Abstract
Studies assessing the effect of vitamin C and E co-supplementation on levels of circulating C-reactive protein (CRP) show contradictory results. We carried out a systematic review and meta-analysis of randomized controlled trials (RCTs) to assess the effect of vitamin C and E co-supplementation on CRP. A systematic search was carried out using PubMed, Scopus, Ovid, Cochrane, Embase, and the Web of Science without any language or time restriction (until 31 March 2019) to retrieve RCTs that examined the effect of vitamin C and E co-supplementation on CRP. A meta-analysis was carried out using a random effects model, and I2 indexes were used to evaluate the heterogeneity. The search yielded 5,134 publications, including 8 eligible RCTs. The results indicate that vitamin C and E co-supplementation does not significantly impact levels of serum CRP [weighted mean difference and 95% confidence interval with random effects model analysis: −0.22 mg/L (−0.85, 0.41), P=0.5]. Subgroup analysis demonstrated that vitamin C and E co-supplementation significantly reduced serum CRP in participants ≥30 years of age, but significantly increased serum CRP in participants <30 years of age. The results of this meta-analysis indicate beneficial effects of vitamins C and E co-supplementation on CRP in participants ≥30 years of age, and not in younger participants. To confirm these results, further well-designed RCTs are needed.
Keywords: C-reactive protein, meta-analysis, vitamins C, vitamin E
INTRODUCTION
C-reactive protein (CRP) is a member of the pentraxin family of proteins and a common acute-phase reactant (Mantovani et al., 2008). Serum CRP rise in response to pro-inflammatory biomarkers such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β (Du Clos and Mold, 2004; Asbaghi et al., 2019; Amani et al., 2018). Levels of serum CRP rise and fall more rapidly than other inflammatory markers, allowing CRP to be a useful marker for following the clinical status of a disease and treatment response (Du Clos and Mold, 2004). Multiple prospective epidemiological studies have reported that CRP is an important biomarker for predicting the incident of stroke, myocardial infarction, peripheral arterial disease, and sudden cardiac death (Ridker, 2003; Choghakhori et al., 2017). These clinical data combined with experimental and laboratory evidence suggest that atherothrombosis in addition to being a lipid accumulation disorder, is associated with chronic inflammation (Ridker, 2003; Malavazos et al., 2007), and that CRP as an inflammatory marker is a stronger biomarker for predicting cardiovascular disorders than low-density lipoprotein cholesterol (Ridker, 2003; Alipour et al., 2018). The evidence suggests that assessing CRP in addition to evaluating cholesterol might be an inexpensive and useful method to improve prediction of cardiovascular disease risk.
Multiple studies have shown vitamin E and vitamin C both possess antioxidant properties (Thosar et al., 2015; Karademirci et al., 2018). In addition, data suggest these vitamins both have immunomodulatory roles, acting through altering redox-sensitive transcription factors such as peroxisome proliferator-activated receptors and nuclear factor-κB (NF-κB) (Reiter et al., 2007; Carr and Maggini, 2017). Several studies have demonstrated anti-inflammatory effects of vitamin E supplementation alone and in combination with vitamin C (Rizzo et al., 2008). However, results of studies that assessed the effect of vitamin C and E co-supplementation on circulating levels of CRP are contradictory. A study by Ullegaddi et al. (2005) indicated that co-supplementation reduced serum concentrations of CRP within 90 days. However, a study by Arruda et al. (2013) indicated that 1,400 mg vitamin C plus 800 mg vitamin E increased levels of serum CRP in patients with sickle cell anemia. In response to the inconsistent results reported in the literature, we carried out a systematic review and Meta-analysis of randomized clinical trials (RCTs) to assess the effect of vitamin C and E co-supplementation on CRP.
MATERIALS AND METHODS
A systematic review and Meta-analysis was carried out according to the guidelines of the 2009 preferred reporting items for systematic reviews and Meta-analysis (PRISMA) statement (Moher et al., 2009).
Search strategy
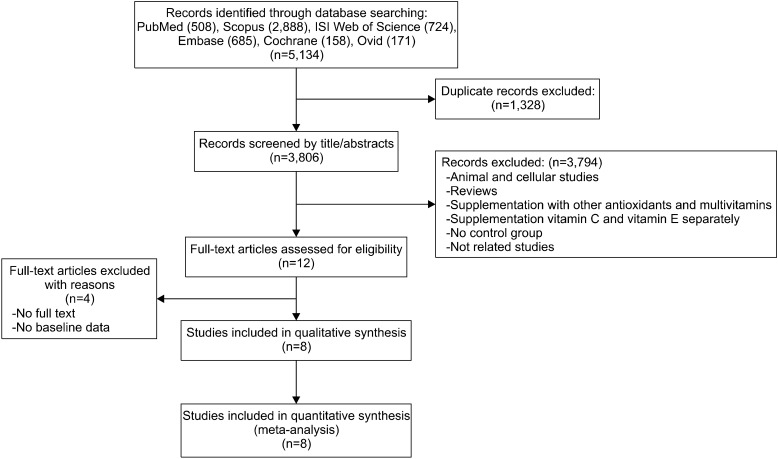
In order to find articles related to the effect of vitamin C and E co-supplementation on CRP, online databases, including PubMed, Scopus, Ovid, Cochrane, Embase, and the Web of Science, were systematically searched without any language and time restriction until 31 March 2019, with the following search terms in titles, abstracts, and keywords [(vitamin C, vit C, ascorbic acid, or ascorbate) and (vitamin E, vit E, alphα-tocopherol, or tocopherol), and (CRP, c-reactive protein, c reactive protein, HSCRP, hs-CRP, high-sensitivity CRP, high sensitivity CRP, inflammation, or inflammatory)]. Further, a manual search and reference lists check of all included studies and related reviews was carried out to identify additional relevant articles. Initially, we identified 5,134 studies, of which 8 unique studies met the inclusion criteria and were selected for inclusion in this Meta-analysis. The process of study selection is shown in the flow diagram (Fig. 1). Reference management was carried out using EndNote X7.5.
Fig. 1.
Flow diagram of the literature search.
Study selection
The inclusion criteria for study selection were as follows: 1) RCT of oral vitamin C and E co-supplementation, 2) trials which reported mean or median values at baseline and at the end of supplementation in both intervention and control groups with reported standard deviation (SD), standard error of the mean, or 95% confidence interval (CI). The exclusion criteria were as follows: 1) studies with no placebo group, 2) case-control, cross-sectional, cohort design, conference papers, and review studies, 3) studies which used a combination of other vitamins and minerals, 4) studies conducted in animal models, 5) studies that were not available, 6) trials in which the study duration was less than 1 week.
Data extraction and quality assessment
After removing duplicates, the titles and abstracts of all studies were independently assessed by two reviewers to identify potentially relevant studies for full text evaluation. Finally, the full texts of selected articles were reviewed to determine whether the article qualified for inclusion. Any disagreement between the two reviewers was resolved by consulting a third author. Using a standardized data collection form, the following data were abstracted: last name of the first author, publication year and country, health status, gender and mean age of the participants, study design, sample size, type of intervention, dosage and duration of the intervention, and main outcome. Any disagreements arising during the data extraction were settled by a third author.
The quality of each included study was assessed using the 5-point scale Jadad score (Jadad et al., 1996). This scale assesses the quality of RCTs and includes random allocation (up to 2 points), double blinding (up to 2 points), and description of withdrawals and dropouts (up to 1 point). Studies with scores of 3 and higher are generally considered to be high quality studies.
Data synthesis and statistical analysis
Changes in the mean and SD of variables between baseline and post intervention were used in the Meta-analysis. In cases when the SD of the change was not reported, SD was calculated using following formula: SD = square root [(SD pre-intervention)2 + (SD post-intervention)2 − (2R ×SD pre-intervention ×SD post-intervention)]. A correlation coefficient of 0.8 was assumed as the R-value in the formula above. For some studies, the reported SE was converted to SD by multiplying standard error with , where n is the sample size of each group. If the variables were reported in median and range (or 95% CI), mean and SD were estimated according to the method by Hozo et al. (2005). When the variables were reported in a graphic form, GetData Graph Digitizer software 2.24 (Fedorov, 2008) was used to digitize and extract the data. A fixed effect model was used to assess the pooled effect size. When heterogeneity was presented, a random effect model was used. We performed subgroup analysis based on gender, health status, age, dose, study quality, study duration, and baseline levels of CRP to detect possible sources of heterogeneity. We used median age (30 years of age) for the subgroup analysis. Therefore, we performed subgroup analysis for participants <30 and ≥30 years of age. Publication bias was assessed using visual assessment of funnel plots, Beg tests and Egger’s regression asymmetry tests. We performed sensitivity analysis (metaninf analysis) by conducting a one-study remove (leave-one-out) approach, to estimate the impact of each trial on the pooled effect size. Between-study heterogeneity was examined using Q tests and I-square (I2) tests (Higgins et al., 2003). All analyses were conducted using STATA ver. 12 (StataCorp., College Station, TX, USA).
RESULTS
Study selection
The initial search yielded 508, 158, 724, 685, 2,888, and 171 citations in PubMed, Cochrane Library, Web of Science, Embase, Scopus, and Ovid papers, respectively. No study was found while searching the reference lists. Of these, 1,328 articles were excluded due to duplication. The titles and abstracts of 3,806 articles were subsequently reviewed and 3,794 studies were excluded due to the following reasons: animal studies, reviews, supplementation with other antioxidants and multivitamins, no placebo group, and unrelated studies. Thereafter, the full texts of 12 studies were assessed for eligibility and 8 were included in this Meta-analysis.
Study characteristics
All 8 included studies (Bruunsgaard et al., 2003; Tahir et al., 2005; Ullegaddi et al., 2005; Bloomer et al., 2007; Peairs and Rankin, 2008; Barker et al., 2011; Arruda et al., 2013; Osman et al., 2016) were double blinded and placebo-controlled trials. The durations of intervention ranged from 7 to 1,151 days. Data are pooled to include 186 participants in the intervention arm and 213 participants in the control arm; participants’ age ranged from 22 to 79 years. Three studies were conducted in the USA (Bloomer et al., 2007; Peairs and Rankin, 2008; Barker et al., 2011), one in Brazil (Arruda et al., 2013), one in Denmark (Bruunsgaard et al., 2003), one in Ireland (Tahir et al., 2005), one in United Kingdom (Ullegaddi et al., 2005) and one in Malaysia (Osman et al., 2016). All the included studies had parallel designs. Five studies include participants of both genders and three studies enrolled only males. Basis on the Jaded scale, 3 studies were of good quality (≥3) but 5 studies had quality scores of less than 3 (Table 1). The vitamin doses also varied between studies. For vitamin C, doses ranged from 500 mg/d to 1,400 mg/d, and for vitamin E doses ranged between 80 mg/d and 900 mg/d. Some studies were conducted on healthy subjects (Bruunsgaard et al., 2003; Peairs and Rankin, 2008; Bloomer et al., 2007) whereas other studies were conducted on patients. The characteristics of the included studies are summarized in Table 1. Five studies did not find a significant effect of vitamin C and E co-supplementation on CRP, two studies found co-supplementation significantly reduced CRP, and one study showed co-supplementation significantly increased CRP (Table 1).
Table 1.
Characteristics of the selected studies included in this meta-analysis
| References | Country | Health status | Study design | Sample size (control/intervention) | Sex | Intervention (name and daily dose) | Trial duration (day) | Biomarker | Age (control/intervention) | Results (intervention group) | Jaded score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arruda et al. (2013) | Brazil | Sickle cell anemia patients | R/DB/PC | 39/44 | F/M | Vit C/1,400 mg | 90 | CRP | 22/27 | Significant increase | 5 |
| Vit E/800 mg | |||||||||||
| Arruda et al. (2013) | Brazil | Sickle cell anemia patients | R/DB/PC | 39/44 | F/M | Vit C/1,400 mg | 180 | CRP | 22/27 | Significant increase | |
| Vit E/800 mg | |||||||||||
| Barker et al. (2011) | USA | Patient with anterior cruciate ligament injury | R/DB/PC | 9/10 | M | Vit C/1,000 mg | 104 | CRP | 36/32 | No significant effect | 2 |
| Vit E/400 IU | |||||||||||
| Bruunsgaard et al. (2003) | Denmark | Healthy | R/DB/PC | 52/50 | M | Vit C/500 mg | 1,151 | CRP | 59/61 | No significant effect | 2 |
| Vit E/200 mg | |||||||||||
| Peairs and Rankin (2008) | USA | Overweight | R/DB/PC | 8/10 | F/M | Vit C/1,000 mg | 7 | CRP | 29.9/31.6 | Significant decrease | 2 |
| Vit E/800 IU | |||||||||||
| Tahir et al. (2005) | Ireland | Patient with aortic valvular stenosis | R/SB | 20/41 | F/M | Vit C/1,000 mg | 180 | CRP | 70/66 | No significant effect | 2 |
| Vit E/400 IU | |||||||||||
| Ullegaddi et al. (2005) | UK | Acute ischaemic | R/SB | 24/24 | F/M | Vit C/500 mg | 7 | CRP | 79/76 | Significant decrease | 3 |
| Stroke patients within 12 h of symptom onset | Vit E/800 IU | ||||||||||
| Ullegaddi et al. (2005) | UK | Acute ischaemic | R/SB | 24/24 | F/M | Vit C/500 mg | 14 | CRP | 79/76 | Significant decrease | 3 |
| Stroke patients within 12 h of symptom onset | Vit E/800 IU | ||||||||||
| Ullegaddi et al. (2005) | UK | Acute ischaemic | R/SB | 24/24 | F/M | Vit C/500 mg | 90 | CRP | 79/76 | Significant decrease | 3 |
| Stroke patients within 12 h of symptom onset | Vit E/800 IU | ||||||||||
| Osman et al. (2016) | Malaysia | Hyper-cholesterolemic patients | R/DB/PC | 19/19 | F/M | Vit C/500 mg | 360 | CRP | 41.3/42 | No significant effect | 2 |
| Vit E/80 mg | |||||||||||
| Bloomer et al. (2007) | USA | Healthy persons (without prior exercise) | R/DB/PC | 8/7 | M | Vit C/1,000 mg | 14 | CRP | 25/23 | No significant effect | 3 |
| Mixed tocopherol/378 mg | |||||||||||
| Mixed tocotrinols/39.5 mg | |||||||||||
| Bloomer et al. (2007) | USA | Healthy persons (without prior exercise) | R/DB/PC | 8/7 | M | Vit C/1,000 mg | 15 | CRP | 25/23 | No significant effect | 3 |
| Mixed tocopherol/378 mg | |||||||||||
| Mixed tocotrinols/39.5 mg | |||||||||||
| Bloomer et al. (2007) | USA | Healthy persons (without prior exercise) | R/DB/PC | 8/7 | M | Vit C/1,000 mg | 16 | CRP | 25/23 | No significant effect | 3 |
| Mixed tocopherol/378 mg | |||||||||||
| Mixed tocotrinols/39.5 mg | |||||||||||
| Bloomer et al. (2007) | USA | Healthy persons (with prior exercise) | R/DB/PC | 7/8 | M | Vit C/1,000 mg | 14 | CRP | 22/22 | No significant effect | 3 |
| Mixed tocopherol/378 mg | |||||||||||
| Mixed tocotrinols/39.5 mg | |||||||||||
| Bloomer et al. (2007) | USA | Healthy persons (with prior exercise) | R/DB/PC | 7/8 | M | Vit C/1,000 mg | 15 | CRP | 22/22 | No significant effect | 3 |
| Mixed tocopherol/378 mg | |||||||||||
| Mixed tocotrinols/39.5 mg | |||||||||||
| Bloomer et al. (2007) | USA | Healthy persons (with prior exercise) | R/DB/PC | 7/8 | M | Vit C/1,000 mg | 16 | CRP | 22/22 | No significant effect | 3 |
| Mixed tocopherol/378 mg | |||||||||||
| Mixed tocotrinols/39.5 mg |
DB, double-blinded; SB, single-blinded; PC, placebo-controlled; R, randomized; F, female; M, male.
Publication bias and sensitivity analyses
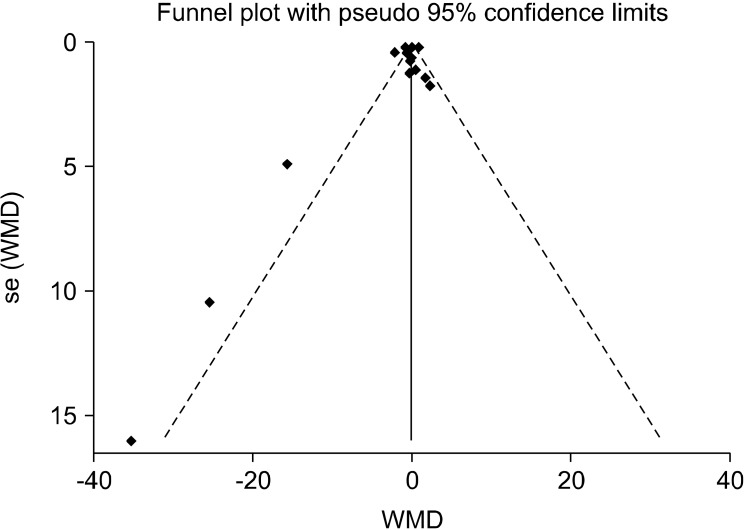
Evaluation of publication bias by funnel plots did not uncover any publication bias within any of the studies (Fig. 2).Further, results from both the Egger’s tests and the Begg’s tests did not indicate any publication bias (P=0.25 and P=0.56, respectively). Metaninf analysis suggested that the elimination of any of the included studies would not alter the final results.
Fig. 2.
Funnel plot of the studies included. WMD, weighted mean difference.
Meta-analysis
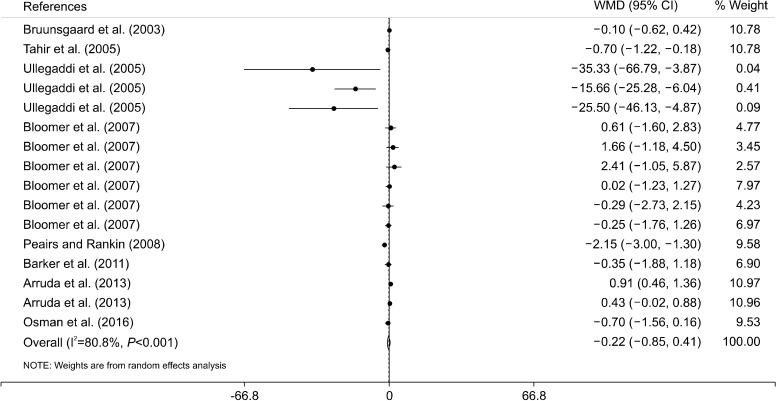
Results of this metα-analysis of 8 clinical trials indicate that vitamin C and E co-supplementation does not significantly impact levels of CRP [weighted mean difference and 95% CI with random effects model analysis: −0.22 mg/L (−0.85, 0.41), P=0.5] (Fig. 3). There was a moderate-to-high level of heterogeneity between the included studies (I2=80.8%, P<0.001). Thus, we conducted subgroup analyses to identify the sources of the heterogeneity. There was no significant effect between subgroups in terms of baseline levels of serum CRP, intervention dose, trial duration, sex, and health status of the participants (Table 2). However, the subgroup analysis based on participant age indicated that vitamin C and E co-supplementation significantly reduced levels of serum CRP in participants aged ≥30 years old of age, and significantly increased levels of serum CRP in participants aged <30 years of age (Table 2). This heterogeneity was not present in male participants aged <30 years of age (Table 2).
Fig. 3.
Forest plot of the random-effects meta-analysis of the effect of vitamin C and E co-supplementation on serum level of C-reactive protein. WMD, weighted mean difference; CI, confidence interval.
Table 2.
Subgroup analyses of the effect of vitamins E and C co-supplementation on serum CRP
| NO | WMD (95% CI) | P within group | P heterogeneity | I2 | |
|---|---|---|---|---|---|
| Baseline CRP (mg/L) | |||||
| ≤3 | 9 | −0.31 (−1.06, 0.44) | 0.42 | <0.001 | 66.1% |
| >3 | 7 | −0.17 (−1.23, 0.88) | 0.74 | <0.001 | 86.3% |
| Trial duration (week) | |||||
| ≤12 | 10 | −0.53 (−2.11, 1.04) | 0.51 | <0.001 | 76.8% |
| >12 | 6 | −0.02 (−0.61, 0.56) | 0.94 | <0.001 | 852% |
| Intervention doses of vitamin C and vitamin E | |||||
| ≥1,000 mg/d vitamin C with ≥700 mg/d vitamin E | 12 | −0.33 (−1.42, 0.76) | 0.55 | <0.001 | 94.1% |
| <1,000 mg/d vitamin C or <700 mg/d vitamin E | 4 | −0.14 (−0.95, 0.66) | 0.73 | <0.001 | 58.4% |
| Sex | |||||
| Male | 8 | −0.02 (−0.43, 0.39) | 0.91 | 0.78 | 0.0% |
| Both sexes | 8 | −0.69 (−1.77, 0.38) | 0.21 | <0.001 | 90.6% |
| Age | |||||
| <30 | 8 | 0.61 (0.31, 0.91) | >0.001 | 0.48 | 0.0% |
| ≥30 | 8 | −1.03 (−1.97, −0.08) | 0.03 | <0.001 | 80.6% |
| Health status | |||||
| Healthy | 8 | −0.17 (−1.09, 0.74) | 0.71 | <0.001 | 70% |
| Patients | 8 | −0.26 (−1.18, 0.66) | 0.56 | <0.001 | 85.5% |
| Jadad score | |||||
| <3 | 5 | −0.79 (−1.48, −0.12) | 0.02 | <0.001 | 75.7% |
| ≥3 | 11 | 0.34 (−0.45, 1.13) | 0.39 | <0.001 | 65.1% |
CRP, C-reactive protein; WMD, weighted mean difference; CI, confidence interval.
DISCUSSION
Results of the present systematic review and metα-analysis demonstrate that vitamin C and E co-supplementation does not significantly impact levels of serum CRP. However, subgroup analysis indicated that co-supplementation differently impacted participants <30 and ≥30 years of age. We showed that vitamin C and E co-supplementation significantly lowered serum CRP in participants ≥30 years of age, but significantly increased serum CRP in participants <30 years of age.
Vitamin E and vitamin C are the most important antioxidants in the human diet. α-Tocopherol is one of the active components of the vitamin E family. During antioxidant activity of vitamin E, α-tocopherol is oxidized to α-tocopheroxyl radicals, which can be regenerated to α-tocopherol by vitamin C (Pryor, 2000). This phenomenon suggests a synergistic function of these vitamins. Previous investigations have reported that vitamin E and C co-supplementation of can increase antioxidant capacity and decrease the amount of lipid peroxidation products (Ullegaddi et al., 2005). A previous study has also reported that the anti-inflammatory effect of antioxidants may also be due to their role in inhibiting oxidative stress (Grimble, 1994). Disturbance to the redox state contribute to immune system activation (Gill et al., 2010), and anti-oxidants such as vitamins C and E prevent up-regulation of cytokines production by oxidants (Grimble, 1994). One of the most well-known inflammatory mediators is CRP. CRP is a protein that binds to C-polysaccharides of the pneumococcal cell wall (Yeh and Willerson, 2003). CRP is part of the innate immune system that can activate the classical complement pathway (Sjöberg et al., 2006), and can bind to damaged cell phospholipids and increase uptake of these cells by macrophages (Du Clos, 2000). Recently, CRP has been shown to possess pro-atherogenic properties. CRP can stimulate endothelial cells to increase secretion of selectins, vascular cell adhesion molecule-1, and monocyte chemotactic protein-1 (Yeh and Willerson, 2003), and can increase expression of pro-inflammatory cytokines such as IL-6 in human endothelial cells (Verma et al., 2002).
However, studies investigating the anti-inflammatory effects of vitamin C and E co-supplementation have published contradictory results. Bruunsgaard et al. (2003) showed that long term co-supplementation does not significantly affect anti-inflammatory responses in healthy men, and does not significantly impact levels of CRP, TNF-α, and IL-6. Further, Osman et al. (2016) showed that supplementation with vitamin E, vitamin C, and vitamin C+E combined in patients with hypercholesterolemia did not significantly impact levels of inflammatory biomarkers such as CRP, TNF-α, and IL-6. However, Ullegaddi et al. (2005) reported that co-supplementation with vitamin C and E lowers CRP after 90 days, whereas, Arruda et al. (2013) reported that co-supplementation with vitamin C and E increases inflammatory markers, such as the neutrophil count and serum CRP. In this metα-analysis, we showed that co-supplementation with vitamin C and E does not significantly affect serum CRP. However, one previous metα-analysis reported that supplementation with vitamin E alone decreases serum CRP (Saboori et al., 2015), and another showed that supplementation with vitamin C alone also decreases serum CRP (Jafarnejad et al., 2018). Previous studies show that serum CRP is associated with age, body fat, and insulin levels (Hughes and Kumari, 2018; Yudkin et al., 1999). Population-based studies have reported a direct correlation between age and serum CRP (Hughes and Kumari, 2018). In addition, body fat can influence levels of serum CRP (Yudkin et al., 1999). IL-6 and TNF-α are secreted by subcutaneous adipose tissues, and are the main stimuli of the CRP synthesis (Du Clos and Mold, 2004). Therefore, serum CRP in healthy populations can be largely influenced by variables that are not subject to modulation by antioxidant supplementation.
Results of our subgroup analysis indicated that co-supplementation with vitamin C and E significantly reduces serum CRP in older participants (≥30 years old). An explanation for this finding may be that oxidative stress increases with age (Judge et al., 2005), thus supplementation with antioxidants such as vitamin C and E reduces inflammatory mediators like calcium pyrophosphate by decreasing oxidative stress. In addition, it has been reported that the prevalence of obesity and being overweight was increase with age (Yamamoto et al., 2011). A relationship between obesity and increased levels of CRP and oxidative stress has also been demonstrated in several studies (Higdon and Frei, 2003). Oxidative stress can activate Toll-like receptors which mediates the inflammatory response and increase secretion of pro-inflammatory cytokines (Gill et al., 2010). Results from our study showed that combined supplementation with vitamin C and E in younger (<30 years old) but not older (≥30 years of age), can increase serum CRP. It has previously been reported that the timing of antioxidant ingestion is an important factor for optimally preventing inflammation (Carroll and Schade, 2003). A study carried out in 2003, demonstrated that co-supplementation with vitamin C and E in the morning may protect against elevations in inflammatory mediators, whereas ingestion of antioxidants immediately before a high-fat meal had no beneficial effects (Carroll and Schade, 2003). Furthermore, it has been reported that vitamin C supplementation increases oxidative stress after eccentric exercise (Childs et al., 2001). Therefore, the reasons for the discrepancy in our results of vitamin C and E co-supplementation between participants of different ages might be due to the differences in the level of oxidative stress at different ages, the timing of antioxidant ingestion, and other possible mechanisms between antioxidants and CRP that are not fully understood. Further studies are required to fully understand the impact of co-supplementation with vitamin C and E.
This systematic review and metα-analysis has several strengths. Firstly, we provide a comprehensive review of vitamin C and E co-supplementation by examining RCTs. Second, this review is the result of an up-to-date literature search of a large number of databases and included eight studies. However, this study has also several limitations. First, our systematic search was not limited to a particular disease, which may have increased heterogeneity. However, we performed subgroup analysis, which decreased heterogeneity in some of the subgroups. Second, although we carried out a comprehensive search, some relevant studies may not have been included. Finally, the strength of the conclusion was limited by the small sample size of the individual studies; however, the results should be helpful to guide future studies.
Our systematic review and metα-analysis indicated that vitamin C and E co-supplementation did not significantly impact levels of serum CRP. However, subgroup analysis indicated that co-supplementation significantly reduced serum CRP in older participants (≥30 years old), and significantly increased serum CRP in participants <30 years of age. Further large-scale RCTs are needed to fully determine the effect of co-supplementation with vitamins C and E on CRP.
Footnotes
AUTHORS’ CONTRIBUTIONS
AA and FF designed the study. FF and OA reviewed and selected the articles. FF and OA extracted needed data from articles. AA performed data analysis and interpretation. AA drafted the manuscript. EF revised the article for important intellectual content.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Alipour M, Malihi R, Hosseini SA, Abbasnezhad A, Ghavami A, Shahmohammadi HA, et al. The effects of catechins on related risk factors with type 2 diabetes: a review. Prog Nutr. 2018;20:12–20. [Google Scholar]
- Amani R, Abbasnezhad A, Hajiani E, Cheraghian B, Abdoli Z, Choghakhori R. Vitamin D3 induced decrease in IL-17 and malondialdehyde, and increase in IL-10 and total antioxidant capacity levels in patients with irritable bowel syndrome. Iran J Immunol. 2018;15:186–196. doi: 10.22034/IJI.2018.39388. [DOI] [PubMed] [Google Scholar]
- Arruda MM, Mecabo G, Rodrigues CA, Matsuda SS, Rabelo IB, Figueiredo MS. Antioxidant vitamins C and E supplementation increases markers of haemolysis in sickle cell anaemia patients: a randomized, double-blind, placebo-controlled trial. Br J Haematol. 2013;160:688–700. doi: 10.1111/bjh.12185. [DOI] [PubMed] [Google Scholar]
- Asbaghi O, Fouladvand F, Gonzalez MJ, Aghamohammadi V, Choghakhori R, Abbasnezhad A. The effect of green tea on C-reactive protein and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Complement Ther Med. 2019;46:210–216. doi: 10.1016/j.ctim.2019.08.019. [DOI] [PubMed] [Google Scholar]
- Barker T, Martins TB, Hill HR, Kjeldsberg CR, Trawick RH, Leonard SW, et al. Vitamins E and C modulate the association between reciprocally regulated cytokines after an anterior cruciate ligament injury and surgery. Am J Phys Med Rehabil. 2011;90:638–647. doi: 10.1097/PHM.0b013e318214e886. [DOI] [PubMed] [Google Scholar]
- Bloomer RJ, Falvo MJ, Schilling BK, Smith WA. Prior exercise and antioxidant supplementation: effect on oxidative stress and muscle injury. J Int Soc Sports Nutr. 2007;4:9. doi: 10.1186/1550-2783-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Poulsen HE, Pedersen BK, Nyyssönen K, Kaikkonen J, Salonen JT. Long-term combined supplementations with α-tocopherol and vitamin C have no detectable anti-inflammatory effects in healthy men. J Nutr. 2003;133:1170–1173. doi: 10.1093/jn/133.4.1170. [DOI] [PubMed] [Google Scholar]
- Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:E1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MF, Schade DS. Timing of antioxidant vitamin ingestion alters postprandial proatherogenic serum markers. Circulation. 2003;108:24–31. doi: 10.1161/01.CIR.0000074221.68903.77. [DOI] [PubMed] [Google Scholar]
- Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/S0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- Choghakhori R, Abbasnezhad A, Amani R, Alipour M. Sex-related differences in clinical symptoms, quality of life, and biochemical factors in irritable bowel syndrome. Dig Dis Sci. 2017;62:1550–1560. doi: 10.1007/s10620-017-4554-6. [DOI] [PubMed] [Google Scholar]
- Du Clos TW, Mold C. C-reactive protein: an activator of innate immunity and a modulator of adaptive immunity. Immunol Res. 2004;30:261–277. doi: 10.1385/IR:30:3:261. [DOI] [PubMed] [Google Scholar]
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- Fedorov S. GetData Graph Digitizer. 2008 Available from: http://www.getdata-graph-digitizer.com/
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimble RF. Nutritional antioxidants and the modulation of inflammation: theory and practice. New Horiz. 1994;2:175–185. [PubMed] [Google Scholar]
- Higdon JV, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler Thromb Vasc Biol. 2003;23:365–367. doi: 10.1161/01.ATV.0000063608.43095.E2. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Kumari M. Age modification of the relationship between C-reactive protein and fatigue: findings from Understanding Society (UKHLS) Psychol Med. 2018;48:1341–1349. doi: 10.1017/S0033291717002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jafarnejad S, Boccardi V, Hosseini B, Taghizadeh M, Hamedifard Z. A meta-analysis of randomized control trials: the impact of vitamin C supplementation on serum CRP and serum hs-CRP concentrations. Curr Pharm Des. 2018;24:3520–3528. doi: 10.2174/1381612824666181017101810. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- Karademirci M, Kutlu R, Kilinc I. Relationship between smoking and total antioxidant status, total oxidant status, oxidative stress index, vit C, vit E. Clin Respir J. 2018;12:2006–2012. doi: 10.1111/crj.12757. [DOI] [PubMed] [Google Scholar]
- Malavazos AE, Corsi MM, Ermetici F, Coman C, Sardanelli F, Rossi A, et al. Proinflammatory cytokines and cardiac abnormalities in uncomplicated obesity: relationship with abdominal fat deposition. Nutr Metab Cardiovasc Dis. 2007;17:294–302. doi: 10.1016/j.numecd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M, Razak A, Rahman T, Muid S, Tengku Ismail T, Ramli A, et al. Effects of adding tocotrienol-tocopherol mixed fraction and vitamin C on inflammatory status in hypercholesterolaemic patients in the low coronary risk category. Biomed Res Ther. 2016;3:557–566. doi: 10.7603/s40730-016-0013-9. [DOI] [Google Scholar]
- Peairs AT, Rankin JW. Inflammatory response to a high-fat, low-carbohydrate weight loss diet: effect of antioxidants. Obesity. 2008;16:1573–1578. doi: 10.1038/oby.2008.252. [DOI] [PubMed] [Google Scholar]
- Pryor WA. Vitamin E and heart disease: basic science to clinical intervention trials. Free Radic Biol Med. 2000;28:141–164. doi: 10.1016/S0891-5849(99)00224-5. [DOI] [PubMed] [Google Scholar]
- Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of α- and γ-tocopherol. Mol Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.CIR.0000053730.47739.3C. [DOI] [PubMed] [Google Scholar]
- Rizzo MR, Abbatecola AM, Barbieri M, Vietri MT, Cioffi M, Grella R, et al. Evidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: impact on insulin action. J Am Coll Nutr. 2008;27:505–511. doi: 10.1080/07315724.2008.10719732. [DOI] [PubMed] [Google Scholar]
- Saboori S, Shab-Bidar S, Speakman JR, Yousefi Rad E, Djafarian K. Effect of vitamin E supplementation on serum C-reactive protein level: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2015;69:867–873. doi: 10.1038/ejcn.2014.296. [DOI] [PubMed] [Google Scholar]
- Sjöberg AP, Trouw LA, McGrath FD, Hack CE, Blom AM. Regulation of complement activation by C-reactive protein: targeting of the inhibitory activity of C4b-binding protein. J Immunol. 2006;176:7612–7620. doi: 10.4049/jimmunol.176.12.7612. [DOI] [PubMed] [Google Scholar]
- Tahir M, Foley B, Pate G, Crean P, Moore D, McCarroll N, et al. Impact of vitamin E and C supplementation on serum adhesion molecules in chronic degenerative aortic stenosis: a randomized controlled trial. Am Heart J. 2005;150:302–306. doi: 10.1016/j.ahj.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Thosar SS, Bielko SL, Wiggins CC, Klaunig JE, Mather KJ, Wallace JP. Antioxidant vitamin C prevents decline in endothelial function during sitting. Med Sci Monit. 2015;21:1015–1021. doi: 10.12659/MSM.893192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullegaddi R, Powers HJ, Gariballa SE. Antioxidant supplementation enhances antioxidant capacity and mitigates oxidative damage following acute ischaemic stroke. Eur J Clin Nutr. 2005;59:1367–1373. doi: 10.1038/sj.ejcn.1602248. [DOI] [PubMed] [Google Scholar]
- Verma S, Li SH, Badiwala MV, Weisel RD, Fedak PW, Li RK, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–1896. doi: 10.1161/01.CIR.0000015126.83143.B4. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Okazaki A, Ohmori S. The relationship between psychosocial stress, age, BMI, CRP, lifestyle, and the metabolic syndrome in apparently healthy subjects. J Physiol Anthropol. 2011;30:15–22. doi: 10.2114/jpa2.30.15. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Willerson JT. Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation. 2003;107:370–371. doi: 10.1161/01.CIR.0000053731.05365.5A. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.ATV.19.4.972. [DOI] [PubMed] [Google Scholar]