Abstract
Simple Summary
The exposure of animals to excessive heat leads to heat stress, heat stroke, or even death. The first negative effects of heat exposure occur in the gut. The elevated temperature leads to damage in intestinal walls and shifts in the composition of intestinal microbiota. In effect, the gut content (mainly intestinal microbiota and their metabolites) leaks through compromised intestinal walls into milieu of the body. Prebiotics (e.g., GOS—galactooligosaccharides) can be used to mitigate the negative effects of the heat stress in poultry. GOS that are delivered in ovo on day 12 of egg incubation stimulates the development of healthy intestinal microbiota in a chicken embryo. Healthy intestinal microbiota enhances the barrier function of the gut and the immune system. Chickens were originally domesticated in southeast Asia and are therefore genetically adapted to handle high temperatures. However, genetic selection towards performance leads to sensitization to high ambient temperature. In this paper, we studied slow-growing chickens with a reputation for heat resistance. We used in ovo stimulation with the GOS prebiotic that was delivered in ovo to promote healthy gut microbiota. In this manner, we combine genetics and environment to describe a model of heat resistance in poultry.
Abstract
Galactooligosaccharides (GOS) that are delivered in ovo improve intestinal microbiota composition and mitigate the negative effects of heat stress in broiler chickens. Hubbard hybrids are slow-growing chickens with a high resistance to heat. In this paper, we determined the impact of GOS delivered in ovo on slow-growing chickens that are challenged with heat. The experiment was a 2 × 2 × 2 factorial design. On day 12 of incubation, GOS (3.5 mg/egg) was delivered into the egg (n = 300). Controls (C) were mock-injected with physiological saline (n = 300). After hatching, the GOS and C groups were split into thermal groups: thermoneutral (TN) and heat stress (HS). HS (30 °C) lasted for 14 days (days 36–50 post-hatching). The spleen (n = 8) was sampled after acute (8.5 h) and chronic (14 days) HS. The gene expression of immune-related (IL-2, IL-4, IL-6, IL-10, IL-12p40, and IL-17) and stress-related genes (HSP25, HSP90AA1, BAG3, CAT, and SOD) was detected with RT-qPCR. Chronic HS up-regulated the expression of the genes: IL-10, IL-12p40, SOD (p < 0.05), and CAT (p < 0.01). GOS delivered in ovo down-regulated IL-4 (acute p < 0.001; chronic p < 0.01), IL-12p40, CAT and SOD (chronic p < 0.05). The obtained results suggest that slow-growing hybrids are resistant to acute heat and tolerant to chronic heat, which can be supported with in ovo GOS administration.
Keywords: GOS, prebiotic, in ovo stimulation, heat, immune response, spleen
1. Introduction
Acute and chronic thermal stress significantly hinders the growth performance of poultry [1]. This is due to the fact that the feed intake of chickens that are reared in intensive poultry farms is negatively correlated with environmental temperature [2]. The main ancestor of the domestic chicken is red junglefowl (Gallus gallus) from the hot jungle climate of Southeast Asia [3]. However, intensive genetic selection for growth and feed efficiency leads to an increased sensitivity of heavy-weight broilers to environmental conditions, including ambient temperature [4]. There is a negative correlation between heat tolerance and growth rate [5]. Fast-growing broilers produce more heat and have a higher heat load [6], which reduces feed intake and growth parameters [7,8,9]. Heat stress (HS) could lead to meat quality issues due to increased ante- and post-mortem glycolytic metabolisms coupled with a reduced protein synthesis and turnover, enhanced fat deposition, and the overproduction of reactive oxygen species [10]. The gastrointestinal tract (GIT) is also very responsive to heat, which alters intestinal microbiota composition [11] and decreases the integrity of the intestinal epithelium [12]. Since exposure to high temperatures is difficult to avoid in intensive production systems, losses in the production and mortality are high [13]. The negative effects of HS in poultry are due to high animal stocking, the insufficient ventilation of poultry houses, as well as geographical factors [14]. Intergovernmental Panel on Climate Change (IPCC) reports clearly show that the climate is warming [15]. Problems related to maintaining optimal temperatures in poultry houses will become more challenging, especially during predicted heat waves, even in moderate climates. HS leads to behavioral, biochemical, and physiological changes. Responses to heat vastly depend on the genetic adaptation of the individual [16] but also on environmental factors, such as intestinal microbiota [17]. Compromised genetic or environmental conditions result in poorer thermoregulation [18].
Heat-resistance depends on the genetic adaptation of the chickens. Native chickens from tropical and sub-tropical regions are more tolerant to high ambient temperatures than fast-growing lines [19,20]. Since they are smaller and lighter and have not been subjected to selective pressure for meat-related traits, they have retained their genetic adaptation to handle high temperatures. Studies on Brazilian breeds (Pelaco and Caneluda) [18] and Egyptian breeds (Fayoumi, Dandarawi, and Sinai) [21] have shown a good tolerance to elevated ambient temperature, manifested by the increased expression of heat shock proteins (HSP). In some countries, native breeds are crossbred with commercial broiler lines to obtain heat-resistant hybrids with good meat production [22]. The hybrids that were used in our experiment are slow-growing free-range chickens that are obtained by crossing a Hubbard RedBro male with a Hubbard JA57 (https://www.hubbardbreeders.com/products/crosses/ja57/) female. These free-range poultry hybrids are distinguished by a good adaptation to a warm climate and a high disease resistance (Federico Sirri, unpublished data).
HS in poultry influences the composition of intestinal microbiota [23], leading to gut dysbiosis [24]. Unstable microbiota impairs the morphology and barrier function of the GIT [25]. Feed additives such as prebiotics support the gut microbiota under stressful conditions [26,27,28]. Prebiotic and probiotic supplementation allow for the maintenance of stable microbiota populations in the gut [29] and prevent “leaky guts” [30]. One of the most efficient ways to improve intestinal microbiota composition in poultry is in ovo stimulation. It allows for the precise introduction of a specific substance directly into the internal environment of the incubating egg. During in ovo stimulation, a prebiotic or synbiotic is injected into the air cell of the incubating egg (in this study, on day 12 of egg incubation) and stimulates the development of indigenous microbiota prior to hatching [31]. The prebiotic supplementation at the embryonic stage supports not only the microbiota development in the growing chickens [32,33,34] but also improves the immune system [35,36], gut morphology [37] and the intestinal barrier function [32].
The positive effect of GOS supplementation on the poultry intestinal microbiome has been demonstrated [38]. Galactooligosaccharides are potent prebiotics that exert beneficial effects on intestinal microbiota in chickens. Particularly interesting is the possibility of using in ovo technology for the early stimulation of the microbial communities with GOS [32,39,40]. The molecular data on broiler chickens have indicated that HS triggers systemic immune and stress responses, which are balanced by GOS that are delivered in ovo [41]. It also mitigates the negative effects of HS in broiler chickens on performance traits, including improved growth efficiency, feed efficiency [39], and meat quality [10]. In this study, we focused on chickens with a different genetic background, i.e., slow-growing hybrids. The aim of this study was to assess the impact of GOS that were delivered in ovo on the modulation of the immune-related and stress-related gene expression signatures in the spleens of slow-growing chickens that were subjected to HS. Hereby, we hypothesize that the genetics of slow-growing chickens combined with in ovo stimulation with GOS will contribute to increased HS resistance.
2. Materials and Methods
2.1. Ethical Statement
The animal procedures were conducted in compliance with decision of the Ethical Committee in Rome (Italy), decision number 503/2016.
2.2. Experimental Trial and Tissue Collection
The experimental material was slow-growing crossbred Hubbard chickens. After 12 days of incubation, 300 eggs were injected with a single dose of 3.5 mg GOS/egg (GOS group) suspended in 0.2 mL of physiological saline into the air chamber. Controls (the C group) were mock-injected with sterile physiological saline (n = 300, volume 0.2 mL/egg). Injection was carried out according to the in ovo procedure [32]. After hatching, the GOS and C groups were divided into two subgroups: maintained in thermoneutral condition (TN) and under the HS condition. In all groups, chicks were sexed and vaccinated against coccidiosis, infectious bronchitis virus, Marek’s disease virus, Newcastle disease, and Gumboro disease, and they received food and water ad libitum. The composition of the diets is presented in Table 1. Male chicks (n = 600, 300 per treatment) were transferred to an environmentally controlled poultry house and divided into 4 groups of 150 chicks/treatment/environmental condition. Each group was composed of 6 replicates of 25 birds each. HS had two forms: acute (on day 36, the temperature in the poultry house was raised to 30 °C for 8.5 h) and chronic (on day 36, the temperature was raised to 30 °C, and these conditions were maintained for 14 days). After that, 8 randomly selected animals from each group with an average body weight were slaughtered and dissected. Fragments of the spleen were collected in the tubes with 3 mL of an RNAlater solution (Invitrogen, Waltham, MA, USA) for RNA stabilization and stored at −80 °C until further processing.
Table 1.
Dietary formulation supplied in slow-growing chickens during three feeding phases.
| Ingredient | Starter (0–14 d) | Grower (15–36 d) | Finisher (37–50 d) |
|---|---|---|---|
| Corn | 42.17 | 34.96 | 12.73 |
| White corn | 0.00 | 0.00 | 15.00 |
| Wheat | 10.00 | 20.00 | 25.01 |
| Sorghum | 0.00 | 0.00 | 5.00 |
| Soybean meal | 23.11 | 20.63 | 17.60 |
| Expanded soybean | 10.00 | 10.00 | 13.00 |
| Sunflower | 3.00 | 3.00 | 3.00 |
| Corn gluten | 4.00 | 3.00 | 0.00 |
| Soybean oil | 3.08 | 4.43 | 5.48 |
| Dicalcium phosphate | 1.52 | 1.20 | 0.57 |
| Calcium carbonate | 0.91 | 0.65 | 0.52 |
| Sodium bicarbonate | 0.15 | 0.10 | 0.15 |
| Salt | 0.27 | 0.27 | 0.25 |
| Coline cloride | 0.10 | 0.10 | 0.10 |
| Lysine solfate | 0.59 | 0.55 | 0.46 |
| Dl-methionine | 0.27 | 0.29 | 0.30 |
| Threonine | 0.15 | 0.14 | 0.14 |
| Enzyme-roxazyme g2g | 0.08 | 0.08 | 0.08 |
| Phytase 0.1% | 0.10 | 0.10 | 0.10 |
| Coccidiostat | |||
| Vit-min premix 1 | 0.50 | 0.50 | 0.50 |
| Dry matter, % | 88.57 | 88.65 | 88.64 |
| Protein, % | 22.70 | 21.49 | 19.74 |
| Lipid, % | 7.06 | 8.24 | 9.74 |
| Fiber, % | 3.08 | 3.04 | 3.07 |
| Ash, % | 5.85 | 5.17 | 4.49 |
| Lys, % | 1.38 | 1.29 | 1.21 |
| Met, % | 0.67 | 0.62 | 0.59 |
| Met + Cys, % | 1.03 | 0.97 | 0.91 |
| Calcium, % | 0.91 | 0.80 | 0.59 |
| Phosphate, % | 0.63 | 0.57 | 0.46 |
| Metabolizable energy (kcal/kg) | 3.076 | 3.168 | 3.264 |
1 Provided the following per kg of diet: vitamin A (retinyl acetate), 13,000 IU; vitamin D3 (cholecalciferol), 4000 IU; vitamin E (DL-α_tocopheryl acetate), 80 IU; vitamin K (menadione sodium bisulfite), 3 mg; riboflavin, 6.0 mg; pantothenic acid, 6.0 mg; niacin, 20 mg; pyridoxine, 2 mg; folic acid, 0.5 mg; biotin, 0.10 mg; thiamine, 2.5 mg; vitamin B12 20 μg; Mn, 100 mg; Zn, 85 mg; Fe, 30 mg; Cu, 10 mg; I, 1.5 mg; Se, 0.2 mg; and ethoxyquin, 100 mg.
2.3. RNA Isolation
Total RNA was isolated from the spleen. Fragments of the spleen tissue were homogenized with the TissueRuptor homogenizer (Qiagen GmbH, Hilden, Germany) in TRIzol® LS Reagent (Ambion/Thermo Fisher Scientific, Valtham, MA, USA). Further steps of RNA isolation and purification were performed with a commercial kit (Universal RNA Purification Kit, EURx, Gdansk, Poland). RNA quality and quantity were evaluated by using electrophoresis in 2% agarose gel and a NanoDrop 2000 spectrophotometer (Scientific Nanodrop Products, Wilmington, NC, USA).
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
Complementary DNA (cDNA) was synthesized by using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Scientific/Fermentas, Vilnius, Lithuania), following the manufacturer’s recommendations. Obtained cDNA was diluted to 70 ng/μL and stored at −20 °C. RT-qPCR reactions were conducted with a total volume of 10 μL. The reaction mixture included Maxima SYBR Green qPCR Master Mix (Thermo Scientific/Fermentas, Vilnius, Lithuania), 1 μM of each primer (Sigma-Aldrich, Schnelldorf, Germany), and 2 μL of diluted cDNA. Thermal cycling was performed in a LightCycler II 480 (Roche Diagnostics, Basel, Switzerland). Each RT-qPCR reaction was conducted in two technical replicates. Gene expression analysis was performed for two gene panels, which were reported earlier [41]. The first gene panel was associated with the immune response and included the genes: IL-2, IL-4, IL-6, IL-10, IL-12p40, and IL-17. The second gene panel was associated with stress response and included the genes: HSP25, HSP90AA1, BAG3, CAT, and SOD. UB and ACTB were used as reference genes. The sequences of the primers that were used in this experiment are presented in Table 2.
Table 2.
List of target genes and primers sequences for RT-qPCR.
| Gene a | NCBI Gene ID | Primer Sequences (5′-3′) | Function b | Ref. |
|---|---|---|---|---|
| Panel 1. Immune-related genes | ||||
| IL-2 | 373958 | F: GCTTATGGAGCATCTCTATCATCA R: GGTGCACTCCTGGGTCTC |
Cytokine important for the proliferation of T and B lymphocytes. Important role in the immune response to antigenic stimuli. | [41] |
| IL-4 | 416330 | F: GCTCTCAGTGCCGCTGATG R: GGAAACCTCTCCCTGGATGTC |
Pleiotropic cytokine produced by activated T cells. B-cell stimulatory factor. | [43] |
| IL-6 | 395337 | F: AGGACGAGATGTGCAAGAAGTTC R: TTGGGCAGGTTGAGGTTGTT |
Cytokine that plays a role in inflammation and the maturation of B cells. Produced at sites of acute and chronic inflammation. | [44] |
| IL-10 | 428264 | F: CATGCTGCTGGGCCTGAA R: CGTCTCCTTGATCTGCTTGATG |
Pleiotropic effects in immunoregulation and inflammation. Inhibits synthesis of cytokines. | [45] |
| IL-12p40 | 404671 | F: TTGCCGAAGAGCACCAGCCG R: CGGTGTGCTCCAGGTCTTGGG |
Can act as a growth factor for activated T and Natural Killer cells. Stimulates production of IFN-gamma. | [46] |
| IL-17 | 395111 | F: GGGATTACAGGATCGATGAGGA R: GAGTTCACGCACCTGGAATG |
Cytotoxic T-lymphocyte-associated protein 8. Proinflammatory cytokine produced by activated T cells. | [41] |
| Panel 2. Stress response genes | ||||
| HSP25 | 428310 | F: CCGTCTTCTGCTGAGAGGAGTG R: ACCGTTGTTCCGTCCCATCAC |
Heat shock protein family B (small) member 9. Response to various cellular stresses. Molecular chaperones which bind to and inhibit irreversible protein aggregation or misfolding under stressful conditions. | [47] |
| HSP90AA1 | 423463 | F: GGTGTTGGTTCCTACTCTGCTTAC R: ACTGCTCATCATCATTGTGCTTGG |
Heat shock protein family class A member 1. Is a molecular chaperone that aids protein folding and quality control for a large proteins. | [47] |
| BAG3 | 423931 | F: AGGGTCGTGCGGATGTGC R: TGTGGTGGCTTAGGCTCTGC |
BAG family molecular chaperone regulator 3. Cellular response to stress. | [47] |
| CAT | 423600 | F: GGGGAGCTGTTTACTGCAAG R: CTTCCATTGGCTATGGCATT |
Catalase a key antioxidant enzyme in the bodies defense against oxidative stress. | [48] |
| SOD1 | 395938 | F: AGGGGGTCATCCACTTCC R: CCCATTTGTGTTGTCTCCAA |
Superoxide Dismutase binds copper and zinc ions. Responsible for destroying free superoxide radicals. | [48] |
| Reference genes | ||||
| ACTB | 396526 | F: CACAGATCATGTTTGAGACCTT R: CATCACAATACCAGTGGTACG |
Beta-actin is highly conserved protein involved in cell motility, structure, integrity and intercellular signaling. Ubiquitously expressed in all eukaryotic cells. | [49] |
| UB | 101747587F | F: GGGATGCAGATCTTCGTGAAA R: CTTGCCAGCAAAGATCAACCTT |
Ubiquitin is associated with protein degradation, DNA repair, cell cycle regulation kinase modification, and regulation of other cell signals pathways. | [49] |
a Annealing temperature for RT-qPCR was 58 °C except from IL-12p40 (65 °C); b gene function derive from GeneCards (http://www.genecards.org).
2.5. Relative Quantification of Gene Expression and Statistical Analysis
The normalization of the expression levels (Ct − cycle threshold) of the target genes was performed with a geometric mean of the two reference genes (UB and ACTB). ∆Ct was calculated by subtracting the Ct of the reference genes from the Ct of the target genes (Ct target − Ct reference). All statistical analyses were based on ∆Ct values. The full-factorial study design allowed us to analyze the impact of GOS that were delivered in ovo and different levels of HS on gene expression signatures in slow-growing hybrid chickens. The first statistical model was two-way ANOVA with interaction, in which in ovo treatment and ambient temperature (acute or chronic HS vs. TN) were considered independent variables (factors). In this analysis, the two time-points of tissue collection (day 36—acute HS and day 50—chronic HS) were independently analyzed. The second statistical model included in ovo treatment and HS (acute vs. chronic HS) as factors. In this model, the datasets from acute and chronic HS were combined. In both ANOVA analyses, the factors (or interaction between them) were considered significant at p < 0.05, p < 0.01, or p < 0.001.
Relative gene expression was calculated with the ∆∆Ct algorithm. In the ∆∆Ct algorithm, a selected a calibrator (control ∆Ct) was subtracted from the ∆Ct of the experimental group. The fold change (FC) of the target gene in the experimental group vs. the control group was calculated according to the formula: 2−∆∆Ct [42]. To visualize the pairwise differences between the treatment groups, the results of the log2 fold change were graphed and compared with a Student’s t-test. The pairwise comparisons were considered significant at p < 0.05. The calculations were performed in MS Excel and SAS Enterprise Guide 9.4 (SAS Institute, Cary, NC, USA). Graphs were drawn by using Graph Pad Prism 7 (GraphPad, La Jolla, CA, USA).
3. Results
3.1. Effects of in ovo Treatment and Thermal Challenge on Gene Expression
The results of two-way ANOVA with interaction, using the first statistical model (GOS vs. C; HS vs. TN; GOS vs. C x HS vs. TN), are presented in Table 3. In acute HS, among the immune-related genes, only IL-4 responded with differential messenger RNA (mRNA) expression to GOS treatment (p < 0.001), temperature (p < 0.05), and the interactions between those two factors (p < 0.01). GOS treatment modulated the expression of the stress-related genes: BAG3, CAT and SOD (p < 0.05). In chronic HS, GOS that were delivered in ovo had immunomodulatory effect on: IL-2 (p < 0.05) and IL-4 (p < 0.001). Temperature significantly modulated the expression of IL-4 (p < 0.01) and IL-12p40 (p < 0.05). GOS treatment, in interaction with the temperature, showed a modulatory effect on stress-related genes: CAT and SOD (p < 0.01).
Table 3.
Effects of in ovo treatment and ambient temperature on gene expression signatures in the spleens of slow-growing chickens.
| Gene | Treatment 1 | Temperature 2 | Treatment × Temperature 3 |
|---|---|---|---|
| Acute HS | |||
| Immune-related panel | |||
| IL-2 | NS | NS | NS |
| IL-4 | <0.001 | <0.05 | <0.01 |
| IL-6 | NS | NS | NS |
| IL-10 | NS | NS | NS |
| IL-12p40 | NS | NS | NS |
| IL-17 | NS | NS | NS |
| Stress-related panel | |||
| BAG3 | <0.05 | NS | NS |
| CAT | <0.05 | NS | NS |
| SOD | <0.05 | NS | NS |
| HSP25 | NS | NS | NS |
| HSP90 | NS | NS | NS |
| Chronic HS | |||
| Immune-related panel | |||
| IL-2 | <0.05 | NS | NS |
| IL-4 | <0.001 | <0.01 | NS |
| IL-6 | NS | NS | NS |
| IL-10 | NS | NS | NS |
| IL-12p40 | NS | <0.05 | NS |
| IL-17 | NS | NS | NS |
| Stress-related panel | |||
| BAG3 | NS | NS | NS |
| CAT | NS | NS | <0.01 |
| SOD | NS | NS | <0.01 |
| HSP25 | NS | NS | NS |
| HSP90 | NS | NS | NS |
Effects: 1 In ovo delivery of galactooligosaccharides (GOS) vs. physiological saline (C); 2 ambient temperature (TN—thermoneutral vs. HS); 3 interaction between in ovo treatment and ambient temperature on immune-related and stress-response genes in chicken spleens. Gene expression analysis was done with RT-qPCR. The significance of effects that were calculated with two-way ANOVA. Significance levels: p < 0.05, p < 0.01 or p < 0.001 (significant), and p > 0.05 (non-significant, NS).
The results of the two-way ANOVA that was performed with the second statistical model (GOS vs. C; acute HS vs. chronic HS; GOS vs. C × acute HS vs. chronic HS) are presented in Table 4. In this statistical model, we evaluated the effects of acute and chronic HS on the associated gene expression in the splenic tissue of slow-growing chickens. In ovo treatment had an effect on gene expression of IL-4 (p < 0.001), which was consistent with the results obtained in the first statistical model. HS (acute vs. chronic) changed the gene expression of IL-2 (p < 0.05) and HSP90 (p < 0.05), which was not observed by comparing TN vs. HS (acute or chronic). The interaction of the two factors significantly affected the stress-related genes CAT and SOD (p < 0.05).
Table 4.
Effects of in ovo treatment and the duration of heat stress (HS) on gene expression signatures in the spleens of slow-growing chickens.
| Gene | Treatment 1 | HS 2 | Treatment × HS 3 |
|---|---|---|---|
| Immune-related panel | |||
| IL-2 | NS | <0.05 | NS |
| IL-4 | <0.001 | NS | NS |
| IL-6 | NS | NS | NS |
| IL-10 | NS | NS | NS |
| IL-12p40 | NS | NS | NS |
| IL-17 | NS | NS | NS |
| Stress-related panel | |||
| BAG3 | NS | NS | NS |
| CAT | NS | NS | <0.05 |
| SOD | NS | NS | <0.05 |
| HSP25 | NS | NS | NS |
| HSP90 | NS | <0.05 | NS |
Effects: 1 In ovo delivery of galactooligosaccharides (GOS) vs. physiological saline (C); 2 HS (acute HS vs. chronic HS); 3 interaction between in ovo treatment and ambient temperature on immune-related and stress-response genes in chicken spleens. Gene expression analysis was done with RT-qPCR. The significance of effects was calculated with two-way ANOVA. Significance levels: p < 0.05, p < 0.01 or p < 0.001 (significant), and p > 0.05 (non-significant, NS).
3.2. Relative Gene Expression Changes in Heat Stress
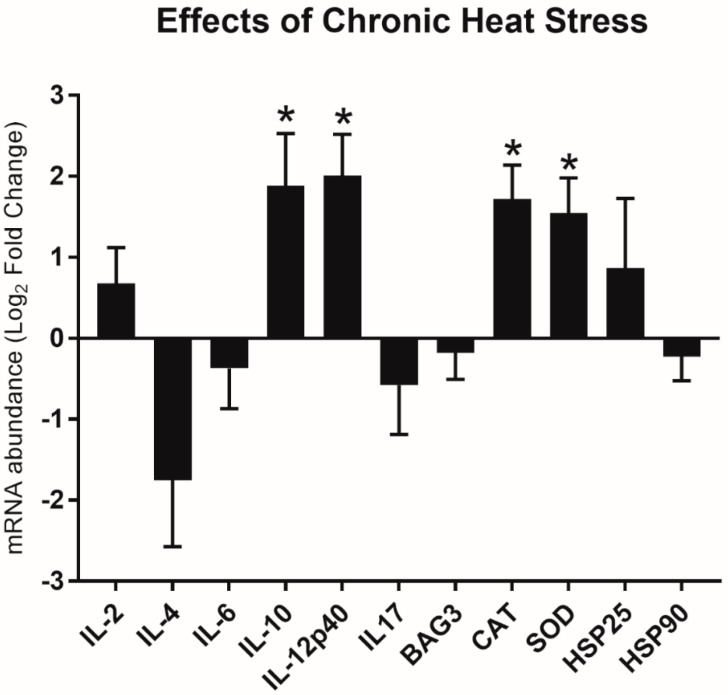
Short-term (acute) heat (TN-C vs. HS-C) did not affect the gene expression signatures that are associated with immune and stress responses in slow-growing chickens. On the other hand, long-term (chronic) heat activated some immune-related and stress-related genes, presented in Figure 1. Chronic HS up-regulated anti-inflammatory (FC log2 of IL-10 = 1.88, p < 0.05) and pro-inflammatory cytokines (FC log2 IL-12p40 = 2.01, p < 0.05). Additionally, oxidative stress was activated in the spleen during chronic HS (FC log2 CAT gene = 1.72, p < 0.01 and FC log2 SOD gene = 1.55, p < 0.05). Surprisingly, the genes encoding chaperones (HSP25 and HSP90) were not activated by either acute or chronic heat (p > 0.05).
Figure 1.
Relative messenger RNA (mRNA) expression of immune-related and stress-related genes in the spleens of slow-growing chickens that were challenged with chronic heat. Gene panel includes: interleukin: IL-2, IL-4. IL-6, IL-10, IL-12p40, and IL-17 and stress-related genes: CAT, SOD, BAG3, HSP25 and HSP90. The x-axis shows a list of genes. The y-axis indicate the relative mRNA abundance of the genes after heat challenge (n = 8). Gene expression analysis was carried out with RT-qPCR. qPCR reactions were performed in triplicate. The geometric mean of the ACTB and UB reference genes was used to calculate delta cycle threshold (dCt) values. The relative gene expression (FC—fold change) was calculated with the delta delta cycle threshold (ddCt) formula and the fold change (FC) was calculated as follows: FC = 2−ΔΔCt. FC values were transformed and presented as Log2FC. The standard error of the means (SEM) shows distribution of the Ct values. Normalized data (dCt values) of thermoneutral control (mock-injected) and heat-stressed control (mock-injected) groups were compared with a Student’s t-test. Significant differences (p < 0.05) are labelled with an asterisk (*). Figures were prepared by using GraphPad Prism 7 (GraphPad, La Jolla, CA, USA).
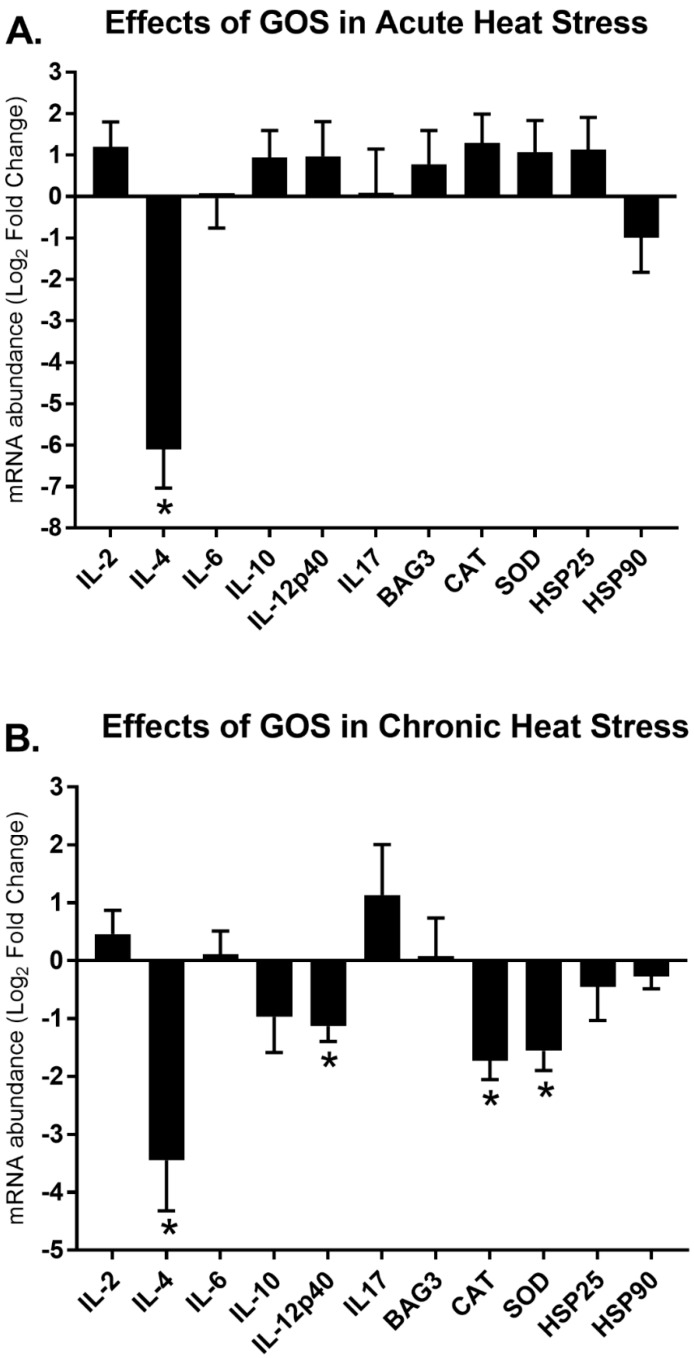
3.3. Effects of GOS Delivered in ovo on Gene Expression Modulation during Acute and Chronic Heat Stress
Figure 2 presents effects of GOS that were delivered in ovo on gene expression signatures during HS. Overall, GOS that were delivered in ovo decreased the splenic expression of the immune-related and stress-related genes during HS (HS-GOS). The most striking effects of GOS that were delivered in ovo on the immune-related gene expression signatures was the down-regulation of IL-4 cytokine during acute (FC log2 IL-4 = −6.10, p < 0.01) and chronic (FC log2 IL-4 = −3.45, p < 0.01) HS. Furthermore, GOS that were delivered in ovo decreased the expression of pro-inflammatory cytokine, IL-12p40, during chronic heat (FC log2 IL-12p40 = −1.13, p < 0.05). Finally, in ovo treatment reduced oxidative stress induced by chronic heat (FC log2 CAT = −1.72, p < 0.05 and SOD = −1.56, p < 0.05). GOS that were delivered in ovo did not modulate expression of the chaperones (p > 0.05).
Figure 2.
Relative mRNA expression of immune-related and stress-related genes in the spleens of slow-growing chickens injected in ovo with GOS and challenged with heat on two levels: A—acute (30 °C for 8.5 h); and B—chronic (30 °C for 14 days). Gene panel includes: interleukin: IL-2, IL-4. IL-6, IL-10, IL-12p40, IL-17 and stress-related genes: CAT, SOD, BAG3, HSP25 and HSP90. X-axis shows a list of genes. Y-axis indicate relative mRNA abundance of the genes after heat challenge (n = 8). Gene expression analysis was carried out with RT-qPCR. qPCR reactions were performed in triplicates. Geometric mean of ACTB and UB reference genes was used to calculate dCt values. The relative gene expression was calculated with ddCt formula (FC = 2−ΔΔCt). FC values were transformed and presented as Log2FC. Standard error of the means (SEM) shows distribution of the Ct values. Normalized data (dCt values) of heat-challenged (A-acute, B-chronic) control (mock-injected) and heat-stressed GOS treatment groups were compared with Student’s t-test. Significant differences (p < 0.05) were labelled with an asterisk (*). Figures were prepared by using GraphPad Prism 7 (GraphPad, La Jolla, CA, USA).
4. Discussion
4.1. Immune-related Gene Expression Signatures
In this study, we determined impact of two factors (i.e., the GOS that were delivered in ovo and HS) on immune-related and stress-related gene expression signatures in the spleens of slow-growing chickens. The spleen is the largest peripheral lymphoid organ and plays a key role in immune responses in chickens [50]. Gene expression signatures that were determined in the splenic tissue informed about the systemic immune response to challenging factors, including heat [51]. The GIT is highly responsive to heat. The detrimental effects of HS on intestinal homeostasis include a reduced nutrient absorption, a disrupted integrity of the intestinal wall, and an activated immune system [52,53]. Intestinal epithelial cells are connected with gap junctions, tight junctions, adherent junctions, and desmosomes [54]. Under the influence of high temperature, the barrier function is compromised, and the intestinal lumen content enters the bloodstream, causing chronic systemic inflammation [25]. The activation of Toll-like receptors (TLR) by microbial signatures triggers myeloid differentiation primary response 88 (MyD88), which in turn induces cytokine secretion [55].
Cytokines are intracellular peptides that serve as immune mediators. During HS, the levels of both pro-inflammatory and anti-inflammatory cytokines increase. This is due to endotoxemia, which is a storm of microbial endotoxin (i.e., LPS—lipopolysaccharides) infiltrating the milieu of the body [56]. In the current study, slow-growing chickens that were subjected to chronic HS demonstrated a significantly higher expression of IL-10 and IL-12p40. IL-10 skews immune responses towards Th2-type (humoral) responses, while IL-12p40 is associated with Th1-type (cellular) immune responses [57]. There is a strong interaction between these cytokines. IL-10 causes a negative regulation of the Th1 response [58]. In this paper, chronic (but not acute) HS increased the level of splenic mRNA encoding both IL-10 and IL-12p40. There are two hypotheses of such the counter-balancing expression of pro- and anti-inflammatory cytokines in the spleens of heat-stressed chickens. First, the IL-10 and IL-12p40 cytokine expression in the spleen can be mediated by the TLR2 signaling pathway, triggered by Gram-positive cell wall components [59]. In heat-stressed individuals, those Gram-positive cell wall components originate from intestinal content, which leaks into the milieu of the body due to increased intestinal permeability [56]. Different bacterial ligands can stimulate TLR2 receptors on different antigen presenting cells (e.g., lymphocyte B, dendritic cells, or macrophages) in the spleen [60]. The gastrointestinal origin of those TLR ligands suggests their variability, and, as such, the ability to activate TLR signaling pathways in different cells. The second hypothesis is associated with endotoxemia that is mediated by LPS influx from the gut due to HS (as mentioned above). Endotoxemia triggers strong pro-inflammatory responses (mediated by IL-12p40) that are balanced by anti-inflammatory IL-10 cytokine [61]. In summary, the activation of two major cytokines in the spleens of slow-growing chickens indicates that the individuals responded to heat with increased pro- and anti-inflammatory immune responses.
GOS that were delivered in ovo balanced the level of IL-10 and IL-12p40 to the baseline (under acute HS) or even down-regulated cytokine expression (under chronic HS). Previously, we have determined that GOS that were delivered in ovo increased the expression of the genes that are involved in the barrier function of the gut of broiler chickens [32]. An improved barrier function allows for a decrease of intestinal permeability due to stress and the influx of antigens into the milieu of the body [62]. In the absence of an antigenic cocktail, TLR-mediated immune responses are not activated. In a broiler study that was conducted with the same experimental design as the current study, IL-12p40 was up-regulated by acute HS, but in ovo GOS stimulation decreased its expression to the level of the control groups [41]. It can be concluded that heat induced mild immune responses in slow-growing chickens, but GOS that were delivered in ovo managed to dampen immune responses under HS.
Interleukin 4 (IL-4) was one of the cytokines that was modulated in slow-growing chickens by both environmental factors, i.e., GOS that were delivered in ovo and HS. Individuals that were treated with GOS under acute and chronic HS expressed a decreased mRNA level of the IL-4 cytokine, confirmed by a significant interaction between the two factors. In our earlier study on broilers [41], the mRNA level of IL-4 was elevated by acute HS (similarly as IL-12p40), but in ovo stimulation with GOS dampened its level to the baseline (i.e., mock-injected controls). In the current study on slow-growing chickens, IL-4 was numerically down-regulated by HS alone, and the down-regulation was further enhanced by the in ovo delivery of GOS (the interaction between treatment and temperature was significant). IL-4 cytokine acts in humoral immunity as a pleiotropic cytokine that is produced in response to receptor activation by Th2-type T cells, basophils, and mast cells [63]. Its major function is regulating antigen-stimulated naïve T cell differentiation and the expression of the specific immunoglobulin E (IgE) and immunoglobulin G (IgG) by B cells [64]. Quinteiro-Filho et al. (2017) found that chronic HS decreased the plasma levels of Immunoglobilin A (IgA) and IgG in broiler chickens [65]. It seems that splenic IL-4 is a good biomarker of the HS response in different chicken genotypes. Early GOS delivery dampens heat-induced Th2 immune responses in slow-growing chickens.
4.2. Stress-related Gene Expression Signatures
Exposure to HS can lead to oxidative stress, which is characterized by the accumulation of reactive oxygen species (ROS) in the cells. The first line of defense is the production of cellular antioxidant enzymes including CAT and SOD, which protect the cells from ROS-induced cellular damage [66]. The current study showed a significant increase in the mRNA expression of CAT and SOD genes during chronic HS. Such increase indicates the activation of the pathways that are associated with counteracting the effects of oxidative stress. HS has been reported to increase the hepatic activity of CAT and SOD enzymes in two broiler chicken genotypes (Ross 308 and Cobb 500) (http://en.aviagen.com/brands/ross/products/ross-308) [67]. Habashy et al. (2018) reported the up-regulation of the mRNA level of SOD (but not CAT) gene under chronic HS (12 days) [68]. Low/basic levels of oxidative stress can play an important role in adapting to stressful environmental conditions [69]. In poultry, antioxidants use ROS to activate the expression of vitagenes, which are responsible for biological adaptation to stress. Vitagenes include the SOD and HSP genes.
In slow-growing chickens, only chronic HS triggered mRNA responses. Long-term HS (14 days) activated anti-inflammatory (IL-10), pro-inflammatory (IL-12p40), and oxidative stress responses (CAT and SOD). Long-term HS can lead to chronic systemic oxidative stress, which is associated with mild subclinical inflammation. This condition, called “OxInflammation,” impairs natural homeostatic adaptation, which leads to stronger systemic inflammation and an increased susceptibility to diseases [70]. If it is not possible to eliminate stressors from the environment, OxInflammation can be reduced by improved acclimatization (e.g., by stimulating intestinal microbiota) and adaptation (e.g., by using slow-growing hybrids).
In this study, both CAT and SOD were up-regulated by chronic (but not acute) HS, but in ovo delivered GOS dampened the mRNA expression of both genes. GOS-stimulated chickens expressed down-regulated signatures of oxidative stress compared to mock-injected birds. Oxidative stress has been recently linked with intestinal microbiota [71]. Apparently, direct contact between intestinal epithelial cells and microbiota induces the production of physiological ROS [72]. Different species of intestinal microbiota induce different levels of ROS production in the gut. For example, Lactobacillus has been reported to trigger ROS production both in vitro and in vivo [72]. We previously determined that GOS that were delivered in ovo elicited bifidogenic effects in broiler chickens (i.e., a higher level of Bifidobacteria), which resulted in a decreased Lactobacilli level in the caecum [32]. We can speculate that GOS that were delivered in ovo had a potent effect on intestinal microbiota composition, which led to a decreased intestinal ROS production under chronic HS.
Splenic HSP genes in slow-growing chickens did not respond to HS, neither acute nor chronic. The up-regulation of HSP is a cellular reaction to reduce the risk of damage (by protein misfolding) during stress. In our earlier study, we observed that the mRNA expression of HSP was triggered in broiler chickens by acute HS, which did not cause any molecular responses in slow-growing chickens (data not presented). This suggests that slow-growing chickens are heat-tolerant, and the mild elevated ambient temperature (30 °C) did not activate HS response via chaperone proteins [73]. Comparative studies on two Brazilians native chicken breeds and commercial line Cobb chickens have shown that HSP70 and HSP90 gene expression differs significantly between breeds. HSP genes were highly up-regulated in native breeds only at a very high ambient temperature (39 °C), which proved them resistant to high temperatures [18].
On a performance level, HS causes losses in feed intake and growth rate. In broiler chickens, chronic HS has been found to significantly reduce (p < 0.01) final body weight (BW) (2.52 kg in TN vs. 3.11 kg in HS) [32,39,40]. In the current study, chronic HS also reduced final BW (p < 0.001), but the difference between TN and HS was almost two times lower than in broiler chickens (2.21 kg in TN vs. 1.94 kg in HS), even with the longer rearing period (42 days in broilers vs. 50 days in slow-growing chickens). The slow-growing crossbreds, which were analyzed in this study, are considered by the Hubbard breeding company as “exceptional in their rusticity.” This indicates better hardiness in comparison to highly selected and fast-growing broilers. The results presented in this paper confirm that slow-growing crossbreds lost only 12% of their total BW due to HS, whereas the losses in fast-growing broilers amounted to 24% of their total BW. GOS that were delivered in ovo improved (p < 0.01) the final BW in broiler chickens (2.76 kg in control chickens vs. 2.89 kg in GOS). In the current study, the final BW of slow-growing chickens was not improved (p > 0.05) by GOS that were delivered in ovo (2.04 kg in control chickens vs. 2.00 kg in GOS) (Federico Sirri, personal communication). The differences in growth rate seemed to have had an impact on the responses to HS in chickens. However, at this point, the data on slow-growing chickens are limited. Therefore, we find it difficult to provide an explanation for why this genotype reacted differently than the highly selected, fast-growing broilers.
5. Conclusions
Slow-growing chickens proved to be well adapted to acute HS, which did not trigger immune-related or stress-related gene expression in the spleen. On the other hand, chronic HS activated genes that are associated with inflammation and oxidative stress (i.e., OxInflammation). GOS that were delivered in ovo mitigated heat-induced OxInflammation and decreased Th2 responses (down-regulation of IL-4). We demonstrated that the genetic adaptation of slow-growing chickens to HS combined with in ovo stimulation with GOS has mitigating effects on the molecular pathways that are associated with immune and stress responses.
Author Contributions
Conceptualization, A.S.; methodology, F.S., M.Z., G.M., A.D. and A.S.; formal analysis, E.P.; investigation, M.Z., F.S., E.P. and A.S.; resources, A.M., F.S., S.T. and A.S.; data curation, E.P., A.D., M.S. and A.S.; writing—original draft preparation, E.P. and A.D.; writing—review and editing, E.P., M.S. and A.S.; visualization, A.S.; supervision, F.S., A.M., G.M. and A.S.; project administration, S.T. and G.M.; funding acquisition, G.M. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by OVOBIOTIC project (grant# RBSI14WZCL) from Ministry of Education, Universities and Research (MIUR) in Rome, Italy and supported by the Polish National Agency for Academic Exchange under Grant No. PPI/APM/2019/1/00003. Aleksandra Skiba and Marika Weitz are kindly acknowledged for their excellent technical assistance with RNA extraction.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Roushdy E.M., Zaglool A.W., El-Tarabany M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018;74:337–343. doi: 10.1016/j.jtherbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Suk Y.O., Washburn K.W. Effects of environment on growth, efficiency of feed utilization, carcass fatness, and their association. Poult. Sci. 1995;74:285–296. doi: 10.3382/ps.0740285. [DOI] [PubMed] [Google Scholar]
- 3.Reay D., Reay D. Climate-Smart Food. Springer International Publishing; New York City, NY, USA: 2019. Climate-Smart Chicken; pp. 107–120. [Google Scholar]
- 4.Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Washburn K.W., Peavey R., Renwick G.M. Relationship of strain variation and feed restriction to variation in blood pressure and response to heat stress. Poult. Sci. 1980;59:2586–2588. doi: 10.3382/ps.0592586. [DOI] [PubMed] [Google Scholar]
- 6.Tallentire C.W., Leinonen I., Kyriazakis I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016:36. doi: 10.1007/s13593-016-0398-2. [DOI] [Google Scholar]
- 7.Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Ijaz A., Sohail A., Shabbir M.Z., Rehman H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012;91:2235–2240. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- 8.Niu Z.Y., Liu F.Z., Yan Q.L., Li W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- 9.Deeb N., Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002;81:293–301. doi: 10.1093/ps/81.3.293. [DOI] [PubMed] [Google Scholar]
- 10.Tavaniello S., Slawinska A., Prioriello D., Petrecca V., Bertocchi M., Zampiga M., Salvatori G., Maiorano G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020;99:612–619. doi: 10.3382/ps/pez556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi D., Bai L., Qu Q., Zhou S., Yang M., Guo S., Li Q., Liu C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019;98:2405–2413. doi: 10.3382/ps/pez026. [DOI] [PubMed] [Google Scholar]
- 12.Dinan T.G., Cryan J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Nienabar J.A., Hahn G.L. Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 2007;52:149–157. doi: 10.1007/s00484-007-0103-x. [DOI] [PubMed] [Google Scholar]
- 14.Nyoni N.M.B., Grab S., Archer E.R.M. Heat stress and chickens: Climate risk effects on rural poultry farming in low-income countries. Clim. Dev. 2019;11:83–90. doi: 10.1080/17565529.2018.1442792. [DOI] [Google Scholar]
- 15.Stocker T.F., Qin D., Plattner G.K., Tignor M., Allen S.K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P.M. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2013. p. 1535. [Google Scholar]
- 16.Taylor N.A.S. Human heat adaptation. Compr. Physiol. 2014;4:325–365. doi: 10.1002/cphy.c130022. [DOI] [PubMed] [Google Scholar]
- 17.Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- 18.Cedraz H., Gromboni J.G.G., Garcia A.A.P., Farias Filho R.V., Souza T.M., De Oliveira E.R., De Oliveira E.B., Do Nascimento C.S., Meneghetti C., Wenceslau A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS ONE. 2017;12:e0186083. doi: 10.1371/journal.pone.0186083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duangjinda M., Tunim S., Duangdaen C., Boonkum W. Hsp70 genotypes and heat tolerance of commercial and native chickens reared in hot and humid conditions. Rev. Bras. Cienc. Avic. 2017;19:7–18. doi: 10.1590/1806-9061-2016-0245. [DOI] [Google Scholar]
- 20.Horst P. Native fowl as reservoir for genomes and major genes with direct and indirect effects on the adaptability and their potential for tropically oriented breeding plans. Arch. Anim. Breed. 1989;53:93–101. [Google Scholar]
- 21.Galal A., Radwan L.M., Rezik H.H., Ayoub H. Expression levels of HSP70 and CPT-1 in three local breeds of chickens reared under normal or heat stress conditions after the introduction of the naked neck gene. J. Therm. Biol. 2019;80:113–118. doi: 10.1016/j.jtherbio.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Aengwanich W. Comparative ability to tolerate heat between Thai indigenous chickens, Thai indigenous chickens crossbred and broilers by using heterophil/lymphocyte ratio. Pakistan J. Biol. Sci. 2007;10:1840–1844. doi: 10.3923/pjbs.2007.1840.1844. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki K., Harasawa R., Yoshitake Y., Mitsuoka T. Effects of crowding and heat stress on intestinal flora, body weight gain, and feed efficiency of growing rats and chicks. Nippon Juigaku Zasshi. Jpn. J. Vet. Sci. 1983;45:331–338. doi: 10.1292/jvms1939.45.331. [DOI] [PubMed] [Google Scholar]
- 24.He J., He Y., Pan D., Cao J., Sun Y., Zeng X. Associations of gut microbiota with heat stress-induced changes of growth, fat deposition, intestinal morphology, and antioxidant capacity in ducks. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tellez G., Jr., Tellez-Isaias G., Dridi S. Heat Stress and Gut Health in Broilers: Role of Tight Junction Proteins. Adv. Food Technol. Nutr. Sci. 2017;3:e1–e4. doi: 10.17140/AFTNSOJ-3-e010. [DOI] [Google Scholar]
- 26.Wang W.C., Yan F.F., Hu J.Y., Amen O.A., Cheng H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018;96:1654–1666. doi: 10.1093/jas/sky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohail M.U., Ijaz A., Yousaf M.S., Ashraf K., Zaneb H., Aleem M., Rehman H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010;89:1934–1938. doi: 10.3382/ps.2010-00751. [DOI] [PubMed] [Google Scholar]
- 28.Zulkifli I., Abdulllah N., Azrin N.M., Ho Y.W. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br. Poult. Sci. 2000;41:593–597. doi: 10.1080/713654979. [DOI] [PubMed] [Google Scholar]
- 29.Baurhoo B., Phillip L., Ruiz-Feria C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007;86:1070–1078. doi: 10.1093/ps/86.6.1070. [DOI] [PubMed] [Google Scholar]
- 30.Ohland C.L., MacNaughton W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298 doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 31.Siwek M., Slawinska A., Stadnicka K., Bogucka J., Dunislawska A., Bednarczyk M. Prebiotics and synbiotics - In ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018:14. doi: 10.1186/s12917-018-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slawinska A., Dunislawska A., Plowiec A., Radomska M., Lachmanska J., Siwek M., Tavaniello S., Maiorano G. Modulation of microbial communities and mucosal gene expression in chicken intestines after galactooligosaccharides delivery In Ovo. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0212318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunislawska A., Slawinska A., Stadnicka K., Bednarczyk M., Gulewicz P., Jozefiak D., Siwek M. Synbiotics for Broiler Chickens—In Vitro Design and Evaluation of the Influence on Host and Selected Microbiota Populations following In Ovo Delivery. PLoS ONE. 2017;12:e0168587. doi: 10.1371/journal.pone.0168587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bednarczyk M., Stadnicka K., Kozłowska I., Abiuso C., Tavaniello S., Dankowiakowska A., Sławińska A., Maiorano G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal. 2016;10:1271–1279. doi: 10.1017/S1751731116000173. [DOI] [PubMed] [Google Scholar]
- 35.Sławinska A., Siwek M., Zylinska J., Bardowski J., Brzezinska J., Gulewicz K.A., Nowak M., Urbanowski M., Płowiec A., Bednarczyk M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biol. 2014;62:277–285. doi: 10.3409/fb62_3.277. [DOI] [PubMed] [Google Scholar]
- 36.Stefaniak T., Madej J.P., Graczyk S., Siwek M., Łukaszewicz E., Kowalczyk A., Sieńczyk M., Bednarczyk M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019;15:105. doi: 10.1186/s12917-019-1850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogucka J., Dankowiakowska A., Elminowska-Wenda G., Sobolewska A., Szczerba A., Bednarczyk M. Effects of prebiotics and synbiotics delivered in ovo on broiler small intestine histomorphology during the first days after hatching. Folia Biol. 2016;64:131–143. doi: 10.3409/fb64_3.131. [DOI] [PubMed] [Google Scholar]
- 38.Hughes R.A., Ali R.A., Mendoza M.A., Hassan H.M., Koci M.D. Impact of dietary galacto-oligosaccharide (GOS) on chicken’s gut microbiota, mucosal gene expression, and Salmonella colonization. Front. Vet. Sci. 2017;4 doi: 10.3389/fvets.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slawinska A., Zampiga M., Sirri F., Meluzzi A., Bertocchi M., Tavaniello S., Maiorano G. Impact of galactooligosaccharides delivered in ovo on mitigating negative effects of heat stress on performance and welfare of broilers. Poult. Sci. 2020;99:407–415. doi: 10.3382/ps/pez512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slawinska A., Plowiec A., Siwek M., Jaroszewski M., Bednarczyk M. Long-Term Transcriptomic Effects of Prebiotics and Synbiotics Delivered In Ovo in Broiler Chickens. PLoS ONE. 2016;11:e0168899. doi: 10.1371/journal.pone.0168899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slawinska A., Mendes S., Dunislawska A., Siwek M., Zampiga M., Sirri F., Meluzzi A., Tavaniello S., Maiorano G. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems. 2019;178:10–15. doi: 10.1016/j.biosystems.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Livak K.J., Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Sławinska A., Siwek M.Z., Bednarczyk M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014;75:997–1003. doi: 10.2460/ajvr.75.11.997. [DOI] [PubMed] [Google Scholar]
- 44.Chiang H.I., Berghman L.R., Zhou H. Inhibition of NF-kB 1 (NF-kBp50) by RNA interference in chicken macrophage HD11 cell line challenged with Salmonella enteritidis. Genet. Mol. Biol. 2009;32:507–515. doi: 10.1590/S1415-47572009000300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothwell L., Young J.R., Zoorob R., Whittaker C.A., Hesketh P., Archer A., Smith A.L., Kaiser P. Cloning and Characterization of Chicken IL-10 and Its Role in the Immune Response to Eimeria maxima. J. Immunol. 2004;173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- 46.Brisbin J.T., Gong J., Parvizi P., Sharif S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010;17:1337–1343. doi: 10.1128/CVI.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S.H., Cheng C.Y., Tang P.C., Chen C.F., Chen H.H., Lee Y.P., Huang S.Y. Differential gene expressions in testes of L2 strain Taiwan country chicken in response to acute heat stress. Theriogenology. 2013;79 doi: 10.1016/j.theriogenology.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 48.El-Deep M.H., Ijiri D., Eid Y.Z., Yamanaka H., Ohtsuka A. Effects of dietary supplementation with Aspergillus Awamorion growth performance and antioxidative status of broiler chickens exposed to high ambient temperature. Egypt. J. Neurol. Psychiatry Neurosurg. 2014;51:281–288. [Google Scholar]
- 49.De Boever S., Vangestel C., De Backer P., Croubels S., Sys S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008;122:312–317. doi: 10.1016/j.vetimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q., Waqas Y., Yang P., Sun X., Liu Y., Ahmed N., Chen B., Li Q., Hu L., Huang Y., et al. Cytological study on the regulation of lymphocyte homing in the chicken spleen during LPS stimulation. Oncotarget. 2017;8:7405–7419. doi: 10.18632/oncotarget.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohtsu H., Yamazaki M., Abe H., Murakami H., Toyomizu M. Heat Stress Modulates Cytokine Gene Expression in the Spleen of Broiler Chickens. J. Poult. Sci. 2015;52:282–287. doi: 10.2141/jpsa.0150062. [DOI] [Google Scholar]
- 52.Liu F., Yin J., Du M., Yan P., Xu J., Zhu X., Yu J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling1. J. Anim. Sci. 2009;87:1941–1949. doi: 10.2527/jas.2008-1624. [DOI] [PubMed] [Google Scholar]
- 53.Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in Susceptibility to Heat Stress along the Chicken Intestine and the Protective Effects of Galacto-Oligosaccharides. PLoS ONE. 2015;10:e0138975. doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karnati H.K., Pasupuleti S.R., Kandi R., Undi R.B., Sahu I., Kannaki T.R., Subbiah M., Gutti R.K. TLR-4 signalling pathway: MyD88 independent pathway up-regulation in chicken breeds upon LPS treatment. Vet. Res. Commun. 2015;39:73–78. doi: 10.1007/s11259-014-9621-2. [DOI] [PubMed] [Google Scholar]
- 56.Heled Y., Fleischmann C., Epstein Y. Cytokines and their role in hyperthermia and heat stroke. J. Basic Clin. Physiol. Pharmacol. 2013;24:85–96. doi: 10.1515/jbcpp-2012-0040. [DOI] [PubMed] [Google Scholar]
- 57.Heufler C., Koch F., Stanzl U., Topar G., Wysocka M., Trinchieri G., Enk A., Steinman R.M., Romani N., Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur. J. Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 58.Ma X., Yan W., Zheng H., Du Q., Zhang L., Ban Y., Li N., Wei F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research. 2015;4:1–13. doi: 10.12688/f1000research.7010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chau T.A., McCully M.L., Brintnell W., An G., Kasper K.J., Vinés E.D., Kubes P., Haeryfar S.M.M., McCormick J.K., Cairns E., et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat. Med. 2009;15:641–648. doi: 10.1038/nm.1965. [DOI] [PubMed] [Google Scholar]
- 60.Duell B.L., Tan C.K., Carey A.J., Wu F., Cripps A.W., Ulett G.C. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol. Med. Microbiol. 2012;64:295–313. doi: 10.1111/j.1574-695X.2012.00931.x. [DOI] [PubMed] [Google Scholar]
- 61.De Waal Malefyt R., Haanen J., Spits H., Koncarolo M.G., Te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., De Vries J.E. Interleukin 10 (il-10) and viral il-10 strongly reduce antigen-specific human t cell proliferation by diminishing the antigen-presenting capacity of monocytes via dowm’egulation of class h major histocompatibility complex expression. J. Exp. Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vancamelbeke M., Vermeire S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silva-Filho J.L., Caruso-Neves C., Pinheiro A.A.S. IL-4: An important cytokine in determining the fate of T cells. Biophys. Rev. 2014;6:111–118. doi: 10.1007/s12551-013-0133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keegan A.D. Encyclopedia of Immunology. Academic Press; Cambridge, MA, USA: 1998. Interleukin 4 Receptor; pp. 1453–1455. [Google Scholar]
- 65.Quinteiro-Filho W.M., Calefi A.S., Cruz D.S.G., Aloia T.P.A., Zager A., Astolfi-Ferreira C.S., Piantino Ferreira J.A., Sharif S., Palermo-Neto J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017;186:19–28. doi: 10.1016/j.vetimm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Halliwell B., Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdo S.E., El-Kassas S., El-Nahas A.F., Mahmoud S. Modulatory Effect of Monochromatic Blue Light on Heat Stress Response in Commercial Broilers. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/1351945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Habashy W.S., Milfort M.C., Rekaya R., Aggrey S.E. Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol. Biol. Rep. 2018;45:389–394. doi: 10.1007/s11033-018-4173-0. [DOI] [PubMed] [Google Scholar]
- 69.Yan L.J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014;2:165–169. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valacchi G., Virgili F., Cervellati C., Pecorelli A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018:9. doi: 10.3389/fphys.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones R.M., Mercante J.W., Neish A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic implications. Curr. Med. Chem. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar A., Wu H., Collier-Hyams L.S., Hansen J.M., Li T., Yamoah K., Pan Z.Q., Jones D.P., Neish A.S. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007;26:4457–4466. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaźmierczuk A., Kiliańska Z.M. The pleiotropic activity of heat-shock proteins. Postep. Hig Med Dosw. 2009;63:502–521. (In Polish) [PubMed] [Google Scholar]