Abstract
Berries are considered an ideal source of polyphenols, especially from the flavonoid group. In this study, we examined the flavonoid content in 16 varieties of Swedish lingonberry, raspberry, blueberry, and strawberry. Nineteen flavonoids were simultaneously quantified using external standards. An additional 29 flavonoids were tentatively identified using MS as no standards were available. Quantification was done using HPLC-UV after optimization of chromatographic and extraction procedures. The method showed high linearity within the range of 2–100 μg/mL (correlation co-efficient >0.999), intra- and inter-day precision of 1.7–7.3% and average recovery above 84% for all compounds. Blueberries and lingonberries were found to contain higher contents of flavonoids (1100 mg/100 g dry weight) than raspberries and strawberries (500 mg/100 g dry weight). Anthocyanins were the dominant flavonoids in all berries. The tentatively characterized compounds contribute 18%, 29%, 61%, and 67% of the total flavonoid content in strawberries, lingonberries, raspberries, and blueberries, respectively. Overall, Swedish berries were shown to be good sources of polyphenols.
Keywords: extraction, flavonoids, HPLC-UV/MS, polyphenols, Swedish berries
1. Introduction
Berries are recognized as a good source of flavonoids. Flavonoids, a primary subgroup of polyphenolic compounds, have been shown to possess potent antioxidant, antimicrobial, and anti-inflammatory properties [1,2,3] and to exhibit beneficial effects against obesity, diabetes, neurodegenerative disorders, and cardiovascular disease [4,5,6,7]. Four flavonoid groups, anthocyanins, flavonols, flavan-3-ols, and proanthocyanidins, commonly exist as plant secondary metabolites in nature. The anthocyanins are pigment compounds in glycosylated forms that affect the colors present in growth periods [8]; the flavonols exist widely in fruits and berries in glycosylated or acetylated forms; the flavan-3-ols include (+)-catechin, (−)-epicatechin, gallocatechin, and epigallocatechin as dominant monomers; and the proanthocyanidins are polymers of A- and B-type, based on the location of interflavan linkages [9]. The structural diversity among flavonoids gives rise to their different chemical characteristics, physiological benefits, and pharmacokinetic behaviors.
Besides flavonoids, berries have also been reported to contain phenolic acids, which occur dominantly in the bound form. However, in some studies, both, flavonoid compounds and phenolic acids were simultaneously extracted using the same extraction method [10,11,12,13]. Complete extraction of flavonoids and free phenolic acids can be achieved using organic solvents [14,15], whereas acid or alkaline hydrolysis at high temperature is normally used for extraction of bound and insoluble phenolic acids [16,17].
With respect to flavonoid quantification, several methods for extraction have been developed. Ultrasonic-assisted extraction methods are recommended owing to the high reproducibility during analysis and low cost in terms of both time and energy [18]. Extraction conditions, which include the type of solvents used for extraction, the ratio of solvent to sample, the number of extraction repetitions, and the duration of extraction, are also factors that affect eventual outcomes [16,19]. For example, Pereira et al. [20] and Kylli et al. [21] even used two-step extraction procedures during quantification of groups of flavonoids in berries. High performance liquid chromatography (HPLC) is a favored and widely applied technique for quantification, and acidified water and acetonitrile are often chosen as the mobile phase [16].
A wide range of flavonoid content (105–1730 mg/100 g fresh weight) in berries has been reported, depending on the type, variety, and growing conditions [22,23,24]. However, information regarding flavonoid composition of some specific berries commonly grown in Sweden is limited (e.g., lingonberry) or lacking (e.g., strawberry variety “Favori”).
The aims of the present study were to (1) optimize a procedure for extraction of flavonoids in berries for analysis using HPLC-UV/MS, and (2) to quantify the flavonoid content in several varieties of Swedish lingonberry, raspberry, blueberry, and strawberry.
2. Materials and Methods
2.1. Reagents, Standards, and Solvents
Nineteen flavonoid compounds (Table 1) were purchased from Extrasynthese (Genay, France). Methanol (HPLC grade, ≥99.9%) was purchased from Honeywell (Seelze, Germany), HPLC-grade acetonitrile from VWR international (Stockholm, Sweden), ethanol (AR, 99.5%) from Solveco (Rosersberg, Sweden), formic acid (ACS, 98–100%) from Merck KGaA (Darmstadt, Germany), and acetone from Sigma-Aldrich (St. Louis, MO, USA).
Table 1.
Retention time, wavelength (λ), regression equation, limit of detection (LOD), and limit of quantification (LOQ) during HPLC-UV of flavonoid standards.
| No. | Compound | Retention Time (min) | λ 1 (nm) | Regression Equation 2 | LOD 3 (μg/mL) | LOQ 3 (μg/mL) | Abbre- Viation |
|---|---|---|---|---|---|---|---|
| 1 | Procyanidin B1 | 9.7 | 280 | y = 11.12x − 1.71 | 0.84 | 2.56 | Pr B1 |
| 2 | (+)-Catechin | 10.1 | 280 | y = 14.10x − 1.72 | 0.67 | 2.02 | (+)-Ca |
| 3 | Delphinidin-3,5-diglucoside | 11.6 | 520 | y = 22.35x − 6.10 | 0.99 | 2.99 | Del-di |
| 4 | Procyanidin B2 | 13.3 | 280 | y = 37.48x − 6.02 | 0.80 | 2.41 | Pr B2 |
| 5 | (-)-Epicatechin | 15.1 | 280 | y = 11.64x − 2.06 | 0.85 | 2.58 | (−)-Epi |
| 6 | Delphinidin-3-O-glucoside | 16.7 | 520 | y = 59.52x − 38.58 | 1.99 | 6.04 | Del-glu |
| 7 | Cyanidin-3-O-galactoside | 17.4 | 520 | y = 64.97x − 12.84 | 0.96 | 2.91 | Cy-gal |
| 8 | Cyanidin-3-O-glucoside | 19.1 | 520 | y = 57.62x − 12.71 | 1.09 | 3.30 | Cy-glu |
| 9 | Cyanidin-3-O-arabinoside | 20.5 | 520 | y = 51.51x − 11.54 | 1.11 | 3.36 | Cy-ara |
| 10 | Pelargonidin-3-O-glucoside | 21.3 | 520 | y = 31.84x − 6.29 | 0.98 | 2.96 | Pel-glu |
| 11 | Petunidin-3-O-glucoside | 21.8 | 520 | y = 56.17x − 29.61 | 1.86 | 5.64 | Pet-glu |
| 12 | Luteolin-8-C-glucoside | 24.9 | 360 | y = 53.77x − 4.83 | 0.49 | 1.49 | Lut-glu |
| 13 | Malvidin-3-O-glucoside | 26.3 | 520 | y = 48.43x − 21.34 | 1.67 | 5.06 | Mal-glu |
| 14 | Procyanidin A2 | 27.0 | 280 | y = 10.64x − 1.78 | 0.92 | 2.80 | Pr A2 |
| 15 | Myricetin-3-O-rhamnoside | 28.8 | 360 | y = 64.46x − 10.05 | 0.77 | 2.34 | My-rha |
| 16 | Quercetin-3-O-galactoside | 30.1 | 360 | y = 30.22x − 4.20 | 0.66 | 2.01 | Qu-gal |
| 17 | Quercetin-3-O-rutinoside | 30.8 | 360 | y = 28.29x − 3.76 | 0.63 | 1.90 | Qu-rut |
| 18 | Quercetin-3-O-rhamnoside | 35.6 | 360 | y = 39.95x − 6.95 | 0.89 | 2.69 | Qu-rha |
| 19 | Kaempferol-3-O-glucoside 4 | 35.6 | 360 | y = 41.27x − 19.89 | 2.16 | 6.54 | Kae-glu |
1 Detection wavelength for each compound according to Vagiri et al. [27]. 2 Linear range for all compounds was tested 2–100 μg/mL, resulting in R2 > 0.999. HPLC conditions as described in the section “Quantification”. 3 Calculated as: LOD = (3.3 × SD)/b; LOQ = (10 × SD)/b, where SD is residual standard deviation of the linear regression and b is slope of the regression line [29]. 4 Kaempferol-3-O-glucoside co-eluted with quercetin-3-O-rhamnoside, so its calibration curve was built separately.
Stock standard solutions were prepared by dissolving the individual flavonoid compounds in methanol to reach a final concentration of 1000 μg/mL. All solutions were kept under nitrogen protection and stored in darkness at 4 °C.
2.2. Berry Samples
Sixteen berry samples commercially available in the Kalmar area, southern Sweden, were purchased in summer 2018. Information regarding variety (except lingonberry) was received from the producers. These comprised wild lingonberries (Vaccinium vitis-idaea) from two producers (unknown varieties, here named L1 and L2), strawberries (Fragaria ananassa) of seven varieties (namely Evie, Faith, Favori, Malwina, Rumba, Salsa, and Sonata), blueberries (Vaccinium myrtillus) of four varieties (Bluecrop, Camelia, Duke, and Legacy), and raspberries (Rubus idaeus) of three varieties (Glen Ample, Kweli, and Versalle).
Based on the popularity of lingonberry in European countries and the diversity of its flavonoid profile [25], one of the lingonberry samples (L1) was selected as the in-house control sample to optimize the extraction method.
All berry samples were separately kept in polyethylene bags and stored at −20 °C before lyophilization. After freeze drying (BenchTop Pro, VirTis, USA), the samples were milled using a laboratory-scale mill (Cyclotec 1093, Tecator, Sweden) and stored at −20 °C until further analysis within a week.
2.3. Sample Extraction
Extraction conditions (i.e., the extraction solvent, the number of extraction repetitions, and the ratio of solvent volume to sample weight) and reconstitution solvent (for dissolving dried extracts prior to injection into HPLC) were optimized based on the method of Latti et al. [26] using the in-house control sample lingonberry L1.
The effects of several types of extraction solvents (aqueous methanol, ethanol, or acetone at a concentration of 50%, 70%, and 100% (v/v) with the addition of formic acid (0%, 1%, 3%, and 5%, v/v)) on flavonoid yield were studied with a solvent volume to a sample weight ratio of 15 μL/mg). To optimize the number of extraction repetitions, flavonoids in the in-house control sample were extracted in four repetitions and the extract from each repetition was analyzed separately. To optimize the reconstitution solvent, the different standard solutions were used. Five different methanol concentrations (100%, 80%, 50%, 40%, and 30% in water, (v/v)) were investigated for reconstitution using standards for individual compounds (10 μg/mL).
In the optimized extraction procedure 210 μL methanol was added to 14 mg freeze-dried berry (n = 3). Samples were sonicated for 15 min prior to centrifugation for 5 min at 13,000× rpm. The supernatants were collected, while the pellets were re-extracted another two times using the same procedure. The combined supernatants were concentrated using SpeedVac (SC100, Thermo Scientific, Waltham, MA, USA) at a medium temperature (43 °C) until dryness. The dry residue was redissolved in methanol/water (40:60, v/v and volume/mass = 30), and filtered through a 0.45 μm Millipore filter (Agilent, St. Clara, CA, USA) before analysis by HPLC-UV.
2.4. Quantification
Quantification of flavonoids was carried out using HPLC-UV/MS (Agilent 1200 series, St. Clara, CA, USA) with the Agilent OpenLab Software Suite Rev. C.01.07. The mass spectrometer (Agilent 6130 Quadrupole, St. Clara, CA, USA) was fitted with electrospray ionization (ESI) and operated in a positive ion mode. Parameters were set as follows: drying gas flow 11.0 L/min, nebulizer pressure 55 psig, drying gas temperature 250 °C, and capillary voltage 3000 V. Mass spectra in the range of mass-to-charge ratio (m/z) 285–670 were collected.
Flavonoids were separated on a 250 mm × 4.6 mm, 3 μm, Luna® Omega C18 column (Phenomenex, Torrance, CA, USA) fitted with a 4 mm × 3.0 mm, C18 Security Guard Cartridge (Phenomenex, Torrance, CA, USA). The column temperature was set to 40 °C, the injection volume to 20 μL, and the flow rate to 1 mL/min. Several mobile phase compositions were investigated: 1%, 3%, 5%, 7%, 10%, and 12% formic acid in water as solvent A; and acetonitrile/methanol/water (90:5:5, 85:7.5:7.5, and 80:10:10 v/v/v) as Solvent B, based on the method of Vagiri et al. [27]. Finally, 3% formic acid in water was employed as solvent A and acetonitrile/methanol/water (80:10:10, v/v/v) as solvent B. The optimized gradient was as follows: 0–45 min, linear gradient from 5% to 29% B; 45–46 min, linear gradient from 29% to 50% B; 46-48 min, 50% B isocratic; 48–49 min, linear gradient from 50% to 5%; 49–55 min, 5% B isocratic.
Quantification was based on an external multilevel calibration curve (n = 6) of 19 flavonoids at 280 nm for flavan-3-ols, 360 nm for flavonols and 520 nm for anthocyanins, according to Vagiri et al. [27]. For tentative identification of further peaks, which according to the literature were expected in the berry extract and where no standards were available, mass spectrometry (Agilent 1200 series, St. Clara, CA, USA) was used; anthocyanins were quantified using UV against cyanidin-3-O-glucoside (520 nm), flavonols against quercetin-3-O-galactoside (360 nm), B-type proanthocyanidin dimers against procyanidin B1 (280 nm), and A-type proanthocyanidin dimers against procyanidin A2 (280 nm) [15,28].
2.5. Quality Control
Linearity of each calibration curve (n = 6) within the range 2–100 μg/mL was evaluated by linear regression analysis. Limit of detection (LOD) and limit of quantification (LOQ) of the compounds identified were determined from the calibration curve data as: LOD = (3.3 × SD)/b; LOQ = (10 × SD)/b, where SD is the residual standard deviation of the linear regression and b is the slope of the regression line [29].
Extraction recovery was investigated by addition of an upper (100% of the expected concentration in samples) and a lower (50% of the expected concentration in samples) level of standards to the in-house control sample prior to extraction. Recovery (R, %) was calculated as: R = 100 × (Cfound−Csample)/Cadded, where Cfound indicates the content measured after addition of standard compounds, Csample indicates the content measured before addition, and Cadded indicates the added amount of standard compounds.
The intra-day variation was calculated from the triplicate assays of the lingonberry extract on the same day (n = 3), while the inter-day variation was measured from assays of the same batch for three separate days (n = 3). The results were expressed as coefficient of variation (CV, %) of means for peak area.
Stability of authentic standard compounds at two different concentrations (5 and 50 μg/mL) was evaluated after storage at 4 °C in darkness for three months. The stability was monitored twice every month for three months by comparing HPLC peaks of standards before storage and after each storage time point.
2.6. Calculations and Statistical Analysis
The total amount of flavonoids (mg/100 g dry weight (dwt) of freeze-dried berries) in each berry species was calculated as the sum of the four subgroups (i.e., anthocyanins, flavonols, flavan-3-ols, and proanthocyanidin dimers), including the tentatively identified compounds. All results were expressed as mean ± SD. Linearity of calibration curves was determined using regression analyses (Excel, Microsoft, Redmond, WA, USA). Flavonoid yield when optimizing extraction was compared using one-way analysis of variance (ANOVA), significance was set to p < 0.05 (Prism 8, GraphPad, La Jolla, CA, USA).
3. Results and Discussion
3.1. Method Optimization for Berry Matrix
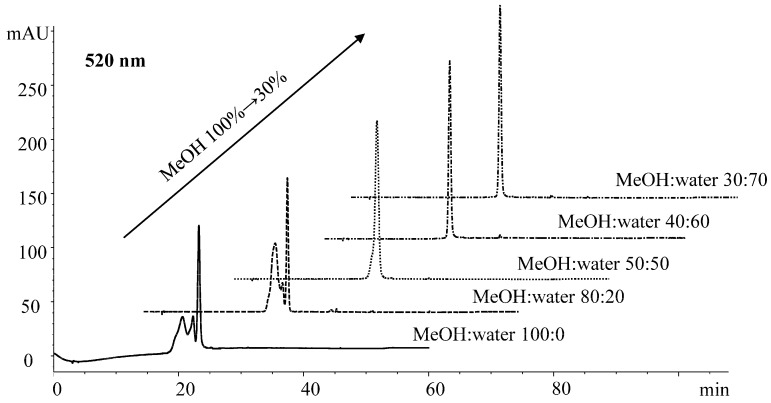
With regard to optimization of the reconstitution solvent for compounds before HPLC quantification, a high percentage of methanol in the reconstitution solvent resulted in distortion of peaks (Figure 1). Using 40% and 30% methanol in water (v/v) as the reconstitution solvent achieved peaks with symmetry around 1.0 without distortion or tailing. The observed solvent effect is in line with findings by Mirali et al. [30] that a high organic proportion in the reconstitution solvent tends to have an adverse effect on chromatography.
Figure 1.
Effect of the reconstitution solvent on peak shape as exemplified by cyandin-3-O-glucoside standard (10 μg/mL) at 520 nm. HPLC conditions as described in Section 2.4.
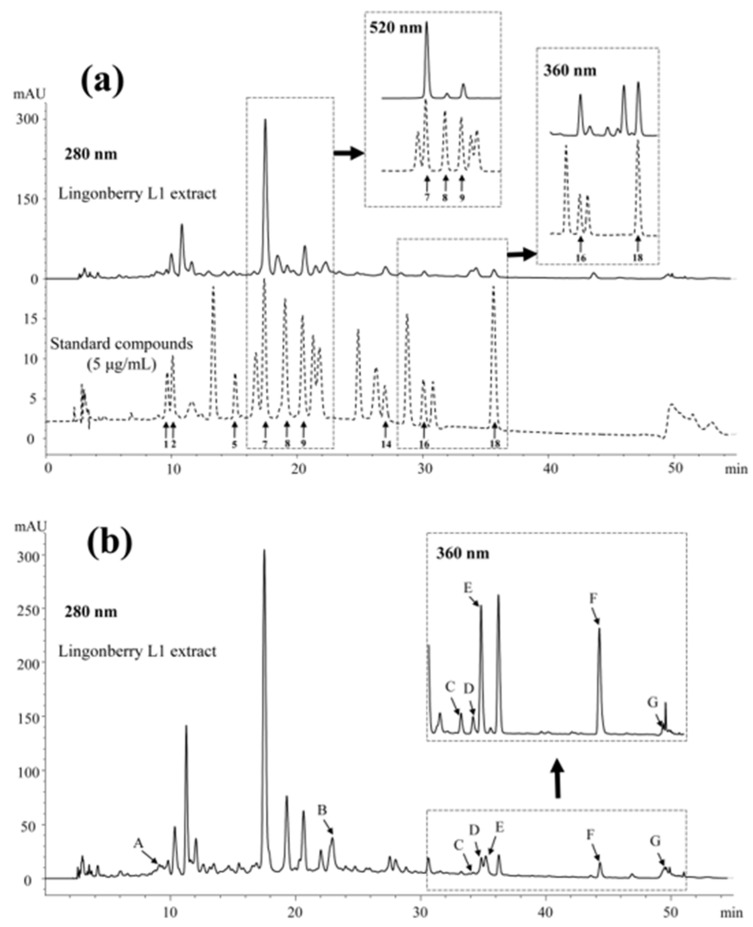
With respect to mobile phase composition, an increase in formic acid proportion up to 12% (v/v) significantly improved peak separation and prevented tailing (Figure S1 in Supplementary Materials). Quercetin-3-O-rhamnoside and kaempferol-3-O-glucoside could not be separated unless the concentration of formic acid was above 5%, while they were completely separated when the concentration was above 7%. Thus use of a higher percentage of formic acid in the mobile phase was preferable in flavonoid analysis, which is in agreement with recommendations by Vagiri et al. [27]. However, considering the recommended working pH of the column (pH 1.5–8.5), a compromise was made and 3% formic acid in water (pH 1.92) was finally selected as solvent A. As for solvent B, use of acetonitrile/methanol/water with composition 80:10:10 (v/v/v) resulted in higher peak resolution (Figure 2) than with the two other solvents (data not shown), and thus it was chosen as the optimal mobile phase B. Using optimized chromatographic conditions, a standard chromatogram was obtained with symmetry of all peaks ranging from 0.8 to 1.0 (Figure 2).
Figure 2.
Chromatograms of lingonberry L1 extract and flavonoid standards (5 μg/mL) at 280 nm. (a) Flavonoids quantified using authentic standards. Peak numbers (1–18) refer to compounds listed in Table 1. (b) Flavonoids tentatively identified and quantified. A, B-type procyanidin; B, A-type procyanidin; C, quercetin-3-O-xyloside; D, quercetin-3-O-arabinoside; E, quercetin-3-O-arabino-furanoside; F, quercetin-3-O-(4’’-HMG)-rhamnoside; G, kaempferol-(HMG)-rhamnoside. The concentration of flavonoids in lingonberry extract ranged from 1.5 to 101.9 μg/mL.
With regard to optimization of the extraction method for a berry matrix, pure methanol showed higher extractability for all flavonoids investigated than ethanol or acetone as extraction solvents (either pure or mixed with water; Figure S2 in Supplementary Materials). An interesting observation was that pure acetone, which showed a remarkably weak extraction ability in our studies, has previously been reported to exhibit strong extraction ability [15,31]. This discrepancy probably resulted from differences in the sample matrices and the water content of the solvent. Fresh and frozen berries were used as sample matrices in the studies by Garcia-Viguera et al. [31] and Kajdzanoska et al. [15], whereas freeze-dried lingonberry samples, which contain little water were employed in the present study. To investigate the effect of acid on extraction, different amounts of formic acid (1–5%) were added to the extraction solvent (pure methanol and aqueous methanol), which negatively affected the total yield of flavonoids (Figure S3 in Supplementary Materials). As for cyanidin-3-O-galactoside, yield was 30% lower on increasing the formic acid concentration to 5%, which might be due to lower stability under the acidic conditions. This finding is consistent with observations by others [15,32,33] who attributed it to instability of flavonoids in extremely acidic environments where hydrolysis, destruction, acetylation, or formylation of polyphenols could occur. Therefore, unacidified methanol was selected as the extraction solvent. In optimization of extraction repetitions, more than 80% of flavonoids in the in-house control sample were found in the first repetition, >10% in the second, less than 5% in the third, and <1% in the fourth (data not shown). Thus, three-repetition extraction, combining supernatants of repetitions 1–3, was selected.
3.2. Quality Control of Quantitative Method
The optimized method provided linearity within the range 2–100 μg/mL, with coefficient of determination (R2) >0.999 for the 19 compounds (Table 1). The LOD for all compounds ranged between 0.5 and 2.0 μg/mL, which was equivalent to 14.7–59.7 μg/g in freeze-dried berry samples. The LOQ ranged from 1.5 to 6.0 μg/mL, which was equivalent to 43.5–181.2 μg/g in freeze-dried berry samples.
Average recovery on adding an upper and lower level of standard (50% and 100% of the expected content) to the in-house control sample ranged between 84% and 103% for both levels (Table S1 in Supplementary Materials), which is an improvement on the previously reported recovery value for myricetin from lingonberries of 53.2% ± 6% [34].
The intra-day (n = 3) and inter-day (n = 3) variation (%, CV for peak area) for individual flavonoids in the in-house control sample was 1.7–5.8% and 1.9–7.3%, respectively.
Standard solutions of all flavonoids investigated (Table 1) maintained stable concentrations of 5 and 50 μg/mL during three months (CV < 10%) at 4 °C, indicating that short-term storage (up to one week) of berry samples in the fridge probably did not significantly affect the outcomes of the analyses.
3.3. Flavonoids in Swedish Berries
The content of flavonoids in the berries, quantified using 19 standards, is shown in Table 2. Three compounds (delphinidin-3,5-diglucoside, procyanidin B2, and luteolin-8-C-glucoside) were not detected in any of the berry samples. An additional 29 flavonoids in the berry samples were tentatively identified using MS (Table 3) [25,35,36,37] and quantified by UV.
Table 2.
Content 1, 2 (mg/100 g dwt) of individual flavonoids in selected berry varieties.
| Lingonberry 3 | Raspberry | Blueberry | Strawberry | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | Kweli | Versalle | Glen Ample | Bluecrop | Duke | Camelia | Legacy | Evie | Favori | Sonata | Faith | Malwina | Salsa | Rumba | |
| Proanthocyanidins | ||||||||||||||||
| Pr B1 | 68.1 ± 0.6 | 111.6 ± 3.8 | n.d. | n.d. | n.d. | 35 ± 1.3 | 19.8 ± 0.2 | 13.9 ± 0.4 | n.d. | 12.4 ± 1.9 | 10.9 ± 0.3 | 15.2 ± 0.1 | 6 ± 0.1 | 11.9 ± 0.1 | 7.9 ± 0.7 | 12.1 ± 1.1 |
| Pr A2 | 51.9 ± 0.0 | 41.8 ± 1.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Flavan-3-ols | ||||||||||||||||
| (+)-Ca | 152.7 ± 2.5 | 243.6 ± 5.9 | 7.4 ± 0.1 | 6.1 ± 0.2 | 4.8 ± 0.2 | 43.1 ± 1.9 | 32 ± 0.6 | 22.6 ± 0.4 | 10.3 ± 0.6 | 38 ± 3.1 | 36.7 ± 1.3 | 45.8 ± 0.5 | 30.5 ± 1.3 | 45.4 ± 1.1 | 32 ± 1 | 48.1 ± 1 |
| (-)-Epi | 38.6 ± 0.4 | 34.1 ± 4.4 | 102.4 ± 4 | 87.1 ± 2.8 | 63.9 ± 7.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Flavonols | ||||||||||||||||
| My-rha | n.d. | n.d. | n.d. | n.d. | n.d. | 2.5 ± 0.1 | 2.7 ± 0.2 | 9.2 ± 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Qu-gal | 35.5 ± 0.0 | 58.8 ± 2.6 | 14.3 ± 0.6 | 19.6 ± 0.3 | 14.9 ± 0.4 | 78.6 ± 1.1 | 67.9 ± 1.6 | 23.7 ± 1.9 | 125.8 ± 3.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Qu-rut | n.d. | n.d. | 4.1 ± 0.1 | 7 ± 0.2 | 9.1 ± 0.3 | 44.7 ± 2 | 32.1 ± 1.3 | 30.4 ± 2.4 | 19.9 ± 0.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Qu-rha | 36.5 ± 0.5 | 46.1 ± 3 | n.d. | n.d. | n.d. | 1.3 ± 0.2 | 1.7 ± 0.2 | 60.2 ± 3.5 | 9.4 ± 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Kae-glu | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 9.4 ± 0.5 | 7.3 ± 0.1 | 8 ± 0.1 | 6.1 ± 0.3 | 5.3 ± 0.1 | 4.3 ± 0.1 | 10.1 ± 0.2 |
| Anthocyanidins | ||||||||||||||||
| Del-glu | n.d. | n.d. | n.d. | n.d. | n.d. | 47 ± 2.4 | 35.1 ± 0.6 | 33.5 ± 1.4 | 5.3 ± 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cy-gal | 308.4 ± 6.1 | 238.9 ± 5.1 | n.d. | n.d. | n.d. | 12.5 ± 0.8 | 10.5 ± 0.2 | 7.8 ± 0.5 | 20.7 ± 1.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cy-glu | 21.1 ± 0.2 | 17.1 ± 0.7 | 74.4 ± 0.8 | 65.2 ± 2.2 | 55.5 ± 2.8 | 7.7 ± 0.7 | 7.2 ± 0.2 | 3.3 ± 0.1 | n.d. | 14.3 ± 0.3 | 11.4 ± 0 | 6.5 ± 0.5 | 4.6 ± 0.1 | 5.3 ± 0.1 | 9.1 ± 0.5 | 7.2 ± 0.6 |
| Cy-ara | 75.3 ± 0.3 | 49.8 ± 0.8 | n.d. | n.d. | n.d. | 67.9 ± 3 | 47.6 ± 1.1 | 86.6 ± 4.2 | 138.4 ± 5.8 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Pel-glu | n.d. | n.d. | 4.5 ± 0.2 | 2.9 ± 0.3 | n.d. | n.d. | n.d. | n.d. | n.d. | 562 ± 3.1 | 487.3 ± 6.9 | 723.9 ± 15 | 184.1 ± 7.3 | 371.4 ± 9.6 | 278.1 ± 7.1 | 353.4 ± 8 |
| Pet-glu | n.d. | n.d. | n.d. | n.d. | n.d. | 37.7 ± 1.9 | 29.6 ± 0.9 | 33.2 ± 1.2 | 4.9 ± 0.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Mal-glu | n.d. | n.d. | n.d. | n.d. | n.d. | 94.3 ± 4.5 | 83.3 ± 1.8 | 88.6 ± 3.9 | 10.5 ± 0.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
1 Values shown are mean ± SD (n = 3). Abbreviations and full names of compounds can be found in Table 1. 2 Delphinidin-3,5-diglucoside, procyanidin B2, and luteolin-8-C-glucoside were not detected in any of the berry samples. 3 Wild lingonberry samples bought from two producers were named L1 and L2 due to a lack of information on variety. Recovery for flavonoids detected in the control sample lingonberry L1 was between 84% and 103% (Table S1 in Supplementary Materials). The moisture content of the berry samples ranged between 80.1% and 87.5% (see Table S2 in Supplementary Materials).
Table 3.
Content (mg/100 g dwt) of tentatively identified flavonoids in all berries.
| Compound | RT 1 (min) |
[M+H] + (m/z 2) | Berry Varieties (mg/100 g dwt) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lingonberry | ||||||||||
| L1 | L2 | |||||||||
| Proanthocyanidins | ||||||||||
| B-type procyanidin 3 | 8.8 | 579 | 46.7 ± 3 | 66 ± 6.4 | ||||||
| A-type procyanidin 4 | 22.2 | 577 | 130 ± 1.5 | 188.6 ± 5.5 | ||||||
| Flavonols 5 | ||||||||||
| Quercetin-3-O-xyloside | 32.7 | 435 | 7.1 ± 0.3 | 10.8 ± 0.5 | ||||||
| Quercetin-3-O-arabinoside | 33.7 | 435 | 6.3 ± 0.1 | 9 ± 0.4 | ||||||
| Quercetin-3-O-arabino-furanoside | 34.3 | 435 | 42.5 ± 1.9 | 56 ± 2.2 | ||||||
| Quercetin-3-O-(4’’-HMG)-rhamnoside 6 | 43.6 | 593 | 41.8 ± 1.1 | 64.3 ± 2.9 | ||||||
| Kaempferol-(HMG)-rhamnoside | 49.6 | 577 | 4.6 ± 0.1 | 3.4 ± 0.2 | ||||||
| Blueberry | ||||||||||
| Bluecrop | Duke | Camelia | Legacy | |||||||
| Anthocyanidins 7 | ||||||||||
| Delphinidin-3-O-galactoside | 15.4 | 465 | 88.8 ± 3.7 | 61.3 ± 1.4 | 110.9 ± 5.4 | 164 ± 6.8 | ||||
| Delphinidin-3-O-arabinoside | 18.4 | 435 | 87.1 ± 5.2 | 67.5 ± 1.3 | 82.3 ± 3.5 | 80.8 ± 2.9 | ||||
| Petunidin-3-O-galactoside | 20.5 | 479 | 60.8 ± 2.7 | 42.6 ± 1 | 77.5 ± 3.7 | 123.8 ± 5.1 | ||||
| Peonidin-3-O-galactoside | 23.2 | 463 | 4.4 ± 0.8 | 3.8 ± 0.2 | 2.1 ± 0.2 | n.d. | ||||
| Petunidin-3-O-arabinoside | 23.6 | 449 | 41.5 ± 2.2 | 28.9 ± 0.8 | 45.8 ± 2.2 | 47.4 ± 1.3 | ||||
| Peonidin-3-O-glucoside | 24.2 | 463 | 5.4 ± 0.7 | 6.1 ± 0.1 | 4.2 ± 0.3 | 0.4 ± 0.4 | ||||
| Malvidin-3-O-galactoside | 25.0 | 493 | 132.9 ± 6.2 | 87.9 ± 2.5 | 187.5 ± 8.7 | 289.8 ± 14 | ||||
| Peonidin-3-O-arabinoside | 25.7 | 433 | 14.2 ± 0.9 | 8.5 ± 0 | 3.7 ± 0.1 | 0.4 ± 0.4 | ||||
| Malvidin-3-O-arabinoside | 28.2 | 463 | 141.7 ± 6.1 | 95.4 ± 2.3 | 121.2 ± 6.7 | 136.5 ± 5.3 | ||||
| Delphinidin-3-acetyl-glucoside | 29.9 | 507 | 16.5 ± 0.6 | 8 ± 0 | 2.4 ± 0 | n.d. | ||||
| Petunidin-3-acetyl-glucoside | 35.0 | 521 | 15.7 ± 0.6 | 8.5 ± 0.6 | n.d. | n.d. | ||||
| Cyanidin-3-malonyl-glucoside | 35.2 | 535 | 27.7 ± 0.9 | 10.3 ± 0.4 | n.d. | n.d. | ||||
| Malvidin-3-acetyl-glucoside | 39.2 | 491 | 43.6 ± 1.4 | 19.9 ± 0.4 | 3.3 ± 0.3 | n.d. | ||||
| Flavonols 5 | ||||||||||
| Myricetin-3-O-arabinoside | 32.3 | 465 | 12.1 ± 0.6 | 10.6 ± 0.3 | n.d. | 8.5 ± 0.6 | ||||
| Quercetin-3-O-arabinoside | 33.6 | 435 | 20.2 ± 0.6 | 19.5 ± 0.7 | 7.3 ± 0.7 | 28.9 ± 1.1 | ||||
| Raspberry | ||||||||||
| Kweli | Versalle | Glen Ample | ||||||||
| Proanthocyanidins | ||||||||||
| B-type procyanidin3 | 11.5 | 579 | 80 ± 1.6 | 123.8 ± 3.6 | 43.1 ± 4.1 | |||||
| Anthocyanidins 7 | ||||||||||
| Cyanidin-3-O-sophoroside | 17.2 | 611 | 192.4 ± 3.2 | 210.9 ± 6.5 | 81.6 ± 4.1 | |||||
| Cyanidin-3-glucosyl-rutinoside | 19.3 | 757 | n.d. | n.d. | 82.9 ± 8.9 | |||||
| Pelargonidin-3-O-rutinoside | 21.6 | 579 | n.d. | n.d. | 51.2 ± 2.5 | |||||
| Strawberry | ||||||||||
| Evie | Favori | Sonata | Faith | Malwina | Salsa | Rumba | ||||
| Proanthocyanidins | ||||||||||
| B-type procyanidin 3 | 8.8 | 579 | 31.5 ± 2.2 | 22.7 ± 2.3 | 34.8 ± 4.6 | 57.7 ± 1.8 | 33.1 ± 1.3 | 65.8 ± 1.6 | 40.2 ± 0.3 | |
| Anthocyanidins 7 | ||||||||||
| Cyanidin-hexose-deoxyhexoside | 24.1 | 595 | 6.8 ± 0.2 | 7.9 ± 1.0 | 9.8 ± 0.2 | 5.7 ± 0.1 | 7.6 ± 0.2 | 11.8 ± 0.4 | 8.8 ± 0.3 | |
| Pelargonidin-3-O-malonylglucoside | 30.6 | 519 | 22.4 ± 0.2 | 20.3 ± 0.2 | 30.1 ± 0.9 | 23.8 ± 0.9 | 12.6 ± 0.4 | 20.3 ± 0.6 | 38.2 ± 0.8 | |
| Flavonols 5 | ||||||||||
| Quercetin-3-O-glucuronide | 30.7 | 479 | 50.7 ± 1.6 | 28.5 ± 0.3 | 21.9 ± 1.4 | 40.5 ± 1.1 | 22.8 ± 0.5 | 39.1 ± 1.7 | 47.8 ± 1.5 | |
1 RT: retention time. 2 m/z: mass-to-charge ratio. 3 Quantified using procyanidin B1 standard. 4 Quantified using procyanidin A2 standard. 5 Quantified using quercetin-3-O-galactoside standard. 6 HMG: 3-hydroxy-3-methylglutaroyl. 7 Quantified using cyanidin-3-O-glucoside standard. The moisture content of the berry samples ranged between 80.1% and 87.5% (see Table S2 in Supplementary Materials).
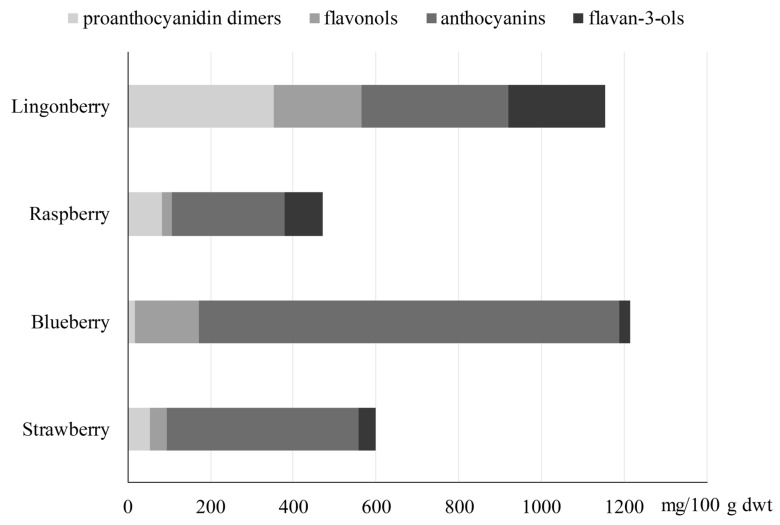
The average content of proanthocyanidin dimers, flavonols, anthocyanins, and flavan-3-ols in lingonberries, raspberries, blueberries, and strawberries is shown in Figure 3. The tentatively characterized compounds contribute 18%, 29%, 61%, and 67% of the total flavonoid content in strawberries, lingonberries, raspberries, and blueberries, respectively. Blueberries and lingonberries contained the highest amount of flavonoids, and anthocyanins were the dominant flavonoids in all berry samples, ranging from 31% to 84%.
Figure 3.
Mean content (mg/100 g dwt) of four subgroups (proanthocyanidins, flavonols, anthocyanins, and flavan-3-ols) of flavonoids in different varieties of lingonberry (n = 2), raspberry (n = 3), blueberry (n = 4), and strawberry (n = 7). Analyses were carried out in triplicate.
In lingonberries, a total of three anthocyanins, seven flavonols, two flavan-3-ols, and four proanthocyanidins were quantified (Table 2 and Table 3). Cyanidin-3-O-galactoside was found to be the most abundant anthocyanin (240–310 mg/100 g dwt) in wild lingonberries, followed by cyanidin-3-O-arabinoside (40–80 mg/100 g dwt) and cyanidin-3-O-glucoside (10–30 mg/100 g dwt) (Table 2). This is in agreement with Latti et al. [38], who reported content in lingonberries of these three compounds of 267, 57, and 15 mg/100 g dwt, respectively. The content of major flavonols, quercetin-3-O-galactoside and quercetin-3-O-rhamnoside detected in lingonberry L2 also agreed with the value reported for lingonberry var. Amberland (60 mg/100 g dwt) [39]. Hellstrom and Mattila [40] reported the presence of flavan-3-ols (14 mg/100 g fresh weight (fwt)), proanthocyanidin dimers (29 mg/100 g fwt), and other proanthocyanidins in lingonberries, which was also confirmed by our findings on flavan-3-ols and proanthocyanidin dimers in lingonberry sample L1.
Five anthocyanins, two flavonols, two flavan-3-ols, and one B-type proanthocyanidin were quantified in the raspberry samples (Table 2 and Table 3), and the dominant anthocyanin was tentatively identified as cyanidin-3-O-sophoroside confirming finding by others [36,41]. Cyanidin-3-glucosylrutinoside and pelargonidin-3-O-rutinoside were only found in Glen Ample in agreement with Sparzak et al. [42], who found differences in polyphenol profile in 11 varieties of red raspberries. Furthermore, the amount of cyanidin-3-O-glucoside in var. Glen Ample (Table 2) was in agreement with previous data (34–60 mg/100 g dwt) [43], as well as (−)-epicatechin [44].
Blueberries contained a greater diversity of flavonoids in various amounts, especially anthocyanin compounds, than other berries (Table 2 and Table 3). For the particular var. Duke, our data on the total anthocyanin content agree with the reported value of 1000 mg/100 g dwt [24,45].
In strawberries, a total of nine flavonoid compounds, comprising two B-type proanthocyanidins, (+)-catechin, four anthocyanins, and two flavonols, were characterized (Table 2 and Table 3). All varieties had a similar flavonoid profile, but the total content varied greatly from 360–750 mg/100 g dwt. The dominant anthocyanin was confirmed to be pelargonidin-3-O-glucoside, but there was an almost four-fold (180–730 mg/100 g dwt) variation between varieties. The dominant flavonol was tentatively identified as quercetin-3-O-glucuronide, confirming previous findings [15,28,46]. Wang et al. [46] studied flavonoids in 14 cultivars of strawberry and found the content of anthocyanins to be 450–1000 μg/g fwt, which was confirmed by our results (220–770 mg/100 g dwt, corresponding to 410–1300 μg/g fwt). Other flavonoid compounds (e.g., kaempferol-3-O-malonylglucoside) have been reported [15,28] but were not detected in our samples, probably due to differences in the varieties tested.
4. Summary
A method enabling the analysis of 45 flavonoid compounds in berry matrices was established. Sixteen flavonoid compounds were quantified with high linearity, precision, and average recovery using external standards. An additional 29 compounds were tentatively identified and quantified using MS. The method was applied for analysis of 16 varieties of Swedish berries.
Both, flavonoid content and pattern were largely dependent on species, but also variety. Blueberries and lingonberries were found to contain 1100 mg/100 g dwt of flavonoids, which is almost twice the content of raspberries and strawberries. Anthocyanins were the dominant flavonoids in all berries. Data should be considered as indicative bearing in mind the limited number of samples and lacking information of postharvest handling.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. A Ph.D. scholarship from China Scholarship Council (CSC) for Liu J. is gratefully acknowledged.
Supplementary Materials
The following are available online at https://www.mdpi.com/2304-8158/9/3/358/s1, Figure S1: Effect of formic acid concentration (1–12%) on separation of flavonoids (5 μg/mL) at 360 nm, Figure S2: Effect of solvent on extraction yield, Figure S3: Effect of formic acid concentration in solvent on extraction yield, Table S1: Recovery rate of different flavonoids in lyophilized lingonberries, Table S2: Moisture content in the 16 berry varieties analyzed.
Author Contributions
Conceptualization, J.L., M.E.H. and C.M.W.; Data curation, J.L.; Formal analysis, J.L.; Investigation, J.L.; Methodology, J.L., M.E.H. and C.M.W.; Resources, M.E.H. and C.M.W.; Software, J.L.; Supervision, M.E.H. and C.M.W.; Validation, J.L.; Visualization, J.L.; Writing—original draft, J.L.; Writing—review and editing, J.L., M.E.H. and C.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Singh J.P., Kaur A., Singh N., Nim L., Shevkani K., Kaur H., Arora D.S. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci. Technol. 2016;65:1025–1030. doi: 10.1016/j.lwt.2015.09.038. [DOI] [Google Scholar]
- 2.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 3.Xiao J.B. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017;57:1874–1905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- 4.Lei F., Zhang X.N., Wang W., Xing D.M., Xie W.D., Su H., Du L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007;31:1023–1029. doi: 10.1038/sj.ijo.0803502. [DOI] [PubMed] [Google Scholar]
- 5.Jennings A., Welch A.A., Spector T., Macgregor A., Cassidy A. Intakes of Anthocyanins and Flavones Are Associated with Biomarkers of insulin Resistance and Inflammation in Women. J. Nutr. 2014;144:202–208. doi: 10.3945/jn.113.184358. [DOI] [PubMed] [Google Scholar]
- 6.Hwang S.L., Shih P.H., Yen G.C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012;60:877–885. doi: 10.1021/jf204452y. [DOI] [PubMed] [Google Scholar]
- 7.Kruger M.J., Davies N., Myburgh K.H., Lecour S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014;59:41–52. doi: 10.1016/j.foodres.2014.01.046. [DOI] [Google Scholar]
- 8.Glover B.J., Martin C. Anthocyanins. Curr. Biol. 2012;22:R147–R150. doi: 10.1016/j.cub.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Ou K.Q., Gu L.W. Absorption and metabolism of proanthocyanidins. J. Funct. Foods. 2014;7:43–53. doi: 10.1016/j.jff.2013.08.004. [DOI] [Google Scholar]
- 10.Sa R.R., Caldas J.D., Santana D.D., Lopes M.V., dos Santos W.N.L., Korn M.G.A., Santos A.D. Multielementar/centesimal composition and determination of bioactive phenolics in dried fruits and capsules containing Goji berries (Lycium barbarum L.) Food Chem. 2019;273:15–23. doi: 10.1016/j.foodchem.2018.05.124. [DOI] [PubMed] [Google Scholar]
- 11.Kylli P., Nohynek L., Puupponen-Pimia R., Westerlund-Wikstrom B., McDougall G., Stewart D., Heinonen M. Rowanberry Phenolics: Compositional Analysis and Bioactivities. J. Agric. Food Chem. 2010;58:11985–11992. doi: 10.1021/jf102739v. [DOI] [PubMed] [Google Scholar]
- 12.de Souza D.R., Willems J.L., Low N.H. Phenolic composition and antioxidant activities of saskatoon berry fruit and pomace. Food Chem. 2019;290:168–177. doi: 10.1016/j.foodchem.2019.03.077. [DOI] [PubMed] [Google Scholar]
- 13.Tian Y., Liimatainen J., Alanne A.L., Lindstedt A., Liu P.Z., Sinkkonen J., Kallio H., Yang B.R. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017;220:266–281. doi: 10.1016/j.foodchem.2016.09.145. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Salas P., Morales-Soto A., Segura-Carretero A., Fernandez-Gutierrez A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules. 2010;15:8813–8826. doi: 10.3390/molecules15128813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajdzanoska M., Petreska J., Stefova M. Comparison of Different Extraction Solvent Mixtures for Characterization of Phenolic Compounds in Strawberries. J. Agric. Food Chem. 2011;59:5272–5278. doi: 10.1021/jf2007826. [DOI] [PubMed] [Google Scholar]
- 16.Khoddami A., Wilkes M.A., Roberts T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.León-González A.J., Truchado P., Tomás-Barberán F.A., López-Lázaro M., Barradas M.C.D., Martín-Cordero C. Phenolic acids, flavonols and anthocyanins in Corema album (L.) D. Don berries. J. Food Compos. Anal. 2013;29:58–63. doi: 10.1016/j.jfca.2012.10.003. [DOI] [Google Scholar]
- 18.Huie C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002;373:23–30. doi: 10.1007/s00216-002-1265-3. [DOI] [PubMed] [Google Scholar]
- 19.Kalia K., Sharma K., Singh H.P., Singh B. Effects of Extraction Methods on Phenolic Contents and Antioxidant Activity in Aerial Parts of Potentilla atrosanguinea Lodd. and Quantification of Its Phenolic Constituents by RP-HPLC. J. Agric. Food Chem. 2008;56:10129–10134. doi: 10.1021/jf802188b. [DOI] [PubMed] [Google Scholar]
- 20.Pereira G.A., Arruda H.S., de Morais D.R., Eberlin M.N., Pastore G.M. Carbohydrates, volatile and phenolic compounds composition, and antioxidant activity of calabura (Muntingia calabura L.) fruit. Food Res. Int. 2018;108:264–273. doi: 10.1016/j.foodres.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Kylli P., Nohynek L., Puupponen-Pimia R., Westerlund-Wikstrom B., Leppanen T., Welling J., Moilanen E., Heinonen M. Lingonberry (Vaccinium vitis-idaea) and European Cranberry (Vaccinium microcarpon) Proanthocyanidins: Isolation, Identification, and Bioactivities. J. Agric. Food Chem. 2011;59:3373–3384. doi: 10.1021/jf104621e. [DOI] [PubMed] [Google Scholar]
- 22.Benvenuti S., Pellati F., Melegari M.A., Bertelli D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004;69:FCT164–FCT169. doi: 10.1111/j.1365-2621.2004.tb13352.x. [DOI] [Google Scholar]
- 23.Kylli P. Ph.D. Thesis. University of Helsinki; Helsinki, Finland: 2010. Berry Phenolics: Isolation, Analysis, Identification, and Antioxidant Properties. [Google Scholar]
- 24.Bunea A., Rugina D.O., Pintea A.M., Sconta Z., Bunea C.I., Socaciu C. Comparative Polyphenolic Content and Antioxidant Activities of Some Wild and Cultivated Blueberries from Romania. Not. Bot. Horti Agrobot. 2011;39:70–76. doi: 10.15835/nbha3926265. [DOI] [Google Scholar]
- 25.Ek S., Kartimo H., Mattila S., Tolonen A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea) J. Agric. Food Chem. 2006;54:9834–9842. doi: 10.1021/jf0623687. [DOI] [PubMed] [Google Scholar]
- 26.Latti A.K., Riihinen K.R., Kainulainen P.S. Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008;56:190–196. doi: 10.1021/jf072857m. [DOI] [PubMed] [Google Scholar]
- 27.Vagiri M., Ekholm A., Andersson S.C., Johansson E., Rumpunen K. An Optimized Method for Analysis of Phenolic Compounds in Buds, Leaves, and Fruits of Black Currant (Ribes nigrum L.) J. Agric. Food Chem. 2012;60:10501–10510. doi: 10.1021/jf303398z. [DOI] [PubMed] [Google Scholar]
- 28.Aaby K., Ekeberg D., Skrede G. Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007;55:4395–4406. doi: 10.1021/jf0702592. [DOI] [PubMed] [Google Scholar]
- 29.Magalhaes S.C.Q., Taveira M., Cabrita A.R.J., Fonseca A.J.M., Valentao P., Andrade P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017;215:177–184. doi: 10.1016/j.foodchem.2016.07.152. [DOI] [PubMed] [Google Scholar]
- 30.Mirali M., Ambrose S.J., Wood S.A., Vandenberg A., Purves R.W. Development of a fast extraction method and optimization of liquid chromatography-mass spectrometry for the analysis of phenolic compounds in lentil seed coats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;969:149–161. doi: 10.1016/j.jchromb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Viguera C., Zafrilla P., Tomas-Barberan F.A. The use of acetone as an extraction solvent for anthocyanins from strawberry fruit. Phytochem. Anal. 1998;9:274–277. doi: 10.1002/(SICI)1099-1565(199811/12)9:6<274::AID-PCA416>3.0.CO;2-G. [DOI] [Google Scholar]
- 32.Bakker J., Timberlake C.F. The Distribution of Anthocyanins in Grape Skin Extracts of Port Wine Cultivars as Determined by High-Performance Liquid-Chromatography. J. Sci. Food Agric. 1985;36:1315–1324. doi: 10.1002/jsfa.2740361217. [DOI] [Google Scholar]
- 33.Revilla E., Ryan J.M., Martin-Ortega G. Comparison of several procedures used for the extraction of anthocyanins from red grapes. J. Agric. Food Chem. 1998;46:4592–4597. doi: 10.1021/jf9804692. [DOI] [Google Scholar]
- 34.Hajazimi E., Landberg R., Zamaratskaia G. Simultaneous determination of flavonols and phenolic acids by HPLC-CoulArray in berries common in the Nordic diet. LWT-Food Sci. Technol. 2016;74:128–134. doi: 10.1016/j.lwt.2016.07.034. [DOI] [Google Scholar]
- 35.Cho M.J., Howard L.R., Prior R.L., Clark J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004;84:1771–1782. doi: 10.1002/jsfa.1885. [DOI] [Google Scholar]
- 36.Maatta-Riihinen K.R., Kamal-Eldin A., Torronen A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (Family Rosaceae) J. Agric. Food Chem. 2004;52:6178–6187. doi: 10.1021/jf049450r. [DOI] [PubMed] [Google Scholar]
- 37.Mullen W., McGinn J., Lean M.E.J., MacLean M.R., Gardner P., Duthie G.G., Yokota T., Crozier A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002;50:5191–5196. doi: 10.1021/jf020140n. [DOI] [PubMed] [Google Scholar]
- 38.Latti A.K., Riihinen K.R., Jaakola L. Phenolic compounds in berries and flowers of a natural hybrid between bilberry and lingonberry (Vaccinium x intermedium Ruthe) Phytochemistry. 2011;72:810–815. doi: 10.1016/j.phytochem.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Zheng W., Wang S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003;51:502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
- 40.Hellstrom J.K., Mattila P.H. HPLC determination of extractable and unextractable proanthocyanidins in plant materials. J. Agric. Food Chem. 2008;56:7617–7624. doi: 10.1021/jf801336s. [DOI] [PubMed] [Google Scholar]
- 41.Fang J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition. 2015;31:1301–1306. doi: 10.1016/j.nut.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Sparzak B., Merino-Arevalo M., Vander Heyden Y., Krauze-Baranowska M., Majdan M., Fecka I., Glod D., Baczek T. HPLC analysis of polyphenols in the fruits of Rubus idaeus L. (Rosaceae) Nat. Prod. Res. 2010;24:1811–1822. doi: 10.1080/14786411003754231. [DOI] [PubMed] [Google Scholar]
- 43.Mazur S.P., Sonsteby A., Nes A., Wold A.B., Foito A., Freitag S., Verrall S., Stewart D., Heide O.M. Effects of Post-Flowering Environmental Variation along an Altitudinal Gradient on Chemical Composition of ‘Glen Ample’ Red Raspberry (Rubus idaeus L.) Eur. J. Hortic. Sci. 2014;79:267–277. [Google Scholar]
- 44.Arts I.C.W., van de Putte B., Hollman P.C.H. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000;48:1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 45.Ochmian I., Kozos K., Chelpinski P., Szczepanek M. Comparison of berry quality in highbush blueberry cultivars grown according to conventional and organic methods. Turk. J. Agric. For. 2015;39:174–181. doi: 10.3906/tar-1404-18. [DOI] [Google Scholar]
- 46.Wang S.Y., Zheng W., Galletta G.J. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agric. Food Chem. 2002;50:6534–6542. doi: 10.1021/jf020614i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.