Abstract
Background
Critically ill newborn infants undergo a variety of painful procedures or experience a variety of painful conditions during their early life in the neonatal unit. In the critically ill paediatric and neonatal population, clonidine is prescribed as an adjunct to opioids or benzodiazepines aiming to reduce the doses of these drugs that are required for analgesia or sedation, or to facilitate weaning from mechanical ventilation. It has been shown that clonidine premedication might have a positive effect on postoperative pain in children.
Objectives
To assess the benefit and harms of clonidine for the prevention or treatment of procedural pain; postoperative pain; or pain associated with clinical conditions in non‐ventilated neonates.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the CENTRAL, MEDLINE via PubMed, Embase, and CINAHL to December 2018. We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials. We ran an updated search from 1 January 2018 to 11 March 2020 in CENTRAL via CRS Web, MEDLINE via Ovid, and CINAHL via EBSCOhost.
Selection criteria
Randomised controlled trials, quasi‐randomised controlled trials, and cluster trials comparing clonidine to placebo or no treatment, opioids, paracetamol, dexmedetomidine, or non‐pharmacological pain‐reducing interventions for the management of procedural pain, postoperative pain, and pain associated with clinical conditions in preterm and term newborns.
Data collection and analysis
Two review authors independently planned to extract data (e.g. number of participants, birth weight, gestational age, modality of administration, and dose of clonidine) and assess the risk of bias (e.g. adequacy of randomisation, blinding, completeness of follow‐up). The primary outcome considered was pain: for procedural pain, the mean values of each analgesia scale assessed during the procedure and at one to two hours after the procedure; for postoperative pain and for pain associated with clinical conditions, the mean values of each analgesia scale assessed at 30 minutes, three hours, and 12 hours after the administration of the intervention. We planned to use the GRADE approach to assess the quality of evidence.
Main results
Our search strategy yielded 3383 references. Two review authors independently assessed all references for inclusion. We did not find any completed studies for inclusion. We excluded three trials where clonidine was administered for spinal anaesthesia.
Authors' conclusions
We did not find any studies that met our inclusion criteria and hence there is no evidence to recommend or refute the use of clonidine for the prevention or treatment of procedural or postoperative pain, or pain associated with clinical conditions in neonates.
Plain language summary
Clonidine for painful procedures and conditions in infants
Review question: does clonidine administration reduce pain in newborns exposed to painful procedures or conditions?
Background: newborns admitted to the hospital are exposed to a number of painful procedures which might include blood sampling, lumbar puncture (a medical procedure where a needle is inserted into the spinal canal, usually to collect cerebrospinal fluid for testing), tubes in the stomach, different types of catheters, and minor surgical procedures such as neonatal circumcision. In addition, newborns might have pain because of clinical conditions, such as, fractured bone, pain following traumatic vaginal birth, disease of the skin (open skin lesions from an inherited skin disorder), or intestines (necrotising enterocolitis (inflammation)). All babies, and especially those born too early (preterm), are very sensitive to pain. Moreover, the experience of repeated episodes of pain might cause problems, such as an altered development of the nervous system, in early life and an increased pain sensitivity when they grow up. Most of the medicines to manage pain and stress in babies have side effects, and might also harm the immature developing brain by inducing death of cells (apoptosis) in the brain.
Study characteristics: we searched for studies up to 11 March 2020. The aim of this review was to assess if clonidine could reduce pain in newborns. This medicine can be given through injections, using a tube in the stomach (nasogastric tube), through the skin, and as spinal anaesthesia. However, the latter is not in the focus of this review as it is used for babies undergoing specific surgical procedures. Clonidine works on the brain modifying responses and reducing stress and agitation. Furthermore, clonidine may reduce pain, especially if used in combination with other medicines.
Key results: we found no studies for our review. Three studies were excluded because clonidine was given for spinal anaesthesia, whereas our review focuses on other indications.
Background
Description of the condition
The importance of pain in neonates was not recognised until the late 1980s when research describing the developmental physiology of nociception emerged (i.e. the sensory nervous system's response to harmful stimuli, frequently manifested as pain) (Anand 1987a; Anand 1987b). In newborn infants, there is an imbalance between the excitatory neural pathways accountable for nociception and the inhibitory neural pathways responsible for localisation and alleviation of noxious stimuli (Fitzgerald 1986). Pain perception develops slowly and advances with postnatal age. In addition, normal brain development is abruptly interrupted by preterm birth, and repetitive painful stimuli may lead to developmental alterations of the nociceptive pathways (Taddio 2009).
Critically ill newborn infants undergo numerous and repeated invasive procedures during their early life in the neonatal intensive care unit (NICU). The 'Epidemiology of Procedural Pain in Neonates' (EPIPPAIN) study reported that 430 preterm and term infants experienced a total of 60,969 first‐attempt procedures during the first two weeks in the NICU. They went through a median of 16 stressful procedures per day, of which 10 were considered stressful and painful (Carbajal 2008). Other investigators have reported a similar number of painful procedures: Barker 1995 reported a mean of 60 painful procures per patient in 54 preterm infants, while Benis 2001 described a total of 5663 procedures in a cohort of 15 preterm infants. In the Johnston 1997 study, a mean of two procedures per patient per day were performed, and some neonates had as many as eight procedures per day during the first week of NICU care. Additionally, in one Dutch cohort of 151 neonates admitted to the NICU, neonates experienced a mean of 14 procedures each day during the first two weeks (Simons 2003).
Despite the growing knowledge about long‐term consequences of neonatal pain and discomfort, a safe and effective strategy to minimise these complications remains a challenge in everyday clinical care (McPherson 2012). Non‐pharmacological support and interventions, such as non‐nutritive sucking and wrapping, are well‐accepted first‐hand strategies, but are insufficient to provide comfort for moderate and severe pain (Brummelte 2012; Golianu 2007).
Oral sucrose and glucose are commonly used in NICUs to provide analgesia or comfort infants, or both, during mild‐to‐moderately painful procedures (Lim 2017). Both have been extensively studied as possible analgesic agents in newborns, however many gaps of knowledge still remain, including appropriate dosing and long‐term consequences (Bueno 2013; Stevens 2016). Nevertheless, neither glucose nor sucrose may be effective for longer or more painful procedures (Costa 2013).
Opioids are the pharmacological agents most commonly administered to treat pain in newborn infants, with fentanyl and morphine most commonly used for procedures such as chest tube insertion, for premedication for intubation or for conditions such as necrotising enterocolitis. The dosage of these drugs varies between studies, the effects are uncertain at procedural pain (Anand 2004; Simons 2003), and the reports of long‐term effects of opioids given during the neonatal period are conflicting (de Graaf 2013; Roze 2008). Rodent models have demonstrated that early opiate exposure diminishes neuronal density and dendritic length (i.e. density and length of brain cells), as well as to increase neuroapoptosis (natural death of cells that occurs during growth or development) (Hammer 1989; Ricalde 1990; Seatriz 1993). Furthermore, rodents exposed to postnatal morphine exhibited reduced brain growth (Zagon 1977), persistently decreased motor activity, and impaired learning ability (Handelmann 1985; Ma 2007; McPherson 2007). Several other pharmacological agents, such as methadone, ketamine, and propofol have been suggested, and used, for analgesia and sedation during neonatal intensive care, but data regarding appropriate dosage and short‐ and long‐term safety in this vulnerable population are currently insufficient, and further research is needed before these drugs are introduced to clinical practice (Allegaert 2007; Anand 2004; Chana 2001; Cravero 2011; Simons 2003).
It has been shown that co‐administration of morphine and paracetamol (acetaminophen) in the management of neonatal postoperative pain may reduce the total amount of opioid needed (Ceelie 2013). However, concerns on neurodevelopment have been raised about the safety of paracetamol (Bauer 2013; Viberg 2014). The use of non‐steroidal anti‐inflammatory agents, such as ibuprofen and indomethacin, is restricted to the pharmacological management of patent ductus arteriosus (i.e. a neonatal heart problem) because of possible adverse effects (e.g. renal insufficiency, platelet dysfunction and pulmonary hypertension) (Ohlsson 2016).
Description of the intervention
A limited experience with alpha2‐agonists (α2‐agonists), mainly clonidine and dexmedetomidine, in term and preterm infants, suggests that they may provide an analgesic and sedative effect. Alpha2‐agonists may induce sedation, provide analgesia, and ameliorate anxiety (Chen 2015; Mantz 2011; Pichot 2012). These effects are mediated through α2‐adrenergic receptor subtype located in the locus coeruleus, which is a nucleus in the pons of the brainstem and the main site for brain synthesis of noradrenaline (norepinephrine). Both clonidine and dexmedetomidine reduce the neuronal activity in the locus coeruleus without affecting the respiratory drive (Hoy 2011). Moreover, it has been suggested that α2‐agonists might have a neuroprotective and anti‐inflammatory effect (Mantz 2011). In animal models of endotoxic shock, both drugs preserve neutrophil function and inhibit the cytokine response (i.e. in cells that regulate the immune response) (Nishina 1999; Taniguchi 2004; Taniguchi 2008). Furthermore, both α2‐agonists protect neurons from damage in vitro and diminish brain lesion size in animal models (Laudenbach 2002; Paris 2006). It is worth noting that one study in newborn rats showed that intrathecal administration (via the spine) of clonidine did not induce signs of spinal histopathology (Walker 2012). The two main adverse effects of α2‐agonists are bradycardia (slow heartbeat) and hypotension (low blood pressure). These are mediated through the α2‐adenoreceptors in the medullary dorsal motor nucleus and motor complex and have been shown to be independent of the sedative effect (Gregoretti 2009; Pichot 2012).
In the critically ill paediatric and neonatal population, clonidine is routinely prescribed as an adjunct to opioids, benzodiazepines, or both, aiming at reducing the doses of these drugs required for analgesia or sedation, to facilitate weaning from mechanical ventilation, or for the management of neonatal abstinence syndrome (Duffett 2012). Furthermore, clonidine has been shown to reduce pain, discomfort, and agitation in a paediatric population following sevoflurane anaesthesia (Tesoro 2005). One Cochrane Review showed that clonidine premedication might have a positive effect on postoperative pain in the paediatric population (neonates not included) (Lambert 2014).
The current literature on practices for procedural and postoperative pain in critically ill newborn infants lacks a comprehensive data summary about the efficacy and safety of clonidine as a potential agent. In 2016, one systematic review was published on clonidine for sedation, analgesia, and iatrogenic drug withdrawal in critically ill infants and children (Capino 2016). However, this review included only mechanically ventilated infants and children. Similarly, one Cochrane Review including one trial (Hünseler 2014) assessed the effects of clonidine in ventilated newborns (Romantsik 2017).
Cochrane Reviews have also focused on pain management with other interventions (e.g. paracetamol (Ohlsson 2016); breastfeeding or breast milk (Shah 2012); and non‐pharmacological management, which included 4905 infants from 63 studies (Pillai Riddell 2015)).
How the intervention might work
Clonidine is a centrally acting α2‐selective adrenergic agonist. It has been suggested that clonidine mediates its sedative effects through the stimulation of the presynaptic α2‐adrenoceptors of the locus coeruleus, leading to a decrease in the release of noradrenaline (Jamadarkhana 2010). As well as exerting a sedative effect, clonidine also acts on the cholinergic, purinergic, and serotonergic pathways, to produce analgesia (Jamadarkhana 2010). This analgesic action is thought to be optimal when combined with other agents, for the management of postoperative pain and pain associated with clinical conditions in neonates. Moreover, the administration of clonidine may exert neuroprotective effects by preventing apoptosis induced by agents such as ketamine (Pontén 2012). The ability of α2‐agonists to protect the neuronal culture from damage in vitro and to reduce the brain lesion size in animal models is promising in the view of neuroprotection (Laudenbach 2002). An expanded description of how clonidine might work in the newborn is provided in a separate review (Romantsik 2017).
Why it is important to do this review
Despite the theoretical advantages of α2‐agonists, the safety and efficacy of their short‐term and long‐term use remain unclear. It is important to note that clonidine is not licensed for use in infants and its effectiveness and safety for pain management in non‐ventilated newborns has not been systematically reviewed.
Clonidine is an α2‐agonist with sedative and analgesic characteristics. In contrast to other analgo‐sedatives, clonidine does not reduce respiratory drive. Clonidine has been shown to be neuroprotective in animal research. For serious painful conditions (e.g. necrotising enterocolitis and postoperative care) the additive use of clonidine might reduce the dose of opioid treatment and subsequent negative effects. However, clonidine pharmacokinetics (PK), pharmacodynamics (PD), pharmacogenetics (PG), or the PK/PD/PG relation has not been tested in this population. It has been used for adults and older children, and the newborn population is treated accordingly. Clonidine was introduced for treatment of hypertension in adults; hypotension and bradycardia are well known adverse effects in that population. The PK, PD, PG, or PK/PD/PG relation needs to be studied in the newborn term and preterm population. Both general vital parameters and specific effects of cerebral activity (electroencephalogram (EEG)) and cerebral haemodynamics (near‐infrared spectrophotometry (NIRS)) are of major interest for the evaluation of the drug effects and adverse effects in this vulnerable population.
Pain and stress are still a problem in the NICU and evidence‐based consensus and clear guidelines are lacking. Clonidine is increasingly used because of the adverse effects of opioids; however, more knowledge about the drug is needed in order to make safe recommendations.
Objectives
To assess the benefit and harms of clonidine for the prevention or treatment of procedural pain; postoperative pain; or pain associated with clinical conditions in non‐ventilated neonates.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials, quasi‐randomised controlled trials, and cluster‐randomised trials. We excluded cross‐over trials.
Types of participants
Full‐term and preterm infants less than 44 weeks' postmenstrual age (PMA) requiring pain management during their hospital stay or as outpatients for:
low intensity pain due to procedures such as: heel lance, venipuncture, lumbar puncture, bladder tap, insertion of nasogastric tube, insertion of venous or arterial catheter/line;
high intensity pain due to procedures such as: insertion of chest drain, or surgery (including neonatal circumcision, any surgery performed in the operating room);
painful clinical conditions: including a fractured long bone, necrotising enterocolitis, open skin lesions from an inherited skin disorder, or pain from an assisted vaginal birth.
We excluded studies where clonidine infusion was administered in ventilated newborns, as this was addressed in another Cochrane Review (Romantsik 2017). However, we included studies where clonidine infusion was administered in ventilated newborns if the intervention specifically aimed to treat procedural or postoperative pain, or pain associated with clinical conditions.
Types of interventions
Clonidine administered at any dose for the prevention or treatment of pain. Clonidine may have been delivered intravenously, orally (or via nasogastric tube), or transdermally. We included studies that reported on single administration of clonidine or multiple (repeated) doses of clonidine over a prolonged period during the initial hospital stay. We excluded studies that compared clonidine with local or regional anaesthesia.
We assessed procedural pain, postoperative pain, and pain associated with clinical conditions in separate comparisons.
Clonidine was compared with placebo or no intervention; opioids; paracetamol; dexmedetomidine; or non‐pharmacological pain‐reducing interventions (e.g. sucrose, glucose, other sweet‐tasting solutions, breast milk, breastfeeding, non‐nutritive sucking, skin‐to‐skin care, or other intervention).
Comparison 1: clonidine compared to placebo or no treatment for the prevention or treatment of procedural, postoperative pain, or pain associated with clinical conditions in neonates.
Comparison 2: clonidine compared to opioids for the prevention or treatment of procedural, postoperative pain, or pain associated with clinical conditions in neonates.
Comparison 3: clonidine compared to paracetamol for the prevention or treatment of procedural, postoperative pain, or pain associated with clinical conditions in neonates.
Comparison 4: clonidine compared to dexmedetomidine for the prevention or treatment of procedural, postoperative pain, or pain associated with clinical conditions in neonates.
Comparison 5: clonidine compared to non‐pharmacological pain‐reducing interventions (e.g. sucrose, glucose, other sweet‐tasting solution, breast milk, breastfeeding, non‐nutritive sucking, skin‐to‐skin care) for the prevention or treatment of procedural, postoperative pain, or pain associated with clinical conditions in neonates.
We planned to perform subgroup analyses according to gestational age (term 37 weeks' gestation or greater); preterm (less than 37 weeks' gestation); extreme preterm infants (less than 28 weeks' gestation); birth weight for gestational age (small, appropriate, large for gestational age); type of pain; dose, duration, and route of clonidine administration; and pharmacological sedation as a co‐intervention (see Subgroup analysis and investigation of heterogeneity).
Types of outcome measures
Primary outcomes
Pain assessed using the pain scales listed in Table 1.
1. Pain scales.
| Pain scale | Population | Type of pain |
| ABC pain scale (Bellieni 2005)a | Preterm and term infants | Procedural pain |
| Astrid Lindgren and Lund Children's Hospitals Pain and Stress Assessment Scale for Preterm and sick Newborn Infants (ALPS‐Neo) (Lundqvist 2014) | Preterm and term infants | Prolonged pain/stress |
| Behavioral Indicators of Infant Pain (BIIP) (Holsti 2008) | Preterm infants | Procedural pain |
| Comfort Neo (van Dijk 2009) | Preterm and term infants | Postoperative and prolonged pain/stress |
| CRIES (Krechel 1995) | Preterm and term infants | Procedural and postoperative pain |
| Douleur Aiguë du Nouveau‐né (DAN) (Acute Pain in Newborn infants, APN, English version) (Carbajal 1997) | Preterm and term infants | Procedural pain |
| Echelle Douleur Incomfort Nouveau‐né (EDIN) (Debillon 2001) | Preterm infants | Prolonged pain |
| 'Faceless' Acute Neonatal pain Scale (FANS) (Milesi 2010) | Preterm and term infants | Procedural pain |
| Neonatal Facial Coding System (NFCS) (Grunau 1998; Peters 2003) | Preterm and term infants | Procedural, postoperative and prolonged pain/stress |
| Neonatal Infant Pain Scale (NIPS) (Lawrence 1993) | Preterm and term infants | Procedural pain |
| Neonatal Pain, Agitation, and Sedation Scale (N‐PASS) (Hummel 2008; Hummel 2010) | Preterm and term infants | Procedural, postoperative and prolonged pain/stress |
| Pain Assessment Tool (PAT) (Hodgkinson 1994; Spence 2005) | Preterm and term infants | Postoperative and prolonged pain/discomfort |
| Preterm Infant Pain Profile (PIPP and PIPP‐R) (Gibbins 2014; Stevens 1996) | Preterm and term infants | Procedural and postoperative pain |
aPublication of development or validation, or both, within parentheses.
For procedural pain, we planned to report the mean values of each analgesia scale assessed during the procedure and at one to two hours after the procedure.
For postoperative pain and for pain associated with clinical conditions, we planned to report the mean values of each analgesia scale assessed: at 30 minutes, three hours, and 12 hours after the administration of the intervention.
Secondary outcomes
For procedural pain – low‐intensity pain.
Completion of the targeted objective (relief of procedural pain) without use of any other agent.
Episodes of apnoea (mean rates of apnoea).
Episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer, occurring during the procedure and at one to two hours after the procedure.
Parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
For procedural pain – high‐intensity pain.
Completion of the targeted objective (relief of procedural pain) without use of any other agent.
Any intraventricular haemorrhage (IVH) (yes/no): any IVH, grades 1 to 4 (according to the Papile 1978 classification); severe IVH (grades 3 and 4).
Episodes of apnoea (mean rates of apnoea).
Episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer, occurring during the procedure and at one to two hours after the procedure.
Altered reactions to painful stimuli following NICU discharge, as reported by study authors.
Parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
For postoperative pain and for pain associated with clinical conditions.
Neonatal mortality.
Mortality during initial hospitalisation.
Completion of the targeted objective (relief of pain associated with clinical conditions) without use of any other agent.
Any IVH (yes/no): any IVH, grades 1 to 4 (according to the Papile 1978 classification); severe IVH (grades 3 and 4).
Cystic periventricular leukomalacia at brain ultrasound in the first month of life (yes/no).
Retinopathy of prematurity (ICROP 1984; yes/no): any; requiring laser therapy.
Duration of mechanical ventilation (days).
Duration of hospital stay (days).
Bronchopulmonary dysplasia/chronic lung disease (yes/no): 28 days; 36 weeks' PMA (Jobe 2001); "physiological definition" (Walsh 2004).
Necrotising enterocolitis (yes/no): any grade; requiring surgery.
Time to full enteral feeding (days).
Episodes of apnoea (mean rates of apnoea).
Episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer, occurring for postoperative or pain associated with clinical conditions, at 30 minutes, three hours, and 12 hours after administration of the intervention.
Altered reactions to painful stimuli following NICU discharge, as reported by study authors.
Parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
-
Major neurodevelopmental disability:
cerebral palsy;
developmental delay (Bayley Mental Developmental Index (Bayley 1993; Bayley 2006), or Griffiths Mental Development Scale assessment more than two standard deviations (SDs) below the mean (Griffiths 1954));
intellectual impairment (intelligence quotient > 2 SD below the mean);
blindness (vision < 6/60 in both eyes);
sensorineural deafness requiring amplification (Jacobs 2013);
we planned to evaluate each of these components as a separate outcome and to extract data on this long‐term outcome from studies that evaluated children after 18 months of chronological age. Data on children aged 18 to 24 months and those aged three to five years were to be assessed separately.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal Standard Search Strategy; neonatal.cochrane.org/resources‐authors/author‐resources‐new‐reviews). We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) and reported the date this was done.
Electronic searches
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 12) in the Cochrane Library; MEDLINE via PubMed (1996 to 3 December 2018); Embase (1980 to 3 December 2018); and CINAHL (1982 to 3 December 2018) using the following search terms: (clonidine[MeSH] OR clonidine OR alpha‐2 agonists), plus database‐specific limiters for randomised controlled trials and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions. We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov (clinicaltrials.gov); the World Health Organization's International Clinical Trials Registry Platform (apps.who.int/trialsearch/), and the ISRCTN Registry (www.isrctn.com/)).
We conducted a comprehensive update search in March 2020 including: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 3) in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1 January 2018 to 11 March 2020); and CINAHL (1 January 2018 to 11 March 2020). We included the search strategies for each database in Appendix 2. We applied no language restrictions.
We searched clinical trial registries for ongoing or recently completed trials. We searched the World Health Organization's International Clinical Trials Registry Platform (apps.who.int/trialsearch/) and the U.S. National Library of Medicine's ClinicalTrials.gov (clinicaltrials.gov) via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry (www.isrctn.com/) for any unique trials not found through the Cochrane CENTRAL search.
Searching other resources
Additionally, we reviewed the reference lists of all identified articles for any relevant articles that were not identified in the primary search.
Data collection and analysis
Selection of studies
Two review authors (OR, MB) independently screened the titles and abstracts to identify potentially relevant citations, retrieved the full texts of all potentially relevant articles, and assessed the eligibility of the studies by filling out eligibility forms designed in accordance with the specified inclusion criteria. We reviewed studies for relevance based on study design, types of participants, interventions, and outcome measures. We resolved any disagreements by discussion and, if necessary, by consulting a third review author (MC). We provided details of studies excluded from the review in the Characteristics of excluded studies table along with the reasons for exclusion. We planned to contact the trial authors if the details of the primary trials were unclear to request further information.
Data extraction and management
Two review authors (OR, MB) planned to independently undertake data abstraction using a data extraction form developed and integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group (EPOC) data collection checklist (Cochrane EPOC 2017).
We planned to extract the following characteristics from each included study:
administrative details: author(s); whether published or unpublished; year of publication; year in which study was conducted; details of other relevant papers cited;
details of study: study design; type, duration, and completeness of follow‐up (e.g. greater than 80%); country and location of study informed consent and ethics approval;
details of participants: birth weight, gestational age, and number of participants;
details of intervention: modality of administration and dose of clonidine;
details of outcomes, as listed in Types of outcome measures.
We planned to resolve any disagreement by discussion between the review authors.
We described any ongoing studies identified, detailing the primary author, research question(s), methods and outcome measures, and an estimate of the reporting date.
When queries arose or when additional data were required, we planned to contact the authors of the trial reports. Two review authors (MC, MB) planned to use Review Manager 5 software to enter all the data (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (OR, MC) planned to independently assess the risk of bias (low, high, or unclear) of all included trials using the Cochrane 'Risk of bias' tool for the following domains (Higgins 2011):
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
any other bias.
We planned to resolve any disagreements by discussion or through a third review author (MB). See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We planned to extract categorical data for each intervention group and calculate risk ratios (RRs) and absolute risk differences (RDs). We planned to obtain means and SDs for continuous data, and perform analyses using mean differences (MDs) when studies used the same pain scale. We planned to calculate standardised mean differences (SMDs) when studies used different pain scales. For each measure of effect, we planned to calculate the corresponding 95% confidence intervals (CIs). We planned to present the number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) when RDs were statistically significant (P < 0.05).
Unit of analysis issues
The unit of analysis was the individual infant. For multiple painful procedures, we considered the first procedure performed in the randomised infant. The unit of analysis for cluster‐randomised trials was the randomised treating centre or cluster. We planned to include cluster‐randomised trials in the analyses, using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from another source, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We planned to contact the original study investigators to request additional data where information about critical and important outcomes was missing (see the list of the clinically relevant outcomes in Data synthesis). We planned to investigate attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals). We planned to perform a sensitivity analysis to evaluate the overall results with and without the inclusion of studies with significant dropout rates. If a study reported outcomes only for participants completing the trial, or only for participants who followed the protocol, we planned to contact the author(s) and ask them to provide additional information to facilitate an intention‐to‐treat analysis; and in instances where this was not possible we would have performed a complete‐case analysis. We planned to address the potential impact of missing data on the findings of the review in the 'Discussion' section.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by comparing the distribution of important participant factors between trials and trial factors (randomisation concealment, blinding of outcome assessment, loss to follow‐up, treatment type, co‐interventions). We planned to assess statistical heterogeneity by examining the I2 statistic (Higgins 2011), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than sampling error.
We planned to interpret the I2 statistic as described by Higgins 2003:
less than 25%: no heterogeneity;
25% to 49%: low heterogeneity;
50% to 74%: moderate heterogeneity;
75% or greater: high heterogeneity.
In addition, we planned to employ the Chi2 test of homogeneity to determine the strength of evidence that heterogeneity is genuine. We planned to explore clinical variation across studies by comparing the distribution of important participant factors among trials and trial factors (randomisation concealment, blinding of outcome assessment, loss to follow‐up, treatment type and co‐interventions). We planned to consider a threshold P value of less than 0.1 as an indicator of whether heterogeneity (genuine variation in effect sizes) is present.
Assessment of reporting biases
We planned to investigate publication bias using funnel plots if at least 10 clinical trials were included in the meta‐analysis (Egger 1997; Higgins 2011)
Data synthesis
We planned to perform statistical analyses according to the recommendations of the Cochrane Neonatal Review Group (neonatal.cochrane.org/en/index.html). We planned to analyse all infants randomised on an intention‐to‐treat basis. For any meta‐analyses we planned to synthesise data using RR, RD, NNTB, NNTH, MD, and 95% CI. We planned to analyse and interpret individual trials separately when we judged meta‐analysis to be inappropriate.
Subgroup analysis and investigation of heterogeneity
We planned to present data from the following subgroups:
gestational age: term infants (37 weeks' gestation or greater); preterm infants (less than 37 weeks' gestation); extreme preterm (less than 28 weeks' gestation);
birth weight: under 1500 g; 1500 g or more;
-
type of pain:
painful procedures: heel lance, venipuncture, lumbar puncture, bladder tap, insertion of nasogastric tube, insertion of venous or arterial catheter/line or chest drain, or surgery (including neonatal circumcision, any surgery performed in the operating room);
painful clinical conditions: including a fractured long bone, necrotising enterocolitis, open skin lesions from an inherited skin disorder, or pain from an assisted vaginal birth;
-
dose of clonidine:
for infusion administration: less than 0.3 μg/kg/hour) versus 0.3 μg/kg/hour to 1 μg/kg/hour versus greater than 1 µg/kg/hour;
for bolus administration: less than 2 μg/kg versus 2 μg/kg to 4 μg/kg versus greater than 4 μg/kg;
duration of treatment (less than 24 hours; one to five days; five days or greater);
route of administration: parenteral; enteral; transdermal;
with versus without pharmacological sedation and pain management as co‐interventions;
within studies that included co‐interventions: studies in which the protocol allowed co‐interventions for sedation and pain management for one or both of the intervention groups; studies in which the protocol mandated sedation with co‐interventions.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the effect of the methodological quality of the trials, checking to ascertain if studies with a high risk of bias overestimated the effect of treatment.
Summary of findings and assessment of the certainty of the evidence
We planned to use the GRADE approach, as outlined in the GRADE Handbook to assess the quality of evidence for the following (clinically relevant) outcomes (gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html; Schünemann 2013):
For procedural pain – low‐intensity pain:
validated pain scale (Table 1);
completion of the targeted objective (relief of procedural pain) without use of any other agent;
episodes of apnoea (mean rates of apnoea);
episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer; occurring during the procedure and at one to two hours after the procedure;
parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
For procedural pain – high‐intensity pain:
validated pain scale (Table 1);
completion of the targeted objective (relief of procedural pain) without use of any other agent;
episodes of apnoea (mean rates of apnoea);
episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer, occurring during the procedure and at one to two hours after the procedure;
parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
For postoperative pain and for pain associated with clinical conditions:
validated pain scale (Table 1);
neonatal mortality;
episodes of apnoea (mean rates of apnoea);
episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer;
parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
Two review authors planned to independently assess the certainty of the evidence for each of the outcomes above. We planned to consider evidence from randomised controlled trials as high certainty but downgrade the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We planned to use GRADEpro GDT to create a 'Summary of findings' table to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence as being one of the following four grades:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
We provided results of the search in the study flow diagram (Figure 1).
1.
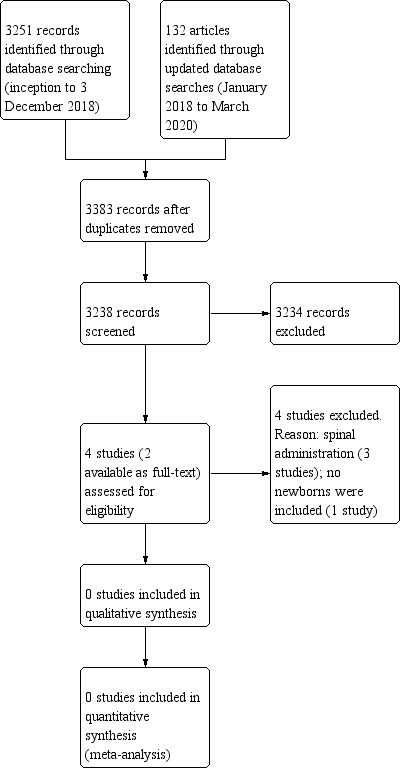
Study flow diagram.
See Characteristics of excluded studies, Characteristics of ongoing studies, and Characteristics of studies awaiting classification tables.
Results of the search
The literature search conducted in December 2018 and March 2020 identified 3383 references after removing duplicates. After screening, we assessed four studies for eligibility: three trials were excluded because of the route of administration (Batra 2010; Delous 2010; Rochette 2004). In addition, one study was classified as 'awaiting classification' because the abstract was not available (we tried to contact the study authors with no success) (Rochette 1998).
Our search of ClinicalTrials.gov identified 27 registered studies, ICTRP identified 10, ISRCTN registry identified one, and Luxid Biopharma Navigator identified 58. Only one ongoing trial was eligible (NCT01075490; see Characteristics of ongoing studies table). However, it is possible that this study has been completed and corresponds to one of the 'Excluded studies' described above (likely Delous 2010).
Included studies
We identified no trials that matched our inclusion criteria.
Excluded studies
We excluded three trials because they administered clonidine for spinal anaesthesia (Batra 2010; Delous 2010; Rochette 2004). In addition, one studies included no newborns (Batra 2010).
Two studies compared clonidine to placebo (Delous 2010; Rochette 2004); Rochette 2004 was a dose‐ranging study with four different doses of clonidine. The third excluded study had four arms: no intervention; clonidine; fentanyl; clonidine plus fentanyl (bupivacaine as co‐intervention in each arm of the study) (Batra 2010).
Risk of bias in included studies
No study met the eligibility criteria of this review.
Effects of interventions
No study met the eligibility criteria of this review.
Discussion
Summary of main results
We identified no eligible randomised controlled trials for inclusion that compared clonidine versus placebo, no intervention, or other interventions for the prevention or treatment of procedural or postoperative pain, or pain associated with clinical conditions in neonates. We excluded three studies that administered clonidine for spinal anaesthesia (Batra 2010; Delous 2010; Rochette 2004). Moreover, there were no newborns in the study of Batra 2010. Two of studies reported longer spinal anaesthesia when clonidine 1 μg/kg was co‐administered (Delous 2010; Rochette 2004).
We identified one ongoing trial (NCT01075490), which might be the protocol of one of the 'Excluded studies' described above (Delous 2010).
Overall completeness and applicability of evidence
We identified no eligible studies for inclusion.
Quality of the evidence
We identified no eligible studies for inclusion.
Potential biases in the review process
We used the standard methods of Cochrane Neonatal to conduct this systematic review. Our inclusive search strategy would theoretically have included all relevant studies. We minimised any potential biases, though the choice of the criteria for considering studies for inclusion in this review (namely Types of interventions) led to the exclusion of three studies where clonidine was administered for spinal anaesthesia (Batra 2010; Delous 2010; Rochette 2004).
Agreements and disagreements with other studies or reviews
One prospective observational study on clonidine in newborns has been reported by the same group as one of the excluded studies in this review (Rochette 2005). The cohort included 124 preterm and term infants undergoing herniorrhaphy under spinal anaesthesia with bupivacaine and clonidine. Apnoea episodes increased in the postoperative period, whereas desaturation episodes and mean arterial pressure were not affected. Short, transient bradycardias occurred in former preterm infants.
One Cochrane Review including 11 studies (neonates not included) on clonidine premedication in children undergoing surgery showed that oral administration of clonidine at 4 μg/kg reduced the need for additional analgesia without the usual adverse effects (Lambert 2014). However, some of the included studies administered atropine prophylactically to prevent bradycardia and hypotension. The interpretation of the effect of clonidine on pain scales was complicated because of the use of different pain scales and heterogeneity in the characteristics of the interventions and study design.
One systematic review of randomised controlled trials reported prolonged duration of analgesia when clonidine was used as an additive in spinal anaesthesia administered to children (neonates not included) (Ansermino 2003). The addition of clonidine 1 μg/kg to 2 μ/kg to local anaesthetic prolonged the duration of analgesia by two to three hours and increased sedation scores. However, there was respiratory depression in some infants. More evidence appears to be available for spinal administration rather than other routes of administration.
Authors' conclusions
Implications for practice.
We found no studies that met our inclusion criteria and hence there is no evidence to recommend or refute the use of clonidine for the prevention or treatment of procedural or postoperative pain, or pain associated with clinical conditions in neonates.
Implications for research.
Future research might assess the effects in the neonatal population, in both term and preterm newborns, by using validated and adequate pain scales. In addition, the use of clonidine might be explored for other routes of administration and in combination with opioids.
What's new
| Date | Event | Description |
|---|---|---|
| 21 April 2020 | Amended | Duplicate text ' Summary of findings and assessment of the certainty of the evidence' removed from Data synthesis section |
Acknowledgements
We thank Matthias Bank (Library and ICT services, Lund University) for designing and running the search strategy.
We thank Colleen Ovelman and Roger Soll for editorial support.
The 'Methods' section of this review is based on a standard template used by Cochrane Neonatal.
We acknowledge the work of Carol Friesen, Information Specialist with Cochrane Neonatal, in conducting the literature searches for the update of this review and of Colleen Ovelman, Managing Editor with Cochrane Neonatal, in peer reviewing the Ovid MEDLINE search strategy.
Appendices
Appendix 1. 2018 Search methods
PubMed, 3 December 2018 #1 (((randomized controlled trial [pt]) OR controlled clinical trial [pt]) OR (((randomized[Title/Abstract] OR randomized[Title/Abstract] OR randomly[Title/Abstract] OR placebo[Title/Abstract] OR trial[Title/Abstract] OR groups[Title/Abstract] OR study[Title/Abstract])) OR drug therapy[MeSH Subheading])) OR cluster[Title/Abstract] 9,569,823
#2 ((((premature OR prematures OR prematurity OR preterms OR preterm OR very low birth OR low birth weight* OR vlbw OR lbw OR "Infant, Extremely Premature"[Mesh] OR "Infant, Premature"[Mesh] OR "Infant, Low Birth Weight"[Mesh])) OR (newborn OR newborns OR neonate OR neonates OR neonatal OR neonat* OR infant OR infants OR infan* OR "Infant, Newborn"[Mesh]))) 1,547,106
#3 (("Clonidine"[Mesh] OR clonidine)) OR ("Adrenergic alpha‐2 Receptor Agonists" [Pharmacological Action] OR "Adrenergic alpha‐2 Receptor Agonists"[Mesh]) 29,248
#4 #1 AND #2 AND #3 840
Embase, 4 December 2018 #1. 'infant'/exp OR infant* OR infan* OR 'neonate'/exp OR neonate* OR neonat* OR 'newborn'/exp OR newborn* OR 'low birth weight'/exp OR 'low birth weight*' OR vlbw OR lbw OR 'very low birth weight'/exp OR 'very low birth' OR 'prematurity'/exp OR prematurity OR premature* OR preterm* 1,662,161
#2. 'clonidine'/exp OR clonidine 42,568
#3. 'alpha 2 adrenergic receptor stimulating agent'/exp 98,020
#4. #2 OR #3 99,698
#5. #1 AND #4 4,232
#6. 'randomized controlled trial'/exp OR 'randomized controlled trial' OR 'controlled clinical trial'/exp OR 'controlled clinical trial' OR randomized:ti,ab OR randomised:ti,ab OR randomly:ti,ab OR placebo:ti,ab OR trial:ti,ab OR study:ti,ab OR 'clinical trial'/exp OR cluster:ti,ab 10,165,392
#7. #1 AND #4 AND #6 1,676
#8. #5 AND 'drug therapy'/lnk 1,868
#9. #1 AND #4 AND ([controlled clinical trial]/lim OR [randomized controlled trial]/lim) 246
#10. #7 OR #8 OR #9 2,814
CINAHL, 6 December 2018
| S1 | (MH "Clonidine") OR clonidine | 1,632 |
| S2 | alpha 2 agonists | 664 |
| S3 | premature* OR prematurity OR preterm* OR very low birth OR low birth weight* OR vlbw OR lbw OR infant OR infan* OR newborn* OR neonate* OR neonat* | 407,226 |
| S4 | (MH "Infant+") OR (MH "Infant, Newborn+") | 217,072 |
| S5 | S3 OR S4 | 407,226 |
| S6 | S1 OR S2 | 2,193 |
| S7 | randomized controlled trial* OR controlled clinical trial OR randomized OR randomised OR randomly OR placebo OR trial OR groups OR study OR PT clinical trial OR clinical trial as topic OR cluster | 2,230,803 |
| S8 | S5 AND S6 AND S7 | 139 |
The Cochrane Library, 6 December 2018
#1 (clonidine):ti,ab,kw (Word variations have been searched) 3,497
#2 MeSH descriptor: [Clonidine] explode all trees 1,809
#3 MeSH descriptor: [Adrenergic alpha‐2 Receptor Agonists] explode all trees 225
#4 #1 OR #2 OR #3 3,636
#5 (infant OR infants OR newborn OR newborns OR neonate OR neonates OR neonatal OR premature OR prematures OR preterm OR preterms OR low birth weight OR vlbw OR lbw):ti,ab,kw (Word variations have been searched) 66,171
#6 #4 AND #5 168
Searches in clinical trial registries, 16 December 2018
Clinicaltrials.gov
Advanced search
Intervention/treatment: clonidine Other terms: premature OR prematurity OR preterms OR preterm OR “very low birth” OR “low birth weight” OR newborn OR newborns OR neonate OR neonates OR infant OR infants No further limits applied 27 results
ICTRP / WHO
Advanced search
Title: premature OR prematurity OR preterms OR preterm OR very low birth OR low birth weight OR newborn OR newborns OR neonate OR neonates OR infant OR infants Intervention: clonidine Ticked box: search for clinical trials in children Recruitment status: ALL, Phases: ALL
Simple search
Infant* AND clonidine
Prematur* AND clonidine
Preterm* AND clonidine
Low birth AND clonidine
Newborn* AND clonidine
Neonate* AND clonidine
10 results
ISRCTN registry Text search: Clonidine AND (premature OR prematurity OR preterms OR preterm OR newborn OR newborns OR neonate OR neonates OR infant OR infants)
1 result
Luxid Biopharma Navigator
"Clonidine" AND (premature OR prematurity OR preterms OR preterm OR newborn OR newborns OR neonate OR neonates OR infant OR infants)
58 results
Appendix 2. 2020 Search methods
The RCT filters have been created using Cochrane's highly sensitive search strategies for identifying randomised trials (Higgins 2019). The neonatal filters were created and tested by the Cochrane Neonatal Information Specialist.
CENTRAL via CRS Web:
Date ranges: 01 January 2018 to 11 March 2020 Terms: 1 MESH DESCRIPTOR Clonidine EXPLODE ALL AND CENTRAL:TARGET 2 clonidine AND CENTRAL:TARGET 3 MESH DESCRIPTOR Adrenergic alpha‐2 Receptor Agonists EXPLODE ALL AND CENTRAL:TARGET 4 (adrenergic ADJ2 "alpha 2" ADJ2 agonist*) AND CENTRAL:TARGET 5 (adrenergic ADJ2 "alpha 2" ADJ2 receptor ADJ2 agonist*) AND CENTRAL:TARGET 6 MESH DESCRIPTOR Adrenergic alpha‐Agonists EXPLODE ALL AND CENTRAL:TARGET 7 (adrenergic ADJ2 alpha ADJ2 agonist*) AND CENTRAL:TARGET 8 (alpha ADJ2 adrenergic ADJ2 receptor ADJ2 agonist*) AND CENTRAL:TARGET 9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 10 MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET 11 infant or infants or infant's or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET 12 #11 OR #10 AND CENTRAL:TARGET 13 #12 AND #9 14 2018 TO 2020:YR AND CENTRAL:TARGET 15 #14 AND #13
MEDLINE via Ovid:
Date ranges: 01 January 2018 to 11 March 2020 Terms: 1. exp Clonidine/ 2. clonidine.mp. 3. exp Adrenergic alpha‐2 Receptor Agonists/ 4. (adrenergic adj2 "alpha 2" adj2 agonist*).mp. 5. (adrenergic adj2 "alpha 2" adj2 receptor adj2 agonist*).mp. 6. exp Adrenergic alpha‐Agonists/ 7. (adrenergic adj2 alpha adj2 agonist*).mp. 8. (alpha adj2 adrenergic adj2 receptor adj2 agonist*).mp. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp infant, newborn/ 11. (newborn* or new born or new borns or newly born or baby* or babies or premature or prematurity or preterm or pre term or low birth weight or low birthweight or VLBW or LBW or infant or infants or 'infant s' or infant's or infantile or infancy or neonat*).ti,ab. 12. 10 or 11 13. randomized controlled trial.pt. 14. controlled clinical trial.pt. 15. randomized.ab. 16. placebo.ab. 17. drug therapy.fs. 18. randomly.ab. 19. trial.ab. 20. groups.ab. 21. or/13‐20 22. exp animals/ not humans.sh. 23. 21 not 22 24. 12 and 23 25. randomi?ed.ti,ab. 26. randomly.ti,ab. 27. trial.ti,ab. 28. groups.ti,ab. 29. ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab. 30. placebo*.ti,ab. 31. 25 or 26 or 27 or 28 or 29 or 30 32. 11 and 31 33. limit 32 to yr="2018 ‐Current" 34. 24 or 33 35. 9 and 34 36. limit 35 to yr="2018 ‐Current"
CINAHL via EBSCOhost:
Date ranges: 01 January 2018 to 11 March 2020 Terms: (clonidine OR (adrenergic AND "alpha 2" AND agonist*) OR (adrenergic AND "alpha 2" AND receptor AND agonist*) OR (adrenergic AND alpha AND agonist*) OR (alpha AND adrenergic AND receptor AND agonist*)) AND (infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Limiters ‐ Published Date: 20180101‐20200331
ISRCTN:
Date ranges: 2018 to 2020 Terms: Clonidine AND (premature OR prematurity OR preterms OR preterm OR newborn OR newborns OR neonate OR neonates OR infant OR infants)
Appendix 3. 'Risk of bias' tool
We planned to use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we planned to seek information regarding the method of randomisation, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We planned to asses each criterion as being at either low, high, or unclear risk of bias. Two review authors separately planned to assess each study and resolve any disagreements through discussion. We planned to add this information to the ‘Characteristics of included studies' table. We planned to evaluate the following issues and enter the findings into the ‘Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we planned to categorise the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we planned to categorise the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
or unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we planned to categorise the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we planned to categorise the methods used to blind outcome assessment. Blinding was to be assessed separately for different outcomes or class of outcomes. We planned to categorise the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we planned to describe the completeness of data including attrition and exclusions from the analysis. We planned to note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We planned to categorise the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
or unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we planned to describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we planned to compare prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we planned to contact study authors to gain access to the study protocol. We planned to assess the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we planned to describe any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We planned to assess whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batra 2010 | No newborns included. |
| Delous 2010 | Spinal administration of clonidine. RCT in 47 preterm and 63 term newborns. Infants were randomised to either clonidine 1 μg/kg or saline. Both groups received spinal anaesthesia with bupivacaine 1 mg/kg. Spinal anaesthesia was prolonged in the clonidine group in both preterm and term infants. Incidence and severity of apnoea did not differ between clonidine and control. Available as an abstract only. |
| Rochette 2004 | Spinal administration of clonidine. RCT in 75 neonates, including 50% of former preterm infants, undergoing elective inguinal herniorrhaphy. Used clonidine 0.25 μg/kg, 0.5 μg/kg, 1 μg/kg, or 2 μg/kg, or no intervention. Both groups received spinal anaesthesia with bupivacaine 1 mg/kg. Infants receiving clonidine 1 μg/kg doubled the duration of spinal block compared to the control group. Infants receiving the highest dose of clonidine (2 μg/kg) were affected more often with transient hypotension and need for caffeine administration. |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Rochette 1998.
| Methods | Not reported in the title of the publication. Abstract and full‐text not available |
| Participants | Newborns |
| Interventions | Clonidine + bupivacaine during spinal anaesthesia |
| Outcomes | Not reported in the title of the publication. Abstract and full‐text not available |
| Notes | We tried to contact the study authors without success. |
Characteristics of ongoing studies [ordered by study ID]
NCT01075490.
| Trial name or title | Neonatal spinal anaesthesia: effects of the addition of clonidine |
| Methods | Randomised controlled trial |
| Participants | Preterm and term infants, requiring inguinal hernia or lower limbs surgery. Sample size: 120 |
| Interventions | Addition of clonidine 1 μg/kg or saline to bupivacaine 1 mg/kg as spinal anaesthesia |
| Outcomes | Primary outcome: number of rescue general anaesthesia Secondary outcomes: apnoea and desaturation occurrence; duration of spinal anaesthesia |
| Starting date | November 2006 |
| Contact information | Alain Rochette, Montpellier, France; E‐mail: a‐rochette@chu‐montpellier.fr |
| Notes | We tried to contact the study authors without success. It is possible that this study has been completed and corresponds to 1 of the 'Excluded studies'. |
Differences between protocol and review
The title has been changed from "Clonidine for painful procedures or conditions in infants" to "Clonidine for pain in non‐ventilated infants" to reflect the broad inclusion criteria for both low‐intensity and high‐intensity painful procedures or conditions (Romantsik 2018).
The search strategy has been updated following the publication of the protocol and is reported in Appendix 1.
The description of the outcomes has been rearranged. We further limited the outcomes in the 'Summary of findings' table based on editorial input.
As of July 2019, Cochrane Neonatal no longer searches Embase for its reviews. Randomised controlled trials and controlled clinical trials from Embase are added to the Cochrane Central Register of Controlled Trials (CENTRAL) via a robust process (see 'How CENTRAL is created' at www.cochranelibrary.com/central/central‐creation). Cochrane Neonatal has validated their searches to ensure that relevant Embase records are found while searching CENTRAL.
Also starting in July 2019, Cochrane Neonatal no longer searches for randomised controlled trials and controlled clinical trials from ClinicalTrials.gov (clinicaltrials.gov) or from World Health Organization's International Clinical Trials Registry Platform (apps.who.int/trialsearch/), as records from both platforms are added to CENTRAL on a monthly basis (see 'How CENTRAL is created' at www.cochranelibrary.com/central/central‐creation). Comprehensive search strategies are executed in CENTRAL to retrieve relevant records. The ISRCTN Registry (at www.isrctn.com/, formerly Controlled‐trials.com), is searched separately.
For the 2020 update, we ran searches in the following databases: CENTRAL via CRS Web, MEDLINE via Ovid, and CINAHL via EBSCOhost. The search strategies are available in Appendix 2. The previous search methods are available in Appendix 1.
Contributions of authors
OR: reviewed the literature and wrote the review.
MC: assisted in the review of literature and in writing of the review.
EN: commented on and reviewed the review.
MB: reviewed the literature and wrote the review.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden.
to OR, EN and MB
-
Istituto Giannina Gaslini, Genoa, Italy.
to MC
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
OR: none.
MC: none.
EN: none.
MB: none.
Edited (no change to conclusions)
References
References to studies excluded from this review
Batra 2010 {published data only}
- Batra YK, Rakesh SV, Panda NB, Lokesh VC, Subramanyam R. Intrathecal clonidine decreases propofol sedation requirements during spinal anesthesia in infants. Paediatric Anaesthesia 2010;20(7):625‐32. [DOI: 10.1111/j.1460-9592.2010.03326.x; PUBMED: 20642661] [DOI] [PubMed] [Google Scholar]
Delous 2010 {published data only}
- Delous E, Rochette A, Bringuier S, Dadure C, Capdevila X. Spinal anaesthesia with clonidine: duration & tolerance: a prospective, randomized, double‐blinded study in 111 neonates. (preliminary results). Regional Anaesthesia and Pain Medicine 2010;35(5):E53‐4. [DOI: 10.1097/AAP.0b013e3181f3582c] [DOI] [Google Scholar]
Rochette 2004 {published data only}
- Rochette A, Raux O, Troncin R, Dadure C, Verdier R, Capdevila X. Clonidine prolongs spinal anaesthesia in newborns: a prospective dose‐ranging study. Anaesthesia and Analgesia 2004;98(1):56‐9. [PUBMED: 14693584] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Rochette 1998 {published data only}
- Rochette A, Raux O, Borry J, Vergnes C, d'Athis F. Clonidine prolongs bupivacaine spinal anaesthesia in newborns. British Journal of Anaesthesia 1998;80 (Suppl 1):141. [Google Scholar]
References to ongoing studies
NCT01075490 {unpublished data only}
- NCT01075490. Neonatal spinal anesthesia: effects of the addition of clonidine. clinicaltrials.gov/show/NCT01075490 (first received 25 February 2010).
Additional references
Allegaert 2007
- Allegaert K, Peeters MY, Verbesselt R, Tibboel D, Naulaers G, Hoon JN, et al. Inter‐individual variability in propofol pharmacokinetics in preterm and term neonates. British Journal of Anaesthesia 2007;99(6):864‐70. [DOI: 10.1093/bja/aem294; PUBMED: 17965417] [DOI] [PubMed] [Google Scholar]
Anand 1987a
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. New England Journal of Medicine 1987;371(21):1321‐9. [DOI: 10.1056/NEJM198711193172105; PUBMED: 3317037] [DOI] [PubMed] [Google Scholar]
Anand 1987b
- Anand KJ, Sippell WG, Aynsley‐Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet 1987;1(8524):62‐6. [PUBMED: 2879174] [DOI] [PubMed] [Google Scholar]
Anand 2004
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363(9422):1673‐82. [DOI: 10.1016/S0140-6736(04)16251-X; PUBMED: 15158628] [DOI] [PubMed] [Google Scholar]
Ansermino 2003
- Ansermino M, Basu R, Vandebeek C, Montgomery C. Nonopioid additives to local anaesthetics for caudal blockade in children: a systematic review. Paediatric Anaesthesia 2003;13(7):561‐73. [PUBMED: 12950855] [DOI] [PubMed] [Google Scholar]
Barker 1995
- Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Archives of Disease in Childhood. Fetal and Neonatal Edition 1995;72(1):F47‐8. [PUBMED: 7743285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 2013
- Bauer AZ, Kriebel D. Prenatal and perinatal analgesic exposure and autism: an ecological link. Environmental Health 2013;12:41. [DOI: 10.1186/1476-069X-12-41; PUBMED: 23656698] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. San Antonio (TX): Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd Edition. San Antonio (TX): Harcourt Assessment, 2006. [Google Scholar]
Bellieni 2005
- Bellieni CV, Bagnoli F, Sisto R, Neri L, Cordelli D, Buonocore G. Development and validation of the ABC pain scale for healthy full‐term babies. Acta Paediatrica 2005;94(10):1432‐6. [PUBMED: 16299876] [DOI] [PubMed] [Google Scholar]
Benis 2001
- Benis MM, Suresh GK. Frequency of invasive procedures in very low birth weight (VLBW) infants in the neonatal intensive care unit (NICU). Pediatric Research 2001; Vol. 49, issue 4:392A.
Brummelte 2012
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, et al. Procedural pain and brain development in premature newborns. Annals of Neurology 2012;71(3):385‐96. [DOI: 10.1002/ana.22267; PUBMED: 22374882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bueno 2013
- Bueno M, Yamada J, Harrison D, Khan S, Ohlsson A, Adams‐Webber T, et al. A systematic review and meta‐analyses of non‐sucrose sweet solutions for pain relief in neonates. Pain Research and Management 2013;18(3):153‐61. [PUBMED: 23748256] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butt 2013
- Butt ML, McGrath JM, Samra HA, Gupta R. An integrative review of parent satisfaction with care provided in the neonatal intensive care unit. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2013;42(1):105‐20. [DOI: 10.1111/1552-6909.12002; PUBMED: 23316895] [DOI] [PubMed] [Google Scholar]
Capino 2016
- Capino AC, Miller JL, Johnson PN. Clonidine for sedation and analgesia and withdrawal in critically ill infants and children. Pharmacotherapy 2016;36(12):1290‐9. [DOI: 10.1002/phar.1850; PUBMED: 27779775] [DOI] [PubMed] [Google Scholar]
Carbajal 1997
- Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier‐Martin M. APN: evaluation behavioral scale of acute pain in newborn infants. Archives de Pediatrie 1997;4(7):623‐8. [PUBMED: 9295899] [DOI] [PubMed] [Google Scholar]
Carbajal 2008
- Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300(1):60‐70. [PUBMED: 18594041] [DOI] [PubMed] [Google Scholar]
Ceelie 2013
- Ceelie I, Wildt SN, Dijk M, Berg MM, Bosch GE, Duivenvoorden HJ, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA 2013;309(2):149‐54. [DOI: 10.1001/jama.2012.148050; PUBMED: 23299606] [DOI] [PubMed] [Google Scholar]
Chana 2001
- Chana SK, Anand KJ. Can we use methadone for analgesia in neonates?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(2):F79‐81. [PUBMED: 11517197] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015
- Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha‐2 agonists for long‐term sedation during mechanical ventilation in critically ill patients. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010269; PUBMED: 25879090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane EPOC 2017
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC resources for review authors, 2017. Available at epoc.cochrane.org/epoc‐resources‐review‐authors (accessed prior to 25 March 2020).
Costa 2013
- Costa MC, Eckert GU, Fortes BG, Fortes Filho JB, Silveira RC, Procianoy RS. Oral glucose for pain relief during examination for retinopathy of prematurity: a masked randomized clinical trial. Clinics 2013;68(2):199‐204. [PUBMED: 23525316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cravero 2011
- Cravero JP, Havidich JE. Pediatric sedation – evolution and revolution. Paediatric Anaesthesia 2011;21(7):800‐9. [DOI: 10.1111/j.1460-9592.2011.03617.x; PUBMED: 21585616] [DOI] [PubMed] [Google Scholar]
de Graaf 2013
- Graaf J, Lingen RA, Valkenburg AJ, Weisglas‐Kuperus N, Groot Jebbink L, Wijnberg‐Williams B, et al. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age?. Pain 2013;154(3):449‐58. [DOI: 10.1016/j.pain.2012.12.006; PUBMED: 23352760] [DOI] [PubMed] [Google Scholar]
Debillon 2001
- Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(1):F36‐41. [PUBMED: 11420320] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffett 2012
- Duffett M, Koop A, Menon K, Meade MO, Cook DJ. Clonidine for the sedation of critically ill children: a systematic review. Journal of Pediatric Intensive Care 2012;1(1):5‐15. [DOI: 10.1002/14651858.CD010269.pub2; PUBMED: 25879090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 1986
- Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Research 1986;389(1‐2):261‐70. [PUBMED: 3948011] [DOI] [PubMed] [Google Scholar]
Gibbins 2014
- Gibbins S, Stevens BJ, Yamada J, Dionne K, Campbell‐Yeo M, Lee G, et al. Validation of the premature infant pain profile‐revised (PIPP‐R). Early Human Development 2014;90(4):189‐93. [DOI: 10.1016/j.earlhumdev.2014.01.005; PUBMED: 24491511] [DOI] [PubMed] [Google Scholar]
Golianu 2007
- Golianu B, Krane E, Seybold J, Almgren C, Anand KJ. Non‐pharmacological techniques for pain management in neonates. Seminars in Perinatology 2007;31(5):318‐22. [DOI: 10.1053/j.semperi.2007.07.007; PUBMED: 17905187] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- Grade Working Group, McMaster University. GRADEpro GDT. Version accessed 10 August 2017. Hamilton (ON): Grade Working Group, McMaster University, 2015.
Gregoretti 2009
- Gregoretti C, Moglia B, Pelosi P, Navalesi P. Clonidine in perioperative medicine and intensive care unit: more than an anti‐hypertensive drug. Current Drug Targets 2009;10(8):799‐814. [PUBMED: 19702526] [DOI] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R. The Abilities of Babies: a Study in Mental Measurement. New York (NY): McGraw‐Hill Book Co. Inc, 1954. [Google Scholar]
Grunau 1998
- Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain 1998;76(3):277‐86. [PUBMED: 9718246] [DOI] [PubMed] [Google Scholar]
Hammer 1989
- Hammer RP Jr, Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicology 1989;10(3):475‐83. [PUBMED: 2696899] [PubMed] [Google Scholar]
Handelmann 1985
- Handelmann GE, Dow‐Edwards D. Modulation of brain development by morphine: effects on central motor systems and behavior. Peptides 1985;6 Suppl 2:29‐34. [PUBMED: 4080616] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hodgkinson 1994
- Hodgkinson K, Bear M, Thorn J, Blaricum S. Measuring pain in neonates: evaluating an instrument and developing a common language. Australian Journal of Advanced Nursing 1994;12(1):17‐22. [PUBMED: 7786451] [PubMed] [Google Scholar]
Holsti 2008
- Holsti L, Grunau RE, Oberlander TF, Osiovich H. Is it painful or not? Discriminant validity of the Behavioral Indicators of Infant Pain (BIIP) scale. Clinical Journal of Pain 2008;24(1):83‐8. [DOI: 10.1097/AJP.0b013e318158c5e5; PUBMED: 18180641] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoy 2011
- Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs 2011;71(11):1481‐501. [DOI: 10.2165/11207190-000000000-00000; PUBMED: 21812509] [DOI] [PubMed] [Google Scholar]
Hummel 2008
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N‐PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology 2008;28(1):55‐60. [DOI: 10.1038/sj.jp.7211861; PUBMED: 18165830] [DOI] [PubMed] [Google Scholar]
Hummel 2010
- Hummel P, Lawlor‐Klean P, Weiss MG. Validity and reliability of the N‐PASS assessment tool with acute pain. Journal of Perinatology 2010;30(7):474‐8. [DOI: 10.1038/jp.2009.185; PUBMED: 19924132] [DOI] [PubMed] [Google Scholar]
Hünseler 2014
- Hünseler C, Balling G, Röhlig C, Blickheuser R, Trieschmann U, Lieser U, et al. Clonidine Study Group. Continuous infusion of clonidine in ventilated newborns and infants: a randomized controlled trial. Pediatric Critical Care Medicine 2014;15(6):511‐22. [DOI: 10.1097/PCC.0000000000000151; PUBMED: 24751788 ] [DOI] [PubMed] [Google Scholar]
ICROP 1984
- Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Pediatrics 1984;74(1):127‐33. [PUBMED: 6547526] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamadarkhana 2010
- Jamadarkhana S, Gopal S. Clonidine in adults as a sedative agent in the intensive care unit. Journal of Anaesthesiology, Clinical Pharmacology 2010;26(4):439‐45. [PUBMED: 21547166] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [DOI: 10.1164/ajrccm.163.7.2011060; PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Johnston 1997
- Johnston CC, Collinge JM, Henderson SJ, Anand KJ. A cross‐sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. Clinical Journal of Pain 1997;13(4):308‐12. [PUBMED: 9430811] [DOI] [PubMed] [Google Scholar]
Krechel 1995
- Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatric Anaesthesia 1995;5(1):53‐61. [PUBMED: 8521311] [DOI] [PubMed] [Google Scholar]
Lambert 2014
- Lambert P, Cyna AM, Knight N, Middleton P. Clonidine premedication for postoperative analgesia in children. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD009633.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laudenbach 2002
- Laudenbach V, Mantz J, Lagercrantz H, Desmonts JM, Evrard P, Gressens P. Effects of alpha(2)‐adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology 2002;96(1):134‐41. [PUBMED: 11753013] [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59‐66. [PUBMED: 8413140] [PubMed] [Google Scholar]
Lim 2017
- Lim Y, Godambe S. Prevention and management of procedural pain in the neonate: an update, American Academy of Pediatrics, 2016. Archives of Disease in Childhood. Education and Practice Edition 2017;102(5):254‐6. [PUBMED: 28724533] [DOI] [PubMed] [Google Scholar]
Lundqvist 2014
- Lundqvist P, Kleberg A, Edberg AK, Larsson BA, Hellström‐Westas L, Norman E. Development and psychometric properties of the Swedish ALPS‐Neo pain and stress assessment scale for newborn infants. Acta Paediatrica 2014;103(8):833‐9. [DOI: 10.1111/apa.12672; PUBMED: 24813238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2007
- Ma MX, Chen YM, He J, Zeng T, Wang JH. Effects of morphine and its withdrawal on Y‐maze spatial recognition memory in mice. Neuroscience 2007;147(4):1059‐65. [DOI: 10.1016/j.neuroscience.2007.05.020; PUBMED: 17601672] [DOI] [PubMed] [Google Scholar]
Mantz 2011
- Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. European Journal of Anaesthesiology 2011;28(1):3‐6. [DOI: 10.1097/EJA.0b013e32833e266d; PUBMED: 20881501] [DOI] [PubMed] [Google Scholar]
McPherson 2007
- McPherson RJ, Gleason C, Mascher‐Denen M, Chan M, Kellert B, Juul SE. A new model of neonatal stress which produces lasting neurobehavioral effects in adult rats. Neonatology 2007;92(1):33‐41. [DOI: 10.1159/000100084; PUBMED: 17596735] [DOI] [PubMed] [Google Scholar]
McPherson 2012
- McPherson C. Sedation and analgesia in mechanically ventilated preterm neonates: continue standard of care or experiment?. Journal of Pediatric Pharmacology and Therapeutics 2012;17(4):351‐64. [PUBMED: 23413121] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milesi 2010
- Milesi C, Cambonie G, Jacquot A, Barbotte E, Mesnage R, Masson F, et al. Validation of a neonatal pain scale adapted to the new practices in caring for preterm newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2010;95(4):F263‐6. [DOI: 10.1136/adc.2008.144758; PUBMED: 19221401] [DOI] [PubMed] [Google Scholar]
Nishina 1999
- Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, et al. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesthesia and Analgesia 1999;88(2):424‐8. [PUBMED: 9972773] [DOI] [PubMed] [Google Scholar]
Ohlsson 2016
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD011219.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Paris 2006
- Paris A, Mantz J, Tonner PH, Hein L, Brede M, Gressens P. The effects of dexmedetomidine on perinatal excitotoxic brain injury are mediated by the alpha2A‐adrenoceptor subtype. Anesthesia and Analgesia 2006;102(2):456‐61. [DOI: 10.1213/01.ane.0000194301.79118.e9; PUBMED: 16428542] [DOI] [PubMed] [Google Scholar]
Peters 2003
- Peters JW, Koot HM, Grunau RE, Boer J, Druenen MJ, Tibboel D, et al. Neonatal Facial Coding System for assessing postoperative pain in infants: item reduction is valid and feasible. Clinical Journal of Pain 2003;19(6):353‐63. [PUBMED: 14600535] [DOI] [PubMed] [Google Scholar]
Pichot 2012
- Pichot C, Ghignone M, Quintin L. Dexmedetomidine and clonidine: from second‐to‐first‐line sedative agents in the critical care setting?. Journal of Intensive Care Medicine 2012;27(4):219‐37. [DOI: 10.1177/0885066610396815; PUBMED: 21525113] [DOI] [PubMed] [Google Scholar]
Pillai Riddell 2015
- Pillai Riddell RR, Racine NM, Turcotte K, Uman LS, Horton RE, Din Osmun L, et al. Non‐pharmacological management of infant and young child procedural pain. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD006275.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pontén 2012
- Pontén E, Viberg H, Gordh T, Eriksson P, Fredriksson A. Clonidine abolishes the adverse effects on apoptosis and behaviour after neonatal ketamine exposure in mice. Acta Anaesthesiologica Scandinavica 2012;56(8):1058‐65. [DOI: 10.1111/j.1399-6576.2012.02722.x; PUBMED: 22694670] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricalde 1990
- Ricalde AA, Hammer RP Jr. Perinatal opiate treatment delays growth of cortical dendrites. Neuroscience Letters 1990;115(2‐3):137‐43. [PUBMED: 2172870] [DOI] [PubMed] [Google Scholar]
Rochette 2005
- Rochette A, Troncin R, Raux O, Dadure C, Lubrano JF, Barbotte E, et al. Clonidine added to bupivacaine in neonatal spinal anesthesia: a prospective comparison in 124 preterm and term infants. Paediatric Anaesthesia 2005;15(12):1072‐7. [DOI: 10.1111/j.1460-9592.2005.01664.x; PUBMED: 16324026] [DOI] [PubMed] [Google Scholar]
Romantsik 2017
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for sedation and analgesia for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD012468.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roze 2008
- Roze JC, Denizot S, Carbajal R, Ancel PY, Kaminski M, Arnaud C, et al. Prolonged sedation and/or analgesia and 5‐year neurodevelopment outcome in very preterm infants: results from the EPIPAGE cohort. Archives of Pediatrics & Adolescent Medicine 2008;162(8):728‐33. [DOI: 10.1001/archpedi.162.8.728; PUBMED: 18678804] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Seatriz 1993
- Seatriz JV, Hammer RP Jr. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Research Bulletin 1993;30(5‐6):523‐7. [PUBMED: 8384517] [DOI] [PubMed] [Google Scholar]
Shah 2012
- Shah PS, Herbozo C, Aliwalas LL, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004950.pub3; PUBMED: 23235618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simons 2003
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003;290(18):2419‐27. [DOI: 10.1001/jama.290.18.2419; PUBMED: 14612478] [DOI] [PubMed] [Google Scholar]
Spence 2005
- Spence K, Gillies D, Harrison D, Johnston L, Nagy S. A reliable pain assessment tool for clinical assessment in the neonatal intensive care unit. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2005;34(1):80‐6. [DOI: 10.1177/0884217504272810; PUBMED: 15673649] [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clinical Journal of Pain 1996;12(1):13‐22. [PUBMED: 8722730] [DOI] [PubMed] [Google Scholar]
Stevens 2016
- Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD001069.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taddio 2009
- Taddio A, Shah V, Atenafu E, Katz J. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain 2009;144(1‐2):43‐8. [DOI: 10.1016/j.pain.2009.02.012; PUBMED: 19329255] [DOI] [PubMed] [Google Scholar]
Taniguchi 2004
- Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin‐induced shock in rats. Critical Care Medicine 2004;32(6):1322‐6. [PUBMED: 15187514] [DOI] [PubMed] [Google Scholar]
Taniguchi 2008
- Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose‐ and time‐related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin‐induced shock in rats. Journal of Anesthesia 2008;22(3):221‐8. [DOI: 10.1007/s00540-008-0611-9; PUBMED: 18685927] [DOI] [PubMed] [Google Scholar]
Tesoro 2005
- Tesoro S, Mezzetti D, Marchesini L, Peduto VA. Clonidine treatment for agitation in children after sevoflurane anesthesia. Anesthesia and Analgesia 2005;101(6):1619‐22. [DOI: 10.1213/01.ANE.0000184204.81877.53; PUBMED: 16301230] [DOI] [PubMed] [Google Scholar]
van Dijk 2009
- Dijk M, Roofthooft DW, Anand KJ, Guldemond F, Graaf J, Simons S, et al. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. Clinical Journal of Pain 2009;25(7):607‐16. [DOI: 10.1097/AJP.0b013e3181a5b52a; PUBMED: 19692803] [DOI] [PubMed] [Google Scholar]
Viberg 2014
- Viberg H, Eriksson P, Gordh T, Fredriksson A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicological Sciences 2014;138(1):139‐47. [DOI: 10.1093/toxsci/kft329; PUBMED: 24361869] [DOI] [PubMed] [Google Scholar]
Walker 2012
- Walker SM, Grafe M, Yaksh TL. Intrathecal clonidine in the neonatal rat: dose‐dependent analgesia and evaluation of spinal apoptosis and toxicity. Anesthesia and Analgesia 2012;115(2):450‐60. [DOI: 10.1213/ANE.0b013e3182501a09; PUBMED: 22467896] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305‐11. [DOI: 10.1542/peds.2004-0204; PUBMED: 15520112] [DOI] [PubMed] [Google Scholar]
Zagon 1977
- Zagon IS, McLaughlin PJ. Morphine and brain growth retardation in the rat. Pharmacology 1977;15(3):276‐82. [DOI: 10.1159/000136699; PUBMED: 866403] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Romantsik 2018
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for painful procedures or conditions in infants. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD013104] [DOI] [Google Scholar]


