mellitus. J Clin Lipidol. 2017;11:362–368. doi: 10.1016/j.jacl.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018;93:741–752. doi: 10.1016/j.kint.2017.08.033. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Uchida J, Izumi H, Tsukamoto Y, Inoue G, Watanabe Y, et al. A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Intern Med. 2014;53:13–19. doi: 10.2169/internalmedicine.53.0861. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Ge Q, Ding Y, Qu H, Wei H, Wu R, et al. Relationship between serum adipsin and the first phase of glucose-stimulated insulin secretion in individuals with different glucose tolerance. J Diabetes Investig. 2018;9:1128–1134. doi: 10.1111/jdi.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn C, McPhee J, Harris N, Williden M, Prendergast K, Schofield G. A 12-week low-carbohydrate, high-fat diet improves metabolic health outcomes over a control diet in a randomised controlled trial with overweight defence force personnel. Appl Physiol Nutr Metab. 2017;42:1158–1164. doi: 10.1139/apnm-2017-0260. [DOI] [PubMed] [Google Scholar]