Abstract
This study examined the bioactive components of Eucalyptus camaldulensis aqueous leaf extracts and their protective effects on liver and renal function in a Plasmodium berghei-induced albino mouse model of malarial infection. The results showed that E. camaldulensis extracts are rich in phytochemicals, including flavonoids, phenols, saponin, terpenes, and tannin. Four days after infection with malaria, elevated parasitemia levels in untreated control mice dropped by 4.57%. Administration of E. camaldulensis extracts at doses of 100, 200, and 300 mg/kg significantly decreased parasitemia levels by 17.39, 61.88, and 60.53%, respectively (all P<0.05), relative to untreated control mice; however, standard antimalarial drugs were more efficacious and reduced parasitemia by 86.73%. Treatment with both E. camaldulensis extracts (100∼300 mg/kg) and standard antimalarial drugs significantly decreased malarial-induced physiological imbalances in liver and renal biomarkers, and serum electrolytes in malaria-infected mice compared with controls (P<0.05). The therapeutic effect of E. camaldulensis was greatest at a dose of 200 and 300 mg/kg. These findings indicate that E. camaldulensis aqueous leaf extracts could protect against malarial-induced aberrations in liver and renal function whilst exhibiting anti-malarial effects, and could explain its use as an antimalarial remedy in traditional medicine.
Keywords: antimalarial potential, Eucalyptus camaldulensis, liver protection, phytochemical compositions, renal protection
INTRODUCTION
Malaria is a disease caused by the parasite, Plasmodium. Malaria is a global health problem, with an estimated two million cases and over five hundred deaths reported each year, a high percentage of which occur in the sub-Saharan Africa (WHO, 2014). Malaria in humans is caused by the Plasmodium species Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, and Plasmodium vivax. A recent study has shown two non-recombining species of Ovale exist (Ovale curtisi and Ovale wallikeri) (Muller et al., 2017), which are non-sympatric in nature (Oguike et al., 2011). Malaria in children in sub-Saharan Africa is primarily caused by P. falciparum (Urdaneta et al., 2001). P. falciparum is also the most abundant Plasmodium species in Africa and is responsible for about 98% of malaria death in Nigeria (WHO, 2014). Malaria in Nigeria accounts for a quarter of all cases in Africa, and has about 30∼50% morbidity and 25% mortality in infants (Okumu et al., 2013). P. falciparum is also the cause of almost all the symptoms of severe malaria (NPC-NMCP, 2012). Anopheles gambiae is the major carrier of malaria, particularly in Nigeria. Anopheles melas is popularly located in the coastal areas (Okumu et al., 2013). Malarial infection is usually associated with production of huge quantities of reactive oxygen species (ROS) by both the host and parasite, therefore inducing oxidative stress in the host system. If left untreated, prolonged infection can therefore result in further systemic complications and even death (Adebayo et al., 2017; Anigboro et al., 2018a).
In order to safe guard people, several drugs have been manufactured that are all effective within certain time frames. However, constant use of these drugs leads in resistance by P. falciparum. Although these drugs are effective against the parasite, most are too expensive for the common man who lives below average. Distribution of the drugs is also another important factor since they cannot be accessed in remote villages. Further, several fake drugs that are harmful to several organs of the body have been produced, increasing the need to focus efforts on the use of medicinal herbs for treatment of malaria (Anigboro et al., 2014; Ancheria et al., 2018). The efficacy of some of these herbs has been shown, however only a few has received attention regarding their individual safety for human organs (Avwioroko et al., 2015; Tonukari et al., 2015).
The family of Eucalyptus (Myrtaceous) plants are source of biologically active compounds, such as steroids, tannins, polyphenolics, glycosides, terpenes, alkaloids, flavonoids, saponins, lignins, vitamin C, fatty acids, phenolics, triterpenoid, flavones, anthocyanin, anthraquinone, coumarins, cardiac glycosides, terpenoids, and polyphenols, including flavonoids, phloroglucinol derivatives, and volatile oils (Mohameda and Ibrahimb, 2007; Kumar and Laxmidhar, 2011; Elaissi et al., 2012; Ghalem and Mohamed, 2014). These compounds have been employed locally for the treatment of ailments, such as wounds, tumors, dyspepsia, and malaria (Yang et al., 2004; Mohameda and Ibrahimb, 2007; Damjanović-Vratnica et al., 2011; Musa et al., 2011; Yuvneet et al., 2013; Boukhatem et al., 2014; Godghate and Sawant, 2014; Mubarak et al., 2015; Anigboro et al., 2018b).
This study was carried out to examine the bioactive components of Eucalyptus camaldulensis aqueous leaf extracts and their protective effects on liver and renal functions in individuals infected with malaria using a Plasmodium berghei-induced albino mouse model of malarial infection.
MATERIALS AND METHODS
Plant collection
Fresh E. camaldulensis leaves were collected from the wide growing habitat in Oviorie-Ovu, Ethiope East Local Government Area, Delta State, Nigeria and were verified by the Botany Department, Faculty of Sciences, Delta State University, Abraka, Delta State, Nigeria. The leaves were air-dried to crisp in a laboratory at 28±2℃ for two weeks until a constant weight. Leaves were then ground to a fine powder using sterilized machine (SBM-2977, M-Tech, Osaka, Japan).
Preparation of the extracts
One hundred grams of powdered leaves was weighed and soaked for 24 h in 400 mL of distilled water in a conical flask. The aqueous extract was then sieved and concentrated at 45℃ to a paste-like solution using rotary evaporator. The extract was further concentrated until dry by using a water bath. The extract was kept in an air-tight container and stored at 4℃ for later use.
Experimental design
Animal acclimatization: Thirty-six adult albino male mice with weight 25 to 30 g were bred and purchased from the animal unit, Faculty of Basic Medical Sciences, Laboratory Animal Facility, Delta State University, Abraka, Delta State, Nigeria. Mice were maintained in normal and standard laboratory conditions of room temperature (24±2℃), a relative humidity (46±6%), a 12 h light/dark cycle, and with adequate ventilation for the duration of the experiment. The animals were first acclimatized for 10∼14 days. During this period, mice were allowed uninhibited access to food and water before they were randomly divided into different groups. Mice received the standard grower’s mash diet (Top Feed, Sapele, Delta State, Nigeria).
All experimental animals received normal feed and water ad libitum during the period of the experiments. The experiments were conducted in compliance with “Guide for Care and Use of Laboratory Animals” of the Department of Biochemistry, Delta State University (approval no.: BCH/REC/2018/006).
Collection and inoculation of experimental animals: Malarial parasites (P. berghei) were obtained from the Nigerian Institute of Medical Research Yaba, Lagos State, Nigeria. Mice were infected with parasites by obtaining parasitized blood from the cut tail tip of an infected (donor) mice. The experimental design was as follows:
Group NC: Control Group (uninfected mice injected with phosphate buffered saline).
Group PC: Untreated malarial infected mice group.
Group C1: Malarial infected mice treated with 100 mg/kg of E. camaldulensis.
Group C2: Malarial infected mice treated with 200 mg/kg of E. camaldulensis.
Group C3: Malarial infected mice treated with 300 mg/kg of E. camaldulensis.
Group STD: Malarial infected mice treated with a standard antimalarial drug (0.68/8.4 mg/kg of Artemether/Lumefantrine).
Sample collection: Mice were sacrificed and blood samples were obtained by ocular puncture using capillary tubes into lithium heparin sample containers. Blood samples were centrifuged at 4,000 rpm for 10 min, and the serum was collected and preserved in a refrigerator at 4℃ for biochemical analysis.
Haematological/biochemical analyses
Whole blood samples were used to determine the degree of parasitemia (malarial parasite load) in infected mice, both before and after treatment. Biochemical analyses were carried out on serum samples collected to estimate the activities/levels of major liver function biomakers, serum electrolytes and renal function biomarkers such as: alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP) activity, total protein (TP), total bilirubin (TB), direct bilirubin (DB), serum sodium, chloride, phosphorous, chloride, urea, uric acid, and serum creatinine levels. Assays were carried out using previously described standard procedures (Reitman and Frankel, 1957; Szasz, 1974; Annino and Giese, 1976; Doumas et al., 1981; Onyema et al., 2006).
Statistical analysis
All data are expressed as mean±standard error of mean (SEM). Statistical comparisons were performed by one-way analysis of variance (ANOVA) followed by Dunnett’s or Tukey’s post-hoc tests using GraphPad Prism software (version 8.0.2, GraphPad Prism Software, San Diego, CA, USA). Difference were considered significant at P<0.05.
RESULTS
Phytochemical constituents
The qualitative and quantitative analysis of the aqueous leaf extracts of E. camaldulensis showed that it is rich in several phytochemicals, including polyphenols, saponins, and flavonoids (Table 1 and 2).
Table 1.
Qualitative analysis of phytochemical constituents of Eucalyptus camaldulensis
| Phytochemical | Eucalyptus camaldulensis (red gum) |
|---|---|
| Saponin | ++ |
| Tannin | +++ |
| Terpenes | +++ |
| Flavonoid | + |
| Phlobatannins | + |
| Alkaloid | − |
| Gycosides | − |
| Resin | +++ |
| Phenol | +++ |
| Micronutrients | − |
| Steroids | − |
| Proteins | − |
| Carbohydrates | ++ |
| Amino acids | − |
| Fixed oil and fats | + |
| Non reducing sugar | +++ |
+, mildly present; ++, highly present; +++, more highly present; −, absent or non-detectable.
Changes in parasitemia cell level
The changes in the amount of parasitemia cells in normal and P. berghei malaria-infected mice at day 0, before infection and after treatment are as showed in Table 3. A noticeable elevation in parasitemia level in control mice was observed at day 4 compared with baseline (0 day). Infected mice who received E. camaldulensis and the standard antimalarial drug had reduced parasitemea levels. Mice in group C2 has a significantly higher parasitemia count compared with the other groups. Administration of the plant extracts at 100 mg/kg, 200 mg/kg, 300 mg/kg, and the standard antimalarial drug remarkably decreased parasitemia levels in these groups.
Table 3.
Antimalarial effect of Eucalyptus camaldulensis aqueous extracts on mice infected with Plasmodium berghei
| Group | Parasitemia day 0 | Parasitemia day 4 | Percent parasitemia (%) on day 4 | Percent decrease in parasitemia (%) on day 4 |
|---|---|---|---|---|
| NC | 0 | 0 | 0 | 0 |
| PC | 17.50±0.49a | 16.70±1.44a | 95.43±8.62a | 4.57 |
| C1 | 11.50±1.02b | 9.50±2.14b | 82.61±22.53b | 17.39 |
| C2 | 12.25±0.76b | 4.67±0.33c | 38.12±7.07c | 61.88 |
| C3 | 11.40±0.98b | 4.50±0.41c | 39.47±9.11c | 60.53 |
| STD | 11.30±1.43b | 1.50±0.11d | 13.27±7.33d | 86.73 |
Values are expressed as mean±SEM (n=6).
Values not sharing a common letter (a-d) differ significantly at P<0.05.
NC, normal control group; PC, untreated P. berghei-infected mice; C1, infected mice treated with E. camaldulensis (100 mg/kg); C2, infected mice treated with E. camaldulensis (200 mg/kg); C3, infected mice treated with E. camaldulensis (300 mg/kg) aqueous; STD, infected mice treated with standard antimalarial drug.
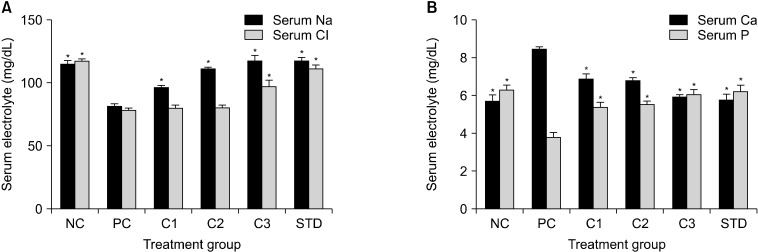
Malaria-induced alterations in serum electrolytes
There was a significant (P<0.05) decrease in serum levels of sodium, chloride, and serum phosphorus in PC compared with those in the NC (Fig. 1A and 1B). Administration of E. camaldulensis (especially at 300 mg/kg) and the standard antimalarial drug, significantly (P<0.05) elevated levels of these electrolytes compared with mice in the PC group. In contrast, serum calcium levels were significantly (P<0.05) increased in the PC group compared with both the NC group and all groups that received E. camaldulensis extracts or antimalarial drugs (Fig. 1B).
Fig. 1.
Effect of Eucalyptus camaldulensis aqueous extracts on levels of (A) serum Na and Cl and (B) serum Ca and P levels in Plasmodium berghei-infected mice. NC, normal control group; PC, untreated P. berghei-infected alone; C1, infected mice treated with E. camaldulensis (100 mg/kg); C2, infected mice treated with E. camaldulensis (200 mg/kg); C3, infected mice treated with E. camaldulensis (300 mg/kg) aqueous; STD, infected mice treated with standard antimalarial drug. *Significantly differed from group PC at P<0.05 using Dunnett’s post-hoc test.
Malaria-induced alterations in liver and renal function biomarkers
Induction of malaria significantly (P<0.05) increased the activities of liver function biomarkers in the PC group compared with the mice in the NC group (Table 4). However, the elevated activities of liver function enzyme biomarkers (serum ALT, AST, ALP, and GGT) significantly (P<0.05) decreased upon administration of different doses of the E. camaldulensis extract (groups C1, C2, and C3) compared with the PC group. A similar trend was also observed in TB and DB levels in mice in groups C1, C2, C3, and STD compared with the PC group (Table 4).
Table 4.
Effect of Eucalyptus camaldulensis aqueous extract treatment on levels of liver function biomarkers in malaria-induced mice
| Group | ALT (U/L) | AST (U/L) | ALP (U/L) | GGT (U/L) | TB (mg/dL) | DB (mg/dL) |
|---|---|---|---|---|---|---|
| NC | 15.30±0.14b | 46.66±0.58b | 40.92±6.04c | 63.00±2.00c | 0.72±0.07b | 0.72±0.14ns |
| PC | 22.80±4.33a | 58.10±0.82a | 60.88±17.9a | 87.50±14.8a | 2.95±2.75a | 1.45±0.91 |
| C1 | 16.95±1.48b | 41.52±7.65c | 59.99±17.5a | 76.50±8.69b | 1.55±1.17a | 0.92±0.15 |
| C2 | 16.62±0.68b | 39.32±6.17c | 49.88±3.43b | 79.00±6.21b | 0.77±0.88b | 0.82±0.45 |
| C3 | 16.83±1.75b | 37.76±10.8c | 45.53±9.56c | 66.33±5.03c | 0.76±1.36b | 0.88±0.56 |
| STD | 10.80±1.00c | 23.65±4.21d | 42.94±4.02c | 69.00±4.96c | 1.07±0.70b | 0.97±0.17 |
Values are expressed as mean±SEM (n=6).
Values not sharing a common letter (a-d) differ significantly at P<0.05.
Not significant.
NC, normal control group; PC, untreated Plasmodium berghei-infected alone; C1, infected mice treated with E. camaldulensis (100 mg/kg); C2, infected mice treated with E. camaldulensis (200 mg/kg); C3, infected mice treated with E. camaldulensis (300 mg/kg) aqueous; STD, infected mice treated with standard antimalarial drug; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatise; GGT, γ-glutamyl transferase; TB, total bilirubin; DB, direct bilirubin.
The kidney function biomarkers investigated (serum urea, uric acid, and creatinine levels) were also markedly increased (P<0.05) in untreated PC compared with the NC, extract-treated (C1, C2, and C3) and STD groups (Table 5).
Table 5.
Effect of Eucalyptus camaldulensis aqueous extract treatment on levels of serum creatinine, urea, and uric acid in malaria-induced mice
| Group | Creatinine (mg/dL) | Urea (mg/dL) | Uric acid (mg/dL) |
|---|---|---|---|
| NC | 1.14±0.39d | 24.32±2.41e | 2.25±1.73d |
| PC | 2.20±0.70a | 50.18±22.1a | 4.90±0.49a |
| C1 | 1.90±0.39a | 40.04±3.15b | 4.30±2.46b |
| C2 | 2.07±0.09a | 36.85±9.19c | 3.85±0.62c |
| C3 | 1.63±0.20b | 30.98±15.3d | 3.36±0.55c |
| STD | 1.52±0.27c | 26.54±8.25e | 2.30±0.29d |
Values are expressed as mean±SEM (n=6).
Values not sharing a common letter (a-e) differ significantly at P<0.05.
NC, normal control group; PC, untreated Plasmodium berghei-infected alone; C1, infected mice treated with E. camaldulensis (100 mg/kg); C2, infected mice treated with E. camaldulensis (200 mg/kg); C3, infected mice treated with E. camaldulensis (300 mg/kg) aqueous; STD, infected mice treated with standard antimalarial drug.
DISCUSSION
In this study, the abundance of various phytochemicals detected qualitatively and quantitatively in E. camaldulensis support its medicinal value. Glycoside, micronutrients, proteins, and amino acids were not detected in the plant extracts, supporting its medicinal efficacy and established pathways of action against several diseases (Iwalewa et al., 2003; Mohammadpour et al., 2013; Orororo et al., 2014; Getahun, 2016). Detection of tannins, alkaloids, and terpenoids strengthens its medicinal value (Iwalewa et al., 2003). Mice infected with malarial parasites and treated with E. camaldulensis extracts showed impeded parasite growth compared with parasite-infected controls.
The average amount of parasitaemia observed in STD group was 13.27%, thus there was little parasite development. In mice treated with E. camaldulensis, the two most effective doses (group C2 and C3) resulted in an average of 38.80% parasitaemia. Although, orthodox antimalarial drugs are most effective, management of malaria is associated with numerous challenges such as drug resistance, being of substandard quality, having a high cost, and not being readily available to the common man, especially those in remote villages; therefore, use of these plant extracts should be encouraged. Since E. camaldulensis extracts (group C2 and C3) suppressed parasitaemia to a mean value of 38.80%, E. camaldulensis could serve as a replacement for conventional antimalarial drugs that lose their effectiveness due to drug resistance.
The severity of malaria infections can be determined by both renal and hepatic malfunction. The clinical manifestation of renal involvement is accompanied by infection with P. falciparum and P. malariae (Naqvi et al., 2003), and may be accountable for an immune complex-mediated glomerular disease resulting in nephrotic syndrome. Other consequences range from urinary sediment abnormalities, mild proteinuria, and electrolyte alterations, to serious renal failure with metabolic acidosis (Padhi and Mishra, 2013). In addition, renal tubular aberrations are related to P. falciparum infection, and complications may include mild to severe tubular necrosis and acute renal failure, followed by frequent oliguria and hypercatabolism (Gomes et al., 2011).
This study demonstrated that E. camaldulensis leaf extracts generally reduced the activities of liver function enzymes (serum ALP, AST, ALT, and GGT) and levels of kidney function biomarkers (serum creatinine, serum urea, and uric acid). Further, these extracts possess blood schizontocidal activity, which is in line with results from suppressive tests (Table 4 and 5). When mice infected with P. berghei were treated with anti-malarial drugs, parasitemia was reduced to almost unnoticeable levels (Rathnapala et al., 2017). We also noticed a remarkable elevation in the levels of serum ALP, ALT, and AST activity in mice infected with P. berghei compared with both the control and treated groups. Similar to the renal function biomarkers (such as serum creatinine, urea, and serum uric acid levels) elevated levels of liver function enzymes, such as serum ALT, AST, ALP, and GGT, are indicative of impaired organ function. In the present study, administration of E. camaldulensis (especially at a dose of 300 mg/kg) and standard antimalarial drugs (Artemether/Lumefantrine) markedly decreased the activities of serum ALP, ALT, and AST compared with mice in the untreated malaria-infected group. This indicates the plant extracts have ameliorative effects on malaria-induced liver function impairment. Creatinine and urea are non-protein nitrogenous wastes that are removed from the body through glomerular filtration. Creatinine is produced internally by creatine kinase in muscle (Onyesom et al. 2015). Measurements of these compounds in serum (creatinine, urea, and uric acid) alone, or alongside some electrolytes, has been described in literature for assessing renal function impairment (Yakubu et al., 2003; Burtis, 2008; Tavafi and Ahmadvand, 2011; Onyesom et al., 2015; Łanocha-Arendarczyk et al., 2018; Nwankpa et al., 2018). In this study, there was a noticeable rise in levels of serum urea, uric acid, and creatinine in mice infected with P. berghei compared with mice in the control and treated groups, probably due to inflammation and interstitial nephritis induced by malarial parasites. The observed modulatory nature of the extracts on the activities of liver enzymes and levels of kidney biomarkers may be due to destruction of the parasites and/or antioxidant properties of the plant extracts, perhaps originating from its active components (phytochemicals) (Gomes et al., 2011; Nutham et al., 2015). It has also been reported that some plants exhibit antiplasmodial activity by thwarting protein synthesis (Rathnapala et al., 2017), perhaps due to the presence of certain phytochemical constituents. Vitamins A, C, and E and flavonoids may act as primary antioxidant or free radical scavengers that fight against oxidative damage induced by the malaria parasite. Hence, E. camaldulensis extracts containing appreciable amounts of these phytochemicals might show antiplasmodial activity via any of these mechanisms.
The human body requires electrolytes for its normal physiological functions (Sitprija, 2008; Rath and Sahu, 2016; Nwankwo et al., 2017). In this study, malaria-induced alterations in levels of serum electrolytes (serum sodium, chloride, calcium, and phosphorus) were ameliorated in mice treated with various concentrations of E. camaldulensis extracts and the standard antimalarial drug compared with mice in the healthy control group (Fig. 1A and 1B). Some symptoms, such as twitching and weakness (fatigue), are sometimes associated with electrolyte imbalance; however, if left unchecked, seizures, and alterations in heart rhythm may follow (Sitprija, 2008). This could be because muscles and neurons depend on the balance and movement of electrolytes through bodily fluids within, outside, or between cells for proper function (Sitprija, 2008; Rath and Sahu, 2016; Nwankwo et al., 2017).
The findings of this research show that aqueous leaf extracts of E. camaldulensis reduce elevated levels of serum creatinine, urea, and uric acid, and decrease elevated levels of liver biomarkers in malaria-induced mice. These findings suggest the potential clinical importance of E. camaldulensis against malaria, and show aqueous leaf extracts may ameliorate or protect against impairment of liver and renal functions, which are sometimes associated with malarial parasite attack in some subjects. Although the extracts also showed ameliorative effects against changes in serum electrolytes levels, its antimalarial effect was most significant at a dose range of 200∼300 mg/kg body weight. Based on these findings, we conclude that subjects who use E. camaldulensis locally for treatment of malaria would benefit from both its antimalarial effects and its ameliorative effect against impairment of liver and kidney functions caused by malarial parasites during critical stages of the infection.
Table 2.
Total phenol and flavonoid contents of Eucalyptus camaldulensis
| Phytoconstituent | Eucalyptus camaldulensis |
|---|---|
| Total phenol (g/100 g DW) | 1.52±0.63 |
| Total flavonoid (μg CE/g) | 67.91±5.01 |
Values are expressed as mean±SEM.
DW, dry weight; CE, catechin equivalent.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Adebayo JO, Adewole KE, Krettli AU. Cysteine-stabilised peptide extract of Morinda lucida (Benth) leaf exhibits antimalarial activity and augments antioxidant defense system in P. berghei-infected mice. J Ethnopharmacol. 2017;207:118–128. doi: 10.1016/j.jep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- Ancheria R, Singh J, Khichi M, Chaturvedi S, Khan S. Herbal approaches for malaria: a review. Asian J Pharm Res Dev. 2018;5:1–10. [Google Scholar]
- Anigboro AA, Avwioroko OJ, Ohwokevwo OA, Nzor JN. Phytochemical constituents, antidiabetic and ameliorative effects of Polyalthia longifiola leaf extract in alloxan-induced diabetic rats. J Appl Sci Environ Manage. 2018a;22:933–998. doi: 10.4314/jasem.v22i6.25. [DOI] [Google Scholar]
- Anigboro AA, Avwioroko OJ, Tonukari NJ. Brillantasia patula aqueous leaf extract averts hyperglycermia, lipid peroxidation, and alterations in hematological parameters in alloxan-induced diabetic rats. Int J Biomed Sci Eng. 2018b;6:43–51. doi: 10.11648/j.ijbse.20180602.14. [DOI] [Google Scholar]
- Anigboro AA, Onakurhefe P, Tonukari NJ, Avwioroko OJ, Egbeme E. Quantitative determination of some phytochemicals (phenol, flavonoid, saponin and alkaloid) in twenty-two Nigerian medicinal plants. Niger J Sci Environ. 2014;13:86–93. [Google Scholar]
- Annino JS, Giese RW. Clinical chemistry. Principles and procedures. 4th ed. Little, Brown and Company; Boston, MA, USA: 1976. p. 412. [Google Scholar]
- Avwioroko OJ, Tonukari NJ, Asagba SO. Biochemical characterization of crude α-amylase of Aspergillus spp. associated with the spoilage of cassava (Manihot esculenta) tubers and processed products in Nigeria. Adv Biochem. 2015;3:15–23. doi: 10.11648/j.ab.20150301.14. [DOI] [Google Scholar]
- Boukhatem MN, Amine FM, Kameli A, Saidi F, Walid K, Mohamed SB. Quality assessment of the essential oil from Eucalyptus globulus Labill of Blida (Algeria) origin. Int Lett Chem Phys Astron. 2014;36:303–315. doi: 10.18052/www.scipress.com/ILCPA.36.303. [DOI] [Google Scholar]
- Burtis CA. Kidney function and disease. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Fundamentals of Clinical Chemistry. 6th ed. Saunders Elsevier; St. Louis, MO, USA: 2008. pp. 360–659. [Google Scholar]
- Damjanović-Vratnica B, Đakov T, Šuković D, Damjanović J. Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill. from Montenegro. Czech J Food Sci. 2011;29:277–284. doi: 10.17221/114/2009-CJFS. [DOI] [Google Scholar]
- Doumas BT, Bayse DD, Carter RJ, Peters T, Schaffer R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem. 1981;27:1642–1650. doi: 10.1093/clinchem/27.10.1642. [DOI] [PubMed] [Google Scholar]
- Elaissi A, Rouis Z, Salem NAB, Mabrouk S, ben Salem Y, Salah KBH, et al. Chemical composition of 8 eucalyptus species' essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement Altern Med. 2012;12:81. doi: 10.1186/1472-6882-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getahun YW. Efficacy of natural oils from eucalyptus species against cochineal insect. Int J Eng Dev Res. 2016;4:738–746. [Google Scholar]
- Ghalem BR, Mohamed B. Antibacterial activity of essential oil of North West Algerian Eucalyptus camaldulensis against Escherichia coli and Staphylococcus aureus. J Coastal Life Med. 2014;2:799–804. [Google Scholar]
- Godghate AG, Sawant RS. Secondary metabolites determinations qualitatively from bark of Butea monosperma and Eucalyptus globulus. Int J Sci Environ Technol. 2014;3:497–501. [Google Scholar]
- Gomes AP, Vitorino RR, Costa A, Mendonça EG, Oliveira MGA, Siqueira BR. Severe Plasmodium falciparum malaria. Rev Bras Ter Intensive. 2011;23:358–369. doi: 10.1590/S0103-507X2011000300015. [DOI] [PubMed] [Google Scholar]
- Iwalewa EO, Iwalewa OJ, Adeboye JO. Analgesic, antipyretic, anti-inflammatory effects of methanol, chloroform and ether extracts of Vernonia cinerea less leaf. J Ethnopharmacol. 2003;86:229–234. doi: 10.1016/S0378-8741(03)00081-3. [DOI] [PubMed] [Google Scholar]
- Kumar HD, Laxmidhar S. A review on phytochemical and pharmacological of Eucalyptus globulus: a multipurpose tree. Int J Res Ayurveda Pharm. 2011;2:1527–1530. [Google Scholar]
- Łanocha-Arendarczyk N, Baranowska-Bosiacka I, Kot K, Pilarczyk B, Tomza-Marciniak A, Kabat-Koperska J, et al. Biochemical profile, liver and kidney selenium (Se) status during acanthamoebiasis in a mouse model. Folia Biol. 2018;66:33–40. doi: 10.3409/fb_66-1.04. [DOI] [Google Scholar]
- Mohameda GA, Ibrahimb SRM. Eucalyptone G, a new phloroglucinol derivative and other constituents from Eucalyptus globulus Labill. Arch Org Chem. 2007;2007:281–291. doi: 10.3998/ark.5550190.0008.f27. [DOI] [Google Scholar]
- Mohammadpour LL, Babak B, Hosseini BSA, Mahmood A, Moghimi BR. Essential oils composition and antibacterial activities of Eucalyptus camaldulensis Dehn. Med Plants-Int J Phytomed Relat Ind. 2013;5:214–218. [Google Scholar]
- Mubarak EE, Ali LZ, Ahmed IFA, Ahmed ABA, Taha RM. Essential oil compositions and cytotoxicity from various organs of Eucalyptus camaldulensis. Int J Agric Biol. 2015;17:320–326. [Google Scholar]
- Muller GC, Junnila A, Traore MM, Traore SF, Doumbia S, Sissoko F, et al. The invasive shrub Prosopis juliflora enhances the malaria parasite transmission capacity of Anopheles mosquitoes: a habitat manipulation experiment. Malar J. 2017;16:237. doi: 10.1186/s12936-017-1878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa DA, Nwodo FOC, Ojogbane E. Phytochemical, antibacterial and toxicity studies of the aqueous extract of Euclayptus camaldulensis Dehnh. Asian J Plant Sci Res. 2011;1:1–10. [Google Scholar]
- Naqvi R, Ahmad E, Akhtar F, Naqvi A, Rizvi A. Outcome in severe acute renal failure associated with malaria. Nephrol Dial Transplant. 2003;18:1820–1823. doi: 10.1093/ndt/gfg260. [DOI] [PubMed] [Google Scholar]
- NPC-NMCP. Nigeria malaria indicator survey 2010: final report. National Population Commission, National Malaria Control Programme and ICF International; Abuja, Nigeria: 2012. pp. 1–123. [Google Scholar]
- Nutham N, Sakulmettatham S, Klongthalay S, Chutoam P, Somsak V. Protective effects of Tinospora crispa stem extract on renal damage and hemolysis during Plasmodium berghei infection in mice. J Pathog. 2015;2015:738608. doi: 10.1155/2015/738608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwankpa P, Ekweogu CN, Egwurugwu JN, Chukwuemeka OG, Etteh CC, Ugwuezumba PC, et al. Assessment of kidney function indices in male albino Wistar rats administered ethanol stem extract of Dennettia tripetala (pepper fruit) Biochem Pharmacol. 2018;7:1000242 [Google Scholar]
- Nwankwo NE, Egbuonu ACC, Nduka FO, Nwodo OFC. Effect of seed extract of Picralima nitida on haematological parameters of malaria-infected albino mice and its interference with the serum electrolyte levels. Ife J Sci. 2017;19:379–388. doi: 10.4314/ijs.v19i2.18. [DOI] [Google Scholar]
- Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, et al. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol. 2011;41:677–683. doi: 10.1016/j.ijpara.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu FO, Mbeyela E, Lingamba G, Moore J, Ntamatungiro AJ, Kavishe DR, et al. Comparative field evaluation of combinations of long-lasting insecticide treated nets and indoor residual spraying, relative to either method alone, for malaria prevention in an area where the main vector is Anopheles arabiensis. Parasit Vectors. 2013;6:46. doi: 10.1186/1756-3305-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–24. [PubMed] [Google Scholar]
- Onyesom I, Onumaechi IF, Ehiwario J, Dagana R. Antiplasmodial activity of Phyllanthus amarus preserves renal function in Plasmodium berghei infected mice. Eur J Med Plants. 2015;5:109–116. doi: 10.9734/EJMP/2015/13230. [DOI] [Google Scholar]
- Orororo OC, Tonukari NJ, Avwioroko OJ, Ezedom T. Effect of supplementation of animal feed with dried cassava (Manihot esculenta) peels, and stems of Vernonia amydalina and Pennisetum purpereum on some biochemical parameters in pigs. Niger Soc Exp Biol. 2014;14:177–183. [Google Scholar]
- Padhi RK, Mishra S. Incidence of renal involvement in malaria in children of Odisha. Int Scholarly Res Not. 2013;2013:573735. doi: 10.5402/2013/573735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath D, Sahu MC. The clinical and biochemical features of complicated falciparum malarial nephropathy. J Taibah Univ Med Sci. 2016;12:110–114. doi: 10.1016/j.jtumed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnapala UL, Goodman CD, McFadden GI. A novel genetic technique in Plasmodium berghei allows liver stage analysis of genes required for mosquito stage development and demonstrates that de novo heme synthesis is essential for liver stage development in the malaria parasite. PLoS Pathog. 2017;13:e1006396. doi: 10.1371/journal.ppat.1006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Sitprija V. Altered fluid, electrolyte and mineral status in tropical disease, with an emphasis on malaria and leptospirosis. Nat Clin Pract Nephrol. 2008;4:91–101. doi: 10.1038/ncpneph0695. [DOI] [PubMed] [Google Scholar]
- Szasz G. New substrates for measuring gamma-glutamyl transpeptidase activity. Z Klin Chem Klin Biochem. 1974;12:228. [PubMed] [Google Scholar]
- Tavafi M, Ahmadvand H. Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell. 2011;43:392–397. doi: 10.1016/j.tice.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Tonukari NJ, Avwioroko OJ, Anigboro AA. Effect of preservation on the chlorophyll content, phytochemicals, and antioxidant capacity of two different varieties of pumpkin (Telfairia occidentalis) leaves. Niger J Technol Res. 2015;10:9–16. doi: 10.4314/njtr.v10i1.3. [DOI] [Google Scholar]
- Urdaneta L, Lal A, Barnabe C, Oury B, Goldman I, Ayala FJ, et al. Evidence for clonal propagation in natural isolates of Plasmodium falciparum from Venezuela. Proc Natl Acad Sci USA. 2001;98:6725–6729. doi: 10.1073/pnas.111144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World malaria report 2010. World Health Organization; Geneva, Switzerland: 2014. pp. 1–204. [Google Scholar]
- Yakubu MT, Bilbis LS, Lawal M, Akanji MA. Evaluation of selected parameters of rat liver and kidney function following repeated administration of yohimbine. Biokemistri. 2003;15:50–56. [Google Scholar]
- Yang YC, Lee HS, Clark JM, Ahn YJ. Insecticidal activity of plant essential oils against Pediculus humanus capitis (Anoplura: Pediculidae) J Med Entomol. 2004;41:699–704. doi: 10.1603/0022-2585-41.4.699. [DOI] [PubMed] [Google Scholar]
- Yuvneet R, Navneet K, Deepa A, Rajandeep K, Hatish P. Phytochemical analysis and antimicrobial activity of methanolic extract of Eucalyptus globules. J Microbiol Biotech Res. 2013;3:77–82. [Google Scholar]