Abstract
Simple Summary
Milk somatic cell count, referring to the total number of somatic cells per milliliter of bovine milk, changes regularly during the lactation cycle. The somatic cell count of healthy cows is usually higher in late lactation than in peak lactation. When the inflammatory response in dairy cow mammary gland becomes more intense, the milk somatic cell count increases together with the reduction of milk quality and yield. Autoimmunity was thought to play an important role in the prevention of mastitis in late lactation of dairy cattle. However, the underlying mechanisms related to the gene expression levels during the process remain unknown. In this study, transcriptome sequencing was performed to screen the differentially expressed genes related to the inflammation and immunity in healthy Chinese Holstein mammary glands. Our findings are helpful to understand the physiological functions of mammary inflammation of Chinese Holstein during late lactation.
Abstract
Somatic cell count (SCC) in milk is widely used in the dairy industry, as an indicator of the health of mammary gland. While the SCC of dairy cattle was higher in late lactation than in peak lactation, its association with gene expressions of mammary gland were largely unknown. In this study, a transcriptomic sequencing approach and bioinformatics analysis were used to investigate the differential expressed genes (DEGs) associated with inflammation and immunity between peak and late periods of lactation in Chinese Holstein. A total of 446 DEGs (padj < 0.05 and fold change >2) were identified, 50 of which belonged to seven pathways and five terms related to inflammation and immunity. Our data suggested that the activation of nuclear transcription factor-κB (NF-κB) pathway and Toll-like receptor signaling pathway caused inflammatory response, and the activation of chemokine signaling pathway and cytokine–cytokine receptor interaction signaling pathway caused a protective immune response to ensure dairy cows health during late lactation. Our findings deepen the understanding of the molecular mechanism and physiological functions of mammary inflammation in Chinese Holstein during late lactation.
Keywords: Chinese Holstein, transcriptome, lactation initiation, mammary gland, differentially expressed genes
1. Introduction
The somatic cell count (SCC) referred to as the total number of somatic cells per milliliter of milk is widely used to measure the health status of dairy cows and the quality of milk [1]. In general, the quality and yield of milk was negatively associated with SCC [2], which is an important indicator of clinical and subclinical bovine mastitis. The United States stipulates that SCC in bovine milk must be less than 750,000/mL, while the European Union is stricter, which adheres to SCC in bovine milk must be less than 400,000/mL, and China’s standard is that SCC in bovine milk must be less than 500,000/mL [3,4].
Previous studies have focused on the interfering factors of SCC and its association with the quality and yield of milk [2,5,6]. Several studies have demonstrated that the SCC was higher in late lactation than that in peak lactation [7,8,9,10,11]. Moreover, the autoimmune reaction and the innate immune response of dairy cattle protect them from mastitis during lactation, especially in the late stage [12], the changes in gene expression level in mammary gland tissue at different stages of lactation are largely unknown. In recent years, transcriptome technology was used to detect changes in dairy cow gene expression level, however, most sampling methods cannot eliminate individual differences [13,14].
The purpose of this study is to screen differential expression genes (DEGs) related to inflammation and immunity in healthy Chinese Holstein during the peak and late lactation period by transcriptome sequencing without slaughter.
2. Materials and Methods
2.1. Ethics Statement
This study was performed in strict accordance with the Regulations of the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, revised in 2004) and approved by the Institutional Animal Care and Use Committee (IACUC) of the Yangzhou University Animal Experiments Ethics Committee (Permit Number: SYXK (Su) IACUC 2012-0029). All animals were reared in compliance with national regulations and according to procedures approved by the veterinary services of China.
2.2. Animals Selection and Samples Collection
A total of 33 healthy Chinese Holstein used in this study were selected from the experimental farm of Yangzhou University. All the animals with similar body weights (628.33 ± 20.05 kg) in the second lactation period did not have a history of mastitis, and fed with total mixed ration (TMR), including 23% alfalfa hay and 7% Chinese wild rye hay with a forage-to-concentrate ratio of 45:55 [15]. The milk yield of each individual was recorded twice per day. Milk samples were collected into 5 mL (for bacterial isolation and identification) and 50 mL (for the determination of SCC) from the left anterior mammary region on the 90th, 150th, 210th, and 270th day of lactation and transported on ice to the lab within 2 h. Three Chinese Holstein (A, B, C) were randomly selected to obtain a biopsy: 1−2 g mammary gland tissue in the same quarter that was providing the milk sample by surgical methods in vivo on the 90th and 270th day of lactation [16,17]. The skin of the selected biopsy site was shaved and disinfected with ethanol (75%), then anaesthetized with SU-MIAN-XIN (846 compound anesthetic agent, 35 mg, intravenously) and injected subcutaneously with 1 mL of procaine. A 1.5 cm incision was made at the midpoint of the selected quarter. The connective tissue was blunt-dissected away to expose the mammary parenchymal tissue using disinfectant shears and tweezers. The mammary tissues biopsy (1–2 g) was obtained and washed with diethylpyrocarbonate (DEPC)-treated double-distilled water. Then, mammary tissues were immediately frozen in liquid nitrogen until RNA isolation. While obtaining mammary tissue samples, 11-mm Michel wound clips (#9534503, Henry Stein, Inc., Melville, NY, USA) were used to close the skin incision. Then, the skin incision was covered with iodine ointment (#1048023, Povidone Iodine Ointment, Guangdong qingfa pharmaceutical co. LTD, Guangzhou, China).
2.3. Microbiological Study
Upon arrival, bacterial isolation was performed as described with minor modification [18]. In brief, 2 mL of milk was diluted into 2 mL of phosphate buffer saline (PBS) and 100 μL of diluent was plated onto a blood agar and a MacConkey plate, and incubated at 37 °C aerobically for 24–48 h. Based on the morphology of colonies, one of identical colony of each sample was expended cultured in nutrient broth at 37 °C. After 24–48 h incubation, 200 μL of culture was used for DNA extraction with the Roche High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany). Extracted DNA was subjected to a PCR assay with a broad-range PCR primer targeting the 16S rDNA gene of pathogenic and nonpathogenic bacteria [19]: Forward primer = 5′- AGAGTTTGATCCTGGCTCAG -3′; reverse primer = 5′- TACGGCTACCTTGTTACGACT -3′. The amplicons were sequenced (Genscript, Nanjing, China) and the BLASTn was performed to determine the bacterial species.
2.4. Determination and Analysis of SCC in Milk Samples
The 20-mL milk samples with 0.015 g of potassium dichromate were sent to Nanjing Agricultural University to determine SCC (Agricultural Product Safety Testing Center of Nanjing Agricultural University, Jiangsu Province, China). Further analysis was performed with SCS calculated with SCC as described [20].
2.5. Total RNA Extraction and cDNA Library Construction
Total RNA was extracted using the mirVana™ miRNA Isolation Kit (Ambion-1561) following the manufacturer’s protocol. RNA integrity was evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The samples with RNA Integrity Number (RIN) ≥ 7 were subjected to the subsequent analysis.
The libraries were constructed using TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, USA, RS-122-2101) according to the manufacturer’s instructions. Then, these libraries were sequenced on the Illumina sequencing platform (HiSeq™ 2500) and 125 bp paired-end reads were generated.
Raw reads were processed using NGS QC Toolkit [20,21]. The reads containing ploy-N and the low-quality reads were removed to obtain the clean reads. Then, the clean reads were mapped to reference bovine genome UMD3.1 using Bowtie2 2.3.5.1 [21,22] and TopHat 2.1.1 [22,23].
2.6. Gene Expression Level Analysis
Gene expression was calculated using the FPKM method, which is the number of fragments per kilobase length from a gene in each million fragments [24]. The read counts of each gene were obtained by HtSeq-count 0.9.1 [25]. PCA analysis was performed using the gene expression profiles. Genes were divided into high (≥500 FPKM), medium (≥10 to 500 FPKM), and low expression (<10 FPKM) [26]. DEGs were identified using the DESeq R package (1.18.0) (2012) functions estimate size factors and nbinom test [27]. False discovery rate (FDR, padj) <0.05 and fold change >2 was set as the threshold for DEGs.
2.7. Functional Annotation and Pathway Analysis of DEGs
Hierarchical cluster analysis of DEGs was performed to explore transcripts expression pattern. DAVID 6.8 (https://david.ncifcrf.gov/) [28] were used for GO (gene ontology) annotation analyses of DEGs. KEGG pathways analyses of DEGs were implemented by KOBAS 3.0 online program (http://kobas.cbi.pku.edu.cn/index.php) [29]. GO enrichment and KEGG pathway enrichment analysis of DEGs were, respectively, performed using R based on the hypergeometric distribution. The calculation for this was formula (1), where N is the number of genes with a pathway annotation in all genes; n is the number of differentially expressed genes in N; M is the number of genes annotated as a particular pathway in all genes; and m is the number of differentially expressed genes annotated as a particular pathway. GO terms and KEGG pathways with padj < 0.05 were significantly enriched in DEGs.
| (1) |
2.8. PPI Network Construction and Analysis
A protein–protein interaction (PPI) network was created using Cytoscape v3.7.2 to further understand and predict the biological activity of the identified DEGs related to inflammation and immunity based on GO and KEGG enrichment analysis [30]. The DEGs’ encoding proteins and their interacting partners were computed from the String v11.0 database for PPI network construction [31]. This PPI network was subsequently visualized in Cytoscape.
2.9. Validation of Sequencing Data by qRT-PCR
Ten DEGs were selected from DEGs at random to validate the transcriptome sequencing results. The primers (Table 1) used for quantification in the study were designed using Primer-BLAST on the NCBI website. In all cases, primers designed for quantitative real-time PCR (qRT-PCR) spanned exon–exon boundaries. In the study, ribosomal protein S9 (RPS9) [32] and β-actin were used as the housekeeping gene. qRT-PCR was performed using the Light Cycler® 480 System (Roche, Hercules, CA, USA) with SYBR Green PCR Master Mix (TaKaRa SYBR® PrimeScript™ RT-PCR Kit, Dalian, China) according to the manufacturer’s instructions (n = 9 experiments, three replicates per experiment). Relative expression was calculated using the 2−ΔΔCt method [33]. qRT-PCR response procedures for: 40 cycles of 95 °C for 30 s, 95 °C for 10 s, 60 °C for 30 s.
Table 1.
Primers used in quantitative real-time PCR.
| Gene | Forward Primers (5′-3′) | Reverse Primers (5′-3′) | Length (bp) | GenBank ID |
|---|---|---|---|---|
| SLC11A2 | AGTTGACCTCCCTGGACATCT | CACGTTCGGAGGAACACTGG | 132 | NM_001101103.1 |
| CD40 | GAACAACACGTGGGGACGAA | CCGCTTCTTGGTTATGTTCCTG | 147 | NM_001105611.2 |
| ICAM1 | GGAGGTGCCGGAATATCAAT | GGCCCACTTCCTCCTTGATTA | 139 | NM_174348.2 |
| CCR1 | TCCGACTCACTCAGGACCTT | CCACGGGTCAAGGGAAATGT | 146 | NM_001077839.1 |
| IL1R2 | ACTGAAGGTGAAAGGCCTGG | CGAAGGTGGACACACCCATT | 150 | NM_001046210.2 |
| ATP1A2 | AGCTGTGGTCATCGTCACTG | TCCGCGTTGATCTGCATCTT | 138 | NM_001081524.1 |
| FXYD2 | TATGGACAGGTGGTACCTGGG | CAGCGGAATCTTTTGCTGAGG | 150 | NM_174320.4 |
| SLC30A1 | TCACGCTACCACCATTCAGC | TTTCCAGACTGGGCTTGTGG | 135 | NM_001205893.2 |
| CCL28 | AAGCAGCCAAGAAAGAGGCT | CCTCTGTGCAGCTTCATCTGT | 150 | NM_001101163.1 |
| TGFB2 | ACCCTCGGAAAATGCCATCC | GCACTCTGGCTTTTGGGTTC | 149 | NM_001113252.1 |
| RPS9 | CCTCGACCAAGAGCTGAAG | CCTCCAGACCTCACGTTTGTTC | 62 | NM_001034034.2 |
| β-actin | CATCCTGACCCTCAAGTA | CTCGTTGTAGAAGGTGTG | 91 | NM_173979.3 |
2.10. Statistical Analysis
All the statistical analyses were performed with Software Package for Social Sciences (SPSS) Version 19.0 (IBM, Among, New York, NY, USA). The differences in SCS of milk collected during different days of lactation was compared with one-way analysis of variance (ANOVA) with comparison among means made by Duncan’s multiple range test. The day of lactation was set as the X (independent) variable and the SCS in milk was set as the Y (outcome) variable. The relative expressions of mRNA were analyzed by using independent sample T-test and mean plots ± 95% confidence intervals. The Pearson correlation coefficient analysis were performed to compare the data obtained from transcriptome sequencing and qRT-PCR. Each biological repetition was carried out involving three replicates. All data were presented as the mean ± standard error (SE) and considered statistically significant when p < 0.05.
3. Results
3.1. Microbiological Analysis
Among the milk samples from enrolled cows, 91.67% (22/24) of them were positive for culturing, whereas 8.33% (2/24) of them yielded no growth. The sterile growth was from the milk on the 90th and 270th day of lactation of the same individual. One bacterial specie, Escherichia coli was identified in the positive cultures.
3.2. Daily Milk Yield and SCC in Milk Samples
Daily milk yield, SCC, and SCS were shown in Table 2. The daily milk yield of all the tested animals showed a continuous decline during the trial period. SCC and SCS of Chinese Holstein showed an increasing trend during the test period. Compared with the 90th day of lactation, the SCS of the 33 animals increased significantly on the 270th day of lactation.
Table 2.
Milk yield, somatic cell count, somatic cell score in different test days (means ± SE).
| Test Days | 90 | 150 | 210 | 270 |
|---|---|---|---|---|
| Daily milk yield (Kg) | 34.40 ± 0.05 a | 33.17 ± 0.04 b | 29.62 ± 0.04 c | 26.51 ± 0.04 d |
| Somatic cell count (SCC) (104) | 24.03 | 24.00 | 32.00 | 46.98 |
| Somatic cell score (SCS) | 4.26 ± 0.01 c | 4.26 ± 0.01 c | 4.68 ± 0.01 b | 5.23 ± 0.01 a |
Note: Different letters a, b, c in the same row differ significantly (p < 0.05) by Duncan’s test. The numbers in line 4 reflected a mean.
3.3. Analysis of cDNA Libraries
Transcriptome sequencing results and quality parameters were shown in Table 3. After the quality control of sequencing data, the number of clean bases accounted for more than 98.05% of the raw bases. The sequences with Q30 and above accounted for more than 95.92%. The GC content reached between 47.50% and 49.00%. The results of comparison between sequencing data and genome information were shown in Table 4. The total reads of each sample were more than 90.90% compatible with the bovine reference genome.
Table 3.
Basic information of sequencing reads and bases.
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Valid Ratio (Base) |
Q30 | GC |
|---|---|---|---|---|---|---|---|
| A-90 | 61,255,240 | 7.66 Gb | 60,490,684 | 7.56 Gb | 98.72% | 97.14% | 48.50% |
| B-90 | 61,664,866 | 7.71 Gb | 60,994,408 | 7.62 Gb | 98.89% | 97.33% | 47.50% |
| C-90 | 59,050,772 | 7.38 Gb | 58,314,034 | 7.29 Gb | 98.72% | 97.11% | 49.00% |
| A-270 | 71,589,742 | 8.95 Gb | 70,550,840 | 8.82 Gb | 98.53% | 96.48% | 48.50% |
| B-270 | 77,932,606 | 9.74 Gb | 76,857,188 | 9.61 Gb | 98.60% | 96.44% | 49.00% |
| C-270 | 64,104,356 | 8.01 Gb | 62,867,970 | 7.86 Gb | 98.05% | 95.92% | 49.00% |
Table 4.
Statistics of total reads and mapped reads.
| Item | A-90 | B-90 | C-90 | A-270 | B-270 | C-270 |
|---|---|---|---|---|---|---|
| Total reads | 60,490,684 | 60,994,408 | 58,314,034 | 70,550,840 | 76,857,188 | 62,867,970 |
| Total mapped | 54,988,154 (90.90%) |
56,242,133 (92.21%) |
54,192,238 (92.93%) |
64,298,141 (91.14%) |
69,765,025 (90.77%) |
58,484,736 (93.03%) |
3.4. Gene Expression in Different Samples
Two clusters were found: Peak lactation and late lactation (Figure S1). The same genes from different dairy cows in same stages could be classified into the same clusters, indicating that the main distinctions in the mRNA expression profiles occurred in the different stage. Total expressed genes are classified into high (≥500 FPKM), medium (≥10 to 500 FPKM), and low (<10 FPKM) expression (Table 5).
Table 5.
Statistics of gene expression in samples.
| Gene Expression | A-90 | B-90 | C-90 | A-270 | B-270 | C-270 |
|---|---|---|---|---|---|---|
| High expression genes (≥500 FPKM) | 82 | 61 | 81 | 63 | 79 | 89 |
| Medium expression genes (≥10 to 500 FPKM) | 4294 | 3207 | 5687 | 3709 | 4947 | 5641 |
| Low expression genes (<10 FPKM) | 11,585 | 12,311 | 10,692 | 11,962 | 11,404 | 10,490 |
| Nonexpressed genes | 5535 | 5917 | 5036 | 5542 | 4846 | 5056 |
| Total expressed genes | 15,961 | 15,579 | 16,460 | 15,734 | 16,430 | 16,220 |
3.5. Screening of Differentially Expressed Genes
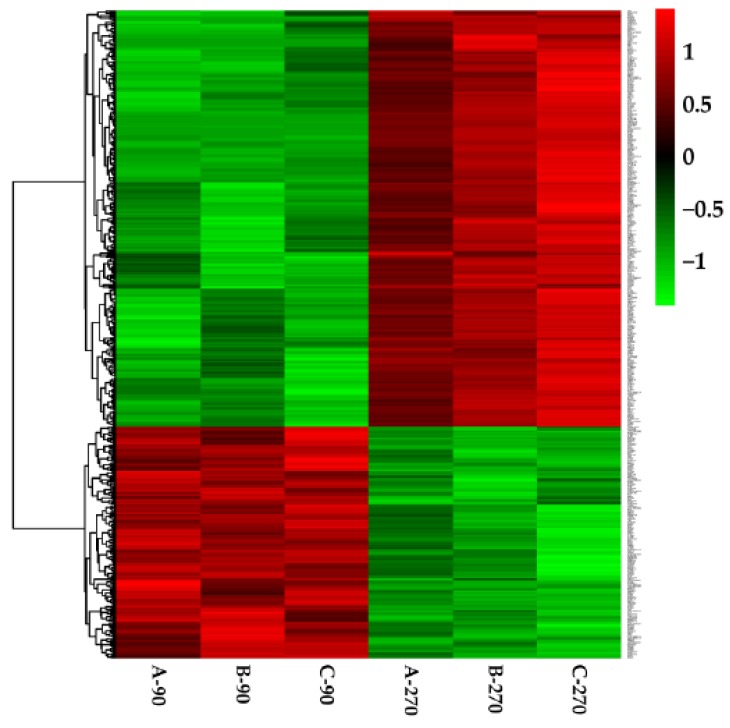
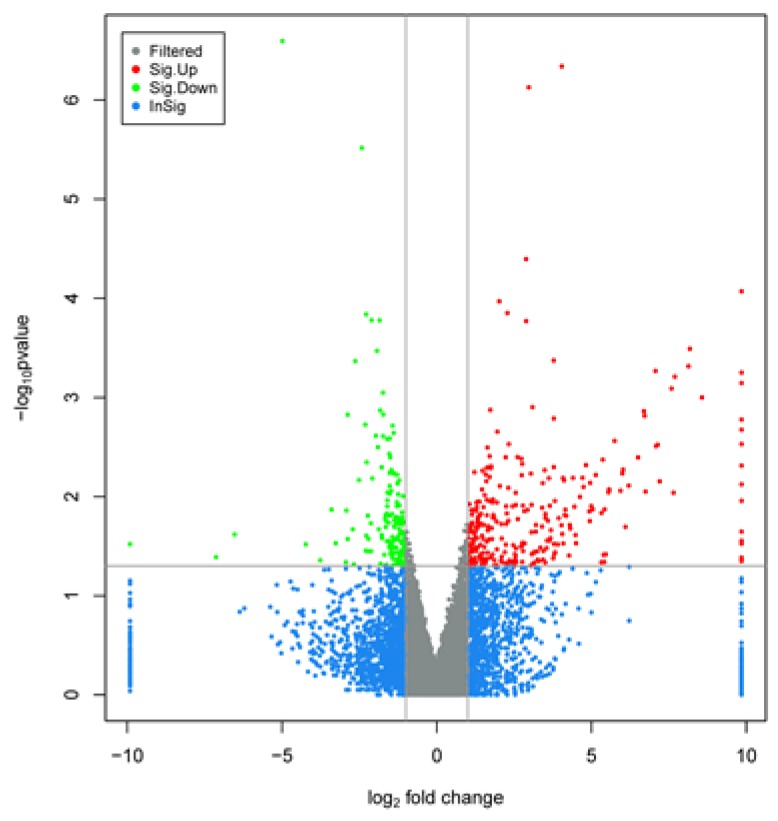
There were 291 upregulated genes and 155 downregulated genes were identified during late lactation in bovine mammary tissues, compared to peak lactation (Figure 1 and Figure 2).
Figure 1.
Heat map of the differentially expressed genes. Green indicates lower expression genes and red indicates higher expression genes.
Figure 2.
Volcano plot displaying differential expressed genes in bovine mammary tissues during peak (A-90, B-90, C-90) and late (A-270, B-270, C-270) lactation. The y-axis corresponded to the mean expression value of log10(p-value), and the x-axis displayed the log2 fold change value. The red and green dots represented the significant differentially expressed gene (padj < 0.05) in bovine mammary tissue during peak and late lactation; the blue and grey dots represented the transcripts whose expression levels did not reach statistical significance in bovine mammary tissue during peak and late lactation.
3.6. GO and KEGG Enrichment Analysis of DEGs
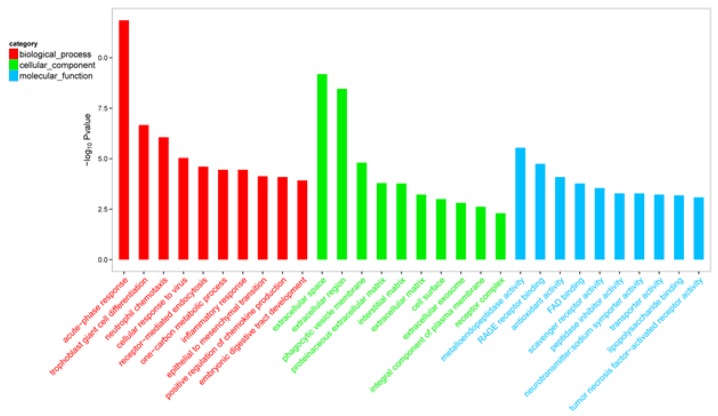
DEGs were classified by GO enrichment according to biological process, cellular component (GO-CC), and molecular function (GO-MF). The top 10 terms of GO-BP, GO-CC, and GO-MF were shown in Figure 3, respectively. Among significantly enriched (padj < 0.05) top 10 GO-BP terms, there were five GO-BP terms related to inflammation and immune, including 30 genes (Table 6).
Figure 3.
Gene ontology functional enrichment analysis of differentially expressed genes. Only top 10 significant biological process, cellular component, and molecular function terms were listed, respectively.
Table 6.
Significantly enriched gene ontology (GO) terms related to inflammation and immunity.
| Term ID | Term | padj | Gene Name | Number of Genes |
|---|---|---|---|---|
| GO:0006953 | Acute-phase response | <0.001 | M-SAA3.2; ORM1; SERPINF2; SAA3; LBP; IL6; CD163; HP; IL1RN | 18 |
| GO:0030593 | Neutrophil chemotaxis | <0.001 | PDE4B; CCL19; CCL20; S100A8; S100A9; CXCL8; CSF3R; TREM1 | 8 |
| GO:0098586 | Cellular response to virus | <0.001 | IKBKE; CCL19; GLI2 | 6 |
| GO:0006954 | Inflammatory response | < 0.001 | RELT; S100A12; TNFRSF6B; OLR1; CCL19; CCL20; CCR1; MEFV; CASP4; SLC11A1; CXCL8; GGT5; CD40; TLR2 | 7 |
| GO:0032722 | Positive regulation of chemokine production | <0.001 | LBP; IL6; TLR2 | 9 |
Note: The gene in bold indicated that the gene was downregulated on the 270th day of lactation. Other genes were upregulated on the 270th day of lactation.
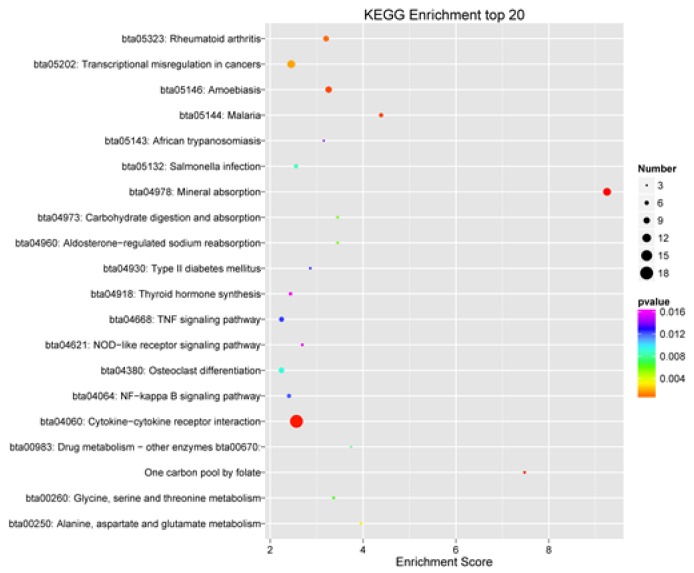
KEGG enrichment analysis of DEGs revealed 43 significantly enriched pathways. The top 20 significantly enriched pathways (padj < 0.05) were listed in Figure 4. Seven of the 43 significantly enriched pathways (padj < 0.05) were associated with inflammation and immune response, including 33 genes (Table 7).
Figure 4.
Top 20 significant pathways of Kyoto Encyclopedia of Genes and Genomes enrichment analysis of differentially expressed genes.
Table 7.
Significantly enriched pathways related to inflammation and immunity.
| KEGG-Pathway | Signal Path | padj | Gene Name | Number of Genes |
|---|---|---|---|---|
| bta04060 | Cytokine–cytokine receptor interaction | <0.001 | CCL19; CCL20; CCL28; CCR1; CD40; CSF3R; CXCL2; CXCL8; CXCR1; CXCR2; EDA; IL1R2; IL6; LIF; OSMR; RELT; TGFB2; TNFRSF6B | 18 |
| bta05323 | Rheumatoid arthritis | 0.001 | ACP5; ATP6V0D2; CCL20; CXCL8; ICAM1; IL6; TGFB2; TLR2 | 8 |
| bta04064 | NF-kappa B signaling pathway | 0.012 | BCL2A1; CCL19; CD40; CXCL8; ICAM1; LBP | 6 |
| bta04668 | TNF signaling pathway | 0.013 | CCL20; CXCL2; ICAM1; IL6; LIF; MMP9; SOCS3 | 7 |
| bta04620 | Toll-like receptor signaling pathway | 0.019 | CD40; CXCL8; IKBKE; IL6; LBP; TLR2 | 6 |
| bta04142 | Lysosome | 0.024 | ABCA2; ACP5; ATP6V0D2; CD68; CTSC; SLC11A1; SLC11A2 | 7 |
| bta04062 | Chemokine signaling pathway | 0.035 | CCL19; CCL20; CCL28; CCR1; CXCL2; CXCL8; CXCR1; CXCR2; HCK | 9 |
Note: The gene name in bold indicated that the gene was downregulated on the 270th day of lactation. Other genes were upregulated on the 270th day of lactation.
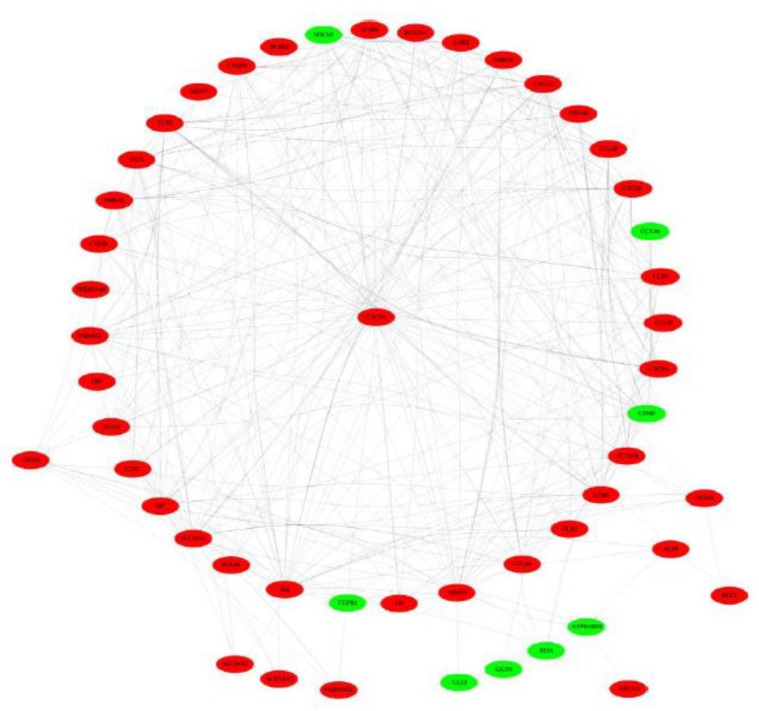
3.7. PPI Network Analysis
The PPI network of DEGs related to inflammation and immunity (Figure 5) revealed the biological activity and interactive relationship of their encoding proteins.
Figure 5.
A protein–protein interaction network of differentially expressed genes related to inflammation and immunity. The node in red and green, respectively indicated that the gene was upregulated and downregulated in on late lactation compared with peak lactation.
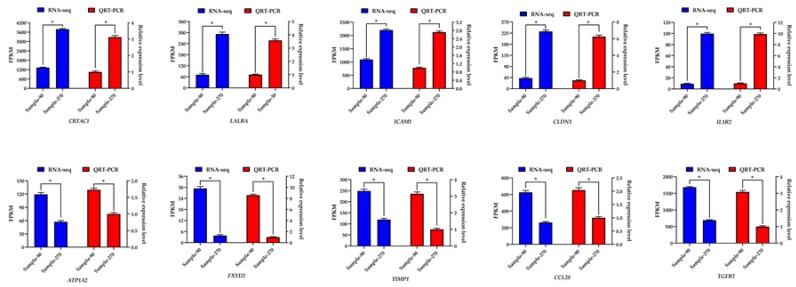
3.8. Verification Results of qRT-PCR
The results showed that the genes expression trends were consistent between sequencing data and qRT-PCR results. Moreover, the correlation coefficient of the sequencing data and qRT-PCR result using β-actin as reference gene reached 0.992 (R2 = 0.984) (Figure 6), which was highly significant (p < 0.000). The correlation coefficient of the sequencing data and qRT-PCR result using RPS9 as reference gene reached 0.981 (R2 = 0.962) (Figure S2), which was highly significant (p < 0.000) too. The reliability of the sequencing data was high.
Figure 6.
Expression level of ten differentially expressed genes detected by qRT-PCR using β-actin as reference gene and RNA-Seq. “*”: p < 0.05.
4. Discussion
The SCS of milk at 270th day of lactation was significantly higher than that at 90th day of lactation. There were five GO-BP terms and seven pathways (padj < 0.05) related to inflammation and immune response. The innate immune system of bovine mammary gland is composed of teat duct, body fluid, and immune cells [34]. The teat duct is closed in nonlactation, scilicet, a physical barrier separates pathogens from bovine mammary gland. At the late stage of lactation, with the increasing times of milking, the pathogens can easily enter the bovine mammary gland due to the mechanical injury to the nipples of cow [35]. Therefore, some pathogens enter bovine mammary gland and the SCC of milk in late lactation was higher than that in peak lactation. In this study, the expression level of Toll-like receptor 2 (TLR2) was significantly upregulated in order to receive more signals released by intruders to activate the Toll-like signaling pathway, inflammatory response term, and positive regulation of chemokine production term. The activated TLR2 induced the activation of MYD88 innate immune signal transduction adaptor (MyD88) [36,37], which can activate the NF-κB signaling pathway to produce nuclear transcription signaling, cytokines, and chemokines [38]. From that moment, the chemokine signaling pathway and the cytokine–cytokine receptor interaction were activated. Cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) were responsible for amplifying signals, while C-X-C motif chemokine ligand 8 (CXCL8) was bound to its specific receptor chemokine (C-X-C motif) receptor 1 (CXCR1) and C-X-C motif chemokine receptor 2 (CXCR2). Neutrophils rapidly respond to chemotactic signals and migrate to inflammatory sites to kill pathogens [39]. Meanwhile, the neutrophil chemotaxis term was activated. Compared with peak lactation, the expression levels of CXCL8, CXCR1, CXCR2, TNF-α, and IL-1β were upregulated at late lactation. These results were consistent with Gilbert et al. [38].
The study of Gilbert et al. [38] showed that E. coli crude lipopolysaccharide (LPS) preparation stimulated TLR2 and Toll-like receptor 4 (TLR4) in bovine mammary epithelial cells. In this study, there was no significant difference in the expression levels of TLR4. We found that there was also no significant difference in the expression levels of the potential downstream genes of TLR4: Interferon regulatory factor 3 (IRF3), C-X-C motif chemokine ligand 10 (CXCL10), chemokine (C-C motif) ligand 2 (CCL2), C-C motif chemokine ligand 5 (CCL5). The analysis of DEGs associated with inflammation and immunity indicated that the analyzed cows have a localized inflammatory reaction, which was consistent with the determination of SCC. In addition, Tahir Usman et al. [40] found that interleukin 17F (IL-17F) and interleukin 17A (IL-17A) could be powerful candidate genes of mastitis resistance and the significant single nucleotide polymorphisms (SNPs) might be useful genetic markers against mastitis in both dairy and dual-purpose cattle. In this study, the expression levels of IL-17F and IL-17A did not change significantly.
Zinc finger protein A20 (A20), encoded by TNF-α induced protein 3 (TNFAIP3), is a deubiquitinase that can be induced by TNF-α and IL-1β and then transcribed rapidly [36,37,41,42]. Ubiquitin is activated by the ubiquitin-activating enzymes (E1) in the presence of ATP and binds to E1. The activated ubiquitin molecule then transfers to the ubiquitin binding enzyme (E2). Ubiquitin ligases (E3) attract ubiquitin-E2 complexes and substrate proteins, and ubiquitin is transferred from E2 to the substrate. Substrate-ubiquitin complexes are removed by deubiquitinating enzyme B [38,43]. In this study, the expression level of TNFAIP3 was significantly higher at late lactation compared with peak, namely A20 acted on deubiquitinated proteins such as tumor necrosis factor receptor-associated protein 6 (TRAF6) and receptor-interacting protein 1 (RIP1) in the NF-κB signaling pathway, thereby negatively regulating the NF-κB signaling pathway, inhibiting the production of inflammatory signals, and ultimately reducing the damage caused by excessive inflammation [44]. The results showed that there was no significant difference in the expression levels of TRAF6 and RIP1 during the two test periods, indicating that the activation and inhibition of NF-κB signaling pathway belonged to the category of innate immune of bovine mammary gland and did not lead to mastitis and autoimmune diseases of the analyzed cows.
Solute carrier family 11 member 1 (SLC11A1) is known as natural resistance related macrophage protein. SLC11A1 is mainly distributed in the mammalian reticuloendothelial system, especially in macrophage phagocytic lysosome membrane. When pathogens invade cells, they activate pattern-recognition receptors, such as Toll-like receptors [45]. Endosomes are formed after the pathogens endocytosis by macrophages. SLC11A1, significantly upregulated at the late lactation, transported the metal ions necessary for the survival of pathogens out of the endosome, thus killing the pathogens [46]. Studies on dairy cows have shown that SLC11A1 is resistant to a variety of pathogens [47,48,49,50]. Joo et al. have proved that the mRNA expression of SLC11A1 in resistant dairy cows was significantly higher than that in susceptible dairy cows. Mastitis resistant dairy cows can be selected according to the difference in SLC11A1 expression [51].
5. Conclusions
In this study, a total of 446 DEGs were identified in the mammary tissue of late lactation (high SCC period) and peak lactation (low SCC period) of Chinese Holstein. Functional analysis showed that 50 DEGs related to immunity and inflammation such as TLR2, TNF-α, IL-1, CXCR1, CXCR2, CXCL8, TNFAIP3, TRAF6, RIP1, and SLC11A1. Further studies are warranted to further explore the molecular mechanism of inflammation and immune response regarding to these DEGs.
Acknowledgments
The authors would like to thank all members of this work for their advice and technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/10/3/510/s1, Figure S1: PCA analysis of the mRNA of genes. Figure S2: Expression level of ten differentially expressed genes detected by qRT-PCR using RPS9 as reference gene and RNA-Seq.
Author Contributions
Conceptualization, Z.Y. and J.J.L.; data curation, Z.H.; formal analysis, Y.F.; funding acquisition, Z.Y. and Y.Y.; investigation, Z.H.; methodology, Y.F.; resources, Y.F.; software, Y.F.; validation, Z.Y.; writing—original draft, Z.H.; writing—review and editing, Z.Y. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Research Program of Jiangsu Province (BK20190881), the China Postdoctoral Science Foundation (2019M650126), the National Natural Science Foundation of China (31872324), Jiangsu Agriculture Science and Technology Innovation Fund (CX (17) 1005), and the Jiangsu Modern Dairy Industry Technology System (JATS (2018) 300).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sarikaya H., Bruckmaier R.M. Importance of the sampled milk fraction for the prediction of total quarter somatic cell count. J. Dairy Sci. 2006;89:4246–4250. doi: 10.3168/jds.S0022-0302(06)72470-5. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y., Ryan C., Barbano D.M., Galton D.M., Rudan M.A., Boor K.J. Effects of somatic cell count on quality and shelf-life of pasteurized fluid milk. J. Dairy Sci. 2000;83:264–274. doi: 10.3168/jds.S0022-0302(00)74873-9. [DOI] [PubMed] [Google Scholar]
- 3.Helgren J.M., Reinemann D.J. Survey of milk quality on US dairy farms utilizing automatic milking systems. Trans. Asabe. 2006;49:551–556. doi: 10.13031/2013.20398. [DOI] [Google Scholar]
- 4.Yang H.Y., Jian G., Liu X.Q., Su J.L., Bo H. Rapid detection of milk somatic cell counts and its clinical significance. Eur. Spine J. 2010;46 doi: 10.1007/s00586-015-4120-x. [DOI] [Google Scholar]
- 5.Eltahawy A.S., Elfar A.H. Influences of somatic cell count on milk composition and dairy farm profitability. Int. J. Dairy Technol. 2010;63:463–469. doi: 10.1111/j.1471-0307.2010.00597.x. [DOI] [Google Scholar]
- 6.Li N., Richoux R., Boutinaud M., Martin P., Gagnaire V. Erratum to: Role of somatic cells on dairy processes and products: A review. Dairy Sci. Technol. 2014;94:517–538. doi: 10.1007/s13594-014-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auldist M.J., Coats S., Rogers G.L., Mcdowell G.H. Changes in the composition of milk from healthy and mastitic dairy cows during the lactation cycle. Aust. J. Exp. Agric. 1995;35:427–436. doi: 10.1071/EA9950427. [DOI] [Google Scholar]
- 8.Hagnestam-Nielsen C., Emanuelson U., Berglund B., Strandberg E. Relationship between somatic cell count and milk yield in different stages of lactation. J. Dairy Sci. 2009;92:3124–3133. doi: 10.3168/jds.2008-1719. [DOI] [PubMed] [Google Scholar]
- 9.Ng-Kwai-Hang K.F., Hayes J.F., Moxley J.E., Monardes H.G. Variability of test-day milk production and composition and relations of somatic cell counts with yield and compositional changes of bovine milk. J. Dairy Sci. 1984;67:361–366. doi: 10.3168/jds.S0022-0302(84)81309-0. [DOI] [PubMed] [Google Scholar]
- 10.Dai W.T., Zou Y.X., White R.R., Liu J.X., Liu H.Y. Transcriptomic profiles of the bovine mammary gland during lactation and the dry period. Funct. Integr. Genom. 2018;18:125–140. doi: 10.1007/s10142-017-0580-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu D.-D., Zhang T.-Z., Sun W., Hu S.-X., Yun H.-Q., Chi H.-H., Han L.-Q., Li X.-H. Effects of days in milk(DIM) on production performance and correlation analysis in primiparous and older holstein cows. China Dairy Cattle. 2018;11 doi: 10.19305/j.cnki.11-3009/s.2018.11.006. [DOI] [Google Scholar]
- 12.Mao Y., Yang Z., Wang X., Hua J., Zhu J., Zhang X. The relationship among milking traits and udder traits SCC in milk for Chinese holstein in south. China Dairy Cattle. 2002;2 doi: 10.3969/j.issn.1004-4264.2002.02.006. [DOI] [Google Scholar]
- 13.Mccoard S.A., Hayashi A.A., Sciascia Q., Rounce J., Sinclair B., Mcnabb W.C., Roy N.C. Mammary transcriptome analysis of lactating dairy cows following administration of bovine growth hormone. Animal. 2016;10:2008–2017. doi: 10.1017/S1751731116000987. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y., Lv H., Jiang M., Zhou J., Song S., Hou X. Functional analysis of the dairy cow mammary transcriptome between early lactation and mid-dry period. JDR. 2019;86:63–67. doi: 10.1017/S0022029919000049. [DOI] [PubMed] [Google Scholar]
- 15.Bauersachs S., Simintiras C.A., Sturmey R.G., Krebs S., Bick J., Blum H., Wolf E., Lonergan P., Forde N. Effect of metabolic status on conceptus-maternal interactions on Day 19 in dairy cattle: II. Effects on the endometrial transcriptome. Biol. Reprod. 2017;97 doi: 10.1093/biolre/iox095. [DOI] [PubMed] [Google Scholar]
- 16.Piantoni P., Wang P., Drackley J.K., Hurley W.L., Loor J.J. Expression of metabolic, tissue remodeling, oxidative stress, and inflammatory pathways in mammary tissue during involution in lactating dairy cows. Bioinform. Biol. Insights. 2010;4:BBI.S5850. doi: 10.4137/BBI.S5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C., Cai W., Zhou C., Yin H., Zhang Z., Loor J.J., Sun D., Zhang Q., Liu J., Zhang S. RNA-Seq reveals 10 novel promising candidate genes affecting milk protein concentration in the Chinese Holstein population. Sci. Rep. 2016;6:26813. doi: 10.1038/srep26813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J., Barkema H.W., Zhang L., Liu G., Deng Z., Cai L., Shan R., Zhang S., Zou J., Kastelic J.P. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J. Dairy Sci. 2017;100:4797–4806. doi: 10.3168/jds.2016-12334. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Zhou M., Hardwidge P.R., Cui H., Zhu G. Isolation and characterization of N-acyl homoserine lactone-producing bacteria from cattle rumen and swine intestines. Front. Cell. Infect. Microbiol. 2018;8:155. doi: 10.3389/fcimb.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y., Fan W., Mao Y., Yang Z., Lu G., Zhang R., Zhang H., Szeto C., Wang C. Bovine leukemia virus infection in cattle of China: Association with reduced milk production and increased somatic cell score. J. Dairy Sci. 2016;99:3688–3697. doi: 10.3168/jds.2015-10580. [DOI] [PubMed] [Google Scholar]
- 21.Patel R.K., Mukesh J. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., Van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon A., Paul Theodor P., Wolfgang H. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crisà A., Ferrè F., Chillemi G., Moioli B. RNA-Sequencing for profiling goat milk transcriptome in colostrum and mature milk. BMC Vet. Res. 2016;12:264. doi: 10.1186/s12917-016-0881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren P., Meng Y., Li B., Ma X., Si E., Lai Y., Wang J., Yao L., Yang K., Shang X. Molecular mechanisms of acclimatization to phosphorus starvation and recovery underlying full-length transcriptome profiling in barley (Hordeum vulgare L.) Front. Plant Sci. 2018;9:500. doi: 10.3389/fpls.2018.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Da W.H., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 29.Xie C., Mao X., Huang J., Ding Y., Wu J., Dong S., Kong L., Gao G., Li C.-Y., Wei L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. NAR. 2011;39:316–322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Zhao X., Wang J., Zong M., Yang H. Bioinformatics analysis of gene expression profile data to screen key genes involved in pulmonary sarcoidosis. Gene. 2017;596:98–104. doi: 10.1016/j.gene.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Bionaz M., Loor J.J. Identification of reference genes for quantitative real-time PCR in the bovine mammary gland during the lactation cycle. Physiol. Genom. 2007;29:312–319. doi: 10.1152/physiolgenomics.00223.2006. [DOI] [PubMed] [Google Scholar]
- 33.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Wellnitz O., Bruckmaier R.M. The innate immune response of the bovine mammary gland to bacterial infection. Vet. J. 2012;192:148–152. doi: 10.1016/j.tvjl.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Hospido A., Sonesson U. The environmental impact of mastitis: A case study of dairy herds. Sci. Total Environ. 2005;343:71–82. doi: 10.1016/j.scitotenv.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Hirotani T., Yamamoto M., Kumagai Y., Uematsu S., Kawase I., Takeuchi O., Akira S. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem. Biophys. Res. Commun. 2005;328:383–392. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z., Xue Y., Hu Z., Zhou F., Ma B., Long T., Xue Q., Liu H. Toll-like receptor 2 gene polymorphisms in Chinese Holstein cattle and their associations with bovine tuberculosis. Vet. Immunol. Immunopathol. 2017;186:51–54. doi: 10.1016/j.vetimm.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert F.B., Cunha P., Jensen K., Glass E.J., Foucras G., Robert-Granié C., Rupp R., Rainard P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013;44:40. doi: 10.1186/1297-9716-44-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerard C., Rollins B.J. Chemokines and disease. Nat. Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 40.Usman T., Wang Y., Liu C., He Y., Wang X., Dong Y., Wu H., Liu A., Yu Y. Novel SNPs in IL-17F and IL-17A genes associated with somatic cell count in Chinese Holstein and Inner-Mongolia Sanhe cattle. J. Anim. Sci. Biotechnol. 2017;8:5. doi: 10.1186/s40104-016-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf F.W., Marks R.M., Sarma V., Byers M.G., Katz R.W., Shows T.B., Dixit V.M. Characterization of a novel tumor necrosis factor-alpha-induced endothelial primary response gene. J. Biol. Chem. 1992;267:1317–1326. doi: 10.1111/j.1432-1033.1992.tb19862.x. [DOI] [PubMed] [Google Scholar]
- 42.Dixit V.M., Green S., Sarma V., Holzman L.B., Wolf F.W., O’Rourke K., Ward P.A., Prochownik E.V., Marks R.M. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J. Biol. Chem. 1990;265:2973–2978. doi: 10.1002/bip.360290316. [DOI] [PubMed] [Google Scholar]
- 43.Yun-Cai L., Josef P., Michael K. Immunity by ubiquitylation: A reversible process of modification. Nat. Rev. Immunol. 2005;5:941–952. doi: 10.1038/nri1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harhaj E.W., Dixit V.M. Regulation of NF-κB by deubiquitinases. Immunol. Rev. 2012;246:107–124. doi: 10.1111/j.1600-065X.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shelly S., Lukinova N., Bambina S., Berman A., Cherry S. Autophagy is an essential component of drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Supek F., Supekova L., Nelson H., Nelson N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl. Acad. Sci. USA. 1996;93:5105–5110. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar N., Mitra A., Ganguly I., Singh R., Deb S.M., Srivastava S.K., Sharma A. Lack of association of brucellosis resistance with (GT)(13) microsatellite allele at 3′UTR of NRAMP1 gene in Indian zebu (Bos indicus) and crossbred (Bos indicus × Bos taurus) cattle. Vet. Microbiol. 2005;111:139–143. doi: 10.1016/j.vetmic.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Pereira-Suárez A.L., Estrada-Chávez C., Arriaga-Díaz C., Espinosa-Cueto P., Mancilla R. Coexpression of NRAMP1, iNOS, and nitrotyrosine in bovine tuberculosis. Vet. Pathol. 2006;43:709–717. doi: 10.1354/vp.43-5-709. [DOI] [PubMed] [Google Scholar]
- 49.Pinedo P.J., Buergelt C.D., Donovan G.A., Melendez P., Morel L., Wu R., Langaee T.Y., Rae D.O. Candidate gene polymorphisms (BoIFNG, TLR4, SLC11A1) as risk factors for paratuberculosis infection in cattle. Prev. Vet. Med. 2009;91:189–196. doi: 10.1016/j.prevetmed.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Hébert A., Sayasith K., Sénéchal S., Dubreuil P., Lagacé J. Demonstration of intracellular Staphylococcus aureus in bovine mastitis alveolar cells and macrophages isolated from naturally infected cow milk. FEMS Microbiol. Lett. 2000;193:57–62. doi: 10.1016/S0378-1097(00)00455-9. [DOI] [PubMed] [Google Scholar]
- 51.Joo Y.S., Moon J.S., Fox L.K., Suh G.H., Kwon N.H., Kim S.H., Park Y.H. Comparison of natural resistance-associated macrophage protein (NRAMP)1 expression between cows with high and low milk somatic cells counts. Asian Australas. J. Anim. Sci. 2003;16:1830–1836. doi: 10.5713/ajas.2003.1830. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.