Abstract
Campylobacter jejuni, a zoonotic pathogen that frequently colonizes poultry, possesses two Microbial Surface Components Recognizing Adhesive Matrix Molecule(s) (MSCRAMMs) termed CadF and FlpA that bind to the glycoprotein fibronectin (FN). Previous to this study, it was not known whether the CadF and FlpA proteins were functionally redundant or if both were required to potentiate host cell binding and signaling processes. We addressed these questions by generating a complete repertoire of cadF and flpA mutants and complemented isolates, and performing multiple phenotypic assays. Both CadF and FlpA were found to be necessary for the maximal binding of C. jejuni to FN and to host cells. In addition, both CadF and FlpA are required for the delivery of the C. jejuni Cia effector proteins into the cytosol of host target cells, which in turn activates the MAPK signaling pathway (Erk 1/2) that is required for the C. jejuni invasion of host cells. These data demonstrate the non-redundant and bi-functional nature of these two C. jejuni FN-binding proteins. Taken together, the C. jejuni CadF and FlpA adhesins facilitate the binding of C. jejuni to the host cells, permit delivery of effector proteins into the cytosol of a host target cell, and aid in the rewiring of host cell signaling pathways to alter host cell behavior.
Keywords: pathogenesis, bacteria–host cell interactions, MSCRAMM, fibronectin, adhesion, virulence determinants, effector proteins
1. Introduction
Fibronectin (FN)-binding proteins (FNBPs) are members of the Microbial Surface Components Recognizing Adhesive Matrix Molecule(s) (MSCRAMMs) family. These proteins are bacterial surface proteins that mediate the attachment of bacteria to FN and can promote adhesion to cells. Given that FNBPs frequently contribute in establishing an infection, a significant amount of effort has been put forth to identify and characterize these proteins in both Gram-positive and Gram-negative bacteria. For example, Staphylococcus aureus possesses fibronectin-binding protein A (FNBPA), fibronectin-binding protein B (FNBPB), and ClfA [1,2], and Streptococcus pyogenes possesses fibronectin-binding protein I (SfbI), fibronectin-binding protein F (SfbF), and M protein [3]. FNBPs have also been identified in Gram-negative pathogens, such as ShdA in Salmonella enterica serotype Typhimurium [4], YadA in Yersinia spp. [5], BBK32, RevA, and RevB in Borrelia burgdorferi [6,7], and TP0136 in Treponema pallidum [8]. Defining the role of MSCRAMMs in promoting bacteria–host interactions has provided a better understanding of the process of microbial colonization and disease.
Campylobacter jejuni is a Gram-negative, curve-shaped, motile, microaerophilic bacterium. This organism is one of the leading bacterial causes of diarrhea in the United States and accounts for 5–14% of diarrheal cases worldwide [9]. In the United States, there are approximately 1.4–2.3 million Campylobacter infections each year [10]. Most cases of the disease result from the handling and consumption of undercooked poultry products and from foods cross-contaminated with raw poultry products. Campylobacteriosis generally occurs 2–5 days after ingestion. C. jejuni infected individuals suffer from fever, nausea, malaise, abdominal pain, and loose to watery stools, which may contain blood and/or fecal leukocytes [11]. In addition, infection with particular strains of C. jejuni correlates with a higher incidence of Guillain–Barré syndrome (GBS), Miller Fisher syndrome, reactive arthritis, and post-infectious irritable bowel syndrome (PI-IBS) [12,13,14]. C. jejuni is the most common antecedent to GBS. The cost associated with treating acute C. jejuni infections and GBS in the U.S. is estimated to be $1.2 billion per year [14]. Generally, C. jejuni infection occurs when the motile bacterium attaches to, invades, and propagates within host intestinal tissues, which results in blood and mucus in the stool [11,15]. Scientists have identified several C. jejuni adhesins that play a critical role in disease symptoms and progression [16,17]. However, further research is necessary to understand Campylobacter virulence and to develop novel therapeutic and immune augmentation strategies that overcome the shortcomings inherent with traditional antibiotics.
C. jejuni possesses at least two outer membrane-embedded FNBPs, termed CadF for Campylobacter adhesion to fibronectin and FlpA for fibronectin-like protein A (FlpA) [16,17]. The cadF gene, which is 960 bp and located first in a bicistronic operon, encodes a 37 kDa protein [16]. The protein possesses a signal peptide sequence, an outer membrane (OM) channel superfamily domain, and an outer membrane protein A (OmpA)-like domain. The flpA gene, which is 1236 bp and located second in a polycistronic operon containing three genes, encodes a 46 kDa protein [18]. The FlpA protein contains a signal peptide sequence and three FN-type III domains. Recombinant CadF and FlpA proteins have been purified and demonstrated to bind to FN in a dose-dependent and saturable fashion [18,19]. CadF mediates binding to FN via a four amino acid motif (Phe-Arg-Leu-Ser) [20], whereas the FN-binding site within FlpA has been localized to a span of nine amino acids (Trp-Arg-Pro-His-Pro-Asp-Phe-Arg-Val) [19]. As mentioned above, disruption of either cadF or flpA results in C. jejuni mutants impaired in their ability to bind to cultured epithelial cells [16,18]. Moreover, C. jejuni cadF and flpA deletion mutants demonstrate a marked impairment in their ability to colonize chickens [17,21]. Studies have yet to be performed to examine the phenotypic properties (cell adherence and FN-binding) of cadF or flpA expressed singly in a C. jejuni cadF flpA double deletion mutant.
While advances have been made in understanding the biology of C. jejuni, genetic manipulation of this bacterium remains challenging. Perhaps the most significant technical obstacle has been in the functional complementation of specific genes. Possible reasons for the lack of functional complementation include aberrant levels of gene expression and protein synthesis due to inappropriate gene copy numbers or alterations in the topological characteristics of the DNA. It has become common for researchers to insert a wild-type copy of the gene into the chromosome (in cis) for complementation. Karlyshev and Wren [22] published an efficient method for gene insertion into the C. jejuni chromosome, whereby the gene of interest is inserted into an rRNA gene cluster by homologous recombination. The benefits of this method are that 1) only one suicide vector needs to be generated for use with multiple C. jejuni strains, as the rRNA gene sequences are conserved among strains and 2) there is little to no effect on bacterial growth and cell function unless the cloned gene itself has a deleterious effect.
Genetic redundancy is where two or more genes encode proteins with the same apparent function. A widespread view is that such redundancy is not evolutionarily stable. However, in the instance where both proteins function with high efficiency or the two proteins interact with the same target but have different functional outcomes, genetic redundancy is evolutionarily stable. The question that arises when two genes encode proteins with similar functions is whether a given protein is ‘necessary’ or ‘sufficient’ for a phenotype. Although ample evidence exists that CadF and FlpA are FNBPs and bona fide adhesins, it has been challenging to address the contribution of each protein individually. The obstacle in addressing the contribution of CadF or FlpA is that it requires the complementation of a cadF flpA double deletion mutant with cadF, flpA, or both genes. More specifically, it has not been possible to generate a complementation vector harboring the cadF gene because the expression of the entire cadF coding sequence in E. coli is toxic. Here we describe a novel approach for the complementation of the cadF gene. We utilized this method to complement a C. jejuni cadF flpA double deletion mutant with cadF, flpA, and both cadF and flpA to assess CadF and FlpA function in the context of a viable bacterium. We believe that the genetic approach described herein can be applied to other C. jejuni genes that have been challenging to complement, as well as to other bacteria where E. coli is used as an intermediate host to generate the vector for functional complementation studies. In a broader context, the findings from this study are widely applicable to understanding disease regulation by FNBPs present in other Gram-positive and Gram-negative bacteria.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
A complete list of the C. jejuni isolates used in this study is provided in Table 1. The C. jejuni 81-176 wild-type strain was grown on Mueller–Hinton (MH) agar plates supplemented with 5% citrated bovine blood (MH-blood) and 2 μg/mL of tetracycline (Tet) or in MH broth with tetracycline in a microaerobic (5% O2, 10% CO2, 85% N2) atmosphere at 37 °C. C. jejuni mutants and complemented isolates were selected on MH blood agar plates with appropriate antibiotics: chloramphenicol (Cm, 8 μg/mL), hygromycin B (Hygro, 250 μg/mL), kanamycin sulfate (Kan, 50 μg/mL), spectinomycin (Spec, 200 μg/mL), and tetracycline (Tet, 2 μg/mL). All cultures were passaged every 24 to 48 h. Escherichia coli Stellar™ cells were maintained on Luria–Bertani (LB) agar plates or in LB broth aerobically at 37 °C. For chemical transformation, E. coli competent cells were grown in SOC medium before plating on LB agar plates supplemented with appropriate antibiotics: chloramphenicol (Cm, 20 μg/mL), hygromycin B (Hygro, 250 μg/mL), kanamycin sulfate (Kan, 50 μg/mL), and spectinomycin (Spec, 100 μg/mL).
Table 1.
C. jejuni isolates used in this study.
| Isolate | Description | Plasmid Used to Generate the Isolate | Antibiotic Resistance 1 | Reference |
|---|---|---|---|---|
| 81-176 | Wild-type strain | None | TetR | [23] |
| ΔflgL | flgL deletion mutant | pBSK-Kan2-flgL-CAT | TetR CmR | [24] |
| ΔcadF | cadF deletion mutant | pBSK-Kan2-cadF-CAT | TetR CmR | This study |
| ΔflpA | flpA deletion mutant | pBSK-flpA-Kan2 | TetR KanR | This study |
| ΔcadF ΔflpA | cadF flpA double deletion mutant | pBSK-flpA-Kan2 | TetR CmR KanR | This study |
| ΔcadF::cadFNP-FLAG | cadF deletion mutant with promoterless cadF gene fused to a FLAG-tag | prRNA-HygroR-cadFNP-FLAG | TetR CmR HygroR | This study |
| ΔcadF::PcadF-400 bp cadF-FLAG | cadF deletion mutant harboring a wild-type copy of cadF | prRNA-SpecR-PcadF cadF400 bp | TetR CmR HygroR SpecR | This study |
| ΔcadF::PcadF-334 bp cadF-FLAG | cadF deletion mutant harboring a wild-type copy of cadF | prRNA-SpecR-PcadF cadF334 bp | TetR CmR HygroR SpecR | This study |
| ΔcadF::PcadF-283 bp cadF-FLAG | cadF deletion mutant harboring a wild-type copy of cadF | prRNA-SpecR-PcadF cadF283 bp | TetR CmR HygroR SpecR | This study |
| ΔflpA::PflpA flpA | flpA deletion mutant harboring a wild-type copy of flpA | prRNA-HygroR-PflpA flpA | TetR KanR HygroR | This study |
| ΔcadF ΔflpA::cadFNP-FLAG | cadF flpA double deletion mutant with a promoterless cadF gene fused to a FLAG-tag | prRNA-HygroR-cadFNP-FLAG | TetR CmR KanR HygroR | This study |
| ΔcadF ΔflpA::PcadF-400 bp cadF-FLAG | cadF flpA double deletion mutant harboring a wild-type copy of cadF | prRNA-SpecR-PcadF cadF400 bp | TetR CmR KanR HygroR SpecR | This study |
| ΔcadF ΔflpA::PcadF-334 bp cadF-FLAG | cadF flpA double deletion mutant harboring a wild-type copy of cadF | prRNA-SpecR-PcadF cadF334 bp | TetR CmR KanR HygroR SpecR | This study |
| ΔcadF ΔflpA::PcadF-283 bp cadF-FLAG | cadF flpA double deletion mutant harboring a wild-type copy of cadF | prRNA-SpecR-PcadF cadF283 bp | TetR CmR KanR HygroR SpecR | This study |
| ΔcadF ΔflpA::PflpA flpA | cadF flpA double deletion mutant harboring a wild-type copy of flpA | prRNA-HygroR-PflpA flpA | TetR CmR KanR HygroR | This study |
| ΔcadF ΔflpA::PcadF cadF-FLAG-PflpA flpA | cadF flpA double deletion mutant harboring a wild-type copies of cadF and flpA | prRNA-SpecR-PflpA flpA-PcadF cadF400 bp | TetR CmR KanR HygroR SpecR | This study |
| 81-176 ciaD-ACD | Wild-type strain harboring ciaD-ACD fusion vector | pRY111-HygroR-PcysM ciaD-ACD | TetR HygroR | This study |
| ΔcadF ciaD-ACD | cadF deletion mutant harboring ciaD-ACD fusion vector | pRY111-HygroR-PcysM ciaD-ACD | TetR CmR HygroR | This study |
| ΔflpA ciaD-ACD | flpA deletion mutant harboring ciaD-ACD fusion vector | pRY111-HygroR-PcysM ciaD-ACD | TetR KanR HygroR | This study |
| ΔcadF ΔflpA ciaD-ACD | cadF flpA double deletion mutant harboring ciaD-ACD fusion vector | pRY111-HygroR-PcysM ciaD-ACD | TetR CmR KanR HygroR | This study |
| ΔflgL ciaD-ACD | flgL deletion mutant harboring ciaD-ACD fusion vector | pRY111-HygroR-PcysM ciaD-ACD | TetR CmR HygroR | This study |
1 CmR: Chloramphenicol; HygroR: Hygromycin; KanR: Kanamycin; SpecR: Spectinomycin; TetR: Tetracycline.
2.2. Generation of C. jejuni 81-176 cadF, flpA and cadF flpA Deletion Mutants
All plasmids used in this study are listed in Table 2. The primers used in this study are listed in Table 3. A C. jejuni 81-176 cadF deletion mutant (ΔcadF) was generated by homologous recombination using a suicide vector harboring a deleted copy of the cadF gene. The 5′ and 3′ flanking regions of the cadF gene were PCR amplified with primer pairs MEK4067/MEK4068 and MEK4063/MEK4064, respectively. The chloramphenicol resistance cassette (CAT) was PCR amplified from the C. jejuni–E. coli pRY111 shuttle vector with the MEK4065 and MEK4066 primers. The 5′ cadF flanking region, chloramphenicol resistance cassette, and 3′ cadF flanking region were cloned into the suicide plasmid pBSK-Kan2 using the Clontech In-Fusion HD cloning kit (Takara Bio USA, Inc., Mountain View, CA, USA), resulting in the plasmid pBSK-Kan2-cadF-CAT. This suicide vector was electroporated into the C. jejuni 81-176 wild-type strain, and chloramphenicol-resistant (CmR) transformants were selected on MH blood agar plates supplemented by chloramphenicol.
Table 2.
Bacterial plasmids used in this study.
| Plasmid | Description | Antibiotic Resistance 1 | Reference |
|---|---|---|---|
| pBSK-Kan2 | pBlueScript II SK (+) cloning vector with the aphA-3 gene cassette encoding KanR (Kan2) replacing the original ampicillin resistance cassette | KanR | [25] |
| pBSK-Kan2-cadF-CAT | pBSK-Kan2 suicide plasmid harboring 5′ and 3′ cadF flanking fragments with a chloramphenicol resistance cassette (CAT) | CmR KanR | This study |
| pBSK-flpA-Kan2 | pBSK suicide plasmid harboring 5′ and 3′ flpA flanking fragments with the C. jejuni kanamycin resistance gene (aphA-3, Kan2) | AmpR KanR | This study |
| prRNA-HygroR | pBSK-Kan2 vector harboring the 16S rRNA and the 23S rRNA gene fragments with a hygromycin B resistance cassette | KanR HygroR | [26] |
| prRNA-HygroR-cadFNP-FLAG | prRNA-HygroR suicide vector harboring the promoterless cadF gene fused with a FLAG-tag | KanR HygroR | This study |
| prRNA-SpecR-PcadF cadF400 bp | prRNA-SpecR suicide vector harboring 400 bp of the cadF gene at the 5′ end with its native promoter | KanR SpecR | This study |
| prRNA-SpecR-PcadF cadF334 bp | prRNA-SpecR suicide vector harboring 334 bp of the cadF gene at the 5′ end with its native promoter | KanR SpecR | This study |
| prRNA-SpecR-PcadF cadF283 bp | prRNA-SpecR suicide vector harboring 283 bp of the cadF gene at the 5′ end with its native promoter | KanR SpecR | This study |
| prRNA-HygroR-PflpA flpA | prRNA- HygroR suicide vector harboring the flpA gene with its native promoter | KanR HygroR | This study |
| prRNA-SpecR-PflpA flpA-PcadF cadF400 bp | prRNA-SpecR suicide vector harboring the flpA gene and 400 bp of the cadF gene at the 5′ end with their native promoters | KanR SpecR | This study |
| pRY111-HygroR-PcysM ciaD-ACD | pRY111-HygroR shuttle vector harboring the ciaD gene with a C. jejuni cysM promoter and fused with the adenylate cyclase domain (ACD) coding sequence | HygroR | This study |
1 AmpR: Ampicillin; CmR: Chloramphenicol; HygroR: Hygromycin; KanR: Kanamycin; SpecR: Spectinomycin.
Table 3.
Primers used in this study.
| Primer ID | Oligo Name | Sequence 5′-3′ | Purpose |
|---|---|---|---|
| MEK4063 | 81-176-CadF-Dwn-RV | TAT AGG GCG AAT TGG GTA CCG CAG CCT CAT TTC CGT CC | cadF mutant construction |
| MEK4064 | 81-176-CadF-Dwn-FW | GAT CGG ATC CCC TCG CTC AAG CAA TGA CAC | cadF mutant construction |
| MEK4065 | 81-176-CadF-CAT-RV | TTG AGC GAG GGG ATC CGA TCT GCG CCC TTT AGT | cadF mutant construction |
| MEK4066 | 81-176-CadF-CAT-FW | TTG GCA AGT GGC TAG CGT GTT CCT TTC CAA GTT AAT TGC G | cadF mutant construction |
| MEK4067 | 81-176-CadF-Up-RV | ACA CGC TAG CCA CTT GCC AAA CCT AAA CAT AAT A | cadF mutant construction |
| MEK4068 | 81-176-CadF-Up-FW | GGG AAC AAA AGC TGG AGC TCC AGT TAG AGG TAT GCT TCC TA | cadF mutant construction |
| MEK4069 | 81-176-FlpA-Dwn-FW | TAT AGG GCG AAT TGG GTA CCT CTG CTC TAT TTT TTT CAA ATC C | flpA mutant construction |
| MEK4070 | 81-176-FlpA-Dwn-RV | GCT TGG ATC CAG AAC CTT CAA GCA AAG TTA AGG | flpA mutant construction |
| MEK4071 | 81-176-FlpA-Kan-FW | TGA AGG TTC TGG ATC CAA GCT TTT TAG ACA TCT AAA TCT AGG TAC TA | flpA mutant construction |
| MEK4072 | 81-176-FlpA-Kan-RV | AAA GAT TTC GGC TAG CGA TAA ACC CAG CGA ACC | flpA mutant construction |
| MEK4073 | 81-176-FlpA-Up-FW | TAT CGC TAG CCG AAA TCT TTT CAT CAT TCT CTC C | flpA mutant construction |
| MEK4074 | 81-176-FlpA-Up-RV | GGG AAC AAA AGC TGG AGC TCA ACT TTT TTA GTA GAT GAA AAT TCA AGG | flpA mutant construction |
| MEK4507 | noProm-CadF-XbaI-FW | ATA TTC TAG AAT GAA AAA AAT ATT ATT ATG TTT AGG TTT GGC AAG TGT TTT ATT CAG TGC | cadF complement first construct |
| MEK4508 | CadF-nostop-BamHI-RV | ATA TAT GGA TCC TCT TAA AAT AAA TTT AGC ATC CAC TCT TCT ATT ATC CGC TCT ACC TTC | cadF complement first construct |
| MEK4509 | rRNA-up-SpecR-XbaI-FW | TGG ATC ACC TCC TTT CTA GAG CTG TTT TTT ACT TGA TAT TGT TTT TTA AAT ATG CTA AAA TTA GGC GTT TC | cadF complement second construct |
| MEK4518 | pCadF-SpecR-SacII-RV | GCT TCT TCC CGC GGT TGC TAC TCT GTT CTA AGT AAT TCC TCA ATT TGT TTT TTC | cadF complement second construct |
| MEK4541 | pCadF-SacII-FW | GAG TAG CAA CCG CGG GAA GAA GCC CAC AAT TCT CTA AAC G | cadF complement second construct |
| MEK4542 | CadF-400bp-SacI-RV | GAA CAA AAG CTG GAG CTC ATT TTA CAC CCG CGC CAT AAT GTC C | cadF complement second construct |
| MEK4543 | CadF-334bp-SacI-RV | GAA CAA AAG CTG GAG CTC CCT CAT ATC CTC CAC CTG CTA AAC C | cadF complement second construct |
| MEK4544 | CadF-283bp-SacI-RV | GAA CAA AAG CTG GAG CTC CAA TAC CTT TAA TAG CAC TCA AAT AAG TTC TTG TAA TAT C | cadF complement second construct |
| MEK4602 | FlpA-Comp-XbaI-FW | GTA TTC TAG AAC AGG AAG AAC TCA TCA AAT TAG AGC | flpA complement first construct |
| MEK4603 | FlpA-Comp-BamHI-RV | ACT CGG ATC CCC AAA GAA ATT CAA CAT CAT CCT TGC | flpA complement first construct |
| MEK4676 | FlpA-Comp-SacII-FW | GTA TCC GCG GAC AGG AAG AAC TCA TCA AAT TAG AGC | flpA complement second construct |
| MEK4677 | FlpA-Comp-SacII-RV | ACT CCC GCG GCC AAA GAA ATT CAA CAT CAT CCT TGC | flpA complement second construct |
| MEK4721 | pRY111-HygR-NcoI-FW | ATA GAA GAT CTC CAT GGA CTA AAG CTC TTG CCC AAG AAG ATT ACG | ciaD-ACD construct |
| MEK4722 | pRY111-HygR-NcoI-RV | GAC AAA CTG GGC CAT GGG ATT TAT CAT GCC TTT CTT TGT CTG TAT TCT CTC | ciaD-ACD construct |
To generate a C. jejuni 81-176 flpA deletion mutant (ΔflpA), another suicide vector was created that harbors the 5′ flanking region of the flpA gene followed by a kanamycin resistance cassette (Kan2) and the 3′ flanking region of flpA. The 5′ and 3′ flanking regions of the flpA gene were PCR amplified with primer pairs MEK4073/MEK4074 and MEK4069/MEK4070, respectively. The aphA-3 gene encoding Kan2 was PCR amplified from the pBSK-Kan2 suicide vector with the MEK4071 and MEK4072 primers. In-fusion cloning was used to clone these three DNA fragments into the suicide plasmid pBSK, resulting in the pBSK-flpA-Kan2 plasmid. This suicide vector was electroporated into the C. jejuni 81-176 wild-type strain, and kanamycin-resistant (KanR) transformants were selected on a MH blood agar plate supplemented with kanamycin. The pBSK-flpA-Kan2 plasmid was also incorporated into the C. jejuni 81-176 ΔcadF mutant to generate a C. jejuni 81-176 cadF flpA double deletion mutant (ΔcadF ΔflpA). The C. jejuni ΔcadF, ΔflpA, and ΔcadF ΔflpA mutants were confirmed by PCR using gene-specific primers.
2.3. Generation of C. jejuni cadF Complemented Isolates
The complementation of the ΔcadF mutant was achieved using a two-step electroporation with a series of different constructs. The first construct that was generated contained the promoterless cadF gene fused to a FLAG-tag. Briefly, the cadF coding sequence was PCR amplified from the C. jejuni 81-176 wild-type strain with the MEK4507 and MEK4508 primers. The amplified product was cloned between the 16S rRNA and 23S rRNA genes contained in the prRNA-HygroR suicide vector using in-fusion cloning, resulting in the prRNA-HygroR-cadFNP-FLAG vector. This vector was electroporated into the ΔcadF mutant, resulting in a ΔcadF::promoterless cadF-FLAG-tag isolate (ΔcadF::cadFNP-FLAG isolate). All transformants were selected on MH blood agar plates supplemented with hygromycin B and confirmed by PCR.
This ΔcadF::cadFNP-FLAG isolate was electroporated a second time with one of the three constructs generated below. The three different constructs contained various lengths of the cadF 5′ coding sequence and were each generated using in-fusion cloning of three individual fragments. The first (variable) fragment contained the native cadF promoter of 154 bp and either 283 bp, 334 bp, or 400 bp of the cadF gene (PcadF cadF283 bp, PcadF cadF334 bp, and PcadF cadF400 bp) and was generated by PCR amplification using primers MEK4541/MEK4542 (437 bp), MEK4541/MEK4543 (488 bp), or MEK4541/MEK4544 (554 bp), respectively. The second fragment contained the aad9 spectinomycin resistance gene [27] driven by the C. jejuni hupB promoter (gBlocks Gene Fragment, Integrated DNA Technologies, San Diego, CA, USA) and was amplified with the MEK4509 and MEK4518 primers. The third fragment contained the 23S rRNA gene and was PCR amplified from the prRNA-HygroR vector. The final constructs were designated prRNA-SpecR-PcadF cadF400 bp, prRNA-SpecR-PcadF cadF334 bp, and prRNA-SpecR-PcadF cadF283 bp, respectively. These three plasmids were individually transformed into the C. jejuni ΔcadF::cadFNP-FLAG isolate by electroporation, and spectinomycin-resistant (SpecR) transformants were selected on MH blood agar plates supplemented with spectinomycin. All three electroporations resulted in individual transformants that possessed a wild-type copy of the cadF gene expressed from its native promoter (ΔcadF::PcadF-400 bp cadF-FLAG, ΔcadF::PcadF-334 bp cadF-FLAG, ΔcadF::PcadF-283 bp cadF-FLAG isolates). A similar strategy was used to complement the ΔcadF ΔflpA double mutant, where the prRNA-HygroR-cadFNP-FLAG construct was used to generate the intermediate C. jejuni ΔcadF ΔflpA::cadFNP-FLAG isolate. This intermediate isolate was used for the incorporation of vectors prRNA-SpecR-PcadF cadF400 bp, prRNA-SpecR-PcadF cadF334 bp, and prRNA-SpecR-PcadF cadF283 bp to generate C. jejuni ΔcadF ΔflpA:: PcadF cadF-FLAG isolates.
2.4. Generation of C. jejuni flpA Complemented Isolates
Two different constructs containing the flpA gene and promoter were used to functionally complement C. jejuni mutants. The prRNA-HygroR-PflpA flpA suicide vector was introduced into the ΔflpA mutant and ΔcadF ΔflpA double mutant. Briefly, a 1459 bp DNA fragment containing the flpA gene was PCR amplified from the C. jejuni 81-176 wild-type strain with the MEK4602 and MEK4603 primers. This fragment contained the flpA gene (1236 bp) and the flpA promoter sequence (223 bp). The fragment was cloned in between the 16S and 23S rRNA genes at the XbaI-BamHI site of the prRNA-HygroR vector, resulting in the plasmid prRNA-HygroR-PflpA flpA. This suicide vector was introduced into the mutants by electroporation, and transformants were selected on MH blood agar plates supplemented with hygromycin B. All HygroR transformants were determined to contain the flpA gene by PCR using gene-specific primers.
The prRNA-SpecR-PflpA flpA-PcadF cadF400 bp suicide vector was introduced into the ΔcadF ΔflpA double mutant. Briefly, the flpA gene and promoter were amplified by PCR from C. jejuni 81-176 wild-type strain with the MEK4676 and MEK4677 primers. The fragment was cloned into the SacII site located between cadF promoter and spectinomycin resistance cassette of the prRNA-SpecR-PcadF cadF400 bp vector, generating the plasmid prRNA-SpecR-PflpA flpA-PcadF cadF400 bp. The vector was electroporated into the C. jejuni ΔcadF ΔflpA::cadFNP-FLAG isolate, and transformants were selected on MH blood agar plates supplemented with spectinomycin.
2.5. Electroporation and Strain Confirmation (PCR and Sequencing)
The electroporations were conducted using a Bio-Rad E. coli Pulser (Bio-Rad, Hercules, CA, USA) and 2 mm gap cuvettes at the 2.5 kV voltage setting. Following electroporation, bacterial suspensions were spread onto MH-blood agar plates for 24 h. Transformants were isolated by streaking the bacterial cells onto MH blood agar plates containing the appropriate antibiotic. The resultant colonies were picked and confirmed by PCR amplification using gene-specific primers.
2.6. Gel Electrophoresis and Immunoblotting
Bacterial whole-cell lysates (WCL; OD540 equivalent = 0.1 per lane) were separated in SDS 12.5% polyacrylamide gel electrophoresis (PAGE) gels using the discontinuous buffer system described by Laemmli [28]. The proteins were either stained with Coomassie Brilliant Blue R-250 (CBB-R250) or electrophoretically transferred to polyvinylidene fluoride membranes (PVDF; Immobilon P; Millipore Corp., Bedford, MA, USA). The blots were incubated with either a rabbit α-CadF serum (1:4000, K6928) or a rabbit α-FlpA serum (1:4000, L0850) in Tris-buffered saline with 0.1% Tween 20 (TBS-T) containing 9% nonfat dry milk (NFDM). Bound antibodies were detected with peroxidase-conjugated goat α-rabbit immunoglobulin G (Sigma-Aldrich, St. Louis, MO, USA). Immunoblot development was done by chemiluminescence (ECL Prime, GE Healthcare Biosciences, Piscataway, NJ, USA), and reactive proteins were visualized with a GE LAS-4000 mini (GE Healthcare).
2.7. Preparation of Outer Membrane Proteins
Outer membrane protein (OMP) extracts were prepared using N-lauroylsarcosine (Sigma-Aldrich), as outlined previously [29]. The extracts were washed with 50 mM Tris (pH 7.5), suspended in the same buffer, and stored at −20 °C. The protein concentration of each OMP extract was determined by the bicinchoninic acid (BCA) assay.
2.8. Adherence Assay
A stock culture of INT 407 cells (ATCC CCL 6) was cultured in Minimal Essential Media (MEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Premium Grade FBS, 100% US Origin, VWR, Product No. 97068-085) and 1 mM sodium pyruvate (Corning Inc., Manassas, VA, USA) at 37 °C in a 5% CO2 incubator. Adherence assays were performed as outlined elsewhere [30], with one modification—the bacteria were suspended in MEM without FBS. All assays in this study were performed at a multiplicity of infection (MOI) ranging between 50 and 500 to one to ensure reproducibility and repeated a minimum of three times. Regardless of the MOI, the trends of the isolates relative to one another were the same.
2.9. Biotinylation of FN and FN-Binding Assay
Human FN (Sigma-Aldrich) was suspended in 1 mL phosphate buffered saline (PBS) to a concentration of 5 µM, and biotinylated by adding 1 mg of Sulfo-NHS-LC-Biotin for 3 h at room temperature. Excess biotin was removed using a Zeba desalting column (40k MWCO, Thermo Fisher Scientific, Waltham, MA, USA) and dialyzing overnight in PBS (40k MWCO) at 4 °C. The C. jejuni isolates were grown overnight in MH broth, pelleted, and resuspended to an OD540 = 0.3 in PBS (~3 × 108 CFU/mL). A suspension containing 80 µL of bacteria in PBS and 20 µL of biotinylated FN was mixed, incubated for 1 h at room temperature, and C. jejuni pelleted by centrifugation. The amount of biotinylated FN in the supernatant was determined using a Pierce Biotin Quantitation Kit, as outlined by the manufacturer (Thermo Fisher Scientific).
2.10. Enzyme-Linked Immunosorbent Assays (ELISAs) with CadF and FlpA Peptides
CadF, FlpA, and scrambled peptides were chemically synthesized by GenMed Synthesis Inc. (San Antonio, TX, USA) and were made up of an N-terminal Nano-tag [31] and 28 amino acids of the respective proteins. The peptide sequences are:
(1) CadF: DVEAWLGARVPLVETSGGFGHYGAGVKFRLSDSLALRLETRDQ;
(2) FlpA: DVEAWLGARVPLVETNRIKLIWRPHPDFRVDSYIIERTKGDDK; and
(3) Scrambled: DVEAWLGARVPLVETPGLGALKDSGHHDGDLSIRSRRFFPRDH,
where the residues in bold highlight the FN-binding domains. To assess FN-binding, 1 µg/well of each peptide was used to coat the well of a polycarbonate 96-well tray in a 0.1 M carbonate buffer (pH 9.5) overnight at 4 °C. Wells were then washed with PBS and blocked with 0.1% bovine serum albumin (BSA) in PBS for 2 h. Wells were washed with PBS, and a two-fold dilution series of FN was added to the wells ranging from 100 to 1.5625 µg/well. FN was either dissolved in PBS alone or in PBS containing 4 M urea. The plate was incubated overnight at 4 °C. In the experiments testing different FN domains, either the 45 kDa fragment (Cat# F0162, Sigma-Aldrich) or the 30 kDa fragment (Cat# F9911, Sigma-Aldrich) was added in place of whole FN in PBS. Wells were washed and probed with α-FN antibody (Cat# F3648, 1:1000, Sigma-Aldrich) in PBS with 0.1% BSA for 2 h at room temperature. The wells were washed, and a secondary α-rabbit HRP antibody (Cat# A6154, 1:4000, Sigma-Aldrich) in PBS with 0.1% BSA was added for an additional 2 h. After extensive washing, TMB substrate (ThermoFisher Scientific) was added to the wells and incubated for approximately 25 min before quenching with 2.5 M H2SO4. Trays were read at 450 nm in a BioTek ELx808IU plate reader (BioTek Instruments Inc., Winooski, VT, USA). All samples were assayed three times on independent days and blanked against wells that were coated with BSA. Because the data were combined from independent assays, absorbance is reported as a percentage of relative absorbance where 0% represents the blank value for a well, and 100% represents the maximum value in a well.
2.11. Immunoblot Analysis of Total-Erk 1/2 and Phospho-Erk 1/2 in C. jejuni Infected INT 407 Cells
The level of total-Erk 1/2 and phospho-Erk 1/2 in C. jejuni infected INT 407 cells was determined by immunoblot analysis. Briefly, INT 407 cells were seeded at a density of 1 × 105 in 24-well tissue culture trays (BD Falcon, Franklin Lakes, NJ, USA, Cat # 353047). The following day, the cells were serum-starved for 4 h. Each well was infected with C. jejuni and incubated for 60 min. Following incubation, the media was removed, INT 407 cells were lysed using 2× sample buffer, and lysates were collected and analyzed by SDS-PAGE coupled with immunoblot analysis. The proteins were transferred to a PVDF membrane and probed with a total-Erk 1/2 antibody (Santa Cruz, Dallas, TX, USA, Cat # sc-94) at a 1:500 dilution and a phospho-Erk 1/2 antibody (Cell Signaling Technology, Danvers, MA, USA, Cat # 4377) at a 1:1000 dilution. Following overnight incubation at 4 °C, the blots were rinsed and incubated for 1 h with horseradish peroxidase-conjugated α-rabbit IgG or α-mouse IgG (Sigma) secondary antibodies at a 1:2000 dilution. Blots were developed using ECL prime reagent (GE Healthcare) and imaged using a LAS 4000 mini (GE healthcare). Bands were quantified using ImageJ. Densitometry analysis was performed to determine the ratio of phospho-protein to total protein (i.e., p-Erk 1/2 to total Erk 1/2). Four negative controls were used in all assays: (1) uninfected cells; (2) cells infected with a C. jejuni ciaD deletion mutant (defective in secretion of the CiaD effector protein from the flagellum); (3) cells infected with a C. jejuni flgL deletion mutant (non-motile, defective in protein secretion from the flagellum); and (4) cells treated with PD98059 (an Erk 1/2 inhibitor). A 50 mM stock solution of Erk 1/2 inhibitor PD98059 (Selleck, Houston, TX, USA, Cat # S1177) was prepared in dimethyl sulfoxide (DMSO) and used at a final concentration of 50 μM. This inhibitor was added to the INT 407 cells 30 min prior to infection and maintained throughout the assay. No significant death of INT 407 cells was observed with the PD98059 treatment, as judged by trypan blue staining (not shown).
2.12. Adenylate Cyclase Domain Reporter Delivery Assays
The ciaD-ACD shuttle plasmid harbors a copy of the ciaD gene fused to the adenylate cyclase domain (ACD) of the Bordetella pertussis CyaA protein and is expressed from the C. jejuni cysM promoter. The CysM protein, which is a 32.4 kDa cytoplasmic protein (O-acetylserine sulfhydrylase B) involved in cysteine biosynthesis, is constitutively synthesized in C. jejuni [32]. The ciaD-ACD shuttle plasmid, used previously [33], was modified by replacing the chloramphenicol resistance gene with the hygromycin resistance gene using the MEK4721 and MEK4722 primers and in-fusion cloning, generating the plasmid pRY111-HygroR-PcysM ciaD-ACD. This modification was needed, as some of the C. jejuni isolates used were chloramphenicol resistant and required a different antibiotic resistance for selection. The ciaD-ACD shuttle plasmid (pRY111-HygroR-PcysM ciaD-ACD) was transformed into the C. jejuni wild-type strain, ΔcadF mutant, ΔflpA mutant, ΔcadF ΔflpA mutant, and ΔflgL mutant by conjugation [33]. SDS-PAGE and immunoblots were performed with a rabbit CyaA IgG polyclonal antibody (sc-33620, Santa Cruz Biotechnology) to confirm the C. jejuni transformants. The ACD delivery assay was then performed with INT 407 cells, as outlined elsewhere with modifications [33]. Briefly, INT 407 cells were seeded into each well of a 6-well tissue culture tray at 1.6 × 106 cells/well. The following day, the cells were rinsed one time with tissue culture medium and infected with a 3 mL suspension of C. jejuni (OD540 = 0.3–0.35) in 1% FBS-MEM. The trays were centrifuged for 5 min at 600× g to promote bacteria–host cell contact followed by incubation for 45 min at 37 °C in a humidified, 5% CO2 incubator. After incubation, the cells were lysed with 0.1 M HCl and 0.1% Triton X-100. Cell lysates were centrifuged for 5 min at 13,000× g, and the clarified supernatants were collected in separate tubes. The cAMP level for each sample was assessed using the Direct cAMP ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA) according to the manufacturer’s specifications. Each isolate was tested in biological triplicate on different days for the delivery of the CiaD-ACD fusion protein into the cytosol of the cells.
2.13. Motility Assays
Motility assays were performed as outlined elsewhere [34]. Briefly, C. jejuni were grown to mid-log phase overnight in MH broth, pelleted by centrifugation, and suspended in MH broth to an OD540 = 1.0. Five microliters of each culture were spotted onto a MH plate containing 0.4% agar, and the plate was incubated for 48 h in a microaerobic chamber at 37 °C. The isolate was judged to be motile if the bacterial growth (migration) was visible beyond the edge of the inoculation spot zone. The plates were imaged with an ImageQuant LAS-4000 mini, and the zone of motility was measured using the ImageJ software. The isolates were tested for motility on six different days. Regardless of the day, the results were consistent.
2.14. Statistical Analysis
All statistical analysis was performed with GraphPad Prism 6.0g (La Jolla, CA, USA). The specific statistical tests are indicated in the figure legends.
3. Results
3.1. Introduction of a Wild-Type Copy of the cadF Gene into the C. jejuni Chromosome
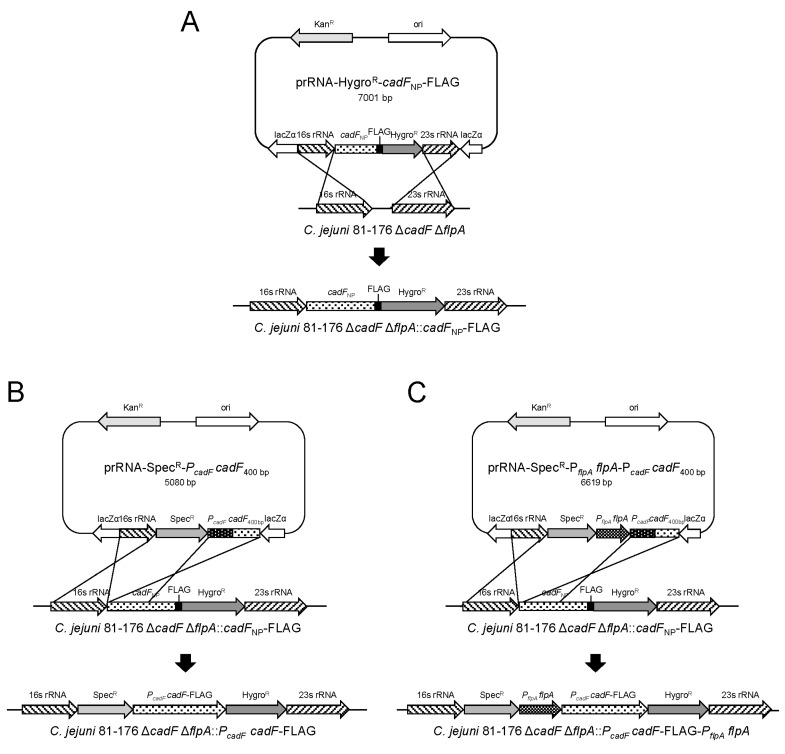
To the best of our knowledge, a C. jejuni cadF deletion mutant (ΔcadF) has yet to be complemented primarily due to the difficulty in generating a shuttle vector; the expression of the full-length cadF gene from its endogenous or a heterologous promoter is toxic to E. coli. We utilized a two-step procedure whereby a fragment harboring the promoterless cadF gene (cadFNP-FLAG) was first inserted into the 16S and 23S rRNA gene cluster of C. jejuni, followed by the introduction of a second fragment harboring the cadF promoter upstream of the promoterless cadF gene (Figure 1). First, a C. jejuni ΔcadF mutant harboring a promoterless cadF gene fused with a FLAG-tag inserted into the rRNA gene cluster (C. jejuni isolate designation: ΔcadF::cadFNP-FLAG). Then, three additional constructs were generated that contained the C. jejuni 16S rRNA gene, the spectinomycin antibiotic resistance gene, and the endogenous cadF promoter plus a 5′-end portion of the cadF gene. The prRNA-SpecR vector harboring the cadF promoter (PcadF) and 5′-end gene fragments (283 bp, 334 bp, or 400 bp) (cadF283 bp, cadF334 bp, or cadF400 bp) were introduced into the C. jejuni by electroporation, and SpecR clones were selected on agar plates. The primary reason for amplifying the different 5′-end gene fragments was in case one or more of the partial gene fragments proved to be toxic when expressed in E. coli; nevertheless, all three fragments were successfully cloned into the prRNA-SpecR vector. The C. jejuni HygroR SpecR transformants were confirmed to contain the entire cadF gene with its endogenous promoter inserted between the 16S and 23S rRNA genes by PCR analysis (C. jejuni isolate designation: ΔcadF::PcadF cadF-FLAG) (data not shown). A similar approach was taken to introduce the wild-type copy of cadF in ΔcadF ΔflpA double mutant (C. jejuni isolate designation: ΔcadF ΔflpA::PcadF cadF-FLAG). The innovative aspect of this approach is that the construct containing the coding sequence of the cadF gene did not contain its endogenous promoter, so it was not expressed in E. coli.
Figure 1.
Two-step suicide vector strategy for the introduction of a wild-type copy of the cadF and flpA genes into the C. jejuni chromosome. (A) In the first step, a promoterless cadF gene fused to a FLAG-tag was cloned into the prRNA-HygroR vector, resulting in the prRNA-HygroR-cadFNP-FLAG construct. This construct was introduced into a C. jejuni ΔcadF mutant or ΔcadF ΔflpA double mutant by electroporation to generate the isolates that have promoterless cadF gene inserted between the 16S rRNA and 23S rRNA genes in the chromosome. (B) The second step includes the cloning of DNA fragments containing the cadF promoter (PcadF) and a portion of cadF coding sequence from the 5′-end (283 bp, 334 bp, or 400 bp) into a prRNA vector with the 16S rRNA gene and the spectinomycin resistance cassette. Here, the representative image shows the insertion of the cadF promoter and the 400 bp of cadF 5′-end into the C. jejuni ΔcadF ΔflpA::cadFNP-FLAG strain. A cadF-complemented isolate was generated by recombination with the 16S rRNA gene and the DNA sequence containing the 5′-end of the cadF gene. The HygroR SpecR transformants were confirmed to contain the entire cadF gene with its endogenous promoter inserted between the 16S and 23S rRNA genes by PCR analysis. (C) A similar approach was taken to complement a ΔcadF ΔflpA mutant with wild-type copies of cadF and flpA. In the first step, the promoterless cadF gene was incorporated in the rRNA region of the chromosome of the C. jejuni ΔcadF ΔflpA mutant. In the second step, the entire flpA gene with its native promoter, the promoter of cadF, and a portion of the cadF coding sequence from the 5′-end (234 bp, 334 bp, or 400 bp) were inserted in the rRNA region to generate the cadF flpA double complement isolate.
3.2. Complementation of cadF in a C. jejuni cadF Deletion Mutant
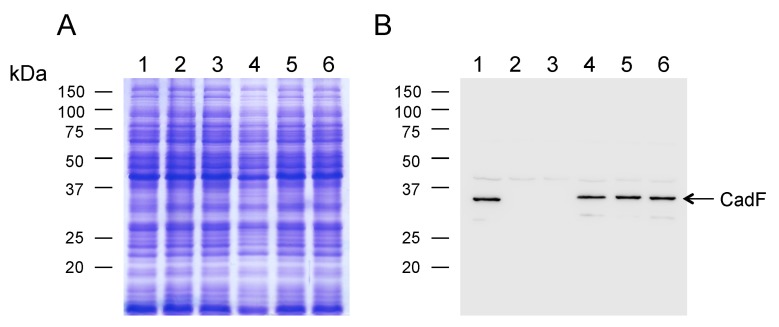
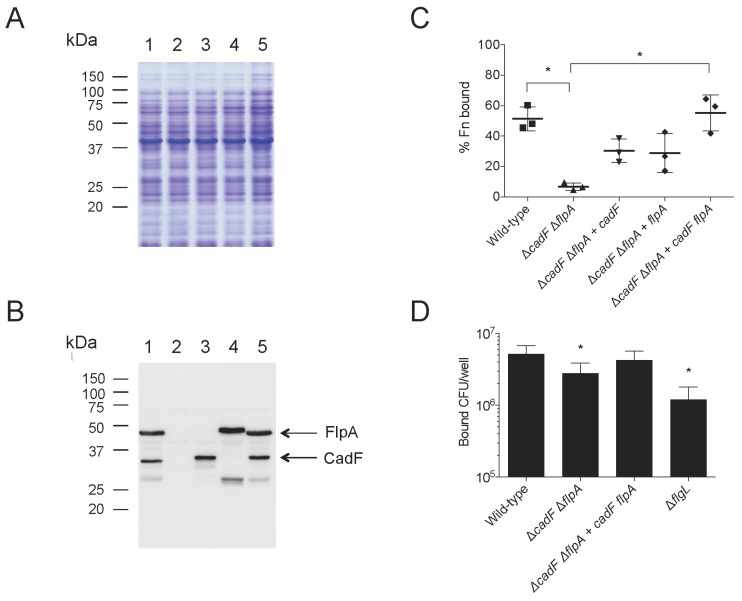
Protein synthesis from the cadF gene in the background of the C. jejuni ΔcadF mutant was examined by SDS-PAGE coupled with immunoblot analysis. A representative gel and immunoblot of the transformants is shown in Figure 2. The positive control consisted of the C. jejuni wild-type strain. The negative controls consisted of two ΔcadF mutants, one being the ΔcadF mutant and the other being the ΔcadF mutant harboring the promoterless cadF gene (ΔcadF::cadFNP-FLAG). A 37 kDa protein was readily observed in the C. jejuni wild-type strain and the three C. jejuni ΔcadF::PcadF cadF-FLAG tagged transformants, as judged by immunoblot analysis using the rabbit α-CadF serum. This finding demonstrates that it is possible to get efficient gene expression and protein synthesis by the integration of a gene with its endogenous promoter within the rRNA gene cluster. Noteworthily, the 37 kDa protein was also detected in the three C. jejuni ΔcadF::PcadF cadF-FLAG transformants by immunoblot analysis using an α-FLAG antibody (not shown), as each contained a FLAG-tag. The addition of an epitope tag provides an additional tool for either the detection or the isolation of a protein.
Figure 2.
Electrophoretic and immunoblot analysis of C. jejuni ΔcadF transformants. Bacterial whole-cell lysates were separated in SDS 12.5% polyacrylamide gels and either: (A) stained with Coomassie Brilliant Blue R-250 or (B) transferred to polyvinylidene fluoride membranes and probed with a rabbit α-CadF serum. The positive control consisted of a C. jejuni wild-type strain, and the negative controls consisted of a mutant with a cadF gene deletion (ΔcadF) and a mutant with a promoterless cadF gene fused to a FLAG-tag (ΔcadF::cadFNP-FLAG). The arrow indicates the CadF protein. Lanes: C. jejuni strains (1) 81-176 (wild-type); (2) ΔcadF; (3) ΔcadF::cadFNP-FLAG; (4) ΔcadF::PcadF-283 bp cadF-FLAG; (5) ΔcadF::PcadF-334 bp cadF-FLAG; and (6) ΔcadF::PcadF-400 bp cadF-FLAG. Molecular mass standards (in kDa) are on the left.
3.3. Complementation of cadF and flpA in a C. jejuni cadF flpA Double Deletion Mutant
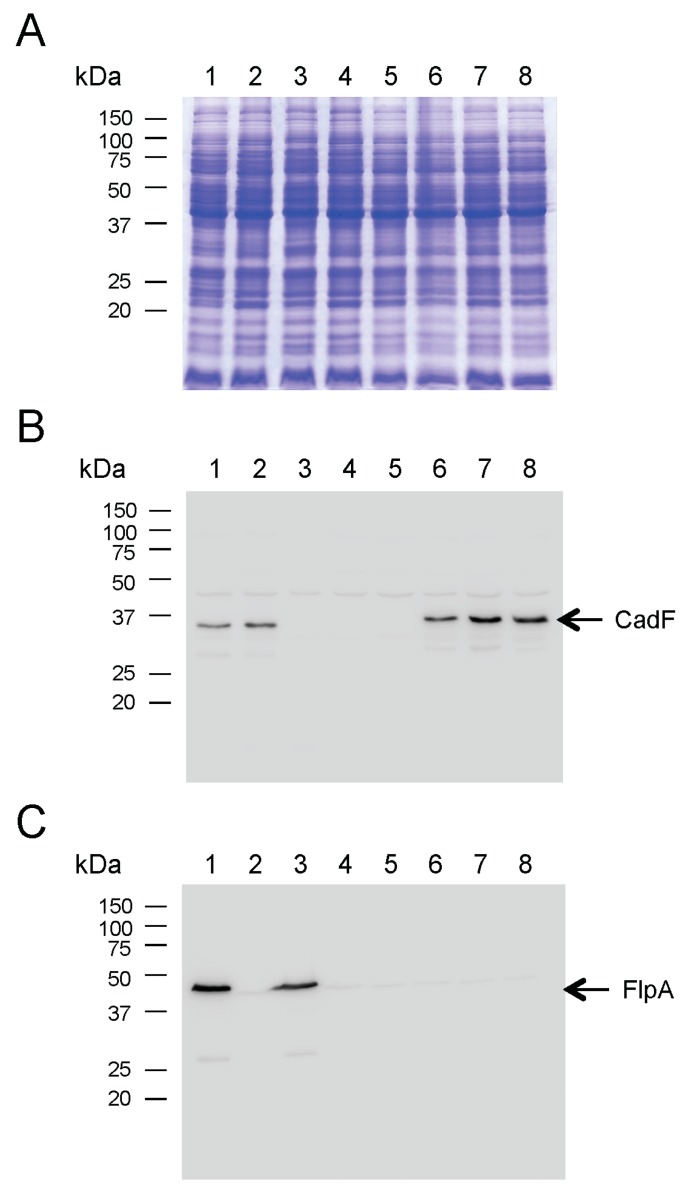
The ability to restore cadF gene expression in a C. jejuni ΔcadF mutant prompted additional experiments using an identical approach in the C. jejuni cadF flpA double deletion mutant (ΔcadF ΔflpA). A representative gel and immunoblot of the transformants is shown in Figure 3. The C. jejuni wild-type strain was included as a positive control. The C. jejuni ΔcadF, ΔflpA, and ΔcadF ΔflpA mutants were included as negative controls. A 37 kDa immunoreactive band was detected with the rabbit α-CadF serum in the C. jejuni wild-type strain, ΔflpA, and three C. jejuni ΔcadF ΔflpA::PcadF cadF-FLAG transformants. In addition, a 46 kDa immunoreactive band was detected with the rabbit α-FlpA serum in only the C. jejuni wild-type strain and ΔcadF isolate. The immunoblot analysis indicated that the two-step strategy was successful in restoring cadF gene expression in the C. jejuni ΔcadF ΔflpA isolate.
Figure 3.
Electrophoretic and immunoblot analysis of C. jejuni ΔcadF ΔflpA transformants. Bacterial whole-cell lysates were separated in SDS 12.5% polyacrylamide gels and either: (A) stained with Coomassie Brilliant Blue R-250, (B) transferred to polyvinylidene fluoride (PVDF) membranes and probed with a rabbit α-CadF serum, or (C) transferred to PVDF membranes and immunoblot with a rabbit α-FlpA serum. The positive control consisted of a C. jejuni wild-type strain. The negative controls consisted of a ΔcadF ΔflpA mutant (lane 4) and the ΔcadF ΔflpA::cadFNP-FLAG isolate (lane 5). The arrows indicate the CadF protein (Panel B) and the FlpA protein (Panel C). Lanes: C. jejuni strains (1) 81-176 (wild-type); (2) ΔflpA; (3) ΔcadF; (4) ΔcadF ΔflpA; (5) ΔcadF ΔflpA::cadFNP-FLAG; (6) ΔcadF ΔflpA::PcadF-283 bp cadF-FLAG; (7) ΔcadF ΔflpA::PcadF-334 bp cadF-FLAG; and (8) ΔcadF ΔflpA::PcadF-400 bp cadF-FLAG. Molecular mass standards (in kDa) are on the left.
3.4. CadF and FlpA Localize in the Outer Membrane of the C. jejuni Transformants
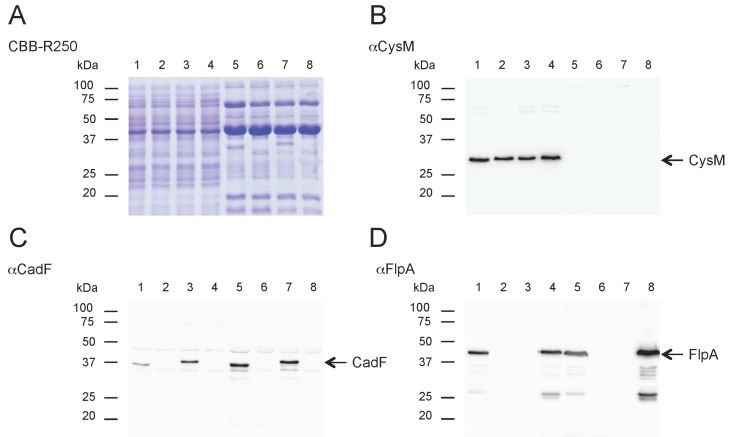
To address the localization of the CadF and FlpA proteins in the C. jejuni transformed isolates, C. jejuni whole-cell lysates (WCLs) and outer membrane protein (OMP) extracts were prepared from the: (1) 81-176 wild-type strain, (2) ΔcadF ΔflpA mutant, (3) ΔcadF ΔflpA::PcadF cadF-FLAG isolate, (4) ΔcadF ΔflpA::PflpA flpA isolate; and (5) ΔcadF ΔflpA::PcadF cadF-FLAG-PflpA flpA isolate. SDS-PAGE and immunoblot analyses were performed as described in the “Materials and Methods” section. The CadF and FlpA proteins were readily detected in the WCLs and OMP extracts of the appropriate isolates using the rabbit α-CadF and α-FlpA sera (Figure 4). To ensure that the OMP extracts were not contaminated with cytoplasmic proteins, the samples were probed with a rabbit α-C. jejuni CysM-specific serum. CysM is a 32.4 kDa cytoplasmic protein involved in cysteine biosynthesis [32]. In contrast to the WCLs, CysM was not detected in the OMP extracts of any of the C. jejuni isolates. Collectively, these data indicate that the CadF and FlpA proteins are located in the outer membrane of the C. jejuni transformed isolates.
Figure 4.
CadF and FlpA are localized in the outer membrane protein (OMP) extracts of the C. jejuni transformed isolates. C. jejuni whole-cell lysates (WCL) and OMP extracts from C. jejuni wild-type, the ∆cadF ∆flpA isolate, and the ∆cadF ∆flpA isolate complemented with cadF or flpA were subjected to SDS-PAGE and immunoblot analysis with rabbit α-CadF, α-FlpA, and α-CysM-specific sera. Panels: (A) proteins were stained with Coomassie Brilliant Blue R-250 (CBB-R250) to show equal loading; (B) immunoblot probed with the α-CysM serum, a cytosolic protein used to assess the purity of the OMP extracts; (C) immunoblot probed with the α-CadF serum; and (D) immunoblot probed with the α-FlpA serum. Lanes: C. jejuni strains (1) 81-176 (wild-type), WCL; (2) ∆cadF ∆flpA, WCL; (3) ∆cadF ∆flpA::PcadF-400 bp cadF-FLAG, WCL; (4) ∆cadF ∆flpA::PflpA flpA, WCL; (5) 81-176 (wild-type), OMP; (6) ∆cadF ∆flpA, OMP; (7) ∆cadF ∆flpA::PcadF-400 bp cadF-FLAG, OMP; (8) ∆cadF ∆flpA::PflpA flpA, OMP. The position of the CysM (32 kDa), CadF (37 kDa), and FlpA (46 kDa) proteins are highlighted with arrows. The positions of the molecular mass standards (in kDa) are indicated on the left.
3.5. CadF and FlpA are Required for Binding of C. jejuni to Epithelial Cells
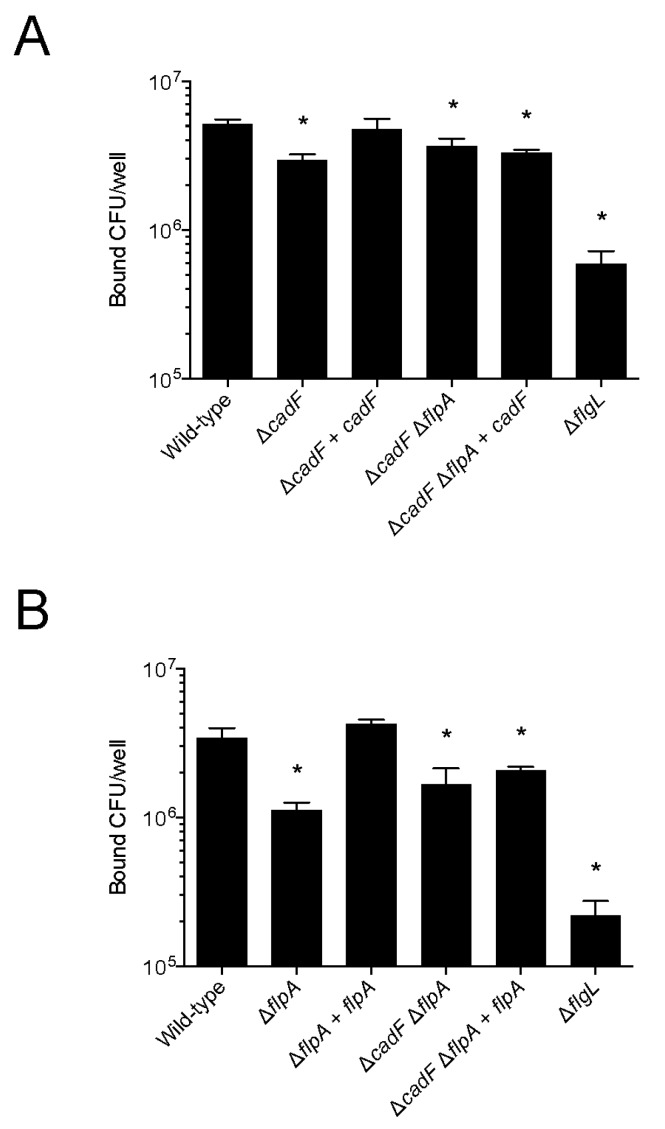
The goal of this study was to determine the individual contribution of the CadF and FlpA proteins in C. jejuni–host cell interactions. Previous work has revealed that the deletion of either the cadF gene or the flpA gene results in the reduction of C. jejuni binding to host epithelial cells [18,30]. In this study, we wanted to know whether the incorporation of a wild-type copy of either a cadF or flpA could restore the defect in host cell binding of the C. jejuni ΔcadF ΔflpA double mutant. This question was experimentally addressed by performing a binding assay with INT 407 cells and the C. jejuni wild-type strain, ΔcadF mutant, ΔflpA mutant, ΔcadF ΔflpA mutant, complemented cadF isolate (ΔcadF ΔflpA::PcadF cadF-FLAG), and complemented flpA isolate (ΔcadF ΔflpA::PflpA flpA) (Figure 5). As expected, the ΔcadF mutant harboring a wild-type copy of the cadF gene restored the level of cell binding to that observed with the wild-type strain (Figure 5A). Similarly, the ΔflpA mutant harboring a wild-type copy of the flpA gene also restored the defect of cell binding (Figure 5B). However, neither cadF nor flpA rescued the binding defect of the ΔcadF ΔflpA mutant to that observed with the wild-type strain (Figure 5A,B). This suggests both CadF and FlpA are required for the binding of C. jejuni to the host cells.
Figure 5.
C. jejuni adherence to INT 407 cells requires both CadF and FlpA. In vitro adherence assays were performed with human INT 407 epithelial cells and the C. jejuni 81-176 wild-type strain, C. jejuni ΔcadF and ΔflpA mutants, and complemented strains as outlined in the “Materials and Methods” section. (A) Transformation of a C. jejuni ΔcadF mutant (ΔcadF::cadFNP-FLAG) but not the ΔcadF ΔflpA mutant restores binding to the level observed with the wild-type strain. (B) Transformation of a C. jejuni flpA deletion mutant (ΔflpA) but not the ΔcadF ΔflpA mutant restores binding to the level observed with the wild-type strain. Values represent the number of adherent bacteria ± standard deviation. The asterisks indicate that the number of bacteria bound to the INT 407 cells was statistically different (* p < 0.05) from that of the C. jejuni wild-type strain, as judged by one-way ANOVA followed by Dunnett’s multiple comparisons test.
3.6. CadF and FlpA are Cooperative in the Binding to Soluble FN and Epithelial Cells
To determine the contribution of CadF and FlpA in C. jejuni binding to FN, it was necessary to introduce a wild-type copy of both the cadF and flpA genes into the ΔcadF ΔflpA double mutant. The strategy that proved to be successful was the introduction of the flpA gene, upstream of the cadF gene, into the rRNA cluster using the prRNA-SpecR-PflpA flpA-PcadF cadF400 bp suicide vector (Figure 1). The resultant colonies were TetR, KanR, CmR, HygroR, and SpecR. A representative gel and immunoblot of the transformants are shown in Figure 6, Panels A and B. Importantly, a 46 kDa immunoreactive band was detected with the α-FlpA serum in the C. jejuni wild-type strain, the ΔcadF ΔflpA mutant complemented with the flpA genes (∆cadF ∆flpA::PflpA flpA), and the ΔcadF ΔflpA mutant complemented with the cadF and flpA gene (ΔcadF ΔflpA::PcadF cadF-FLAG-PflpA flpA isolate). The positive control consisted of the C. jejuni wild-type strain, and the negative control consisted of the C. jejuni ΔcadF ΔflpA mutant. Based on the immunoblot analysis, we now possessed three C. jejuni isolates: (1) the ΔcadF ΔflpA mutant containing the cadF gene (ΔcadF flpA::PcadF cadF); (2) the ΔcadF ΔflpA mutant containing the flpA gene (ΔcadF flpA::PflpA flpA); and (3) the ΔcadF ΔflpA mutant containing both the cadF and flpA genes (ΔcadF ΔflpA:: PcadF cadF-PflpA flpA).
Figure 6.
Maximal C. jejuni binding to soluble fibronectin (FN) and epithelial cells requires both CadF and FlpA. Bacterial whole-cell lysates were separated in SDS 12.5% polyacrylamide gels and either: (A) stained with Coomassie Brilliant Blue R-250 or (B) transferred to polyvinylidene fluoride membranes and probed with a mixture of the rabbit α-CadF serum and rabbit α-FlpA serum. The positive control consisted of a C. jejuni wild-type strain. Lanes: C. jejuni strains (1) 81-176 (wild-type); (2) ∆cadF ∆flpA; (3) ∆cadF ∆flpA::PcadF cadF; (4) ∆cadF ∆flpA::PflpA flp: and (5) ∆cadF ∆flpA::PcadF cadF-PflpA flpA. (C) C. jejuni binding to soluble FN. C. jejuni were incubated with biotin-labeled FN for one hour, pelleted, and the amount of FN that remained in the solution was assayed. The concentration of biotin-labeled FN was determined using a Pierce biotin quantitation kit, as described in the “Materials and Methods” section. Each dot on the chart represents data from independent assays on independent days. The scale is set such that 100% represents complete FN pulldown (measured by assaying PBS only with no FN), and 0% represents no pulldown (measured by assaying FN only, in the absence of C. jejuni). The asterisk indicates that the amount of FN-binding was significantly different than the ∆cadF ∆flpA mutant as tested by one-way ANOVA followed by a post-hoc Dunnett’s analysis (* p < 0.05). (D) C. jejuni binding to epithelial cells. In vitro adherence assays were performed with human INT 407 epithelial cells and the C. jejuni 81-176 wild-type strain, ΔcadF ΔflpA mutant, ΔcadF ΔflpA mutant complemented with both cadF and flpA, and the ΔflgL mutant. Transformation of a C. jejuni ΔcadF ΔflpA mutant with both cadF and flpA restores binding to the level observed with the wild-type strain. Values represent the number of adherent bacteria ± standard deviation. The asterisks indicate that the number of bacteria bound to the INT 407 cells was statistically different (* p < 0.05) from that of the C. jejuni wild-type strain, as judged by one-way ANOVA followed by Dunnett’s multiple comparisons test.
To determine whether CadF, FlpA, or both CadF and FlpA are required for C. jejuni to bind FN at a maximum level (i.e., a level equivalent to that of a wild-type isolate), we compared the FN-binding activity of the ΔcadF ΔflpA isolate, ΔcadF ΔflpA::PcadF cadF isolate, ΔcadF ΔflpA::PflpA flpA isolate, and the ΔcadF ΔflpA:: PcadF cadF-PflpA flpA isolate to a wild-type isolate (Figure 6C). The C. jejuni isolates were incubated with biotin-labeled FN for one hour to allow the soluble FN to bind to C. jejuni. Quantification of unbound biotin-labeled FN that remained in the solution revealed that neither a C. jejuni ΔcadF ΔflpA::PcadF cadF nor a C. jejuni ΔcadF ΔflpA::PflpA flpA isolate was able to bind FN to the same extent as observed with the wild-type strain. However, the binding of the C. jejuni ΔcadF ΔflpA::PcadF cadF-PflpA flpA isolate to FN was indistinguishable from that of the wild-type strain. Based on this finding, we performed a binding assay with INT 407 epithelial cells and compared the binding activity of the ΔcadF ΔflpA isolate, and the ΔcadF ΔflpA:: PcadF cadF-PflpA flpA isolate to a wild-type isolate (Figure 6D). Similar to FN-binding, the C. jejuni ΔcadF ΔflpA::PcadF cadF-PflpA flpA isolate restored the level of cell binding to that we observed with the wild-type strain. In summary, the FN-binding assay clearly demonstrates that both CadF and FlpA are required for maximal C. jejuni binding to FN and epithelial cells.
3.7. CadF and FlpA Bind to the 40–45 kDa Fragment of FN
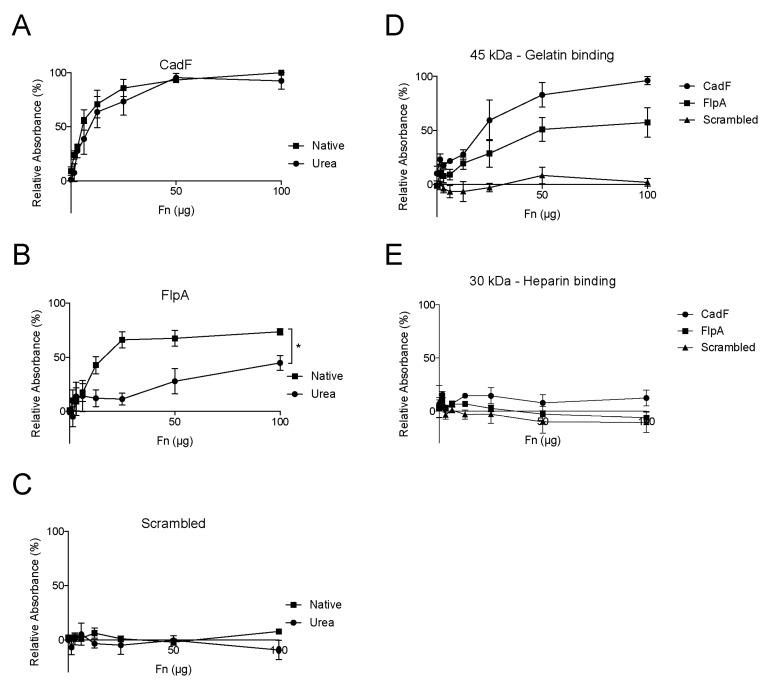
FN is a complex glycoprotein composed of multiple repeats (Supplemental Figure S1). Soluble FN has a compact, folded quaternary structure, stabilized through ionic interactions [35]. However, the quaternary structure of FN can be perturbed in response to binding events, including the binding of FN to the surface of cells for fibril assembly and in response to binding by bacterial adhesins [36]. In the laboratory, mild denaturants can destabilize the interionic interactions, leading to an extended (unfolded) conformation [35]. ELISAs were performed to determine if CadF and FlpA displayed different binding properties to folded versus unfolded FN (Figure 7A–C). Wells were coated with peptides derived from the CadF or FlpA binding domains, and a range of FN concentrations were added to the wells in the presence or absence of 4 M urea. The CadF peptide binds to FN in a saturation-dependent manner in both the presence and absence of urea. In contrast, the FlpA peptide bound to FN in a saturation-dependent manner only in the absence of urea. The simplest explanation for this finding is that the optimal binding of FlpA requires FN to be in a folded, or more native, conformational state.
Figure 7.
Peptides derived from the binding domains of CadF and FlpA bind to folded fibronectin (FN), specifically in the 45 kDa gelatin-binding region. ELISAs were done using peptides derived from the FN-binding domains of CadF, FlpA, and a scrambled peptide to determine the ability to bind to FN in the presence or absence of 4 M urea. Wells were coated with the peptide, and a range of FN concentrations were added to the wells. (A) The CadF peptide binds to FN in a saturation-dependent manner in both the presence and absence of urea. (B) The FlpA peptide only binds FN in a saturation-dependent manner in the absence of urea. (C) The scrambled peptide, which is a randomized stretch of amino acids drawn from the CadF and FlpA peptides, did not bind to FN. (D) Neither the CadF, FlpA, nor scrambled peptide bound to the 30 kDa fragment of FN. (E) Both the CadF and FlpA peptides, but not the scrambled peptide, bound to the 45 kDa fragment of FN. The data are presented as a percentage of relative absorbance, where zero is the assay background derived from bovine serum albumin (BSA)-coated wells, and 100% is the highest value in the given assay. The data represents the mean ± the standard deviation of three independent biological replicates. A summary statistic was calculated from the area under the curve (AUC) in the presence and absence of urea and compared using a paired Student’s t-test. The asterisk indicates that the AUC was significantly different in the presence and absence of urea (* p < 0.05).
Previous work has demonstrated that FNBPs from Gram-positive and Gram-negative pathogens target different regions of the FN molecule. Indeed, many pathogens target the N-terminal 70 kDa fragment that can be obtained by cathepsin D digestion of FN. Tryptic digestion of the 70 kDa fragment yields the 29–30 kDa N-terminal domain (NTD) harboring the heparin-binding domain and the 40–45 kDa NTD harboring the gelatin-binding domain (Supplemental Figure S1). Previous work has revealed that FlpA binds to the 40–45 kDa fragment. To determine if CadF binds N-terminus of FN, ELISAs were performed using the FlpA peptide as a positive control (Figure 7D,E). Similar to FlpA, CadF exhibited dose-dependent and saturable binding to wells coated with the 45 kDa NTD and a low level of binding (nonspecific) to wells coated with the 30 kDa NTD. Collectively, these data indicate that both CadF and FlpA bind to the 45 kDa NTD but, based on the saturation curves generated in the presence and absence of denaturant (urea), they likely bind to different regions within the FN 45 kDa NTD fragment. Noteworthily, the 40–45 kDa NTD is comprised of four FN I and two FN II repeats (FN I6–FN II1–2–FN I7–9), where FN II2 forms a tight complex with FN I6 [37,38,39]. Interestingly, the 40–45 kDa NTD has been proposed to undergo major conformational rearrangements [36].
3.8. CadF and FlpA Binding to Host-Associated Cellular FN Triggers Host Cell Activation of Erk 1/2
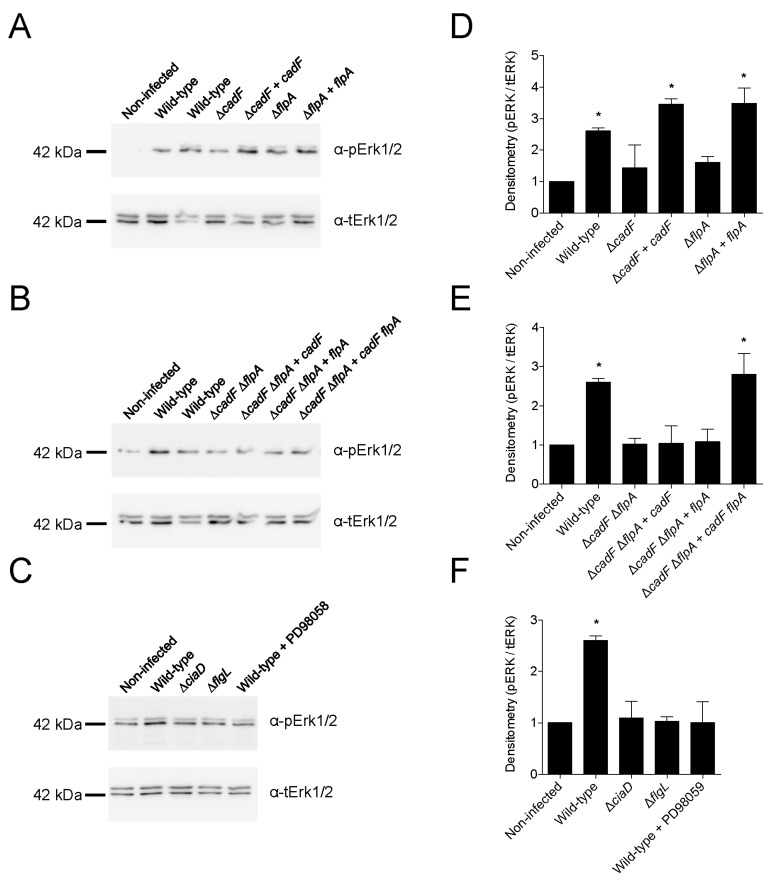
C. jejuni internalization into host cells is a bacterial-driven process that requires activation of the mitogen-activated protein kinase (MAPK) pathway. More specifically, activated (phosphorylated) Erk 1/2 activates cortactin, which, in turn, binds to the actin remodeling proteins N-WASP and WAVE2 to promote membrane ruffling [40]. To address if the CadF and FlpA proteins are necessary for the activation of the host cell kinase Erk 1/2, INT 407 cells were infected with a C. jejuni wild-type strain (positive control), the cadF and flpA mutants and complemented isolates, and a ΔflgL mutant (negative control). The flgL gene encodes the hook-filament junction protein, and the FlgL protein is required for both bacterial motility and Campylobacter effector protein (Cia protein) export from the flagellar T3SS [33]. The results of the assays are shown in Figure 8. The infection of INT 407 cells with the C. jejuni wild-type strain resulted in a significant increase in phosphorylated Erk 1/2 when compared to uninfected cells. In contrast to the wild-type isolate, phosphorylated Erk 1/2 was not increased in cells infected with the ΔcadF mutant, ΔflpA mutant, ΔcadF ΔflpA mutant, nor the ΔcadF ΔflpA mutant harboring a wild-type copy of the cadF or flpA gene (Figure 8A,B,D,E). The necessity of CadF and FlpA for Erk 1/2 activation was demonstrated using complemented isolates (Figure 8, Panels A, B, D, and E). Previous work has demonstrated that the C. jejuni CiaD effector protein, which is secreted from the flagellar T3SS, is required for maximal cell invasion via Erk 1/2 activation and for C. jejuni disease in a mouse model [40,41]. As expected, Erk 1/2 activation was blunted in the INT 407 cells inoculated with the C. jejuni ciaD deletion mutant (ΔciaD) and flgL deletion mutant (ΔflgL) (Figure 8C,F). Finally, the inoculation of the INT 407 cells with the C. jejuni wild-type strain in the presence of the MEK 1/2 inhibitor PD98059 (a potent inhibitor of Erk 1/2 activation) prevented Erk 1/2 activation (Figure 8C,F). These results demonstrate that the C. jejuni CadF and FlpA proteins are both necessary for maximal Erk 1/2 activation.
Figure 8.
Maximal activation of Erk 1/2 requires the C. jejuni CadF and FlpA proteins. INT 407 cells were infected with C. jejuni and whole-cell lysates harvested after 60 min incubation as outlined in the “Materials and Methods” section (Panels A, B, and C). Immunoblots were probed with a phospho-specific antibody to Erk 1/2 (Mr = 42 and 44 kDa). Blots were stripped and re-probed with a total α-Erk 1/2 antibody for a loading control. (Panels D, E, and F) Densitometry was performed on the immunoblots to determine the level of Erk 1/2 activation. The value obtained for INT 407 cells, in the absence of C. jejuni infection, was established as a value of one. Please note two wells were infected with the C. jejuni wild-type isolate in the top two blots (Panels A and B). The asterisks indicate a significant difference in values compared to the control (INT 407 cells only), as judged by one-way ANOVA followed by a post-hoc Dunnett’s analysis (* p < 0.05). Error bars represent standard deviations of samples from three biological replicates.
3.9. C. jejuni Effector Protein Delivery to Host Cells Requires CadF and FlpA
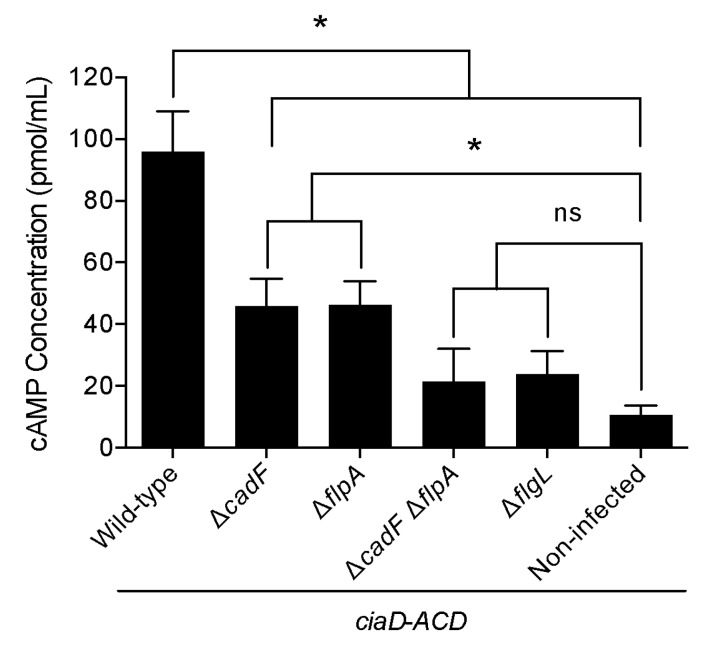
C. jejuni synthesizes at least eight flagellar-dependent secreted virulence proteins, known as the Campylobacter invasion antigens (Cia), that are delivered to the cytosol of host cells where they modify signaling processes [42,43]. We speculated that the MAPK pathway (Erk 1/2) was not activated in INT 407 cells infected with the ΔcadF mutant, ΔflpA mutant, and ΔcadF ΔflpA mutant because, in the absence of effective binding, C. jejuni cannot deliver effector proteins into the cells. To determine if Cia protein delivery to host cells requires bacteria–host cell contact mediated by the CadF and/or FlpA FNBPs, we used isolates transformed with the ciaD-ACD construct coupled with the ACD reporter assay. We specifically chose to use a CiaD-ACD fusion protein for these assays because CiaD is the most extensively characterized Cia protein and, as mentioned previously, CiaD is required for maximal Erk 1/2 activation [40,41]. A shuttle vector harboring the ciaD-ACD construct was introduced into the C. jejuni wild-type strain and mutants by conjugation. All C. jejuni transformants were confirmed to produce the CiaD-ACD fusion protein (C. jejuni wild-type strain (positive control), ΔcadF mutant, ΔflpA mutant, ΔcadF ΔflpA mutant, and ΔflgL mutant (secretion-negative, negative control)) (Supplemental Figure S2). In addition, all C. jejuni transformants, except for the ΔflgL mutant, were motile (Supplemental Figure S3). The ability of the C. jejuni transformants to deliver the CiaD-ACD protein to INT 407 cells was determined after a 45 min infection. As expected, infection with the C. jejuni wild-type strain harboring ciaD-ACD resulted in a significant increase in cAMP compared to the ΔflgL secretion-negative mutant (Figure 9). Regarding the C. jejuni FN-binding mutants, infection of the cells with the wild-type strain resulted in a significant increase in cAMP compared to the ΔcadF, ΔflpA, and ΔcadF ΔflpA mutants. Notably, infection of the cells with the ΔcadF mutant as well as the ΔflpA mutant resulted in a moderate increase in cAMP when compared to the ΔcadF ΔflpA double mutant (Figure 9). Collectively, these results demonstrate that the CiaD effector protein requires the CadF and FlpA proteins to be effectively delivered to the cytosol of host epithelial cells.
Figure 9.
Efficient delivery of the C. jejuni CiaD effector protein to host cells requires the CadF and FlpA adhesins. A C. jejuni wild-type strain, ΔcadF mutant, ΔflpA mutant, ΔcadF ΔflpA mutant, and ΔflgL mutant (negative control) were transformed with shuttle vector harboring ciaD fused to the adenylate cyclase domain (ciaD-ACD). The delivery of the CiaD-ACD fusion proteins to the cytosol of human INT 407 cells was determined using the adenylate cyclase domain (ACD) delivery assay, as described in the “Materials and Methods” section. The asterisk indicates that the amount of cAMP produced in cells infected with the C. jejuni wild-type strain was significantly greater than the value obtained from a given mutant, as judged by one-way ANOVA followed by Tukey’s analysis (* p< 0.05). The amount of cAMP produced in cells infected with the C. jejuni ΔcadF mutant and ΔflpA mutant was also significantly greater than the non-infected samples (* p< 0.05). The values obtained from the C. jejuni ΔcadF ΔflpA mutant, and ΔflgL mutant showed no statistical significance compared to non-infected samples as judged by one-way ANOVA followed by Tukey’s analysis (* p< 0.05; ns, non-significant). The data represent the mean ± standard deviation of the values from three biological replicates.
4. Discussion
C. jejuni synthesizes a repertoire of adhesins (i.e., CadF, FlpA, JlpA, etc.) to promote cell adherence. Among these adhesins, CadF and FlpA are two surface-exposed proteins that bind to the extracellular matrix glycoprotein FN. Previously, both CadF and FlpA were reported to promote C. jejuni binding to cultured epithelial cells and were shown to be required for C. jejuni to colonize chickens [17,21]. However, the individual role of CadF and FlpA in binding to FN and host cells was not known previous to this study, in part, because researchers had difficulty in generating a CadF complemented isolate. In this study, we utilized a two-step cloning strategy to complement a C. jejuni ΔcadF mutant. The experiments performed herein revealed that: (1) neither CadF nor FlpA alone could restore the defect in binding of C. jejuni ΔcadF ΔflpA double mutant to host cells; (2) CadF and FlpA binding to FN is additive; (3) both CadF and FlpA are necessary for C. jejuni activation of the MAPK signaling pathway (Erk 1/2); and (4) both CadF and FlpA are required for the delivery of the Cia effector proteins into the cytosol of a host cell. A model depicting the C. jejuni CadF- and FlpA-driven processes is shown in Figure 10. Noteworthily, both CadF and FlpA proteins are conserved among C. jejuni isolates, implying that these two proteins contribute to C. jejuni pathogenesis [16,18].
Figure 10.
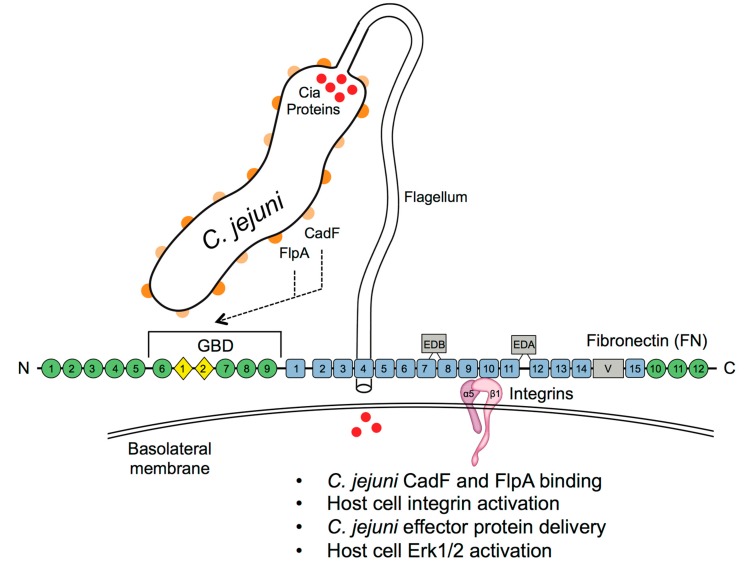
A model of C. jejuni interaction with host cells. C. jejuni drives the host cell processes by forming the three-component bridge between the CadF and FlpA proteins, host cell-associated fibronectin (FN), and α5β1 integrin. While only a single FN molecule and a single α5β1 integrin are shown for simplicity, C. jejuni-induced host cell signaling requires multiple FN molecules and the occupancy and clustering of multiple α5β1 integrin heterodimers for the maximal stimulation of cellular signaling pathways. FN is composed of 12 FN type I repeats (FN I, green circles), 2 FN type II repeats (FN II, yellow diamonds), and 15–18 FN type III repeats (FN III, blue squares). Additionally, Extra Domains A and B (EDA and EDB) are shown and the variable (V) region that connects two type III repeats. Both CadF and FlpA proteins bind to the 45 kDa N-terminal domain (NTD) of FN that harbors the gelatin-binding domain (GBD). However, CadF and FlpA likely bind to different regions of the 45 kDa fragment based on ELISAs performed in the presence or absence of 4 M urea (see the text for details). Intimate contact between C. jejuni and the host cells by CadF and FlpA facilitates the delivery of the C. jejuni effector protein CiaD, which is necessary for Erk 1/2 activation. Together, CadF and FlpA set the stage for host cell invasion by binding to FN associated with the α5β1 integrins on the basolateral surface of cells (sites of focal adhesions) and to deliver effectors that stimulate host cellular signaling pathways.
Many bacterial genes and gene fragments have been found to be lethal to E. coli when expressed [44]. This introduces a technical limitation when studying these genes and their products. Since the discovery of CadF, researchers have been unable to complement a C. jejuni cadF mutant due to the inability to construct a complementation vector. In this study, we developed an innovative approach to complement the cadF deletion mutant. Our approach involved generating a suicide vector with a wild-type copy of the cadF gene without its promoter in E. coli. The promoterless cadF gene was then introduced into the rRNA cluster of the C. jejuni ΔcadF mutant. A second transformation was used to introduce the cadF promoter, thereby restoring the expression of the cadF gene from its native promoter. The introduction of both fragments into the rRNA region was dependent on homologous recombination using flanking sequences and the use of antibiotic resistance genes to select for the desired recombinants. We propose that the strategy used in this study for complementation can be used for other C. jejuni genes that, when expressed in E. coli, are toxic. We also believe that this cloning strategy should be broadly applicable to other bacteria.
In accordance with previous studies, we found that a deletion of the cadF gene, flpA gene, or both genes significantly reduced the number of C. jejuni bound to INT 407 cells. Moreover, the CadF and FlpA proteins, which share only 25.6% similarity at the amino acid level, cannot functionally complement one another as evident in the phenotypic assays performed in this study (e.g., facilitating cell adherence, promoting FN binding, stimulating cell signaling, and enabling effector delivery to host cells). This finding is consistent with the fact that the CadF and FlpA proteins have distinct FN-binding domains [19,20]. Noteworthily, deduced amino acid sequences of the S. aureus FNBPA and FNBPB share a high level (74.2%) of similarity [45], and that complementation of a fnbA fnbB double mutant with either fnbA or fnbB fully restores the FN-binding activity [46]. Collectively, the data indicate that CadF and FlpA work together to maximize the binding of C. jejuni to host-associated FN.
The FN molecule is composed of multiple copies of three repeats or modules, each having a well-organized secondary and tertiary structure [36]. In plasma, FN is present in a compact form where the quaternary structure is facilitated through the interactions of specific modules. However, the binding of FN to cells or, more relevant to this study, the binding of bacterial FNBPs to FN, results in perturbation of the quaternary structure and an extended molecule (unfolded conformation). Moreover, studies have revealed that bacterial FNBPs bind to different sites (domains) within the FN molecule [38]. The 29 kDa N-terminal fragment of FN is the most prominent and canonical FN region targeted by bacterial FNBPs, such as FNBPA and FNBPB in S. aureus, and F1 and Sfb1 in S. pyogenes [47,48]. The 29 kDa fragment contains five FN I repeats (FN I1–5) and harbors the heparin-binding domain. The adjacent 40–45 kDa fragment contains the gelatin-binding domain (GBD)/collagen-binding domain and functions as the non-canonical binding site for select bacterial FNBPs, including the F1 and Sfb1 from S. pyogenes, which also bind to the 29 kDa fragment [49]. The 40–45 kDa FN fragment is comprised of four FN I and two FN II repeats in the following order: FN I6–FN II1–2–FN I7–9. Bacterial FNBPs also target the 120–140 kDa central fragment of FN (FN III9–10, FN III12). For example, FbpA and FbpB from Clostridium perfringens bind to FN III9–10, and Embp from Staphylococcus epidermidis binds to FN III12 [50,51]. The differences in the CadF and FlpA FN-binding domains prompted experiments to determine the region that CadF binds to FN. Prior to this study, FlpA was reported to bind to the FN fragment harboring the GBD. Our study revealed that both CadF and FlpA bind to this 45 kDa fragment of FN. However, differences were apparent in the binding properties of the CadF and FlpA peptides. Although the saturation curve for CadF binding to FN was nearly identical in the absence of urea (folded conformation) and presence of urea (unfolded conformation), the binding of the FlpA peptide was noticeably different in the absence versus the presence of urea. These findings suggest that FlpA binds to a conformation-dependent site that is disrupted when FN is unfolded. As mentioned above, FN is folded when secreted into the extracellular space but, upon binding to the α5β1 integrins, it unfolds. The extended form of FN exposes cryptic binding sites that promote the assembly of FN molecules into fibrils [52]. We speculate that, even though CadF and FlpA both bind to the 45 kDa GBD fragment, they modulate the behavior of FN in different ways. Additional studies are required to determine if CadF and FlpA binding to FN is sequential, or if the binding of one of adhesin can potentiate the binding of the second adhesin.
Bacteria commonly invade (nonphagocytic) epithelial cells via a zipper (exclusive) or a trigger (inclusive) mechanism [53]. The zipper mechanism involves the interaction of bacterial adhesins binding to host cell receptors, resulting in the activation of host cell signaling cascades and bacterial internalization. The trigger mechanism involves the translocation of bacterial effectors into the cytosol of a host cell, which stimulates host cell signaling cascades and massive cytoskeleton rearrangement that drives the formation of membrane ruffles, ultimately leading to bacterial uptake. S. aureus uses the FNBPs to facilitate cell internalization by a zipper mechanism [54]. The FNBP-mediated mechanism of S. aureus internalization requires FN as a bridging molecule and the α5β1 integrin as a host cell receptor, resulting in signal transduction, tyrosine kinase activity, and cytoskeletal rearrangements [38,55]. In S. aureus, fnbA and fnbB single mutants show no significant reduction in adhesion to FN [46], but both FNBPs are required for severe disease in mice [56]. Compared with other known and putative C. jejuni adhesins, a distinguishing feature of the CadF and FlpA proteins is that they promote cellular invasion by binding to FN bound to α5β1 integrins on the basolateral surface of a cell [57,58,59]. However, in the early 1990s, it was also reported that C. jejuni invasion, but not cell adherence, was significantly reduced in the presence of chloramphenicol, a selective inhibitor of bacterial protein synthesis [60]. Later it was found that C. jejuni secretes effector proteins, albeit via its flagellum rather than a dedicated secretory system, and that knockouts in genes encoding the effector proteins blunted C. jejuni host cell invasion [25,40,41,42]. Together, these findings indicate that C. jejuni utilizes aspects of both the zipper and trigger mechanisms—in essence, a hybrid of the two processes [61].
The finding that both CadF and FlpA are required for maximal binding to FN raised the possibility that both proteins were required for the delivery of the Cia effector proteins into the cytosol of a host target cell. The experiments performed in this study revealed that CadF and FlpA are bi-functional proteins that act in a cooperative manner to first bind to host cells and then to stimulate signal transduction pathways, including the MAPK pathway, involved in C. jejuni cell invasion. More precisely, the delivery of the CiaD effector protein to the cytosol of host cells stimulates Erk 1/2 activation, which, in turn, phosphorylates (activates) cortactin [40,41]. Activated cortactin promotes membrane ruffles by binding to the actin remodeling proteins N-WASP and WAVE2 [62]. In support of this model of C. jejuni cell invasion, mutagenesis studies have also indicated that CadF and an intact flagellum are involved in Rho GTPase activation and host cell invasion [63,64]. Our findings support a binding and effector mechanism of C. jejuni cell invasion, whereby the CadF and FlpA proteins facilitate the delivery of effector proteins.
In a recent study, the contribution of the CadF adhesin to disease was assessed by infecting abiotic (gnotobiotic) IL-10−/− mice with the C. jejuni 81-176 wild-type strain and with a cadF deletion mutant [65]. In this model, the animals were inoculated with C. jejuni (109 CFU on two consecutive days), and disease parameters were assessed daily until six days post-infection. Pathology was scored based on the presence of blood in the stool, diarrhea, and animal behavior. Interestingly, the median pathology score at day six in the mice infected with the ΔcadF mutant was approximately eight, while the wild-type infected mice had more severe symptoms with a median pathology score of 12. In addition, there was attenuated intestinal tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) responses during infection with the ΔcadF mutant compared to the wild-type strain. Thus, the ΔcadF mutant was blunted in clinical symptoms in mice administered high bacterial doses (i.e., an inoculum that a human is unlikely to encounter). The diminished clinical symptoms observed in the mice inoculated with the ΔcadF mutant are consistent with a model whereby the CadF protein contributes to disease by facilitating cell adherence and delivery of the effector proteins. However, given that C. jejuni possesses multiple adhesins and that cell adherence is multifactorial, it is possible to bypass the necessity for the intimate cell adherence via CadF, and possibly FlpA, by using high/repeated doses of mutant bacteria. Noteworthily, previous work has also demonstrated that the CadF adhesin, as well as intact flagella, is necessary for Cdc42 and Rac1 activation (GTP-bound) in human INT 407 cells [66,67]. Cdc42 and Rac1 are the primary host cell Rho GTPases involved in C. jejuni invasion. We have also reported that Rac1-GTP production is also diminished in human INT 407 cells infected with C. jejuni flpA and cadF flpA double mutant [19]. Given the findings from this study, which demonstrate that CadF and FlpA work together in facilitating cell adherence, promoting FN binding, stimulating cell signaling, and enabling effector delivery to host cells, studies are warranted to determine if disease symptoms arise in animals inoculated with a C. jejuni cadF flpA double mutant.
5. Conclusion
In agreement with previous studies, this study further cements the concept that CadF and FlpA are the major FNBPs that promote C. jejuni attachment to host cells. With the complementation of cadF in ΔcadF and ΔcadF ΔflpA isolates, we demonstrated that cadF or flpA alone are unable to fully restore the defect in host cell FN-binding. Our data also suggest that CadF and FlpA bind to different sites within the GBD fragment of FN, indicating the cooperative role of these two proteins. Together, the CadF and FlpA adhesins facilitate the binding of C. jejuni to host cells, permit the delivery of C. jejuni effector proteins into the cytosol of a host target cell, and aid in the rewiring of host cell signaling pathways that alter host cell behavior. C. jejuni utilizes the unique binding and effector mechanism, where the coordinated action of the CadF and FlpA FNBPs and Cia effector proteins promote the C. jejuni invasion of cells, and modulates host cell signaling. On a final note, we hope that the genetic strategy described herein for gene complementation can be applied to genetic studies with C. jejuni and other bacteria with less well-developed genetic systems.
Acknowledgments
We thank Colby M. Corneau for his assistance with SDS-PAGE and immunoblots. We also thank Christopher R. Gourley and Courtney M. Klappenbach for proofreading the manuscript.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/2076-2607/8/3/389/s1.
Author Contributions
Conceptualization, M.E.K., P.K.T., and N.M.N.; methodology, M.E.K., P.K.T., and N.M.N.; formal analysis, M.E.K., P.K.T., N.M.N., and K.L.T.; investigation, M.E.K., P.K.T., N.M.N., and K.L.T.; data curation, M.E.K.; writing—original draft preparation, M.E.K. and P.K.T.; writing—review and editing, P.K.T., N.M.N., K.L.T., and M.E.K.; funding acquisition, M.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the National Institutes of Health (Award Number R01AI125356) to M.E.K.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, data collection, data analyses, writing of the manuscript, or in the decision to publish. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Froman G., Switalski L.M., Speziale P., Hook M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J. Biol. Chem. 1987;262:6564–6571. [PubMed] [Google Scholar]
- 2.McDevitt D., Francois P., Vaudaux P., Foster T.J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 3.Talay S.R., Valentin-Weigand P., Timmis K.N., Chhatwal G.S. Domain structure and conserved epitopes of Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol. Microbiol. 1994;13:531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 4.Kingsley R.A., Santos R.L., Keestra A.M., Adams L.G., Baumler A.J. Salmonella enterica serotype Typhimurium ShdA is an outer membrane fibronectin-binding protein that is expressed in the intestine. Mol. Microbiol. 2002;43:895–905. doi: 10.1046/j.1365-2958.2002.02805.x. [DOI] [PubMed] [Google Scholar]
- 5.Tertti R., Skurnik M., Vartio T., Kuusela P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect. Immun. 1992;60:3021–3024. doi: 10.1128/IAI.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Probert W.S., Johnson B.J. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol. Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 7.Brissette C.A., Bykowski T., Cooley A.E., Bowman A., Stevenson B. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun. 2009;77:2802–2812. doi: 10.1128/IAI.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinkman M.B., McGill M.A., Pettersson J., Rogers A., Matejkova P., Smajs D., Weinstock G.M., Norris S.J., Palzkill T. A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin. Infect. Immun. 2008;76:1848–1857. doi: 10.1128/IAI.01424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altekruse S.F., Stern N.J., Fields P.I., Swerdlow D.L. Campylobacter jejuni—An emerging foodborne pathogen. Emerg. Infect. Dis. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel M.C., Vugia D.J., Shallow S., Marcus R., Segler S., McGivern T., Kassenborg H., Reilly K., Kennedy M., Angulo F., et al. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin. Infect. Dis. 2004;38:S165–S174. doi: 10.1086/381583. [DOI] [PubMed] [Google Scholar]
- 11.Blaser M.J., Berkowitz I.D., LaForce F.M., Cravens J., Reller L.B., Wang W.L. Campylobacter enteritis: Clinical and epidemiologic features. Ann. Intern. Med. 1979;91:179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- 12.Allos B.M. Campylobacter jejuni infections: Update on emerging issues and trends. Clin. Infect. Dis. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 13.Schwerer B. Antibodies against gangliosides: A link between preceding infection and immunopathogenesis of Guillain-Barré syndrome. Microbes Infect. 2002;4:373–384. doi: 10.1016/S1286-4579(02)01550-2. [DOI] [PubMed] [Google Scholar]
- 14.Frenzen P.D. Economic cost of Guillain-Barré syndrome in the United States. Neurology. 2008;71:21–27. doi: 10.1212/01.wnl.0000316393.54258.d1. [DOI] [PubMed] [Google Scholar]
- 15.Black R.E., Levine M.M., Clements M.L., Hughes T.P., Blaser M.J. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 16.Konkel M.E., Garvis S.G., Tipton S.L., Anderson D.E., Jr., Cieplak W., Jr. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 1997;24:953–963. doi: 10.1046/j.1365-2958.1997.4031771.x. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan R.C., Neal-McKinney J.M., Dhillon A.S., Miller W.G., Konkel M.E. Examination of Campylobacter jejuni putative adhesins leads to the identification of a new protein, designated FlpA, required for chicken colonization. Infect. Immun. 2009;77:2399–2407. doi: 10.1128/IAI.01266-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konkel M.E., Larson C.L., Flanagan R.C. Campylobacter jejuni FlpA binds fibronectin and is required for maximal host cell adherence. J. Bacteriol. 2010;192:68–76. doi: 10.1128/JB.00969-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson C.L., Samuelson D.R., Eucker T.P., O’Loughlin J.L., Konkel M.E. The fibronectin-binding motif within FlpA facilitates Campylobacter jejuni adherence to host cell and activation of host cell signaling. Emerg. Microbes Infect. 2013;2:e65. doi: 10.1038/emi.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konkel M.E., Christensen J.E., Keech A.M., Monteville M.R., Klena J.D., Garvis S.G. Identification of a fibronectin-binding domain within the Campylobacter jejuni CadF protein. Mol. Microbiol. 2005;57:1022–1035. doi: 10.1111/j.1365-2958.2005.04744.x. [DOI] [PubMed] [Google Scholar]
- 21.Ziprin R.L., Young C.R., Stanker L.H., Hume M.E., Konkel M.E. The absence of cecal colonization of chicks by a mutant of Campylobacter jejuni not expressing bacterial fibronectin-binding protein. Avian Dis. 1999;43:586–589. doi: 10.2307/1592660. [DOI] [PubMed] [Google Scholar]
- 22.Karlyshev A.V., Wren B.W. Development and application of an insertional system for gene delivery and expression in Campylobacter jejuni. Appl. Environ. Microbiol. 2005;71:4004–4013. doi: 10.1128/AEM.71.7.4004-4013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korlath J.A., Osterholm M.T., Judy L.A., Forfang J.C., Robinson R.A. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J. Infect. Dis. 1985;152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- 24.Negretti N.M., Clair G., Talukdar P.K., Gourley C.R., Huynh S., Adkins J.N., Parker C.T., Corneau C.M., Konkel M.E. Campylobacter jejuni demonstrates conserved proteomic and transcriptomic responses when co-cultured with human INT 407 and Caco-2 epithelial cells. Front. Microbiol. 2019;10:755. doi: 10.3389/fmicb.2019.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen J.E., Pacheco S.A., Konkel M.E. Identification of a Campylobacter jejuni-secreted protein required for maximal invasion of host cells. Mol. Microbiol. 2009;73:650–662. doi: 10.1111/j.1365-2958.2009.06797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gourley C.R., Negretti N.M., Konkel M.E. The food-borne pathogen Campylobacter jejuni depends on the AddAB DNA repair system to defend against bile in the intestinal environment. Sci. Rep. 2017;7:14777. doi: 10.1038/s41598-017-14646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nirdnoy W., Mason C.J., Guerry P. Mosaic structure of a multiple-drug-resistant, conjugative plasmid from Campylobacter jejuni. Antimicrob. Agents Chemother. 2005;49:2454–2459. doi: 10.1128/AAC.49.6.2454-2459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Shoaf-Sweeney K.D., Larson C.L., Tang X., Konkel M.E. Identification of Campylobacter jejuni proteins recognized by maternal antibodies of chickens. Appl. Environ. Microbiol. 2008;74:6867–6875. doi: 10.1128/AEM.01097-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monteville M.R., Yoon J.E., Konkel M.E. Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology. 2003;149:153–165. doi: 10.1099/mic.0.25820-0. [DOI] [PubMed] [Google Scholar]
- 31.Lamla T., Erdmann V.A. The Nano-tag, a streptavidin-binding peptide for the purification and detection of recombinant proteins. Protein Expr. Purif. 2004;33:39–47. doi: 10.1016/j.pep.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Garvis S.G., Tipton S.L., Konkel M.E. Identification of a functional homolog of the Escherichia coli and Salmonella typhimurium cysM gene encoding O-acetylserine sulfhydrylase B in Campylobacter jejuni. Gene. 1997;185:63–67. doi: 10.1016/S0378-1119(96)00631-2. [DOI] [PubMed] [Google Scholar]
- 33.Neal-McKinney J.M., Konkel M.E. The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells. Front. Cell. Infect. Microbiol. 2012;2:31. doi: 10.3389/fcimb.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neal-McKinney J.M., Christensen J.E., Konkel M.E. Amino-terminal residues dictate the export efficiency of the Campylobacter jejuni filament proteins via the flagellum. Mol. Microbiol. 2010;76:918–931. doi: 10.1111/j.1365-2958.2010.07144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith M.L., Gourdon D., Little W.C., Kubow K.E., Eguiluz R.A., Luna-Morris S., Vogel V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer L.M., Ma W., Mosher D.F. Dynamic structure of plasma fibronectin. Crit. Rev. Biochem. Mol. Biol. 2015;51:213–227. doi: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bocquier A.A., Potts J.R., Pickford A.R., Campbell I.D. Solution structure of a pair of modules from the gelatin-binding domain of fibronectin. Structure. 1999;7:1451–1460. doi: 10.1016/S0969-2126(00)88336-7. [DOI] [PubMed] [Google Scholar]
- 38.Henderson B., Nair S., Pallas J., Williams M.A. Fibronectin: A multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol. Rev. 2011;35:147–200. doi: 10.1111/j.1574-6976.2010.00243.x. [DOI] [PubMed] [Google Scholar]
- 39.Pickford A.R., Smith S.P., Staunton D., Boyd J., Campbell I.D. The hairpin structure of the 6F1 1F2 2F2 fragment from human fibronectin enhances gelatin binding. EMBO J. 2001;20:1519–1529. doi: 10.1093/emboj/20.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuelson D.R., Konkel M.E. Serine phosphorylation of cortactin is required for maximal host cell invasion by Campylobacter jejuni. Cell Commun. Signal. 2013;11:82. doi: 10.1186/1478-811X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuelson D.R., Eucker T.P., Bell J.A., Dybas L., Mansfield L.S., Konkel M.E. The Campylobacter jejuni CiaD effector protein activates MAP kinase signaling pathways and is required for the development of disease. Cell Commun. Signal. 2013;11:79. doi: 10.1186/1478-811X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konkel M.E., Klena J.D., Rivera-Amill V., Monteville M.R., Biswas D., Raphael B., Mickelson J. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 2004;186:3296–3303. doi: 10.1128/JB.186.11.3296-3303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konkel M.E., Kim B.J., Rivera-Amill V., Garvis S.G. Bacterial secreted proteins are required for the internaliztion of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 1999;32:691–701. doi: 10.1046/j.1365-2958.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- 44.Kimelman A., Levy A., Sberro H., Kidron S., Leavitt A., Amitai G., Yoder-Himes D.R., Wurtzel O., Zhu Y., Rubin E.M., et al. A vast collection of microbial genes that are toxic to bacteria. Genome Res. 2012;22:802–809. doi: 10.1101/gr.133850.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke F.M., McCormack N., Rindi S., Speziale P., Foster T.J. Fibronectin-binding protein B variation in Staphylococcus aureus. BMC Microbiol. 2010;10:160. doi: 10.1186/1471-2180-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greene C., McDevitt D., Francois P., Vaudaux P.E., Lew D.P., Foster T.J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz-Linek U., Pilka E.S., Pickford A.R., Kim J.H., Hook M., Campbell I.D., Potts J.R. High affinity streptococcal binding to human fibronectin requires specific recognition of sequential F1 modules. J. Biol. Chem. 2004;279:39017–39025. doi: 10.1074/jbc.M405083200. [DOI] [PubMed] [Google Scholar]
- 48.Joh D., Wann E.R., Kreikemeyer B., Speziale P., Hook M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/S0945-053X(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 49.Sela S., Aviv A., Tovi A., Burstein I., Caparon M.G., Hanski E. Protein F: An adhesin of Streptococcus pyogenes binds fibronectin via two distinct domains. Mol. Microbiol. 1993;10:1049–1055. doi: 10.1111/j.1365-2958.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 50.Katayama S., Tagomori M., Morita N., Yamasaki T., Nariya H., Okada M., Watanabe M., Hitsumoto Y. Determination of the Clostridium perfringens-binding site on fibronectin. Anaerobe. 2015;34:174–181. doi: 10.1016/j.anaerobe.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Christner M., Franke G.C., Schommer N.N., Wendt U., Wegert K., Pehle P., Kroll G., Schulze C., Buck F., Mack D., et al. The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol. Microbiol. 2010;75:187–207. doi: 10.1111/j.1365-2958.2009.06981.x. [DOI] [PubMed] [Google Scholar]
- 52.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cossart P., Sansonetti P.J. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 54.Sinha B., Francois P.P., Nusse O., Foti M., Hartford O.M., Vaudaux P., Foster T.J., Lew D.P., Herrmann M., Krause K.H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 55.Hymes J.P., Klaenhammer T.R. Stuck in the middle: Fibronectin-binding proteins in Gram-positive bacteria. Front. Microbiol. 2016;7:1504. doi: 10.3389/fmicb.2016.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shinji H., Yosizawa Y., Tajima A., Iwase T., Sugimoto S., Seki K., Mizunoe Y. Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect. Immun. 2011;79:2215–2223. doi: 10.1128/IAI.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monteville M.R., Konkel M.E. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect. Immun. 2002;70:6665–6671. doi: 10.1128/IAI.70.12.6665-6671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatayama S., Shimohata T., Amano S., Kido J., Nguyen A.Q., Sato Y., Kanda Y., Tentaku A., Fukushima S., Nakahashi M., et al. Cellular tight junctions prevent effective Campylobacter jejuni invasion and inflammatory barrier disruption promoting bacterial invasion from lateral membrane in polarized intestinal epithelial cells. Front. Cell. Infect. Microbiol. 2018;8:15. doi: 10.3389/fcimb.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouwman L.I., Niewold P., van Putten J.P. Basolateral invasion and trafficking of Campylobacter jejuni in polarized epithelial cells. PLoS ONE. 2013;8:e54759. doi: 10.1371/journal.pone.0054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konkel M.E., Cieplak W., Jr. Altered synthetic response of Campylobacter jejuni to cocultivation with human epithelial cells is associated with enhanced internalization. Infect. Immun. 1992;60:4945–4949. doi: 10.1128/IAI.60.11.4945-4949.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ó’Cróinín T., Backert S. Host epithelial cell invasion by Campylobacter jejuni: Trigger or zipper mechanism? Front. Cell. Infect. Microbiol. 2012;2:25. doi: 10.3389/fcimb.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirkbride K.C., Sung B.H., Sinha S., Weaver A.M. Cortactin: A multifunctional regulator of cellular invasiveness. Cell Adh. Migr. 2011;5:187–198. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eucker T.P., Konkel M.E. The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal Campylobacter jejuni invasion of host cells by stimulating membrane ruffling. Cell. Microbiol. 2012;14:226–238. doi: 10.1111/j.1462-5822.2011.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krause-Gruszczynska M., Rohde M., Hartig R., Genth H., Schmidt G., Keo T., Konig W., Miller W.G., Konkel M.E., Backert S. Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell. Microbiol. 2007;9:2431–2444. doi: 10.1111/j.1462-5822.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt A.M., Escher U., Mousavi S., Tegtmeyer N., Boehm M., Backert S., Bereswill S., Heimesaat M.M. Immunopathological properties of the Campylobacter jejuni flagellins and the adhesin CadF as assessed in a clinical murine infection model. Gut Pathog. 2019;11:24. doi: 10.1186/s13099-019-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boehm M., Krause-Gruszczynska M., Rohde M., Tegtmeyer N., Takahashi S., Oyarzabal O.A., Backert S. Major host factors involved in epithelial cell invasion of Campylobacter jejuni: Role of fibronectin, integrin beta1, FAK, Tiam-1, and DOCK180 in activating Rho GTPase Rac1. Front. Cell. Infect. Microbiol. 2011;1:17. doi: 10.3389/fcimb.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krause-Gruszczynska M., Boehm M., Rohde M., Tegtmeyer N., Takahashi S., Buday L., Oyarzabal O.A., Backert S. The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: Role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2. Cell Commun. Signal. 2011;9:32. doi: 10.1186/1478-811X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.