Abstract
Background
Community transmission of coronavirus 2019 (Covid-19) was detected in the state of Washington in February 2020.
Methods
We identified patients from nine Seattle-area hospitals who were admitted to the intensive care unit (ICU) with confirmed infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Clinical data were obtained through review of medical records. The data reported here are those available through March 23, 2020. Each patient had at least 14 days of follow-up.
Results
We identified 24 patients with confirmed Covid-19. The mean (±SD) age of the patients was 64±18 years, 63% were men, and symptoms began 7±4 days before admission. The most common symptoms were cough and shortness of breath; 50% of patients had fever on admission, and 58% had diabetes mellitus. All the patients were admitted for hypoxemic respiratory failure; 75% (18 patients) needed mechanical ventilation. Most of the patients (17) also had hypotension and needed vasopressors. No patient tested positive for influenza A, influenza B, or other respiratory viruses. Half the patients (12) died between ICU day 1 and day 18, including 4 patients who had a do-not-resuscitate order on admission. Of the 12 surviving patients, 5 were discharged home, 4 were discharged from the ICU but remained in the hospital, and 3 continued to receive mechanical ventilation in the ICU.
Conclusions
During the first 3 weeks of the Covid-19 outbreak in the Seattle area, the most common reasons for admission to the ICU were hypoxemic respiratory failure leading to mechanical ventilation, hypotension requiring vasopressor treatment, or both. Mortality among these critically ill patients was high. (Funded by the National Institutes of Health.)
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the novel coronavirus first detected in Wuhan, China, that causes coronavirus disease 2019 (Covid-19).1 Since initial detection of the virus, more than 400,000 cases of Covid-19 have been confirmed worldwide, with the first reported cases in the United States occurring on January 19, 2020, in the state of Washington.2,3 The number of cases in Washington continues to rise; as of March 27, 2020, there had been 3700 confirmed Covid-19 cases and 175 deaths.4 Most of the cases have been in King County, an urban county that includes Seattle and outlying suburbs and accounts for 1760 Covid-19 cases and 125 deaths. Genomic and epidemiologic analyses of sequenced virus RNA recovered in the western Washington region5 have shown that the spread of SARS-CoV-2 has been the result of local community transmission — meaning that the source of infection cannot be traced back to a known exposure. Person-to-person transmission has also been well documented in skilled nursing and long-term care facilities in the Seattle metropolitan area.
Initial reports from China and Italy suggest high mortality and stressed intensive care unit (ICU) capacity.6,7 Reports describing patients admitted to the ICU with Covid-19 in the United States are limited.8 A better characterization of Covid-19 infection in critically ill patients is important to guide decision making regarding critical care capacity and allocation of resources.9 The aim of our multicenter report is to describe the demographic characteristics, coexisting conditions, imaging findings, and outcomes among critically ill patients with Covid-19 in the Seattle metropolitan area.
Methods
Study Population, Setting, and Data Collection
We included patients with laboratory-confirmed Covid-19 infection who were admitted to nine hospital ICUs in the Seattle region between February 24 and March 9, 2020. A confirmed case of Covid-19 was defined by a positive result on a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab. Only laboratory-confirmed cases were included.
Twenty-four adults (18 years of age or older) were identified from nine hospitals, including three University of Washington (UW) Medicine Hospitals (Harborview Medical Center, UW Medical Center–Montlake, and Northwest campuses), the Virginia Mason Medical Center, and the Swedish Hospitals (First Hill and Cherry Hill). This group represents six of the eight adult acute care hospitals in the city of Seattle; hospitals connected to these systems in suburbs outside Seattle (UW–Valley Medical Center, Swedish–Issaquah, and Swedish–Edmonds) were also included in the group of nine. Pregnant women, prisoners, and children (those younger than 18 years of age) were excluded from the study. The UW institutional review board approved this study with the reliance agreement (also known as an authorization agreement) of other local medical centers. The institutional review board at Swedish Medical Center also independently approved the study. Informed consent was waived, and researchers analyzed only deidentified (anonymized) data.
Data from each institution’s electronic medical record was obtained through a research form in Research Electronic Data Capture software (REDCap, Vanderbilt University). We obtained demographic data, information on clinical symptoms or signs at presentation, and laboratory and radiologic results during ICU admission. All laboratory tests and radiologic assessments, including plain chest radiography and computed tomography of the chest, were performed at the discretion of the treating physician.
Study Definitions
Coexisting conditions were ascertained from physician documentation. Patient data were censored at the time of data cutoff, which occurred on March 23, 2020. Acute respiratory distress syndrome (ARDS) was defined as acute-onset hypoxemia (the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen [Pao2:Fio2], <300) with bilateral pulmonary opacities on chest imaging that were not fully explained by congestive heart failure or other forms of volume overload.10
Specimen Collection and Testing
Clinical specimens for Covid-19 diagnostic testing were obtained in accordance with Centers for Disease Control and Prevention (CDC) guidelines. Initially, all clinical specimens were tested with the assay developed by the CDC, targeting the N1 and N2 genes,11 which was run at the Washington Department of Health State Public Health Laboratory. On March 2, UW began using an assay developed by the UW School of Medicine clinical virology laboratory (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org). The assay is based on the World Health Organization standard, and it targets the SARS-CoV-2 E gene and RdRp gene.12 If both targets are detected, the assay is reported as positive; if one target is detected, the assay is reported as inconclusive and is later confirmed reflexively by the real-time RT-PCR assay developed by the CDC at the Washington State Public Health Laboratory.
Statistical Analysis
Descriptive statistics were used to summarize the data; results are reported as medians and interquartile ranges or means and standard deviations, as appropriate. Categorical variables were summarized as counts and percentages. No imputation was made for missing data. Analysis was performed with Stata 15.1 software (StataCorp).
Results
Demographic and Clinical Characteristics of the Patients
During the period from February 24 through March 9, we identified 24 critically ill patients with confirmed Covid-19 infection at the nine hospitals surveyed. This number included all patients admitted to the ICU under the care of an intensivist at any time during the hospital stay. The demographic and clinical characteristics of the patients are shown in Table 1. The mean (±SD) age of the patients was 64±18 years (range, 23 to 97); 63% were men. The mean duration of symptoms before hospital admission was 7±4 days. None of the patients had recently traveled to a country with known transmission, such as China, South Korea, Iran, or Italy. A slight majority of patients (13 [54%]) had had recent contact with a person known to have been ill, but whether the sick contacts were infected with SARS-CoV-2 was not known because of the limited availability of testing at the time. Sixteen patients (67%) were admitted from home, and 6 patients (25%) were admitted from a skilled nursing facility. The most common symptoms on admission to the hospital were shortness of breath and cough, each of which occurred in 21 patients (88%). Documented fever was present in 12 patients (50%) on presentation at the hospital. Patient-level data are available in the Supplementary Appendix.
Table 1. Clinical Characteristics of the Patients at Baseline.*.
| Characteristic | Patients(N=24) |
|---|---|
| Enrollment site — no. (%) | |
| University of Washington (UW Medical Center–Montlake, Harborview, Northwest, and Valley) | 15 (63) |
| Swedish Medical Center (First Hill, Cherry Hill, Issaquah, and Edmonds) | 8 (33) |
| Virginia Mason Hospital | 1 (4) |
| Admission location — no. (%) | |
| Home | 16 (67) |
| Skilled nursing facility | 6 (25) |
| Hospital transfer | 2 (8) |
| Mean age (range) — yr | 64±18 (23–97) |
| Sex — no. (%) | |
| Male | 15 (63) |
| Female | 9 (38) |
| Body-mass index† | 33.2±7.2 |
| Coexisting disorder — no. (%) | |
| Asthma | 3 (14) |
| Cancer‡ | 0 |
| Chronic dialysis | 0 |
| Chronic kidney disease | 5 (21) |
| Chronic obstructive pulmonary disease | 1 (4) |
| Current or former tobacco smoker§ | 5 (22) |
| Diabetes mellitus | 14 (58) |
| Hemorrhagic or ischemic stroke | 2 (8) |
| Human immunodeficiency virus | 1 (4) |
| Obstructive sleep apnea | 5 (21) |
| Mean duration of symptoms before admission — days | 7±4 |
| Respiratory symptoms — no. (%) | |
| Cough | 21 (88) |
| Shortness of breath | 21 (88) |
| Sore throat | 2 (8) |
| Sputum production | 10 (42) |
| Systemic symptoms — no. (%) | |
| Headache | 2 (8) |
| Rhinorrhea | 4 (17) |
| History of travel and contacts — no. (%) | |
| Travel to country where Covid-19 is endemic within previous 3 mo¶ | 0 |
| Known sick contact | 13 (54) |
| Vital signs on ICU admission — no./total no. (%) | |
| Temperature >100.4°F or 38°C | 12/24 (50) |
| Heart rate >100 beats per min‖ | 11/23 (48) |
| Respiratory rate ≥20 breaths per min‖ | 19/23 (83) |
| Imaging — no. (%) | |
| Chest radiography | 23 (96) |
| Chest computed tomography | 5 (21) |
Plus-minus values are means ±SD. Percentages may not total 100 because of rounding. ICU denotes intensive care unit.
The body-mass index in the weight in kilograms divided by the square of the height in meters. Data on body-mass index were missing for one patient.
Cancer included known lymphoma, leukemia and metastatic cancer.
Data on current or former smoking were missing for one patient.
Countries with endemic Covid-19 disease included China, Iran, Italy, and South Korea.
One patient did not have a heart rate or respiratory rate recorded in the medical chart at admission.
Chronic medical conditions were common in this critically ill population. Fourteen patients (58%) had diabetes mellitus and 5 (21%) had chronic kidney disease; 3 patients (14%) had asthma, and all 3 had recently received, as an outpatient, systemic glucocorticoids for a presumed asthma exacerbation before becoming critically ill. Five patients (22%) were current or former smokers and 1 patient (4%) had chronic obstructive pulmonary disease; 8 patients (33%) had more than one coexisting condition.
Laboratory and Radiologic Findings
Table 2 shows the laboratory and radiologic findings in the patients on admission and during the ICU course. On admission, lymphocytopenia was common (in 75% of the patients), with a median lymphocyte count of 720 per cubic millimeter (interquartile range, 520 to 1375). Arterial lactate was 1.5 mg per deciliter or higher in 8 patients, and hepatic enzymes were 40 U per liter or higher in 9 patients. Troponin concentrations were elevated in 2 patients early in their ICU course (maximum value, 0.80 ng per deciliter).
Table 2. Laboratory Data at Hospital Admission and Imaging Findings.*.
| Laboratory Data | Patients(N=24) |
|---|---|
| On admission | |
| White-cell count | |
| Median (IQR) — per mm3 | 8430 (5625–12,450) |
| Distribution — no. (%) | |
| ≥10,000/mm3 | 9 (38) |
| ≤4000/mm3 | 1 (4) |
| Lymphocyte count | |
| Median (IQR) — per mm3 | 720 (520–1375) |
| ≤1500/mm3 — no. (%) | 18 (75) |
| Aspartate aminotransferase >40 U/liter — no./total no. (%) | 9/22 (41) |
| Alanine aminotransferase >40 U/liter — no./total no. (%) | 7/22 (32) |
| Lactate ≥1.5 mmol/liter — no./total no. (%) | 8/15 (53) |
| During first 3 days in ICU | |
| Highest serum creatinine, median (IQR) — mg/dl | 1.08 (0.79–1.98) |
| Highest troponin ≥0.06 ng/ml — no./total no. (%) | 2/13 (15) |
| Lowest platelet count, median (IQR) — per mm3 | 180,000 (109,000–257,000) |
| Highest bilirubin level, median (IQR) — mg/dl† | 0.6 (0.5–0.7) |
| Creatine kinase ≥100 U/liter — no./total no. (%) | 3/6 (50) |
| Lowest Pao2:Fio2 ratio during mechanical ventilation — median (IQR)‡ | |
| Day 1 | 142 (94–177) |
| Day 2 | 139 (112–171) |
| Day 3 | 134 (108–171) |
| Infection analyses — no. positive/total no. | |
| Blood cultures | 0/20 |
| Sputum cultures | 0/15 |
| Influenza A | 0/23 |
| Influenza B | 0/23 |
| Respiratory syncytial virus | 0/23 |
| Extended-spectrum respiratory viruses | 0/21 |
| Chest radiography findings — no./total no. (%)§ | |
| Clear | 0/23 |
| Bilateral infiltrates | 23/23 (100) |
| Pleural effusion | 0/23 |
| Computed tomography findings — no./total no. (%) | |
| Bilateral ground-glass opacification | 4/5 (80) |
| Nodules | 1/5 (20) |
| Pleural effusions | 0/5 |
Plus-minus values are means ±SD. To convert the values for creatinine to μmol per liter, multiply by 88.4. Fio2 denotes the fraction of inspired oxygen, IQR interquartile range, and Pao2 the partial pressure of arterial oxygen.
Data were available for 23 patients.
Data on Pao2:Fio2 ratio were missing for 2 patients who received mechanical ventilation.
One patient died before chest imaging was completed.
A chest radiograph was obtained in 23 patients (96%) on ICU admission, and all the radiographs showed bilateral pulmonary opacities. No pleural effusions were seen. A computed tomographic (CT) scan of the chest was obtained in 5 patients (21%); four of the scans showed bilateral ground glass opacities, and one showed pulmonary nodules. Figure 1 provides representative images from a single patient, illustrating the rapid evolution of pulmonary opacities and the diffuse findings. A video showing the CT cross-sectional images of the chest is available at NEJM.org.
Figure 1. Chest Radiographs and CT Images of a 55-Year-Old Patient with SARS-CoV-2.
An initial radiograph (anteroposterior view) of the chest at admission (Panel A) shows hazy opacities in the upper and mid lung zones. Another chest radiograph obtained approximately 24 hours after the initial presentation (Panel B) shows worsening multifocal air-space opacities. Axial CT images (Panels C and D) and coronal reformats (Panel E) obtained within 2 hours after the chest radiograph in Panel B show extensive ground glass opacities and occasional foci of consolidation.
Microbiologic Results
The performance of the local UW assay was similar to that of the CDC assay in comparison diagnostic testing. All patients had Covid-19 confirmed by an RT-PCR assay of a nasal swab. The UW assay returned one inconclusive result that was later confirmed as positive by the Washington State Public Health Laboratory.
Of the patients with laboratory-confirmed Covid-19, nearly all (23 patients [96%]) also had nasopharyngeal swabs sent to test for influenza and respiratory syncytial virus, and 21 patients (88%) had an extended-spectrum respiratory viral panel. None of the patients had a coinfection with another virus (see Table S1 in the Supplementary Appendix). Sputum samples from 15 patients were sent for bacterial culture, and all were negative for bacterial growth; blood samples from 20 patients were sent, and all remained negative. Bronchoscopy was performed in 4 patients. Two patients had bronchoscopy performed before diagnosis of Covid-19; bacterial and viral RT-PCR results were negative for other respiratory viruses. Two subjects had bronchoscopy performed late in the hospitalization for mucous plugging, without additional cultures.
Respiratory Failure and Shock
Eighteen patients (75%) received invasive mechanical ventilation (Table 3). For patients who received mechanical ventilation, the Pao2:Fio2 ratios were consistent with moderate-to-severe ARDS. Pulmonary secretions were characterized as either moderate or thick and purulent in 14 patients (77%) during the first 7 days of mechanical ventilation. The median Fio2 on day 1 of mechanical ventilation was 0.9 (interquartile range, 0.7 to 1.0), and the Fio2 improved on day 3 to 0.6 (interquartile range, 0.5 to 0.7). The median driving pressure (the difference between plateau pressure and positive end expiratory pressure [PEEP]) on day 1 of mechanical ventilation was 13 cm of water (interquartile range, 11 to 17). During the first 3 days of mechanical ventilation, the median driving pressure was 12 to 13 cm of water. The median pulmonary compliance on day 1 was 29 ml per cm of water (interquartile range, 25 to 36). The pulmonary compliance improved during the first 3 days of mechanical ventilation, with median compliance on day 3 of 37 ml per cm of water (interquartile range, 25 to 42). Five patients (28%) were placed in a prone position, 7 (39%) received neuromuscular blockade, and 5 (28%) received inhaled pulmonary vasodilators to treat hypoxemic respiratory failure in ARDS. Tracheostomy was not completed in any of the patients.
Table 3. ICU-Level Therapies and Clinical Outcomes.*.
| ICU Therapies | Patients |
|---|---|
| Therapy — no./total no. (%) | |
| High-flow nasal cannula | 10/24 (42) |
| CPAP or noninvasive positive pressure | 0/24 |
| Invasive mechanical ventilation | 18/24 (75) |
| Prone position | 5/18 (28) |
| Neuromuscular blockade | 7/18 (39) |
| Inhaled pulmonary vasodilators | 5/18 (28) |
| Extracorporeal membrane oxygenation | 0/24 |
| Vasopressors | 17/24 (71) |
| Echocardiogram completed | 9/24 (38) |
| Echocardiogram showing new left ventricular dysfunction | 0/9 |
| Characteristics of mechanical ventilation | |
| Moderate or thick and purulent secretions — no./total no.(%)† | 14/18 (77) |
| Day 1 median values | |
| Plateau pressure (IQR) — cm of water‡ | 25 (20–28) |
| Driving pressure (IQR) — cm of water‡ | 13 (11–17) |
| Highest Fio2 — median (IQR) | 0.9 (0.7–1.0) |
| Compliance (IQR) — ml/cm of water§ | 29 (25–36) |
| Day 2 median values | |
| Plateau pressure (IQR) — cm of water‡ | 24 (21–29) |
| Driving pressure (IQR) — cm of water‡ | 13 (12–17) |
| Highest Fio2 — median (IQR) | 0.7 (0.5–0.8) |
| Compliance (IQR) — ml/cm of water¶ | 26 (20–35) |
| Day 3 median values | |
| Plateau pressure (IQR) — cm of water‡ | 22 (19–28) |
| Driving pressure (IQR) — cm of water‡ | 12 (10–14) |
| Highest Fio2 (IQR) — median (IQR) | 0.6 (0.5–0.7) |
| Compliance (IQR) — ml/cm of water¶ | 37 (25–42) |
| Outcomes | |
| Median length of stay (IQR) — days | |
| In hospital | 12 (8–18) |
| In ICU | 9 (4–14) |
| In hospital, survivors | 17 (16–23) |
| In ICU, survivors | 14 (4–17) |
| Median duration of mechanical ventilation (IQR) — days | |
| Overall | 10 (7–12) |
| In patients who were extubated | 11 (7–12) |
| Extubated — no./total no. (%) | 6/18 (33) |
| Died in hospital — no. (%) | 12 (50) |
| Discharged from hospital — no. (%) | 5 (21) |
The driving pressure is the difference between plateau pressure and positive end expiratory pressure [PEEP]. CPAP denotes continuous positive airway pressure.
Moderate or thick and purulent secretions (as compared with no secretions or minimal or thin secretions) were those observed over the first 7 days of mechanical ventilation.
Data were available for 13 of 18 patients who required invasive mechanical ventilation.
Data were available for 9 of 18 patients who required invasive mechanical ventilation.
Data were available for 10 of 18 patients who required invasive mechanical ventilation.
Seventeen patients (71%) presented with concurrent hypotension requiring vasopressors, without clear evidence of secondary infection. Of these patients, 3 (18%) had transient hypotension after intubation; 14 (82%) had hypotension that was unrelated to intubation or that persisted for more than 12 hours after intubation. None of the echocardiograms completed in 9 patients (38%) showed new cardiac dysfunction. Seven patients received compassionate-use remdesivir as antiviral therapy, 1 patient received hydroxychloroquine, and 1 patient received lopinavir–ritonavir; no patient received systemic glucocorticoids or tocilizumab in the ICU.
Outcomes
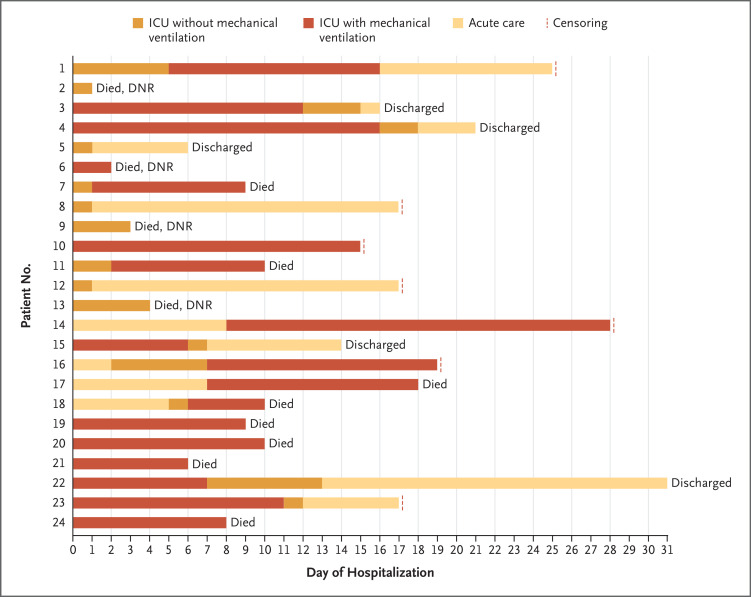
All patients had at least 14 days of hospital follow-up. As of March 23, of the 24 patients, 12 (50%) had died, 4 (17%) had been discharged from the ICU but remained in the hospital, 3 (13%) were receiving mechanical ventilation and were still in the ICU, and 5 (21%) had been discharged from the hospital (Figure 2). A greater percentage of patients over 65 years of age had died than patients under 65 years of age (62% vs. 37%). The 12 deaths included 4 patients (17%) who had do-not-resuscitate orders in place before hospital admission for use in the event of cardiac arrest, and 6 additional patients (25%) had do-not-resuscitate orders that were instituted during their admission.
Figure 2. Outcomes for Individual Patients Included in the Case Series.
Do-not-resuscitate (DNR) designates orders that were in place before hospital admission. As of March 23, 2020, a total of 12 patients (50%) had died. Six patients who had received mechanical ventilation had been extubated and three patients remained intubated. Five patients had been discharged from the hospital. All the patients had at least 14 days of follow up. Dashed red lines indicate censoring of data.
The median length of hospital stay among survivors was 17 days (interquartile range, 16 to 23), and the median length of ICU stay among survivors was 14 days (interquartile range, 4 to 17) (Table 3). The median duration of mechanical ventilation was 10 days (interquartile range, 7 to 12), and 6 patients (33%) had been extubated as of March 23, 2020. Three patients continued to receive ventilatory support, so the durations of mechanical ventilation and ICU stay are likely to be underestimates.
Discussion
This multicenter case series describes 24 critically ill patients who presented with acute hypoxemic respiratory failure and laboratory-confirmed Covid-19 infection. We included all patients with Covid-19 who were admitted to an ICU at nine Seattle-area hospitals between February 24 and March 9, 2020. All the patients had acquired Covid-19 without known exposure to a returning traveler. Coexisting lower respiratory bacterial infections were not identified in sputum and blood cultures obtained early in the clinical course. Overall outcomes were poor in patients who received ICU care.
The patients in our series presented with respiratory symptoms similar to those of patients described in reports from China, which indicates a common host response to SARS-CoV-2. Cough was the most common presenting symptom, as it was in reports from China, and the mean duration of symptoms before ICU admission was 1 week.13 Only half the patients had fever at the time of hospital admission, which suggests that fever may not be a useful criterion to determine the severity of illness and that diagnostic algorithms that require fever for Covid-19 testing may delay diagnosis. The majority of patients had chronic illnesses before their admission to the ICU, most commonly diabetes mellitus and chronic kidney disease. Lymphocytopenia was common on hospital admission, as it was in reports from China.13
The case fatality rate of 50% in this series (to date) is similar to that reported among critically ill patients in Chinese hospitals but lower than that in a single-center experience reported from our area.8,13 Although the case fatality rate was higher in persons 65 years of age or older, it was still substantial (37%) in persons younger than 65 years of age. Our case fatality rate may be an underestimate, given that 3 patients remained intubated at the time data were censored.
Patients who received mechanical ventilation had high oxygen requirements soon after initiation of mechanical ventilation, with plateau pressures (mean, 25 cm of water) and driving pressures (mean, 12 to 13 cm of water) similar to those in populations of patients with ARDS enrolled in clinical trials.14,15 For example, the mean plateau pressure in the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial14 was 25 to 26 cm of water. Of the 18 patients who received invasive mechanical ventilation in this series, 6 had been successfully extubated. The earliest extubation occurred 8 days after initiation of invasive mechanical ventilation, which suggests that acute respiratory failure due to Covid-19 may require prolonged mechanical ventilation lasting days to weeks and that readiness for extubation is unlikely to occur early in patients receiving mechanical ventilation. Of patients who were extubated, the age range was 23 to 88 years, which suggests that age may not be the sole indicator for successful extubation.
A large proportion of patients in this series presented with shock that required vasopressor support. In patients who had an echocardiogram, myocardial dysfunction was uncommon. The lack of bacterial or viral coinfection suggests that the observed shock was directly related to Covid-19. In these preliminary data, Covid-19 infection appears to differ from seasonal influenza, which is commonly associated with bacterial coinfection due to pathogens that colonize the nasopharynx, such as staphylococcus and streptococcus.16 Regarding antiviral interventions, 7 patients received compassionate-use remdesivir, but we have insufficient information to report associated outcomes. Bronchoscopy was performed in a minority of patients and did not appear to change clinical management.
Three patients with mild asthma had received systemic glucocorticoids within 1 week before ICU admission, as outpatients, for presumed asthma exacerbation while they had symptoms of Covid-19. These 3 patients then presented to the hospital again, with severe respiratory failure requiring invasive mechanical ventilation. Previous studies have shown that glucocorticoid treatment for phylogenetically similar viruses, SARS-CoV (2003) and Middle East respiratory syndrome coronavirus (MERS-CoV), was associated with a higher subsequent plasma viral load, longer duration of viremia, and worse clinical outcomes.17-20 These findings are in contrast to those of a recent nonrandomized observational study that suggested that glucocorticoids may be associated with improved clinical outcomes in patients with Covid-19 and ARDS.21 Further research is necessary to determine the role of systemic glucocorticoids in patients with Covid-19 infection.
Our study has several notable limitations. First, some cases had incomplete documentation of clinical symptoms, missing laboratory testing, or both. However, given the need to provide objective data and the urgent timeline, we did not approach patients to obtain additional history or biologic samples for laboratory measurement. Second, 7 patients (29%) remained in hospitals at the time of data censoring on March 23, 2020; as a result, outcomes for those patients were not known. Third, because of our focus on the critical care needs of patients with the greatest severity of illness, our sample size is small. Finally, it is possible that critically ill patients with established goals of care that were not consistent with admission to an ICU were not included in this report. Specifically, this includes patients who received care on the general medical ward that focused on comfort measures only.
This early experience of the Covid-19 pandemic in the United States resembles the experience in other countries, with high mortality for patients requiring care in the ICU. Patients with coexisting conditions and older age are at risk for severe disease and poor outcomes after ICU admission. Better information is needed to inform care for these challenging patients. Our findings also highlight the importance of planning for mass critical care as the need for ICU care and ventilatory support to treat patients with Covid-19 grows rapidly in the United States.22,23
Acknowledgments
We thank Adrienne Meyer from the University of Washington institutional review board for rapidly reviewing and facilitating study protocols across many area hospitals.
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on March 30, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by a grant from the National Institutes of Health (K23DK116967, to Dr. Bhatraju).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.World Health Organization. Novel coronavirus — China. January 12, 2020. (http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/).
- 2.Center for Systems Science and Engineering. Coronavirus COVID-19 global cases. 2019. (https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6).
- 3.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2019 Novel coronavirus outbreak (COVID-19). Seattle: Washington State Department of Health, 2020. (https://www.doh.wa.gov/Emergencies/Coronavirus). [Google Scholar]
- 5.Bedford T. Cryptic transmission of novel coronavirus revealed by genomic epidemiology. March 2, 2020. (https://bedford.io/blog/ncov-cryptic-transmission/).
- 6.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020. February 24 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020. March 13 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 8.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020. March 19 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adalja AA, Toner E, Inglesby TV. Priorities for the US health community responding to COVID-19. JAMA 2020. March 3 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 10.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-2533. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). 2020. (https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html).
- 12.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. DOI: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019;380:1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-2168. [DOI] [PubMed] [Google Scholar]
- 16.Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA 2013;309:275-282. [DOI] [PubMed] [Google Scholar]
- 17.Peiris JSM, Chu CM, Cheng VCC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol 2004;31:304-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018;197:757-767. [DOI] [PubMed] [Google Scholar]
- 20.Lansbury LE, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis. Crit Care Med 2019. November 15 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020. March 13 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandrock CE. Care of the critically ill and injured during pandemics and disasters: groundbreaking results from the Task Force on Mass Critical Care. Chest 2014;146:881-883. [DOI] [PubMed] [Google Scholar]
- 23.Christian MD, Devereaux AV, Dichter JR, Rubinson L, Kissoon N. Introduction and executive summary: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest 2014;146:Suppl:8S-34S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.