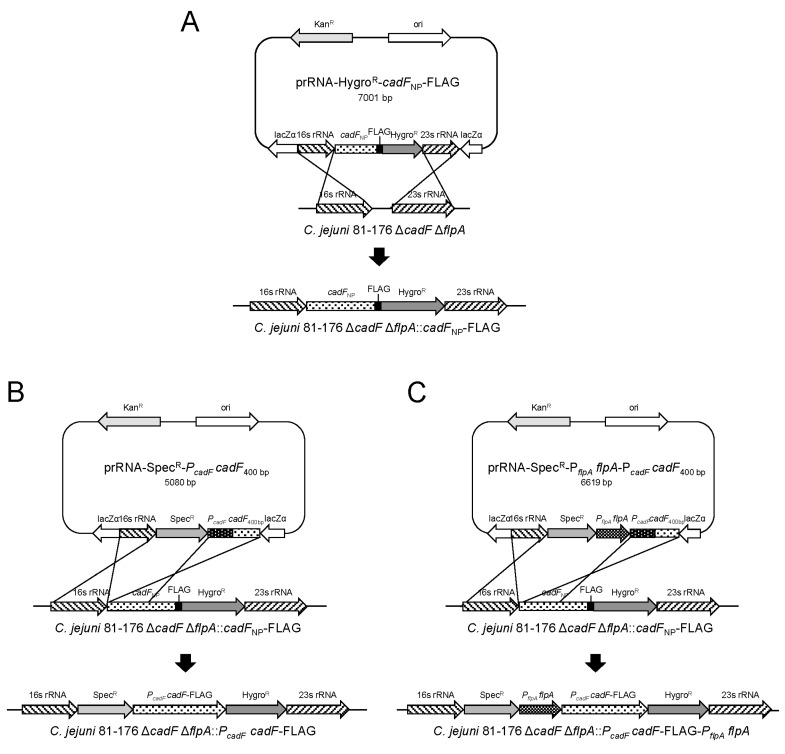
Figure 1.
Two-step suicide vector strategy for the introduction of a wild-type copy of the cadF and flpA genes into the C. jejuni chromosome. (A) In the first step, a promoterless cadF gene fused to a FLAG-tag was cloned into the prRNA-HygroR vector, resulting in the prRNA-HygroR-cadFNP-FLAG construct. This construct was introduced into a C. jejuni ΔcadF mutant or ΔcadF ΔflpA double mutant by electroporation to generate the isolates that have promoterless cadF gene inserted between the 16S rRNA and 23S rRNA genes in the chromosome. (B) The second step includes the cloning of DNA fragments containing the cadF promoter (PcadF) and a portion of cadF coding sequence from the 5′-end (283 bp, 334 bp, or 400 bp) into a prRNA vector with the 16S rRNA gene and the spectinomycin resistance cassette. Here, the representative image shows the insertion of the cadF promoter and the 400 bp of cadF 5′-end into the C. jejuni ΔcadF ΔflpA::cadFNP-FLAG strain. A cadF-complemented isolate was generated by recombination with the 16S rRNA gene and the DNA sequence containing the 5′-end of the cadF gene. The HygroR SpecR transformants were confirmed to contain the entire cadF gene with its endogenous promoter inserted between the 16S and 23S rRNA genes by PCR analysis. (C) A similar approach was taken to complement a ΔcadF ΔflpA mutant with wild-type copies of cadF and flpA. In the first step, the promoterless cadF gene was incorporated in the rRNA region of the chromosome of the C. jejuni ΔcadF ΔflpA mutant. In the second step, the entire flpA gene with its native promoter, the promoter of cadF, and a portion of the cadF coding sequence from the 5′-end (234 bp, 334 bp, or 400 bp) were inserted in the rRNA region to generate the cadF flpA double complement isolate.