Abstract
Salmonella genus represents the most common foodborne pathogens causing morbidity, mortality, and burden of disease in all regions of the world. The introduction of antimicrobial agents and Salmonella-specific phages has been considered as an effective intervention strategy to reduce Salmonella contamination. However, data from the United States, European countries, and low- and middle-income countries indicate that Salmonella cases are still a commonly encountered cause of bacterial foodborne diseases globally. The control programs have not been successful and even led to the emergence of some multidrug-resistant Salmonella strains. It is known that the host immune system is able to effectively prevent microbial invasion and eliminate microorganisms. However, Salmonella has evolved mechanisms of resisting host physical barriers and inhibiting subsequent activation of immune response through their virulence factors. There has been a high interest in understanding how Salmonella interacts with the host. Therefore, in the present review, we characterize the functions of Salmonella virulence genes and particularly focus on the mechanisms of immune escape in light of evidence from the emerging mainstream literature.
Keywords: Salmonella, virulence, immune escape, immune response
1. Introduction
Salmonella is a flagellated rod-shaped Gram-negative facultative anaerobe which infects multiple animal hosts including humans by contaminating a wide variety of foods [1,2,3,4]. Salmonella enterica (S. enterica) is regarded as the most pathogenic species and includes > 2600 serovars characterized until now [5]. With regard to human diseases, Salmonella is divided into two groups: Typhoidal serotypes and thousands of non-typhoidal Salmonella serotypes (NTS). Typhoidal serovars causing typhoid fever include Salmonella enterica serovar Typhi (S. Typhi), Paratyphi (S. Paratyphi), and Sendai (S. Sendai) [6,7]. The most common NTS are serovars Typhimurium (S. Typhimurium), Enteritidis (S. Enteritidis), and Dublin (S. Dublin) [8]. Infection with NTS ordinarily results in gastroenteritis, diarrhea, and fever (almost always present), with a low case fatality [9,10]. In addition to diarrheal disease, non-typhoidal Salmonella infections can invade normally sterile sites, resulting in bacteremia, meningitis, and other focal infections [11,12]. The invasive non-typhoidal Salmonella (iNTS) disease is usually characterized by the presence of the nonspecific fever similar to malaria and other febrile illnesses, resulting in clinically indistinguishable and higher case fatality than the non-invasive infections [11,13]. Different serotypes of Salmonella have different hosts, food sources, and pathogenesis, making their control challenging and complicated serotypes [14,15].
S. Typhi, S. Paratyphi, and S. Sendai are all human restricted [16,17,18]. Following ingestion and overcoming the resident microbiota, Salmonella initially colonizes the distal part of the small intestine [19]. Typhoidal Salmonella (TS) possesses specific virulence factors including typhoid toxin and virulence capsular polysaccharide (Vi antigen) that are involved in the development of symptoms and immune evasion [20,21]. The bacteria invade the intestinal mucosa, potentially through microfold (M) cells, and disseminate to the lymphatics and blood stream via phagocytes and ultimately spread to the spleen and liver [22,23,24]. These pathogens are invasive but do not normally trigger a rapid inflammatory response. Following recovery, some of the infected individuals are likely to become chronic carriers [25]. Typhoid infections are traditionally treated with ampicillin, chloramphenicol, and fluoroquinolones. However, physicians began moving away from commonly prescribed antibiotics due to an increased prevalence of multidrug-resistant (MDR) strains of S. Typhi. The transfer of antimicrobial resistance (AMR) genes between bacteria is commonly facilitated by plasmid or transposon exchange [26]. The AMR genes are generally associated with an IncHI1 plasmid which harbors a composite transposon that can carry multiple resistance genes [26,27].
Unlike TS, NTS has a broad host range. The infections caused by NTS are usually self-limiting and do not proceed beyond the lamina propria, but some iNTS have evolved a number of virulence genes which allow them to invade the intestinal mucosa and proliferate in phagocytes [28,29,30,31,32]. Both NTS and TS rely on two Salmonella pathogenicity islands (SPI) encoded type III secretion systems (T3SS), i.e., T3SS1 and T3SS2, which are essential for Salmonella invasion and dissemination [33]. Shortly after invasion, bacteria spread to systemic sites and cause systemic infection [34]. Fluoroquinolones, chloramphenicol, and oxytetracycline are commonly used to treat NTS infections. NTS develop the drug resistance by plasmids or integrons for destroying the activity of antibacterial drugs [35,36]. Point mutations within certain genes in S. Typhimurium have been identified as a potential cause of drug resistance [37]. Thus, prevention efforts are needed to reduce an unnecessary antimicrobial use in patient care settings and in food animals to help prevent the emergence of the resistance and infections with resistant NTS.
Difficulty in treating Salmonella infections is gradually increasing, and it has now become necessary to develop new treatment strategies. Vaccine development is a potential prospect for Salmonella control. This is particularly relevant given that the few licensed vaccines so far have targeted S. Typhi in people [38]. In essence, the ability to survive and replicate within the host phagocytes largely determines whether Salmonella can disseminate from the colonization site (intestines) to establish a systemic infection. Therefore, focused studies on how Salmonella escapes from host immunity and survives longer periods in short-lived and mobile myeloid cells will add a great value to our understanding of the host-pathogen interactions. In this review, by focusing on enticing findings of past and present studies, we briefly describe the mechanisms used by Salmonella to escape the innate and specific immunity to disseminate and establish infections.
2. Origin, Classification, and Diseases Caused by Salmonella
Since the first Kauffmann-White serotype scheme based on surface molecular antigen variation was published in 1934, serotyping has become the most important tool for identifying and classifying the Salmonella strains for more than 80 years [39,40]. Since 120 to 160 million years, Salmonella has evolved into a complex group of phenotypically diverse serovars. More than 2600 serotypes have been discovered since 1885 alone, resulting in antigenically distinct variants which are pathogenic in more than 100 species including mammals, birds, reptiles, and insects [5,41]. Salmonella genus is divided into two species, i.e., S. enterica and Salmonella bongori [42,43,44]. S. enterica is further classified into seven subspecies, i.e., enterica (I), salamae (II), arizonae (IIIa), diarizonae (IIIb), indica (IV), houtenae (VI), and several serovars previously assigned to the group IV (VII) based on biochemical and genomic modifications [41]. These subspecies are further classified into more than 50 serotypes based on O (somatic) antigen, and into multiple serotypes based on H (flagella) antigens [45]. Intriguingly, strains belonging to the subspecies I cause 99% of human cases of salmonellosis [46,47]. Meanwhile, S. enterica subspecies II, IIIa, IIIb, IV, VI, and S. bongori are usually isolated from cold-blooded animal species and environments but rarely from humans [41]. Recently, it has been proposed that high-throughput DNA sequencing can open a gateway to Salmonella serotyping and can improve our understanding regarding strains of public health relevance [48]. Whole-genome and metagenome sequence data permit the continuation of traditional serovar nomenclature and enhance the ability to infer true phylogenetic relationships between isolates [49].
The major clinical syndromes caused by Salmonella infection in humans are divided into typhoid fever that is predominantly caused by S. Typhi, S. Paratyphi, and S. Sendai [6,7,8], and a range of clinical syndromes including diarrheal disease caused by NTS. Typhoid is a human-restricted and highly adapted invasive disease, while NTS has a wide range of vertebrate hosts and more severe and invasive presentation in immunocompromised adults [50].
Typhoid fever remains a predominant enteric fever worldwide; meanwhile, an increasing incidence of enteric fever caused by S. Paratyphi A is also reported [6,51]. A principle difference between S. Typhi and other strains is the presence of Vi antigen. The Vi antigen is considered to be a virulence factor of S. Typhi, which modulates the different pro-inflammatory signaling pathways and allows S. Typhi to survive and replicate in the host cells, particularly the phagocytes [52]. S. Typhi uses these cells to disseminate to systemic sites of the body, such as the liver, spleen, and bone marrow. It is estimated that 5% of infected individuals will not be able to clear the infection within one year and enter a chronic carrier state where bacteria mainly reside in hepatobiliary tract and gallbladder, and thus increase the risk of cancer development [25,53,54].
NTS is an acute gastroenteritis typically acquired orally through contaminated water, fruits, seafood, vegetables, and meat, especially poultry. Following ingestion through contaminated food or water, its incubation period can vary from 4 to 72 h, and acute symptoms, such as fever, chills, nausea and vomiting, abdominal cramps, and diarrhea can be observed [55,56]. Available data demonstrate that there are estimated 1.3 billion cases of gastroenteritis caused by Salmonella, leading to approximately three million deaths worldwide per year [57,58]. Due to the lack of clean water supplies and proper sanitation, mortality caused by NTS gastroenteritis is mainly observed in the developing countries, but it is also of a considerable importance in the developed countries [58,59]. There are hundreds of NTS serovars that may cause invasive NTS human disease with a varying invasive virulence [60,61]. iNTS disease is caused mainly by S. Typhimurium, S. Enteritidis, and S. Dublin [8]. The iNTS disease burden in Africa is especially caused due to urbanization with large populations living in crowded and insanitary conditions with poor access to potable water [62,63,64]. iNTS is more common amongst people with an impaired immunity, and typically represents a febrile systemic illness and lower respiratory tract disease, commonly attributable to co-infections with HIV or malaria [50,60,65,66].
3. The Virulence-Related Genes of SPI
The specific regions encoding the virulence-related genes distributed in a cluster of Salmonella chromosomes and plasmids are called SPI. To date, 23 SPIs have been identified and characterized [33,67,68]. The five of these, i.e., SPIs-1–5 are common to all serotypes of Salmonella [69,70,71,72,73,74], whereas SPIs-19–23 are absent in both S. Typhi and S. Typhimurium, and are only present in a few S. enterica serovars including Dublin, Gallinarum, and Derby [67,68], and hence will not be discussed here. From SPIs-1–18, only SPI-1, 4, 9, 14, and 18 encoded effectors play an important role in Salmonella invasion into macrophages and epithelial cells. The virulence effectors secreted by SPI-2, 3, 5–8, 10–13, and 16 are implicated in helping Salmonella withstand the acidic environment, accomplishing the intracellular replication, and immune escape from the host. SPI-1 and SPI-2 contain a large number of virulence genes associated with the intracellular pathogenesis and co-encode T3SS, a molecular syringe which transfers the effectors from the bacteria into the host cell cytoplasm, and in turn, the effector manipulates allowing for bacterial invasion and replication in the host cells [75,76,77,78]. To date, over 40 SPI-1 and SPI-2 effectors have been identified in S. Typhimurium, S. Typhi, and S. Paratyphi A (Table 1) [17]. All serovars seem to have a set of “core” effectors, suggesting that they are critical for virulence in different hosts (PipA, PipB, PipB2, SifA, SipA, SipB, SipC, SipD, SopB, SopD, SpiC, SptP, SseF, SseG, SseL, SteA, and SteD). These effectors play diverse roles during infection. In Table 1, we have summarized the major effectors encoded by SPI-1 and SPI-2 in S. Typhimurium, S. Typhi, and S. Paratyphi A, and their functions. Among these 41 effectors identified in S. Typhimurium, 16 are absent in S. Typhi and S. Paratyphi A (AvrA, GogA, GogB, GtgA, GtgE, SlrP, SopA, SopD2, SpvB, SpvC, SpvD, SrfJ, SsaJ, SseJ, SseK2 and SsrA) [17]. Most of these effectors are from SPI-2, indicating that the role of SPI-2 in typhoidal serovars deserves further investigation. This may be related to the broad host range lifestyle of NTS and reflect the host restriction of TS. SopE2, SifB, SsaV, SseB, and SspH2 have a special mention, as they are present in S. Typhi but absent in S. Paratyphi A (Table 1) [17]. These dissimilarities in typhoidal strains may reflect differences between human restricted lifestyle of S. Typhi and S. Paratyphi. SspH2 promotes the colonization of Salmonella in host cells [79]. SopE2 is shown to contribute to generation of a replicative endosomal compartment in enterocytes and facilitate enterocytes invasion in vivo [80]. SpvB interferes with host intracellular iron metabolism via downregulation of nuclear factor erythroid-derived 2-related factor 2 (NRF2) [81]. SifB, a member of Salmonella-induced filaments (SIFs), is necessary for SIF formation and maintaining the integrity of Salmonella-containing vacuoles (SCVs) [82]. However, the potential function of SifB is still unknown [83]. The details regarding effectors are shown in Table 1 and Figure 1.
Table 1.
The functions of SPI-1/2 effectors in S. Typhimurium, S. Typhi, and S. Paratyphi A.
| Effectors | Pathogenicity Island | Function (s) | Key Reference (s) |
|---|---|---|---|
| AvrA | SPI-1/SPI-2 | Stabilizes the intestinal epithelial permeability and tight junctions; cysteine protease; inhibits NF-κB signaling | [84] |
| GogA | SPI-2 | Cleaves the subset of NF-κB subunits; inhibits NF-κB signaling | [85,86] |
| GogB | SPI-2 | Inhibits NF-κB signaling | [85] |
| GtgA | SPI-2 | Inhibits NF-κB signaling | [85] |
| GtgE | SPI-1/SPI-2 | Promotes replication inside murine macrophages | [87] |
| PipA | SPI-2 | Cleaves the subset of NF-κB subunits; inhibits NF-κB signaling | [85] |
| PipB | SPI-2 | Targeted to SIFs | [88] |
| PipB2 | SPI-2 | Resists extraction by high salt, high pH; implicated in recruitment of kinesin-1 to SCV | [89] |
| SifA | SPI-2 | Detoxifies lysosomes; subverts human NLRP3 and NLRC4 inflammasome; required for SCV membrane stability; SIF formation; contributes to T3SS1-independent inflammation | [90] |
| SifB | SPI-2 | Targeted to SIFs | [82,83] |
| SipA | SPI-1 | Enhances actin filament assembly; promotes proliferation of cytosolic Salmonella; disrupts tight junctions; SCV trafficking | [91] |
| SipB | SPI-1 | Cholesterol-binding translocon component; triggers apoptosis via caspase-1 activation in macrophages and DCs | [82] |
| SipC | SPI-1 | Translocon component: mediates effector molecule translocation; promotes actin polymerization and bundling | [92] |
| SipD | SPI-1 | Translocon component | [93] |
| Slrp | SPI-1/SPI-2 | Inhibits the release of IL-1β | [87] |
| SopA | SPI-1 | A HECT-like E3 ubiquitin ligase | [94] |
| SopB | SPI-1 | Modulates SCV trafficking; phosphoinositide phosphatase; involved in phagosomal closure; enhances RhoG activation; disrupts tight junctions; stimulates chloride secretion; prevents apoptosis through activation of Akt | [95] |
| SopD | SPI-1/SPI-2 | SIF formation, prevents accumulation of Rab32 on SCV and SIFs | [87] |
| SopD2 | SPI-2 | Targeted to SIFs and late endosomes | [96] |
| SopE | SPI-1 | Promotes colonization of Salmonella; induces remodeling of actin | [97] |
| SopE2 | SPI-1 | Guanine nucleotide exchange factor for Cdc42; promotes pro-inflammatory signaling | [80] |
| SpiC | SPI-2 | Interferes with vesicular trafficking in host cells to prevent SCV-lysosome fusion | [92] |
| SptP | SPI-1 | Rho GAP domain functions in downregulating host membrane ruffling after entry; tyrosine phosphatase domain acts on ACK; vimentin; and presumably other substrates | [98] |
| SpvB | SPI-2 | Promotes macrophage apoptosis and P-body disassembly | [81,87] |
| SpvC | SPI-1/SPI-2 | Inhibits MAPK signaling | [99] |
| SpvD | SPI-1/SPI-2 | Inhibits NF-κB signaling | [87] |
| SrfJ | SPI-2 | Responses to intracellular conditions | [100] |
| SsaJ | SPI-2 | Prevents the phagocyte NADPH oxidase from trafficking toward SCVs | [101] |
| Ssav | SPI-2 | Prevents the phagocyte NADPH oxidase from trafficking toward SCVs | [102] |
| SseB | SPI-2 | Prevents the phagocyte NADPH oxidase from trafficking toward SCVs | [102] |
| SseF | SPI-2 | Tethers SCV to the Golgi network; contributes to Sif formation; replication of Salmonella in SCV | [87,103] |
| SseG | SPI-2 | Tethers SCV to the Golgi network; contributes to Sif formation; replication of Salmonella in SCV | [87,103] |
| SseJ | SPI-2 | Acyl transferase; cholesterol esterification; SCV membrane dynamics | [87,104] |
| SseK1 | SPI-2 | Inhibits TNFα-stimulated NF-κB signaling | [105] |
| SseK2 | SPI-2 | Related effectors that inhibits NF-κB signaling | [105] |
| SseL | SPI-2 | Inhibits autophagic clearance of cytosolic aggregates; induces late macrophage cell death; inhibits directional migration of macrophages and DCs | [106] |
| SspH2 | SPI-2 | An E3 ubiquitin ligase; activates NOD1 signaling | [79,87] |
| SsrA | SPI-2 | Prevents the phagocyte NADPH oxidase from trafficking toward SCVs | [102] |
| SteA | SPI-1/SPI-2 | SIF formation, vacuolar membrane partitioning | [107] |
| SteC | SPI-2 | Induces assembly of F-actin meshwork around SCV | [108] |
| SteD | SPI-2 | Inhibits antigen presentation and T cell activation | [17] |
NF-κB: Nuclear factor kappa beta; SCV: Salmonella-containing vacuole; NLRP3: the NOD-like receptor family, pyrin domain containing 3; NLRC4: NLR-family CARD-containing protein 4; SIF: Salmonella-induced filament; T3SS1: type III secretion system 1; HECT: homologous to E6-AP carboxy terminus; GAP: GTPase-activating phosphatase; ACK: a Cdc42-associated tyrosine kinase; NADPH: nicotinamide adenine dinucleotide phosphate; TNFα: tumour necrosis factor alpha; DCs: dendritic cells; NOD1: nucleotide-binding oligomerisation domain 1.
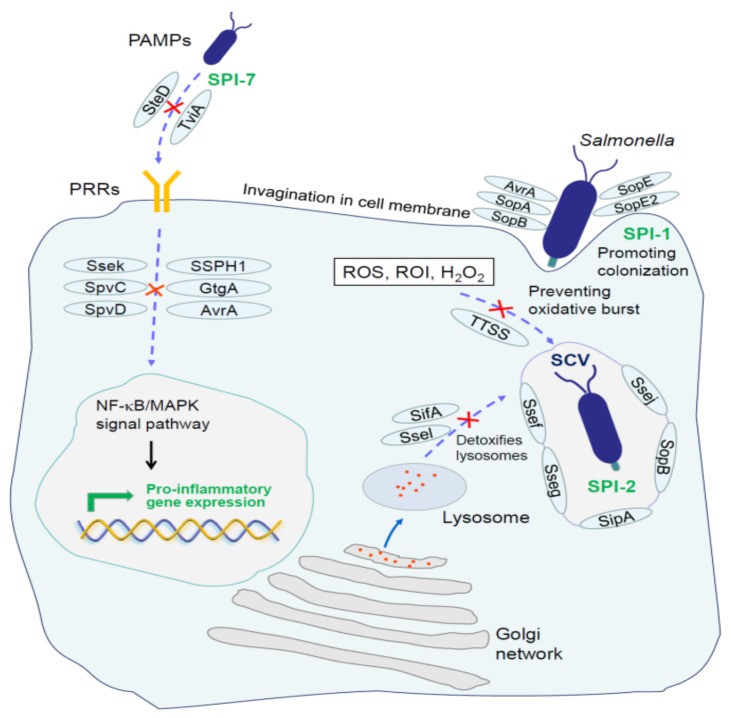
Figure 1.
Role of Salmonella T3SS effectors in epithelial cells and macrophages. SopB, SopD, SopE, and AvrA are essential for membrane invasion during Salmonella infections. SipA, SseJ, SopE2, and SopB are required for biogenesis and correct localization of SCV. SifA and SopD2 contribute to evasion of lysosomal degradation. SPI-7 effector TviA is mainly responsible for masking the surface antigens, leading to the failure of PRRs to recognize them. Several effectors including SseL, GtgA, GogA, PipA, SpvC, and SpvD inhibit the innate immune signaling, and subsequently diminish the production of proinflammatory mediators and result in an inefficient clearance of phagocytized bacteria. Salmonella can also prevent the interaction of NADPH oxidase subunit Cytb558 with SCV and escape from the oxidative burst depends on T3SS. PAMP: Pathogen-associated molecular pattern; PRR: Pattern recognition receptor; ROS: Reactive oxygen species; ROI: Reactive oxygen intermediates; SCV: Salmonella-containing vacuole; SPI: Salmonella pathogenicity islands; NF-κB: Nuclear factor kappa beta; MAPK: Mitogen-activated protein kinase.
SPI-1 is a DNA fragment of around 40 kb with stable genetic traits, and present in all Salmonella. SPI-1 contains the inv, hil, org, spt, spa, sip, iag, iac, prg, sic, and other genes, encoding the regulator and secretory effector proteins of T3SS1. It is worthwhile to mention that not all genes within SPI-1 are associated with the T3SS1, but it has now been demonstrated that at least 29 T3SS1 genes are involved in different encoding functions. The regulators and effectors of T3SS1 are related to Salmonella colonization and invasion into intestinal epithelial cells and lead to necrosis and inflammatory reactions in macrophages [77,109]. Furthermore, these effectors are implicated in regulating the host cell exocytosis, interfering with host signal transduction pathways, and allowing Salmonella localization, survival and proliferation inside the vacuoles [95,110,111]. In addition, four genes, i.e., sit A, sit B, sit C, and sit E, play an important role in full virulence [112].
SPI-2 contains more than 40 genes which constitute four operons. From these, ssa encodes the T3SS2 [101], ssr encodes a secretion system regulator [113], and ssc encodes a molecular chaperone [114,115]. SPI-2-related secretion system T3SS2 delivers more than 20 effectors through the vacuole membrane into the host cytosol [100], playing an essential role during the second stage of host invasion which controls the survival and replication of Salmonella in phagocytes and epithelial cells [116]. At the same time, it allows Salmonella to escape the bactericidal effects of macrophages, and plays an important regulatory role in the progression of systemic infection and intracellular pathogenesis [74,116].
SPI-3, involved in the survival of Salmonella in macrophages, is around 17 kb and contains 10 ORFs constituting six transcription units. The major virulence gene encoded by SPI-3, mgtCB, is a high-affinity Mg2+ uptake system which is required for adaptation to nutritional limitations of the intra-phagosomal habitat [117]. From these, SPI-3 has been implicated in mediating the survival of Salmonella in macrophages and low Mg2+ environments [71].
SPI-4 is a 27 kb region that encodes a type 1 secretion system (T1SS), contributing to the adhesion of Salmonella to epithelial cell surfaces [118,119]. The SPI-4-encoded T1SS consists of five proteins (SiiABCDF) and secretes the giant adhesin SiiE, which is the largest protein in Salmonella, resulting in membrane ruffle formation and uptake of Salmonella [120,121].
SPI-5 is approximately 7 kb and plays a vital role in enteropathogenicity [122]. It encodes at least five genes, i.e., pipA, pipB, pipC, pipD, and sopB. The encoded proteins are related to the intestinal mucosal fluid secretion and inflammatory responses, and are regulated by SPI-1 and SPI-2 T3SS [122,123]. Recent studies on Salmonella have identified additional pathogenicity islands, such as SPI-6-23 [33,124,125,126,127,128,129,130,131,132,133,134]. S. Typhimurium and S. Typhi genomes contain six common SPIs (SPIs-6, 9, 11, 12, 13, and 16). SPI-7, 8, 10, 15, 17, 18 were considered to be present in S. Typhi genome, but absent in S. Typhimurium. SPI-14 is specific to S. Typhimurium [33]. Identification of new islands has improved our understanding regarding the members of Salmonella and their pathogenicity.
SPI-6 is approximately 59 kb and encodes a type 6 secretion system (T6SS) [135]. SPI-6 T6SS contributes to intra-macrophage survival and successful establishment of S. enterica in host gut during infection [136]. The transcriptional repression of the SPI-6 T6SS core component clpV resulted in defective intra-macrophage survival, attenuated virulence, and diminished systemic dissemination [137].
SPI-7 is the largest genomic island around 134 kb in length and encodes important virulence genes, including the major Vi antigen and IVB operon in serovars Typhi, Paratyphi C and some strains of serovar Dublin [130]. These genes benefit bacteria against phagocyte-mediated killing and modulating the innate immune responses [138].
SPI-8 is approximately 6.8 kb region located adjacent to the pheV tRNA gene and encodes a degenerate integrase, two bacteriocin pseudogenes, and intact genes encoding proteins conferring resistance to these bacteriocins [133]. It has been speculated that proteins encoded in SPI-8 could improve bacterial fitness of typhoid serovars in human gut, however, further focused studies are required to support this caveat [133,134].
SPI-9 is about 16 kb island and encodes the type I system that helps in modulation of bacterial adhesion to the epithelial cells similar to the SPI-4 [129]. SPI-10 is approximately 32.8 kb in length, containing the sefB, sefC, sefR, and prpZ genes, which are implicated in the regulation of chaperone protein on mycelia manipulators [125]. From these, prpZ has been implicated in promoting S. Typhi survival in human macrophages [139,140].
SPI-11 includes pagC, pagD, and msgA, which reportedly have important roles related to the survival of S. Typhi in macrophages [141,142]. RaoN, a small RNA encoded within SPI-11, has been shown to be necessary for survival under in vitro stress conditions and contributes to the growth of S. Typhimurium in macrophages [128]. SPI-12, located next to the proL tRNA gene, is approximately 15 kb in S. Typhimurium and 6.3 kb in S. Typhi [33]. Regulation of genes within SPI-12 is conducive to in vivo adaptability [127]. SPI-13 is a 19 kb gene cluster and contributes to the virulence of Salmonella [143]. Recent studies have shown that SPI-13 mediated d-glucuronic acid (DGA) and tyramine (TYR) metabolic pathways can afford nutritional fitness to Salmonella Enteritidis (S. Enteritidis) [143].
SPI-14 is approximately 9 kb and specific to S. Typhimurium. LoiA, a novel virulence-regulating protein encoded in SPI-14, has been shown to be induced under low oxygen conditions and can enhance the ability of S. Typhimurium to invade host epithelial cells [144,145]. SPI-15, 16, and 17 were identified by bioinformatics in 2006 [146]. Studies on these islands are still very limited. SPI-15 is 6.5 kb, inserted near glyU tRNA, and is only present in S. Typhi, and absent in S. Typhimurium [146]. SPI-16 is found in S. Typhimurium and S. Typhi as a 4.5 kb fragment inserted next to argU tRNA. It is required for intestinal persistence of S. Typhimurium in mice [147]. Comparatively, SPI-17 is 5.1 kb long, and inserted in argW tRNA encoding six open reading frames (ORFs) [146]. SPI-18 harbors two ORFs organized into an operon, hlyE and taiA genes, and both are implicated in virulence. TaiA is a novel invasin involved in an increased phagocytosis of S. Typhi by macrophages [148]. HlyE presents a complex regulation network which participates in different stages of infective process. It affects the Ca2+ homeostasis in epithelial cells by induction of slow, intracellular Ca2+ oscillations to control S. Typhi growth in cells [149].
4. Molecular Mechanisms of Salmonella Immune Escape
In healthy individuals, the host body can recognize and clear pathogens through the innate and acquired immunity by a strong host immune response. However, invasive Salmonella can evade the immune surveillance using the sophisticated strategies, and could replicate, survive, and cause the persistent bacterial infections in hosts without even exhibiting the typical clinical symptoms [150]. For example, it has been reported that, in certain cases, patients with typhoid fever may carry bacteria in their gallbladder for the rest of their lives [32]. In general, such infections do not show clinical symptoms, but are a potential threat to the host. These asymptomatic carriers presumably act as reservoirs for a diverse range of S. Typhi strains and may act as a breeding ground for new genotypes [25]. It has been reported that S. Typhi chronic infection facilitates the gallbladder cancer development in humans [151]. S. Typhimurium involved in the persistent infections is also difficult to eliminate, and infected patients often continue shedding these pathogens in the environment, resulting in disease transmission [25,32].
4.1. Escape of Innate Immune System
The innate immune system provides the first line of defense against invading microorganisms by inducing a variety of inflammatory and antimicrobial responses. It is also particularly important in the gastrointestinal tract, where Salmonella is first colonized, to resist against a large variety of pathogenic microorganisms. However, it is not surprising that Salmonella has evolved strategies to overcome and adapt to an inflammatory environment. Intestinal epithelial cells are a primary cellular barrier of the gut and critical for nutrient uptake [152]. The epithelial cells form a continuous intact physical epithelial barrier with interspersing tight junctions (TJs) between each cell. Salmonella may disrupt the TJs structure through SPI-1-secreted effectors resulting in an increased permeability to luminal antigens, degrading the mucosal barrier function [153]. Intestinal microflora play a crucial role in the host defense, and oral probiotics have been shown to increase intestinal antimicrobial activity and paneth cells, which are the main intestinal cells responsible for the production of immunoreactive antimicrobial peptide (AMP) [154]. This peptide helps stabilize the intestinal barrier, while promoting the stability of intestinal microbial flora. Musca domestica cecropin and JH-3 (an analog of hemoglobin peptide P3), as the novel AMPs, were recently found to have an obvious inhibitive effect on S. Typhimurium [155,156]. However, the presence of host AMPs activates the PbgA which is required to maintain PhoPQ system of S. Typhimurium, promoting remodeling of outer membrane and resistance to innate immune AMPs [157]. The transcytosis of Salmonella across the gut epithelium by M cells is important for the induction of efficient immune responses to mucosal antigens in the Peyer’s patches [158]. M cells function as the antigen-sampling cells, selectively transporting Salmonella antigens and delivering the latter to the underlying lymphoid tissues where protective immune responses are initiated [22,159]. Paradoxically, Salmonella exploit M cells as a route for the host invasion. Both S. Typhi [160] and S. Typhimurium [161] selectively target and invade M cells through SPI-1.
During Salmonella invasion of the host cells, its surface pathogen-associated molecular patterns (PAMPs) are recognized by the host cell pattern recognition receptors (PRRs) [152]. The PAMPs which are significantly expressed by Salmonella include: Lipoprotein, curli amyloid fibrils, lipopolysaccharide (LPS), flagellin, and CpG DNA, which are recognized by PRRs. In addition to identifying the PAMPs, PRRs can also recognize the “danger-associated molecular patterns” (DAMPs). During an invasive Salmonella infection, innate immune responses are initiated by PAMPs and DAMPs, leading to the activation and recruitment of neutrophils and macrophages.
Extensively studied PRRs include the Toll-like receptors (TLRs) and NOD-like receptors (NLRs) [162,163,164]. TLRs recognize Salmonella on the cell surface and in endosomes, whereas NLRs detect Salmonella components in the cytosol. In an early stage of Salmonella infection, recognition of ligands by TLRs increases the bactericidal activity of local tissue macrophages, induces the maturation and migration of dendritic cells, and initiates the production of inflammatory cytokines and chemokines [165]. Curli amyloid fibrils are recognized by the TLR2/TLR1 heterodimer complex. It was shown that inability to produce curli fibrils will markedly reduce the ability of HeLa cells to respond to stimulation with intact S. Typhimurium [166]. Moreover, epithelial cells augment the barrier function via recognizing S. Typhimurium curli fibers in the gut by activating TLR2/phosphatidylinositol 3-kinase (PI3K) pathway [167]. In addition to curli fibrils, intact Salmonella contain triacyl lipoproteins that also stimulate responses through the TLR2 receptor [166,168]. TLR4 directly recognizes LPS, one of the main components of Salmonella’s outer membrane, promotes proinflammatory cytokine production, and phagocytic cell recruitment [169]. It is known that LPS is not homogeneous [170]. Additionally, studies have found that structural and chain length differences in LPS between serotypes of Salmonella are sufficient to drive different host immune responses [171,172,173]. S. Typhimurium uses PbgA and PmrA/Pmrb system to influence LPS assembly and drive variable host Type I IFN responses for their survival in various ecological niches [157,173,174,175]. The flagellin and non-methylated CpG sequence in Salmonella DNA are easily recognized by TLR5 [176] and TLR9 [177,178], respectively. Following ligand binding, TLRs engage the signaling adaptors MyD88 and TRIF, which are recruited in the C-terminal domain of TLRs. This recruitment initiates the downstream signaling and subsequently induces the host cells to produce inflammatory factors (interleukin-8, interleukin-10, interferon-α, and others), causing an infiltration of neutrophils to the site of infection and thereby producing an inflammatory response [179]. However, it has been demonstrated that S. Typhi can prevent neutrophil recruitment in the intestinal mucosa by masking its surface antigens with SPI-7 and interfering with TLRs [138,180,181]. Moreover, a SPI-7-encoded regulatory protein TviA can reduce TLR5-mediated inflammatory responses by controlling capsule expression and flagellar movement (Figure 1) [182,183]. These evidences indicate that the encoding genes locus SPI-7 in S. Typhi is a necessary factor for escaping the host inflammatory reactions. Capsules in S. Typhimurium are wrapped around LPS, which also prevents the inflammatory response induced by TLR4 recognition [181]. Even if TLRs successfully identify the PAMPs, Salmonella SPI-2 encoded proteins, i.e., SseL, SpvD, PipA, GogA, GtgA, SpvC, can inhibit the nuclear factor kappa beta (NF-κB), extracellular signal-regulated kinase (Erk), and mitogen-activated protein kinase (MAPK) activation, thus suppressing the transcriptional responses leading to inflammation (Figure 1) [85,106,184,185,186]. SseL acts as a deubiquitinase and prevents the ubiquitination of IkB-α. It results in the inability of IκB-α to dissociate from NF-κB, leaving NF-κB in an inactive state [106]. SpvD targets the NF-κB pathway by interfering with nuclear translocation of p65 [184]. PipA, GogA, and GtgA redundantly target components of NF-κB signaling pathway to inhibit transcriptional responses leading to inflammation [86]. SpvC removes phosphate groups of Erk and p38 MAPKs by phosphothreonine lyase to interfere with the downstream signaling pathways [185,186]. SseK suppresses TNF-α-induced, but not TLR-induced NF-κB, activation and cell death during macrophage infection [105]. Moreover, the effector AvrA transcribed by SPI-1 is able to stabilize the intestinal epithelial permeability and tight junctions of intestinal epithelial cells to mitigate a destructive effect produced by other SPI-1 effectors (i.e., SopB, SopD, SopE, and SopE2) (Figure 1). It was shown that disintegration of tight junctions in the intestinal epithelial cells could enhance the intestinal inflammatory responses. Thus, Salmonella can also avoid the host inflammatory responses through AvrA [84].
Moreover, Salmonella can trigger their own phagocytosis by macrophages [187,188,189,190] and become encapsulated in SCV. The effector SipA, SseJ, SopE2, and SopB are required for biogenesis and correct localization of SCV [80]. SipA provides functional continuity between forced bacterial entry and the intracellular replicative niche by priming the SCV, and the localization of SseJ maintains the membrane integrity and stability of SCV [91,104]. SopB is essential for efficient cytosolic proliferation of Salmonella (Figure 1) [191]. Once Salmonella become established within SCV, they become hidden from many extracellular detection mechanisms. SseF and SseG anchor SCV at the Golgi network and remain in this region during first few rounds of bacterial replication, forming a clustered microcolony of vacuoles (Figure 1) [192]. However, macrophages have evolved NLRs that can recognize the presence of PAMPs in the cytosol [164]. Upon binding to the ligand, the NLRs initiate different signaling cascades. NOD1 and NOD2 interact with a common adaptor protein called receptor-interacting protein 2 (RIP2) to mediate an efficient clearance of Salmonella from mucosal tissue [193,194]. Inflammasome assembly is usually triggered by the cytosolic NLRs which sense dangerous signals. It consists of NLRs, the adaptor proteins apoptosis-associated speck-like protein containing a CARD (ASC) and the effector molecules caspase-1, resulting in caspase-dependent secretion of mature pro-inflammatory cytokines IL-1β, IL-18, and pyroptotic cell death [195,196]. Mouse NLR apoptosis inhibitory protein (NAIP2) and human NAIP can recognize the S. Typhimurium T3SS inner rod component PrgJ, and NAIP5 can recognize S. Typhimurium flagellin D0 domain to induce NLR family CARD-domain containing protein 4 (NLRC4) phosphorylation and caspase-1 activation [197,198,199]. SCV lysis releases bacterium into the macrophage cytosol, where it is detected by the noncanonical inflammasome and eventually induces the pyroptotic death of the host cell [200,201]. However, SPI-2-mediated T3SS2 secrets effectors into the cytoplasm, and these effectors protect against the harmful environmental factors by regulating the vacuoles and intracellular biochemical reactions to facilitate the survival and replication of Salmonella in SCV [82,83,86,87,89,92]. Studies have demonstrated that human macrophage death and IL-1β production are elicited by S. Typhimurium SPI-1 but suppressed by SPI-2 [87,202]. SPI-2 supports the SPI-1-driven active infection of human macrophages and intra-macrophage bacterial survival [202].
Another potential reason that Salmonella induces its own phagocytosis by macrophages may be to avoid the phagocytic killing by neutrophils. This is also supported by the fact that Salmonella has a limited ability to resist the neutrophil-mediated bactericidal effects. Lysosomes in phagocytic cells contain a variety of hydrolases for combating bacteria. Evading lysozyme degradation is an important strategy for the survival of intracellular bacteria. It has been reported that SCV can fuse with lysosomes [203,204]. Interestingly, a Salmonella effector SifA, which is required to maintain the SCV membrane, has the ability to reduce the lysosomal enzyme activity (Figure 1) [90]. In addition to SifA, Salmonella also uses SopD2 to interfere with endosome-to-lysosome trafficking (Figure 1) [96]. Therefore, in order to efficiently kill pathogens in SCV, host cells are required to generate a stronger bactericidal environment.
Oxidative bursting catalyzed by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is induced by phagocytic cells to produce a large number of reactive oxygen intermediates (ROI), such as O2- and H2O2, which are converted into a strong oxidant hypochloric acid and rapidly kill Salmonella [205]. However, Salmonella depends on SPI-2 effectors, i.e., SseB, SsrA, SsaJ, and Ssav for preventing an interaction of NADPH oxidase subunit Cytb558 with SCV to avoid the oxidative burst (Figure 2) [102,206]. In addition, Salmonella can resist the oxidative killing effect of ROI using catalase, antioxidant proteins, and superoxide dismutase [207]. Reactive nitrogen intermediates (RNI) include nitric oxide and its derivatives, such as nitrososulfur compounds, nitrogen peroxide, etc. Reactive nitrogen intermediates can also kill Salmonella through various mechanisms, such as by causing DNA damage, preventing SPI-2 transcription, and inhibiting the PhoP/PhoQ acid-tolerance regulation reaction [208]. However, Salmonella also possesses the NO2- operating system and nitrate reductase for protection against RNI damage [209].
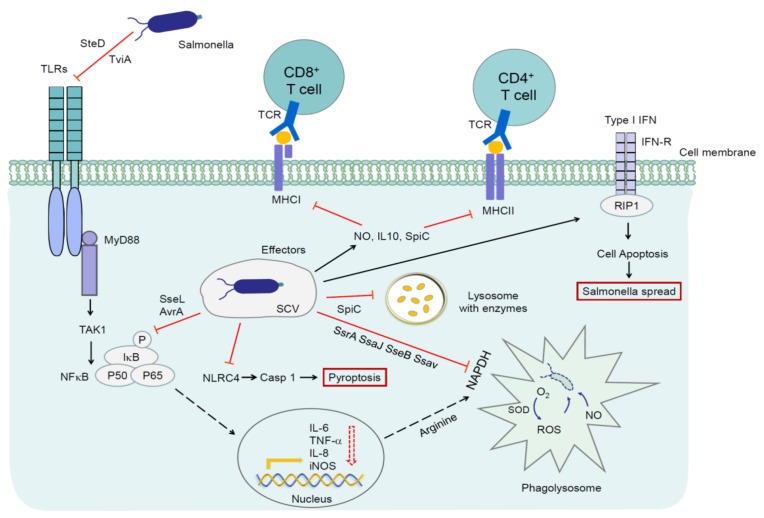
Figure 2.
Mechanisms by which Salmonella escape host immune responses. SPI-2 effector SpiC prevents DCs from presenting antigens to MHCs, and SifA blocks MHC II expression, resulting in an inadequate activation of naive T cells. SPI-2 effector SteD with its chaperone SrcA is a key requirement for Salmonella which suppress T cell activation by forcing an inappropriate ubiquitination of MHC II. SseB, SsrA, SsaJ, Ssav are used for avoiding the oxidative burst. Salmonella also increases IL-10 and NO production and induces the expression of iNOS to inhibit the proliferation of T cells. Furthermore, when SPI2 is activated, the expression of flagellin in the intracellular environment is inhibited, preventing the NLRC4 from recognizing Salmonella. During the course of infection, Salmonella exploits the host type I interferon response to eliminate the macrophages through RIP-dependent cell death and promotes its own survival. TLR: Toll-like receptor; MyD88: Myeloid differentiation primary response gene 88; TAK1: Transformed growth factor kinase 1; IκB-α: NF-κB inhibitor alpha; TCR: T cell receptor; MHC I: Major histocompatibility complex class I; MHC II: Major histocompatibility complex class II; NLRC4: NLR family CARD domain containing 4; CASP1: Caspase 1; IL-6: Interleukin 6; IL-8: Interleukin 8; IL-10: Interleukin 10; TNF-α: Tumor necrosis factor a; iNOS: Inducible nitric oxide synthase; IFN: Interferon; IFN-R: Interferon-a/b receptor; SOD: Superoxide dismutase; NADPH: Nicotinamide adenine dinucleotide phosphate; NO: nitric oxide; RIP: receptor-interacting protein.
Eswarappa and colleagues have shown that most SCVs in macrophages contain only one bacterium. Salmonella replicates in the SCV, and with an increasing bacterial number, one SCV divides into multiple SCVs, which benefits the survival of the intracellular bacteria [210]. On the one hand, it becomes much more difficult for the host cells to combat multiple SCVs compared to a single SCV, as this effort also requires more bactericidal media. In addition, a bacterium occupying a single SCV reduces the competition for nutrients and secretes effectors more efficiently into the cytoplasm [210]. Macrophages provide a safe haven to Salmonella for its survival and proliferation. However, when nutrients in the host cells are depleted, Salmonella is forced to induce the host cell death and search for new a host instead. Salmonella mediates macrophage death through two mechanisms [211]. One of these mechanisms involves the rapid induction of macrophage death. Salmonella expressing the SPI-1 T3SS rapidly trigger caspase-1-dependent apoptosis of infected macrophages [94,212,213]. Murine bone marrow-derived macrophages undergo lysis within 1 h of infection [214]. This rapid activation of programmed macrophage cell death depends on SPI-1 encoded protein SipB, bacterial flagellin, and the T3SS1 export machinery [211,215]. However, the other mechanism is SPI-1-independent, and characterized by a delayed induction of apoptosis to kill infected macrophages as late as 18 h post-infection. A functional T3SS2 and OmpR (ancestral regulator involved in the expression of ssrAB operon located in SPI-2) are required for the delayed induction of apoptosis, and allow Salmonella to spread intercellularly within apoptotic bodies [211,216,217]. Furthermore, past studies have indicated that the rapid and delayed activations of programmed macrophage cell death are independent of each other, since the mutations in SPI-1 do not affect the delayed induction of apoptosis, and the mutations in SPI-2 do not affect rapid induction of apoptosis [211]. The dead or dying macrophages containing Salmonella are engulfed by other macrophages recruited at the site of infection, and these macrophages can again serve as a safe haven for Salmonella to survive, while avoiding the extracellular host defenses [218]. Furthermore, studies have revealed that there are subpopulations of vacuolar and cytosolic Salmonella [219]. Vacuolar Salmonella are T3SS2-induced, whereas cytosolic Salmonella are induced by T3SS1 and flagellated [220]. The release of bacteria from SCV leads to their transcriptional reprogramming and a robust replication in the cytosol that exceeds their replication rate in the SCV [220,221]. However, the permissiveness of Salmonella survival and replication following vacuole lysis is dependent upon the cell type. For instance, Salmonella eventually hyper-replicate in the cytosol of epithelial cells but not in the cytosol of fibroblasts or macrophages [222]. Epithelial cells infected with Salmonella trigger an acute intracellular amino acid starvation, resulting in the induction of xenophagy to protect the host cells from Salmonella, but it is temporally restricted and not absolute [219,223]. Eventually, epithelial cell death via pyroptosis results in cell lysis, proinflammatory cytokine release, and escape of the cytosolic bacteria into the extracellular space, providing a potential mechanism of dissemination [219].
4.2. Escape of Adaptive Immune Responses
As the antigen presenting cells, macrophages and dendritic cells (DCs) can directly recognize the PAMPs in bacteria and present the bacterial antigens to T cells, initiate the proliferation and differentiation of naive T cells into the effector T cells, playing an important role in adaptive immune response against the invading bacteria. Interference with these functions is likely to increase the survival chances and invasion of bacteria in the hosts. Therefore, it seems that manipulating the antigen presentation capability of antigen presenting cells is another important strategy of pathogens for suppressing and escaping the host immune responses [224]. SseI has been shown to block the migration of DCs to lymphocytes [225]. Moreover, the major histocompatibility complex (MHC) plays an important role in combating the Salmonella during the later stages of infection [226,227]. It was shown that in human cells harboring intracellular Salmonella, SPI-2-encoded SifA is responsible for interfering with major histocompatibility complex class II (MHC II) cell surface expression and thereby provides Salmonella with a specific mechanism to evade or delay the host adaptive immune response (Figure 2) [228]. SPI-2-encoded SteD with its chaperone SrcA can force an inappropriate ubiquitination of MHC II to suppress T cell activation (Figure 2) [229,230]. Tobar and colleagues have shown that Salmonella prevents the degradation of lysosomes in DCs by SPI-2 effector SpiC, making it impossible for DCs to bind and present antigens to MHC, thus preventing the differentiation of naive T cells [231] (Figure 2). Once T cells are activated during infection, the majority of both CD4+ and CD8+ T cells have acquired an activated phenotype and an unexpectedly large fraction of these T-cell populations secreted IFN-γ to inhibit bacterial replication [232,233,234,235]. IL-12 has been identified as a major IFN-γ inducer. Interestingly, persons lacking the IL-12 receptor are more susceptible to Salmonella infection [236,237]. TNF-α also controls S. Typhimurium replication levels in persistently infected hosts [234]. Despite a profound activation of both CD4+ and CD8+ populations, expansion of either T-cell population was marginal [238]. Only a moderate (two- to three-fold) expansion of these T-cell populations were observed over several weeks of infection. Salmonella induces the expression of inducible nitric oxide synthase (iNOS) by SPI-2 to inhibit the proliferation and differentiation of T cells [224]. Furthermore, in mice models, S. Typhimurium infection resulted in immunosuppression by increasing IL-10 and nitric oxide (NO) production with immunosuppressive activity [233,239,240,241] (Figure 2).
Both CD4+ and CD8+ T lymphocytes and the humoral immune responses are required to control Salmonella infection [242,243,244,245,246,247,248]. It has been demonstrated that mice continuously infected with Salmonella have higher antibody titers [232] including IgA, IgM, and IgG [232,249], indicating that B cells also play an important role in the host defense. This may represent a deliberate shift from Th1/Th17 to Th2 responses [250]. Moreover, the adaptive immune responses also provide a positive feedback to the innate immune system [251]. This feedback is mediated via cytokines synthesis, leading to an increased number and activation of effector cells, and subsequently producing an increased antimicrobial response.
5. Perspectives
In recent years, different disciplines, such as immunology, microbiology, and cell biology have contributed greatly to our understanding of the interaction between Salmonella and the host. Several studies have revealed complex interactions between microbial pathogens and higher organisms. Future studies will hopefully expand our understanding of an interplay between immunity and bacteria in different infected organs. At present, our understanding of the interaction of Salmonella with innate and adaptive immunity evading the host defense strategies in humans is still incomplete. When the effects of normal microbial colonization flora contained in the host and the diversity of environmental conditions are analyzed, the complexity of the interaction between bacteria and host becomes far greater than our current knowledge in this domain. Invasive diseases caused by Salmonella remain a major factor accounting for the severe death and morbidity rates worldwide. Therefore, the ongoing research focusing on the relationship between Salmonella and the host immunity has the desired potential to explicate complex questions related to the Salmonella–host interactions and improve the prevention and treatment strategies aimed at combating these infectious diseases in the near future.
Acknowledgments
This work was supported by National Key R & D Program of Intergovernmental Key Projects (Grant No: 2018YFE0101700).
Author Contributions
M.W. wrote the initial draft of manuscript. I.H.Q. Critically reviewed, revised, and commented on manuscript. L.W. reviewed the manuscript. G.Z. provided the idea for manuscript. H.H. designed the topics in this review, outlined the synopsis for the manuscript, and reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- 1.De Abrew Abeysundara P., Dhowlaghar N., Nannapaneni R., Schilling M.W., Mahmoud B., Sharma C.S., Ma D.P. Salmonella enterica growth and biofilm formation in flesh and peel cantaloupe extracts on four food-contact surfaces. Int. J. Food Microbiol. 2018;280:17–26. doi: 10.1016/j.ijfoodmicro.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 2.Tadepalli S., Bridges D.F., Driver R., Wu V.C.H. Effectiveness of different antimicrobial washes combined with freezing against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes inoculated on blueberries. Food Microbiol. 2018;74:34–39. doi: 10.1016/j.fm.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Olaimat A.N., Al-Holy M.A., Abu Ghoush M., Al-Nabulsi A.A., Holley R.A. Control of Salmonella enterica and Listeria monocytogenes in hummus using allyl isothiocyanate. Int. J. Food Microbiol. 2018;278:73–80. doi: 10.1016/j.ijfoodmicro.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Parry-Hanson Kunadu A., Holmes M., Miller E.L., Grant A.J. Microbiological quality and antimicrobial resistance characterization of Salmonella spp. in fresh milk value chains in Ghana. Int. J. Food Microbiol. 2018;277:41–49. doi: 10.1016/j.ijfoodmicro.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Jajere S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World. 2019;12:504–521. doi: 10.14202/vetworld.2019.504-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wain J., Hendriksen R.S., Mikoleit M.L., Keddy K.H., Ochiai R.L. Typhoid fever. Lancet. 2015;385:1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PubMed] [Google Scholar]
- 7.Coburn B., Grassl G.A., Finlay B.B. Salmonella, the host and disease: A brief review. Immunol. Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian R., Im J., Lee J.-S., Jeon H.J., Mogeni O.D., Kim J.H., Rakotozandrindrainy R. The global burden and epidemiology of invasive non-typhoidal infections. Hum. Vaccines Immunother. 2019;15:1421–1426. doi: 10.1080/21645515.2018.1504717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsolis R.M., Kingsley R.A., Townsend S.M., Ficht T.A., Adams L.G., Baumler A.J. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv. Exp. Med. Biol. 1999;473:261–274. [PubMed] [Google Scholar]
- 10.Adams D.A., Thomas K.R., Jajosky R.A., Foster L., Sharp P., Onweh D.H., Schley A.W. Summary of Notifiable Infectious Diseases and Conditions—United States, 2014. Morb. Mortal. Wkly. Rep. 2016;63:1–152. doi: 10.15585/mmwr.mm6354a1. [DOI] [PubMed] [Google Scholar]
- 11.Crump J.A., Sjölund-Karlsson M., Gordon M.A., Parry C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015;28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuPont H.L. Clinical practice. Bacterial diarrhea. N. Engl. J. Med. 2009;361:1560–1569. doi: 10.1056/NEJMcp0904162. [DOI] [PubMed] [Google Scholar]
- 13.Stanaway J.D., Parisi A., Sarkar K., Blacker B.F., Reiner R.C., Hay S.I., Nixon M.R., Dolecek C., James S.L., Mokdad A.H., et al. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019;19:1312–1324. doi: 10.1016/S1473-3099(19)30418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhowlaghar N., Bansal M., Schilling M.W., Nannapaneni R. Scanning electron microscopy of Salmonella biofilms on various food-contact surfaces in catfish mucus. Food Microbiol. 2018;74:143–150. doi: 10.1016/j.fm.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Marin C., Torres C., Marco-Jimenez F., Cerda-Cuellar M., Sevilla S., Ayats T., Vega S. Supplementary feeding stations for conservation of vultures could be an important source of monophasic Salmonella typhimurium 1,4,[5],12:i. Sci. Total Environ. 2018;636:449–455. doi: 10.1016/j.scitotenv.2018.04.310. [DOI] [PubMed] [Google Scholar]
- 16.Spanò S. Mechanisms of Salmonella Typhi Host Restriction. Adv. Exp. Med. Biol. 2016;915:283–294. doi: 10.1007/978-3-319-32189-9_17. [DOI] [PubMed] [Google Scholar]
- 17.Johnson R., Mylona E., Frankel G. Typhoidal Salmonella: Distinctive virulence factors and pathogenesis. Cell. Microbiol. 2018;20:e12939. doi: 10.1111/cmi.12939. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y., Lin E., Zou S., Chen C.-L., Chiu C.-H. Complete genome sequence of Salmonella enterica serovar Sendai shows H antigen convergence with S. Miami and recent divergence from S. Paratyphi A. BMC Genom. 2019;20:398. doi: 10.1186/s12864-019-5798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ly K.T., Casanova J.E. Mechanisms of Salmonella entry into host cells. Cell. Microbiol. 2007;9:2103–2111. doi: 10.1111/j.1462-5822.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- 20.Haghjoo E., Galán J.E. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA. 2004;101:4614–4619. doi: 10.1073/pnas.0400932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liston S.D., Ovchinnikova O.G., Whitfield C. Unique lipid anchor attaches Vi antigen capsule to the surface of Salmonella enterica serovar Typhi. Proc. Natl. Acad. Sci. USA. 2016;113:6719–6724. doi: 10.1073/pnas.1524665113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jepson M.A., Clark M.A. The role of M cells in Salmonella infection. Microbes Infect. 2001;3:1183–1190. doi: 10.1016/S1286-4579(01)01478-2. [DOI] [PubMed] [Google Scholar]
- 23.Jones B.D., Falkow S. Salmonellosis: Host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez-Torres A., Fang F.C. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 2000;3:54–59. doi: 10.1016/S1369-5274(99)00051-X. [DOI] [PubMed] [Google Scholar]
- 25.Gunn J.S., Marshall J.M., Baker S., Dongol S., Charles R.C., Ryan E.T. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014;22:648–655. doi: 10.1016/j.tim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klemm E.J., Shakoor S., Page A.J., Qamar F.N., Judge K., Saeed D.K., Wong V.K. Emergence of an Extensively Drug-Resistant Serovar Typhi Clone Harboring a Promiscuous Plasmid Encoding Resistance to Fluoroquinolones and Third-Generation Cephalosporins. MBio. 2018;9 doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt K.E., Phan M.D., Baker S., Duy P.T., Nga T.V.T., Nair S., Turner A.K. Emergence of a globally dominant IncHI1 plasmid type associated with multiple drug resistant typhoid. PLoS Negl. Trop. Dis. 2011;5:e1245. doi: 10.1371/journal.pntd.0001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wotzka S.Y., Nguyen B.D., Hardt W.D. Salmonella Typhimurium Diarrhea Reveals Basic Principles of Enteropathogen Infection and Disease-Promoted DNA Exchange. Cell Host Microbe. 2017;21:443–454. doi: 10.1016/j.chom.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Bumann D., Schothorst J. Intracellular Salmonella metabolism. Cell. Microbiol. 2017;19 doi: 10.1111/cmi.12766. [DOI] [PubMed] [Google Scholar]
- 30.Brumell J.H., Rosenberger C.M., Gotto G.T., Marcus S.L., Finlay B.B. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell. Microbiol. 2001;3:75–84. doi: 10.1046/j.1462-5822.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 31.Wei S., Huang J., Liu Z., Wang M., Zhang B., Lian Z., Guo Y. Differential immune responses of C57BL/6 mice to infection by Salmonella enterica serovar Typhimurium strain SL1344, CVCC541 and CMCC50115. Virulence. 2019;10:248–259. doi: 10.1080/21505594.2019.1597496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monack D.M., Mueller A., Falkow S. Persistent bacterial infections: The interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2004;2:747–765. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 33.Sabbagh S.C., Forest C.G., Lepage C., Leclerc J.-M., Daigle F. So similar, yet so different: Uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010;305:1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 34.Carden S.E., Walker G.T., Honeycutt J., Lugo K., Pham T., Jacobson A., Bouley D. Pseudogenization of the Secreted Effector Gene sseI Confers Rapid Systemic Dissemination of S. Typhimurium ST313 within Migratory Dendritic Cells. Cell Host Microbe. 2017;21:182–194. doi: 10.1016/j.chom.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll L.M., Gaballa A., Guldimann C., Sullivan G., Henderson L.O., Wiedmann M. Identification of Novel Mobilized Colistin Resistance Gene mcr-9 in a Multidrug-Resistant, Colistin-Susceptible Salmonella enterica Serotype Typhimurium Isolate. MBio. 2019;10 doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed H.A., El-Hofy F.I., Shafik S.M., Abdelrahman M.A., Elsaid G.A. Characterization of Virulence-Associated Genes, Antimicrobial Resistance Genes, and Class 1 Integrons in Salmonella enterica serovar Typhimurium Isolates from Chicken Meat and Humans in Egypt. Foodborne Pathog. Dis. 2016;13:281–288. doi: 10.1089/fpd.2015.2097. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins K.L., Arnold C., Threlfall E.J. Rapid detection of gyrA and parC mutations in quinolone-resistant Salmonella enterica using Pyrosequencing technology. J. Microbiol. Methods. 2007;68:163–171. doi: 10.1016/j.mimet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Garmory H.S., Brown K.A., Titball R.W. Salmonella vaccines for use in humans: Present and future perspectives. FEMS Microbiol. Rev. 2002;26:339–353. doi: 10.1111/j.1574-6976.2002.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 39.Edwards P.R., Kauffmann F. A simplification of the Kauffmann-White schema. Am. J. Clin. Pathol. 1952;22:692–697. doi: 10.1093/ajcp/22.7_ts.692. [DOI] [PubMed] [Google Scholar]
- 40.Smith N.H., Selander R.K. Sequence invariance of the antigen-coding central region of the phase 1 flagellar filament gene (fliC) among strains of Salmonella typhimurium. J. Bacteriol. 1990;172:603–609. doi: 10.1128/JB.172.2.603-609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winfield M.D., Groisman E.A. Evolution and Ecology of Salmonella. EcoSal Plus. 2004;1 doi: 10.1128/ecosalplus.6.4.6. [DOI] [PubMed] [Google Scholar]
- 42.Calenge F., Kaiser P., Vignal A., Beaumont C. Genetic control of resistance to salmonellosis and to Salmonella carrier-state in fowl: A review. Genet. Sel. Evol. 2010;42:11. doi: 10.1186/1297-9686-42-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner F.W., Villar R.G., Angulo F.J., Tauxe R., Swaminathan B. Salmonella nomenclature. J. Clin. Microbiol. 2000;38:2465–2467. doi: 10.1128/JCM.38.7.2465-2467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeves M.W., Evins G.M., Heiba A.A., Plikaytis B.D., Farmer J.J. Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J. Clin. Microbiol. 1989;27:313–320. doi: 10.1128/JCM.27.2.313-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J., Nelson K., McWhorter A.C., Whittam T.S., Selander R.K. Recombinational basis of serovar diversity in Salmonella enterica. Proc. Natl. Acad. Sci. USA. 1994;91:2552–2556. doi: 10.1073/pnas.91.7.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beltran P., Musser J.M., Helmuth R., Farmer J.J., Frerichs W.M., Wachsmuth I.K., Ferris K. Toward a population genetic analysis of Salmonella: Genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc. Natl. Acad. Sci. USA. 1988;85:7753–7757. doi: 10.1073/pnas.85.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uzzau S., Brown D.J., Wallis T., Rubino S., Leori G., Bernard S., Casadesus J. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 2000;125:229–255. doi: 10.1017/S0950268899004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bale J., Meunier D., Weill F.-X., dePinna E., Peters T., Nair S. Characterization of new Salmonella serovars by whole-genome sequencing and traditional typing techniques. J. Med. Microbiol. 2016;65:1074–1078. doi: 10.1099/jmm.0.000325. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S., Yin Y., Jones M.B., Zhang Z., Deatherage Kaiser B.L., Dinsmore B.A., Fitzgerald C. Salmonella serotype determination utilizing high-throughput genome sequencing data. J. Clin. Microbiol. 2015;53:1685–1692. doi: 10.1128/JCM.00323-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon M.A. Salmonella infections in immunocompromised adults. J. Infect. 2008;56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Admassu D., Egata G., Teklemariam Z. Prevalence and antimicrobial susceptibility pattern of Salmonella enterica serovar Typhi and Salmonella enterica serovar Paratyphi among febrile patients at Karamara Hospital, Jigjiga, eastern Ethiopia. SAGE Open Med. 2019;7 doi: 10.1177/2050312119837854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schadich E., Dzubak P., Hajduch M. Role of Salmonella Typhi Vi Antigen and Secretory Systems on Immune Response. Curr. Pharm. Design. 2016;22:6251–6260. doi: 10.2174/1381612822666160829142308. [DOI] [PubMed] [Google Scholar]
- 53.Jorge J.F., Costa A.B., Rodrigues J.L., Girao E.S., Luiz R.S., Sousa A.Q., Moore S.R. Salmonella typhi liver abscess overlying a metastatic melanoma. Am. J. Trop. Med. Hyg. 2014;90:716–718. doi: 10.4269/ajtmh.13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagaraja V., Eslick G.D. Systematic review with meta-analysis: The relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment. Pharmacol. Ther. 2014;39:745–750. doi: 10.1111/apt.12655. [DOI] [PubMed] [Google Scholar]
- 55.Chen H.M., Wang Y., Su L.H., Chiu C.H. Nontyphoid salmonella infection: Microbiology, clinical features, and antimicrobial therapy. Pediatr. Neonatol. 2013;54:147–152. doi: 10.1016/j.pedneo.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Lin R., Wan J., Xiong Y., Wu K., Cheong W.C., Zhou G., Wang D. A quantitative study of charge carrier dynamics in well-defined WO3 nanowires and nanosheets: Insight into the crystal facet effect in photocatalysis. J. Am. Chem. Soc. 2018 doi: 10.1021/jacs.8b05293. [DOI] [PubMed] [Google Scholar]
- 57.Kurtz J.R., Goggins J.A., McLachlan J.B. Salmonella infection: Interplay between the bacteria and host immune system. Immunol. Lett. 2017;190:42–50. doi: 10.1016/j.imlet.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molbak K. Human health consequences of antimicrobial drug-resistant Salmonella and other foodborne pathogens. Clin. Infect. Dis. 2005;41:1613–1620. doi: 10.1086/497599. [DOI] [PubMed] [Google Scholar]
- 59.Gal-Mor O., Boyle E.C., Grassl G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014;5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon M.A. Invasive nontyphoidal Salmonella disease: Epidemiology, pathogenesis and diagnosis. Curr. Opin. Infect. Dis. 2011;24:484–489. doi: 10.1097/QCO.0b013e32834a9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones T.F., Ingram L.A., Cieslak P.R., Vugia D.J., Tobin-D’Angelo M., Hurd S., Medus C. Salmonellosis outcomes differ substantially by serotype. J. Infect. Dis. 2008;198:109–114. doi: 10.1086/588823. [DOI] [PubMed] [Google Scholar]
- 62.Feasey N.A., Dougan G., Kingsley R.A., Heyderman R.S., Gordon M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amuasi J.H., May J. Non-typhoidal salmonella: Invasive, lethal, and on the loose. Lancet Infect. Dis. 2019;19:1267–1269. doi: 10.1016/S1473-3099(19)30521-3. [DOI] [PubMed] [Google Scholar]
- 64.Lim S.H., Methé B.A., Knoll B.M., Morris A., Obaro S.K. Invasive non-typhoidal Salmonella in sickle cell disease in Africa: Is increased gut permeability the missing link? J. Transl. Med. 2018;16:239. doi: 10.1186/s12967-018-1622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon M.A., Kankwatira A.M., Mwafulirwa G., Walsh A.L., Hopkins M.J., Parry C.M., Faragher E.B. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: An emerging disease pathogenesis. Clin. Infect. Dis. 2010;50:953–962. doi: 10.1086/651080. [DOI] [PubMed] [Google Scholar]
- 66.Gilchrist J.J., MacLennan C.A. Invasive Nontyphoidal Salmonella Disease in Africa. EcoSal Plus. 2019;8 doi: 10.1128/ecosalplus.ESP-0007-2018. [DOI] [PubMed] [Google Scholar]
- 67.Blondel C.J., Jimenez J.C., Contreras I., Santiviago C.A. Comparative genomic analysis uncovers 3 novel loci encoding type six secretion systems differentially distributed in Salmonella serotypes. BMC Genom. 2009;10:354. doi: 10.1186/1471-2164-10-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sevellec Y., Vignaud M.L., Granier S.A., Lailler R., Feurer C., Le Hello S., Mistou M.Y. Polyphyletic Nature of Salmonella enterica Serotype Derby and Lineage-Specific Host-Association Revealed by Genome-Wide Analysis. Front. Microbiol. 2018;9:891. doi: 10.3389/fmicb.2018.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rice C.J., Ramachandran V.K., Shearer N., Thompson A. Transcriptional and Post-Transcriptional Modulation of SPI1 and SPI2 Expression by ppGpp, RpoS and DksA in Salmonella enterica sv Typhimurium. PLoS ONE. 2015;10:e0127523. doi: 10.1371/journal.pone.0127523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buckner M.M., Croxen M.A., Arena E.T., Finlay B.B. A comprehensive study of the contribution of Salmonella enterica serovar Typhimurium SPI2 effectors to bacterial colonization, survival, and replication in typhoid fever, macrophage, and epithelial cell infection models. Virulence. 2011;2:208–216. doi: 10.4161/viru.2.3.15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blanc-Potard A.B., Solomon F., Kayser J., Groisman E.A. The SPI-3 pathogenicity island of Salmonella enterica. J. Bacteriol. 1999;181:998–1004. doi: 10.1128/JB.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rychlik I., Karasova D., Sebkova A., Volf J., Sisak F., Havlickova H., Kummer V. Virulence potential of five major pathogenicity islands (SPI-1 to SPI-5) of Salmonella enterica serovar Enteritidis for chickens. BMC Microbiol. 2009;9:268. doi: 10.1186/1471-2180-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiss T., Morgan E., Nagy G. Contribution of SPI-4 genes to the virulence of Salmonella enterica. FEMS Microbiol. Lett. 2007;275:153–159. doi: 10.1111/j.1574-6968.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 74.Hensel M. Salmonella pathogenicity island 2. Mol. Microbiol. 2000;36:1015–1023. doi: 10.1046/j.1365-2958.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- 75.Hautefort I., Thompson A., Eriksson-Ygberg S., Parker M.L., Lucchini S., Danino V., Bongaerts R.J. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell. Microbiol. 2008;10:958–984. doi: 10.1111/j.1462-5822.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galan J.E. Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 77.Haraga A., Ohlson M.B., Miller S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 78.Shea J.E., Hensel M., Gleeson C., Holden D.W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shappo M.O.E., Li Q., Lin Z., Hu M., Ren J., Xu Z., Pan Z. SspH2 as anti-inflammatory candidate effector and its contribution in Salmonella Enteritidis virulence. Microb. Pathog. 2020;142:104041. doi: 10.1016/j.micpath.2020.104041. [DOI] [PubMed] [Google Scholar]
- 80.Zhang K., Riba A., Nietschke M., Torow N., Repnik U., Pütz A., Fulde M. Minimal SPI1-T3SS effector requirement for Salmonella enterocyte invasion and intracellular proliferation in vivo. PLoS Pathog. 2018;14:e1006925. doi: 10.1371/journal.ppat.1006925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang S., Deng Q., Sun L., Dong K., Li Y., Wu S., Huang R. Effector SpvB interferes with intracellular iron homeostasis regulation of transcription factor NRF2. FASEB J. 2019;33:13450–13464. doi: 10.1096/fj.201900883RR. [DOI] [PubMed] [Google Scholar]
- 82.Knuff K., Finlay B.B. What the SIF Is Happening-The Role of Intracellular-Induced Filaments. Front. Cell. Infect. Microbiol. 2017;7:335. doi: 10.3389/fcimb.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rajashekar R., Liebl D., Chikkaballi D., Liss V., Hensel M. Live cell imaging reveals novel functions of Salmonella enterica SPI2-T3SS effector proteins in remodeling of the host cell endosomal system. PLoS ONE. 2014;9:e115423. doi: 10.1371/journal.pone.0115423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao A.P., Petrof E.O., Kuppireddi S., Zhao Y., Xia Y., Claud E.C., Sun J. Salmonella type III effector AvrA stabilizes cell tight junctions to inhibit inflammation in intestinal epithelial cells. PLoS ONE. 2008;3:e2369. doi: 10.1371/journal.pone.0002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jennings E., Esposito D., Rittinger K. Structure-function analyses of the bacterial zinc metalloprotease effector protein GtgA uncover key residues required for deactivating NF-kappaB. J. Biol. Chem. 2018;293:15316–15329. doi: 10.1074/jbc.RA118.004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun H., Kamanova J., Lara-Tejero M., Galan J.E. A Family of Salmonella Type III Secretion Effector Proteins Selectively Targets the NF-kappaB Signaling Pathway to Preserve Host Homeostasis. PLoS Pathog. 2016;12:e1005484. doi: 10.1371/journal.ppat.1005484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jennings E., Thurston T.L.M., Holden D.W. Salmonella SPI-2 Type III Secretion System Effectors: Molecular Mechanisms and Physiological Consequences. Cell Host Microbe. 2017;22:217–231. doi: 10.1016/j.chom.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Knodler L.A., Steele-Mortimer O. The Salmonella effector PipB2 affects late endosome/lysosome distribution to mediate Sif extension. Mol. Biol. Cell. 2005;16:4108–4123. doi: 10.1091/mbc.e05-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henry T., Couillault C., Rockenfeller P., Boucrot E., Dumont A., Schroeder N., Hermant A. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc. Natl. Acad. Sci. USA. 2006;103:13497–13502. doi: 10.1073/pnas.0605443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGourty K., Thurston T.L., Matthews S.A., Pinaud L., Mota L.J., Holden D.W. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science. 2012;338:963–967. doi: 10.1126/science.1227037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brawn L.C., Hayward R.D., Koronakis V. Salmonella SPI1 effector SipA persists after entry and cooperates with a SPI2 effector to regulate phagosome maturation and intracellular replication. Cell Host Microbe. 2007;1:63–75. doi: 10.1016/j.chom.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Myeni S.K., Wang L., Zhou D. SipB-SipC complex is essential for translocon formation. PLoS ONE. 2013;8:e60499. doi: 10.1371/journal.pone.0060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glasgow A.A., Wong H.T., Tullman-Ercek D. A Secretion-Amplification Role for Salmonella enterica Translocon Protein SipD. ACS Synth. Biol. 2017;6:1006–1015. doi: 10.1021/acssynbio.6b00335. [DOI] [PubMed] [Google Scholar]
- 94.Kamanova J., Sun H., Lara-Tejero M., Galan J.E. The Salmonella Effector Protein SopA Modulates Innate Immune Responses by Targeting TRIM E3 Ligase Family Members. PLoS Pathog. 2016;12:e1005552. doi: 10.1371/journal.ppat.1005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perrett C.A., Zhou D. Salmonella type III effector SopB modulates host cell exocytosis. Emerg. Microbes Infect. 2013;2:e32. doi: 10.1038/emi.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Costa V.M., Braun V., Landekic M., Shi R., Proteau A., McDonald L., Cygler M. Salmonella Disrupts Host Endocytic Trafficking by SopD2-Mediated Inhibition of Rab7. Cell Rep. 2015;12:1508–1518. doi: 10.1016/j.celrep.2015.07.063. [DOI] [PubMed] [Google Scholar]
- 97.Vonaesch P., Sellin M.E., Cardini S., Singh V., Barthel M., Hardt W.-D. The Salmonella Typhimurium effector protein SopE transiently localizes to the early SCV and contributes to intracellular replication. Cell. Microbiol. 2014;16:1723–1735. doi: 10.1111/cmi.12333. [DOI] [PubMed] [Google Scholar]
- 98.Johnson R., Byrne A., Berger C.N., Klemm E., Crepin V.F., Dougan G., Frankel G. The Type III Secretion System Effector SptP of Salmonella enterica Serovar Typhi. J. Bacteriol. 2017;199 doi: 10.1128/JB.00647-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haneda T., Ishii Y., Shimizu H., Ohshima K., Iida N., Danbara H., Okada N. Salmonella type III effector SpvC, a phosphothreonine lyase, contributes to reduction in inflammatory response during intestinal phase of infection. Cell. Microbiol. 2012;14:485–499. doi: 10.1111/j.1462-5822.2011.01733.x. [DOI] [PubMed] [Google Scholar]
- 100.Figueira R., Holden D.W. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology. 2012;158:1147–1161. doi: 10.1099/mic.0.058115-0. [DOI] [PubMed] [Google Scholar]
- 101.Hensel M., Shea J.E., Raupach B., Monack D., Falkow S., Gleeson C., Kubo T. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella Pathogenicity Island 2. Mol. Microbiol. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 102.Gallois A., Klein J.R., Allen L.A., Jones B.D., Nauseef W.M. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- 103.Abrahams G.L., Müller P., Hensel M. Functional dissection of SseF, a type III effector protein involved in positioning the salmonella-containing vacuole. Traffic. 2006;7:950–965. doi: 10.1111/j.1600-0854.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 104.Kolodziejek A.M., Miller S.I. Salmonella modulation of the phagosome membrane, role of SseJ. Cell. Microbiol. 2015;17:333–341. doi: 10.1111/cmi.12420. [DOI] [PubMed] [Google Scholar]
- 105.Günster R.A., Matthews S.A., Holden D.W., Thurston T.L.M. SseK1 and SseK3 Type III Secretion System Effectors Inhibit NF-κB Signaling and Necroptotic Cell Death in Salmonella-Infected Macrophages. Infect. Immun. 2017;85 doi: 10.1128/IAI.00010-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Le Negrate G., Faustin B., Welsh K., Loeffler M., Krajewska M., Hasegawa P., Mukherjee S. Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-kappaB, suppresses IkappaBalpha ubiquitination and modulates innate immune responses. J. Immunol. 2008;180:5045–5056. doi: 10.4049/jimmunol.180.7.5045. [DOI] [PubMed] [Google Scholar]
- 107.Domingues L., Holden D.W., Mota L.J. The Salmonella effector SteA contributes to the control of membrane dynamics of Salmonella-containing vacuoles. Infect. Immun. 2014;82:2923–2934. doi: 10.1128/IAI.01385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Odendall C., Rolhion N., Förster A., Poh J., Lamont D.J., Liu M., Freemont P.S. The Salmonella kinase SteC targets the MAP kinase MEK to regulate the host actin cytoskeleton. Cell Host Microbe. 2012;12:657–668. doi: 10.1016/j.chom.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chatterjee S., Chaudhury S., McShan A.C., Kaur K., De Guzman R.N. Structure and biophysics of type III secretion in bacteria. Biochemistry. 2013;52:2508–2517. doi: 10.1021/bi400160a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cardenal-Munoz E., Gutierrez G., Ramos-Morales F. Global impact of Salmonella type III secretion effector SteA on host cells. Biochem. Biophys. Res. Commun. 2014;449:419–424. doi: 10.1016/j.bbrc.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 111.Young A.M., Palmer A.E. Methods to Illuminate the Role of Salmonella Effector Proteins during Infection: A Review. Front. Cell. Infect. Microbiol. 2017;7:363. doi: 10.3389/fcimb.2017.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janakiraman A., Slauch J.M. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol. Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- 113.Spector M.P. The starvation-stress response (SSR) of Salmonella. Adv. Microb. Physiol. 1998;40:233–279. doi: 10.1016/s0065-2911(08)60133-2. [DOI] [PubMed] [Google Scholar]
- 114.Hirvas L., Koski P., Vaara M. Identification and sequence analysis of the gene mutated in the conditionally lethal outer membrane permeability mutant SS-C of Salmonella typhimurium. EMBO J. 1991;10:1017–1023. doi: 10.1002/j.1460-2075.1991.tb08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hirvas L., Koski P., Vaara M. Primary structure and expression of the Ssc-protein of Salmonella typhimurium. Biochem. Biophys. Res. Commun. 1990;173:53–59. doi: 10.1016/S0006-291X(05)81020-4. [DOI] [PubMed] [Google Scholar]
- 116.Hensel M., Shea J.E., Waterman S.R., Mundy R., Nikolaus T., Banks G., Vazquez-Torres A. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 117.Blanc-Potard A.B., Groisman E.A. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barlag B., Hensel M. The giant adhesin SiiE of Salmonella enterica. Molecules. 2015;20:1134–1150. doi: 10.3390/molecules20011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kirchweger P., Weiler S., Egerer-Sieber C., Blasl A.-T., Hoffmann S., Schmidt C., Sander N. Structural and functional characterization of SiiA, an auxiliary protein from the SPI4-encoded type 1 secretion system from Salmonella enterica. Mol. Microbiol. 2019;112:1403–1422. doi: 10.1111/mmi.14368. [DOI] [PubMed] [Google Scholar]
- 120.Gerlach R.G., Claudio N., Rohde M., Jackel D., Wagner C., Hensel M. Cooperation of Salmonella pathogenicity islands 1 and 4 is required to breach epithelial barriers. Cell. Microbiol. 2008;10:2364–2376. doi: 10.1111/j.1462-5822.2008.01218.x. [DOI] [PubMed] [Google Scholar]
- 121.Gerlach R.G., Jackel D., Stecher B., Wagner C., Lupas A., Hardt W.D., Hensel M. Salmonella Pathogenicity Island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell. Microbiol. 2007;9:1834–1850. doi: 10.1111/j.1462-5822.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 122.Wood M.W., Jones M.A., Watson P.R., Hedges S., Wallis T.S., Galyov E.E. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol. Microbiol. 1998;29:883–891. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 123.Knodler L.A., Celli J., Hardt W.D., Vallance B.A., Yip C., Finlay B.B. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol. Microbiol. 2002;43:1089–1103. doi: 10.1046/j.1365-2958.2002.02820.x. [DOI] [PubMed] [Google Scholar]
- 124.Cao G., Allard M., Strain E., Stones R., Zhao S., Brown E., Meng J. Genetic diversity of Salmonella pathogenicity islands SPI-5 and SPI-6 in Salmonella Newport. Foodborne Pathog. Dis. 2014;11:798–807. doi: 10.1089/fpd.2014.1784. [DOI] [PubMed] [Google Scholar]
- 125.Saroj S.D., Shashidhar R., Karani M., Bandekar J.R. Distribution of Salmonella pathogenicity island (SPI)-8 and SPI-10 among different serotypes of Salmonella. J. Med. Microbiol. 2008;57:424–427. doi: 10.1099/jmm.0.47630-0. [DOI] [PubMed] [Google Scholar]
- 126.Bueno S.M., Santiviago C.A., Murillo A.A., Fuentes J.A., Trombert A.N., Rodas P.I., Youderian P. Precise excision of the large pathogenicity island, SPI7, in Salmonella enterica serovar Typhi. J. Bacteriol. 2004;186:3202–3213. doi: 10.1128/JB.186.10.3202-3213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tomljenovic-Berube A.M., Henriksbo B., Porwollik S., Cooper C.A., Tuinema B.R., McClelland M., Coombes B.K. Mapping and regulation of genes within Salmonella pathogenicity island 12 that contribute to in vivo fitness of Salmonella enterica Serovar Typhimurium. Infect. Immun. 2013;81:2394–2404. doi: 10.1128/IAI.00067-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee Y.H., Kim S., Helmann J.D., Kim B.H., Park Y.K. RaoN, a small RNA encoded within Salmonella pathogenicity island-11, confers resistance to macrophage-induced stress. Microbiology. 2013;159:1366–1378. doi: 10.1099/mic.0.066688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Velasquez J.C., Hidalgo A.A., Villagra N., Santiviago C.A., Mora G.C., Fuentes J.A. SPI-9 of Salmonella enterica serovar Typhi is constituted by an operon positively regulated by RpoS and contributes to adherence to epithelial cells in culture. Microbiology. 2016;162:1367–1378. doi: 10.1099/mic.0.000319. [DOI] [PubMed] [Google Scholar]
- 130.Pickard D., Wain J., Baker S., Line A., Chohan S., Fookes M., Barron A. Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. J. Bacteriol. 2003;185:5055–5065. doi: 10.1128/JB.185.17.5055-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Desai P.T., Porwollik S., Long F., Cheng P., Wollam A., Bhonagiri-Palsikar V., Hallsworth-Pepin K. Evolutionary Genomics of Salmonella enterica Subspecies. MBio. 2013;4 doi: 10.1128/mBio.00579-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hensel M. Evolution of pathogenicity islands of Salmonella enterica. Int. J. Med. Microbiol. 2004;294:95–102. doi: 10.1016/j.ijmm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 133.Parkhill J., Dougan G., James K.D., Thomson N.R., Pickard D., Wain J., Churcher C. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature. 2001;413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 134.Espinoza R.A., Silva-Valenzuela C.A., Amaya F.A., Urrutia I.M., Contreras I., Santiviago C.A. Differential roles for pathogenicity islands SPI-13 and SPI-8 in the interaction of Salmonella Enteritidis and Salmonella Typhi with murine and human macrophages. Biol. Res. 2017;50:5. doi: 10.1186/s40659-017-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mulder D.T., Cooper C.A., Coombes B.K. Type VI secretion system-associated gene clusters contribute to pathogenesis of Salmonella enterica serovar Typhimurium. Infect. Immun. 2012;80:1996–2007. doi: 10.1128/IAI.06205-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sana T.G., Flaugnatti N., Lugo K.A., Lam L.H., Jacobson A., Baylot V., Durand E. Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl. Acad. Sci. USA. 2016;113:E5044–E5051. doi: 10.1073/pnas.1608858113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang S., Yang D., Wu X., Yi Z., Wang Y., Xin S., Wang D. The ferric uptake regulator represses type VI secretion system function by binding directly to the clpV promoter in Salmonella enterica serovar Typhimurium. Infect. Immun. 2019 doi: 10.1128/IAI.00562-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raffatellu M., Chessa D., Wilson R.P., Dusold R., Rubino S., Baumler A.J. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 2005;73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liaquat S., Sarwar Y. Virulotyping of Salmonella enterica serovar Typhi isolates from Pakistan: Absence of complete SPI-10 in Vi negative isolates. PLoS Negl. Trop. Dis. 2018;12:e0006839. doi: 10.1371/journal.pntd.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Faucher S.P., Viau C., Gros P.P., Daigle F., Le Moual H. The prpZ gene cluster encoding eukaryotic-type Ser/Thr protein kinases and phosphatases is repressed by oxidative stress and involved in Salmonella enterica serovar Typhi survival in human macrophages. FEMS Microbiol. Lett. 2008;281:160–166. doi: 10.1111/j.1574-6968.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 141.Miller S.I., Kukral A.M., Mekalanos J.J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gunn J.S., Alpuche-Aranda C.M., Loomis W.P., Belden W.J., Miller S.I. Characterization of the Salmonella typhimurium pagC/pagD chromosomal region. J. Bacteriol. 1995;177:5040–5047. doi: 10.1128/JB.177.17.5040-5047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elder J.R., Paul N.C., Burin R., Guard J., Shah D.H. Genomic organization and role of SPI-13 in nutritional fitness of Salmonella. Int. J. Med. Microbiol. 2018;308:1043–1052. doi: 10.1016/j.ijmm.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 144.Li H., Li X., Lv R., Jiang X., Cao H., Du Y., Jiang L. Global regulatory function of the low oxygen-induced transcriptional regulator LoiA in Salmonella Typhimurium revealed by RNA sequencing. Biochem. Biophys. Res. Commun. 2018;503:2022–2027. doi: 10.1016/j.bbrc.2018.07.151. [DOI] [PubMed] [Google Scholar]
- 145.Jiang L., Feng L., Yang B., Zhang W., Wang P., Jiang X., Wang L. Signal transduction pathway mediated by the novel regulator LoiA for low oxygen tension induced Salmonella Typhimurium invasion. PLoS Pathog. 2017;13:e1006429. doi: 10.1371/journal.ppat.1006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vernikos G.S., Parkhill J. Interpolated variable order motifs for identification of horizontally acquired DNA: Revisiting the Salmonella pathogenicity islands. Bioinformatics. 2006;22:2196–2203. doi: 10.1093/bioinformatics/btl369. [DOI] [PubMed] [Google Scholar]
- 147.Bogomolnaya L.M., Santiviago C.A., Yang H.J., Baumler A.J., Andrews-Polymenis H.L. ‘Form variation’ of the O12 antigen is critical for persistence of Salmonella Typhimurium in the murine intestine. Mol. Microbiol. 2008;70:1105–1119. doi: 10.1111/j.1365-2958.2008.06461.x. [DOI] [PubMed] [Google Scholar]
- 148.Faucher S.P., Forest C., Beland M., Daigle F. A novel PhoP-regulated locus encoding the cytolysin ClyA and the secreted invasin TaiA of Salmonella enterica serovar Typhi is involved in virulence. Microbiology. 2009;155:477–488. doi: 10.1099/mic.0.022988-0. [DOI] [PubMed] [Google Scholar]
- 149.Jofre M.R., Rodriguez L.M., Villagra N.A., Hidalgo A.A., Mora G.C., Fuentes J.A. RpoS integrates CRP, Fis, and PhoP signaling pathways to control Salmonella Typhi hlyE expression. BMC Microbiol. 2014;14:139. doi: 10.1186/1471-2180-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Levine M.M., Robins-Browne R.M. Factors that explain excretion of enteric pathogens by persons without diarrhea. Clin. Infect. Dis. 2012;55:S303–S311. doi: 10.1093/cid/cis789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Di Domenico E.G., Cavallo I., Pontone M., Toma L., Ensoli F. Biofilm Producing Salmonella Typhi: Chronic Colonization and Development of Gallbladder Cancer. Int. J. Mol. Sci. 2017;18:1887. doi: 10.3390/ijms18091887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Broz P., Ohlson M.B., Monack D.M. Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes. 2012;3:62–70. doi: 10.4161/gmic.19141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boyle E.C., Brown N.F., Finlay B.B. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell. Microbiol. 2006;8:1946–1957. doi: 10.1111/j.1462-5822.2006.00762.x. [DOI] [PubMed] [Google Scholar]
- 154.Cazorla S.I., Maldonado-Galdeano C., Weill R., De Paula J., Perdigon G.D.V. Oral Administration of Probiotics Increases Paneth Cells and Intestinal Antimicrobial Activity. Front. Microbiol. 2018;9:736. doi: 10.3389/fmicb.2018.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang L., Gui S., Liang Z., Liu A., Chen Z., Tang Y., Xiao M. Cecropin (Mdc) Alleviates-Induced Colonic Mucosal Barrier Impairment: Associating With Inflammatory and Oxidative Stress Response, Tight Junction as Well as Intestinal Flora. Front. Microbiol. 2019;10:522. doi: 10.3389/fmicb.2019.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang L., Zhao X., Xia X., Zhu C., Zhang H., Qin W., Xu Y. Inhibitory Effects of Antimicrobial Peptide JH-3 on Serovar Typhimurium Strain CVCC541 Infection-Induced Inflammatory Cytokine Release and Apoptosis in RAW264.7 Cells. Molecules. 2019;24:596. doi: 10.3390/molecules24030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fan J., Petersen E.M., Hinds T.R., Zheng N., Miller S.I. Structure of an Inner Membrane Protein Required for PhoPQ-Regulated Increases in Outer Membrane Cardiolipin. MBio. 2020;11 doi: 10.1128/mBio.03277-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gayet R., Bioley G., Rochereau N., Paul S., Corthesy B. Vaccination against Salmonella Infection: The Mucosal Way. Microbiol. Mol. Biol. Rev. 2017;81 doi: 10.1128/MMBR.00007-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mabbott N.A., Donaldson D.S., Ohno H., Williams I.R., Mahajan A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao L., Ezak T., Li Z.Y., Kawamura Y., Hirose K., Watanabe H. Vi-Suppressed wild strain Salmonella typhi cultured in high osmolarity is hyperinvasive toward epithelial cells and destructive of Peyer’s patches. Microbiol. Immunol. 2001;45:149–158. doi: 10.1111/j.1348-0421.2001.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 161.Wang K.C., Huang C.H., Huang C.J., Fang S.B. Impacts of Salmonella enterica Serovar Typhimurium and Its speG Gene on the Transcriptomes of In Vitro M Cells and Caco-2 Cells. PLoS ONE. 2016;11:e0153444. doi: 10.1371/journal.pone.0153444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lim K.H., Staudt L.M. Toll-like receptor signaling. Cold Spring Harb. Perspect. Biol. 2013;5:a011247. doi: 10.1101/cshperspect.a011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elinav E., Strowig T., Henao-Mejia J., Flavell R.A. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 164.Kanneganti T.D., Lamkanfi M., Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 165.Tam M.A., Rydström A., Sundquist M., Wick M.J. Early cellular responses to Salmonella infection: Dendritic cells, monocytes, and more. Immunol. Rev. 2008;225:140–162. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 166.Tükel C., Nishimori J.H., Wilson R.P., Winter M.G., Keestra A.M., van Putten J.P.M., Bäumler A.J. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cell. Microbiol. 2010;12:1495–1505. doi: 10.1111/j.1462-5822.2010.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Oppong G.O., Rapsinski G.J., Newman T.N., Nishimori J.H., Biesecker S.G., Tükel Ç. Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica serovar Typhimurium curli fibrils in the gut. Infect. Immun. 2013;81:478–486. doi: 10.1128/IAI.00453-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Van Bergenhenegouwen J., Kraneveld A.D., Rutten L., Garssen J., Vos A.P., Hartog A. Lipoproteins attenuate TLR2 and TLR4 activation by bacteria and bacterial ligands with differences in affinity and kinetics. BMC Immunol. 2016;17:42. doi: 10.1186/s12865-016-0180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rathinam V.A.K., Zhao Y., Shao F. Innate immunity to intracellular LPS. Nat. Immunol. 2019;20:527–533. doi: 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vatanen T., Kostic A.D., d’Hennezel E., Siljander H., Franzosa E.A., Yassour M., Kolde R. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mikołajczyk A., Kozłowska A., Gonkowski S. Distribution and Neurochemistry of the Porcine Ileocaecal Valve Projecting Sensory Neurons in the Dorsal Root Ganglia and the Influence of Lipopolysaccharide from Different Serotypes of spp. on the Chemical Coding of DRG Neurons in the Cell Cultures. Int. J. Mol. Sci. 2018;19:2551. doi: 10.3390/ijms19092551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shaio M.F., Rowland H. Bactericidal and opsonizing effects of normal serum on mutant strains of Salmonella typhimurium. Infect. Immun. 1985;49:647–653. doi: 10.1128/IAI.49.3.647-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Avraham R., Haseley N., Brown D., Penaranda C., Jijon H.B., Trombetta J.J., Satija R. Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses. Cell. 2015;162:1309–1321. doi: 10.1016/j.cell.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cian M.B., Giordano N.P., Masilamani R., Minor K.E., Dalebroux Z.D. Salmonella enterica Serovar Typhimurium Uses PbgA/YejM To Regulate Lipopolysaccharide Assembly during Bacteremia. Infect. Immun. 2019;88 doi: 10.1128/IAI.00758-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen H.D., Groisman E.A. The biology of the PmrA/PmrB two-component system: The major regulator of lipopolysaccharide modifications. Annu. Rev. Microbiol. 2013;67 doi: 10.1146/annurev-micro-092412-155751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zeng H., Carlson A.Q., Guo Y., Yu Y., Collier-Hyams L.S., Madara J.L., Gewirtz A.T. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 177.Lahiri A., Lahiri A., Das P., Vani J., Shaila M.S., Chakravortty D. TLR 9 activation in dendritic cells enhances salmonella killing and antigen presentation via involvement of the reactive oxygen species. PLoS ONE. 2010;5:e13772. doi: 10.1371/journal.pone.0013772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tursi S.A., Tükel Ç. Curli-Containing Enteric Biofilms Inside and Out: Matrix Composition, Immune Recognition, and Disease Implications. Microbiol. Mol. Biol. Rev. 2018;82 doi: 10.1128/MMBR.00028-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yamamoto M., Takeda K. Current views of toll-like receptor signaling pathways. Gastroenterol. Res. Pract. 2010;2010:240365. doi: 10.1155/2010/240365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Raffatellu M., Chessa D., Wilson R.P., Tukel C., Akcelik M., Baumler A.J. Capsule-mediated immune evasion: A new hypothesis explaining aspects of typhoid fever pathogenesis. Infect. Immun. 2006;74:19–27. doi: 10.1128/IAI.74.1.19-27.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wilson R.P., Raffatellu M., Chessa D., Winter S.E., Tukel C., Baumler A.J. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell. Microbiol. 2008;10:876–890. doi: 10.1111/j.1462-5822.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 182.Tran Q.T., Gomez G., Khare S., Lawhon S.D., Raffatellu M., Baumler A.J., Ajithdoss D. The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect. Immun. 2010;78:527–535. doi: 10.1128/IAI.00972-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Winter S.E., Raffatellu M., Wilson R.P., Russmann H., Baumler A.J. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell. Microbiol. 2008;10:247–261. doi: 10.1111/j.1462-5822.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 184.Rolhion N., Furniss R.C., Grabe G., Ryan A., Liu M., Matthews S.A., Holden D.W. Inhibition of Nuclear Transport of NF-kB p65 by the Salmonella Type III Secretion System Effector SpvD. PLoS Pathog. 2016;12:e1005653. doi: 10.1371/journal.ppat.1005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mazurkiewicz P., Thomas J., Thompson J.A., Liu M., Arbibe L., Sansonetti P., Holden D.W. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases. Mol. Microbiol. 2008;67:1371–1383. doi: 10.1111/j.1365-2958.2008.06134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li H., Xu H., Zhou Y., Zhang J., Long C., Li S., Chen S. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- 187.Alpuche-Aranda C.M., Racoosin E.L., Swanson J.A., Miller S.I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J. Exp. Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cirillo D.M., Valdivia R.H., Monack D.M., Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 189.Groisman E.A. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thompson J.A., Liu M., Helaine S., Holden D.W. Contribution of the PhoP/Q regulon to survival and replication of Salmonella enterica serovar Typhimurium in macrophages. Microbiology. 2011;157:2084–2093. doi: 10.1099/mic.0.048926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Klein J.A., Grenz J.R., Slauch J.M. Controlled Activity of the Salmonella Invasion-Associated Injectisome Reveals Its Intracellular Role in the Cytosolic Population. MBio. 2017;8 doi: 10.1128/mBio.01931-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yu X.J., Liu M., Holden D.W. Salmonella Effectors SseF and SseG Interact with Mammalian Protein ACBD3 (GCP60) To Anchor Salmonella-Containing Vacuoles at the Golgi Network. MBio. 2016;7 doi: 10.1128/mBio.00474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Keestra A.M., Winter M.G., Klein-Douwel D., Xavier M.N., Winter S.E., Kim A., Tsolis R.M. A Salmonella virulence factor activates the NOD1/NOD2 signaling pathway. MBio. 2011;2 doi: 10.1128/mBio.00266-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Geddes K., Rubino S., Streutker C., Cho J.H., Magalhaes J.G., Le Bourhis L., Selvanantham T. Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect. Immun. 2010;78:5107–5115. doi: 10.1128/IAI.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 196.Ferrand J., Ferrero R.L. Recognition of Extracellular Bacteria by NLRs and Its Role in the Development of Adaptive Immunity. Front. Immunol. 2013;4:344. doi: 10.3389/fimmu.2013.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reyes Ruiz V.M., Ramirez J., Naseer N., Palacio N.M., Siddarthan I.J., Yan B.M., Boyer M.A. Broad detection of bacterial type III secretion system and flagellin proteins by the human NAIP/NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA. 2017;114:13242–13247. doi: 10.1073/pnas.1710433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Matusiak M., Van Opdenbosch N., Vande Walle L., Sirard J.-C., Kanneganti T.-D., Lamkanfi M. Flagellin-induced NLRC4 phosphorylation primes the inflammasome for activation by NAIP5. Proc. Natl. Acad. Sci. USA. 2015;112:1541–1546. doi: 10.1073/pnas.1417945112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Qu Y., Misaghi S., Izrael-Tomasevic A., Newton K., Gilmour L.L., Lamkanfi M., Louie S. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 200.Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 201.Meunier E., Dick M.S., Dreier R.F., Schurmann N., Kenzelmann Broz D., Warming S., Roose-Girma M. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 202.Bierschenk D., Monteleone M., Moghaddas F., Baker P.J., Masters S.L., Boucher D., Schroder K. The Salmonella pathogenicity island-2 subverts human NLRP3 and NLRC4 inflammasome responses. J. Leukoc. Biol. 2019;105:401–410. doi: 10.1002/JLB.MA0318-112RR. [DOI] [PubMed] [Google Scholar]
- 203.Drecktrah D., Knodler L.A., Howe D., Steele-Mortimer O. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic. 2007;8:212–225. doi: 10.1111/j.1600-0854.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Oh Y.K., Alpuche-Aranda C., Berthiaume E., Jinks T., Miller S.I., Swanson J.A. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect. Immun. 1996;64:3877–3883. doi: 10.1128/IAI.64.9.3877-3883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jantsch J., Chikkaballi D., Hensel M. Cellular aspects of immunity to intracellular Salmonella enterica. Immunol. Rev. 2011;240:185–195. doi: 10.1111/j.1600-065X.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- 206.Vazquez-Torres A., Xu Y., Jones-Carson J., Holden D.W., Lucia S.M., Dinauer M.C., Mastroeni P. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 207.Fang F.C. Antimicrobial actions of reactive oxygen species. MBio. 2011;2 doi: 10.1128/mBio.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bourret T.J., Song M., Vazquez-Torres A. Codependent and independent effects of nitric oxide-mediated suppression of PhoPQ and Salmonella pathogenicity island 2 on intracellular Salmonella enterica serovar typhimurium survival. Infect. Immun. 2009;77:5107–5115. doi: 10.1128/IAI.00759-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Henard C.A., Vazquez-Torres A. Nitric oxide and salmonella pathogenesis. Front. Microbiol. 2011;2:84. doi: 10.3389/fmicb.2011.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Eswarappa S.M., Negi V.D., Chakraborty S., Chandrasekhar Sagar B.K., Chakravortty D. Division of the Salmonella-containing vacuole and depletion of acidic lysosomes in Salmonella-infected host cells are novel strategies of Salmonella enterica to avoid lysosomes. Infect. Immun. 2010;78:68–79. doi: 10.1128/IAI.00668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Van der Velden A.W., Lindgren S.W., Worley M.J., Heffron F. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype typhimurium. Infect. Immun. 2000;68:5702–5709. doi: 10.1128/IAI.68.10.5702-5709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fink S.L., Cookson B.T. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 213.Brennan M.A., Cookson B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 214.Miao E.A., Alpuche-Aranda C.M., Dors M., Clark A.E., Bader M.W., Miller S.I., Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 215.Franchi L., Amer A., Body-Malapel M., Kanneganti T.D., Ozoren N., Jagirdar R., Inohara N. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 216.Monack D.M., Detweiler C.S., Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell. Microbiol. 2001;3:825–837. doi: 10.1046/j.1462-5822.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 217.Ygberg S.E., Clements M.O., Rytkonen A., Thompson A., Holden D.W., Hinton J.C., Rhen M. Polynucleotide phosphorylase negatively controls spv virulence gene expression in Salmonella enterica. Infect. Immun. 2006;74:1243–1254. doi: 10.1128/IAI.74.2.1243-1254.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Navarre W.W., Zychlinsky A. Pathogen-induced apoptosis of macrophages: A common end for different pathogenic strategies. Cell. Microbiol. 2000;2:265–273. doi: 10.1046/j.1462-5822.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 219.Knodler L.A. Salmonella enterica: Living a double life in epithelial cells. Curr. Opin. Microbiol. 2015;23:23–31. doi: 10.1016/j.mib.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 220.Knodler L.A., Vallance B.A., Celli J., Winfree S., Hansen B., Montero M., Steele-Mortimer O. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. USA. 2010;107:17733–17738. doi: 10.1073/pnas.1006098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Malik-Kale P., Winfree S., Steele-Mortimer O. The bimodal lifestyle of intracellular Salmonella in epithelial cells: Replication in the cytosol obscures defects in vacuolar replication. PLoS ONE. 2012;7:e38732. doi: 10.1371/journal.pone.0038732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Beuzon C.R., Salcedo S.P., Holden D.W. Growth and killing of a Salmonella enterica serovar Typhimurium sifA mutant strain in the cytosol of different host cell lines. Microbiology. 2002;148:2705–2715. doi: 10.1099/00221287-148-9-2705. [DOI] [PubMed] [Google Scholar]
- 223.Tattoli I., Sorbara M.T., Vuckovic D., Ling A., Soares F., Carneiro L.A.M., Yang C. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe. 2012;11:563–575. doi: 10.1016/j.chom.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 224.Cheminay C., Mohlenbrink A., Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J. Immunol. 2005;174:2892–2899. doi: 10.4049/jimmunol.174.5.2892. [DOI] [PubMed] [Google Scholar]
- 225.Brink T., Leiss V., Siegert P., Jehle D., Ebner J.K., Schwan C., Shymanets A. Salmonella Typhimurium effector SseI inhibits chemotaxis and increases host cell survival by deamidation of heterotrimeric Gi proteins. PLoS Pathog. 2018;14:e1007248. doi: 10.1371/journal.ppat.1007248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hormaeche C.E., Harrington K.A., Joysey H.S. Natural resistance to salmonellae in mice: Control by genes within the major histocompatibility complex. J. Infect. Dis. 1985;152:1050–1056. doi: 10.1093/infdis/152.5.1050. [DOI] [PubMed] [Google Scholar]
- 227.Nauciel C., Ronco E., Guenet J.L., Pla M. Role of H-2 and non-H-2 genes in control of bacterial clearance from the spleen in Salmonella typhimurium-infected mice. Infect. Immun. 1988;56:2407–2411. doi: 10.1128/IAI.56.9.2407-2411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mitchell E.K., Mastroeni P., Kelly A.P., Trowsdale J. Inhibition of cell surface MHC class II expression by Salmonella. Eur. J. Immunol. 2004;34:2559–2567. doi: 10.1002/eji.200425314. [DOI] [PubMed] [Google Scholar]
- 229.Bayer-Santos E., Durkin C.H., Rigano L.A., Kupz A., Alix E., Cerny O., Jennings E. The Salmonella Effector SteD Mediates MARCH8-Dependent Ubiquitination of MHC II Molecules and Inhibits T cell Activation. Cell Host Microbe. 2016;20:584–595. doi: 10.1016/j.chom.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Godlee C., Cerny O., Durkin C.H., Holden D.W. SrcA is a chaperone for the Salmonella SPI-2 type three secretion system effector SteD. Microbiology. 2019;165:15–25. doi: 10.1099/mic.0.000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tobar J.A., Carreno L.J., Bueno S.M., Gonzalez P.A., Mora J.E., Quezada S.A., Kalergis A.M. Virulent Salmonella enterica serovar typhimurium evades adaptive immunity by preventing dendritic cells from activating T cells. Infect. Immun. 2006;74:6438–6448. doi: 10.1128/IAI.00063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Monack D.M., Bouley D.M., Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J. Exp. Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pie S., Matsiota-Bernard P., Truffa-Bachi P., Nauciel C. Gamma interferon and interleukin-10 gene expression in innately susceptible and resistant mice during the early phase of Salmonella typhimurium infection. Infect. Immun. 1996;64:849–854. doi: 10.1128/IAI.64.3.849-854.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 1992;60:450–454. doi: 10.1128/IAI.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mastroeni P., Harrison J.A., Robinson J.H., Clare S., Khan S., Maskell D.J., Dougan G. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: Role of gamma interferon and macrophage activation. Infect. Immun. 1998;66:4767–4776. doi: 10.1128/IAI.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.MacLennan C., Fieschi C., Lammas D.A., Picard C., Dorman S.E., Sanal O., MacLennan J.M. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J. Infect. Dis. 2004;190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 237.Staretz-Haham O., Melamed R., Lifshitz M., Porat N., Fieschi C., Casanova J.L., Levy J. Interleukin-12 receptor beta1 deficiency presenting as recurrent Salmonella infection. Clin. Infect. Dis. 2003;37:137–140. doi: 10.1086/375229. [DOI] [PubMed] [Google Scholar]
- 238.Mittrucker H.W., Kohler A., Kaufmann S.H. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Eisenstein T.K., Huang D., Meissler J.J., Jr., al-Ramadi B. Macrophage nitric oxide mediates immunosuppression in infectious inflammation. Immunobiology. 1994;191:493–502. doi: 10.1016/S0171-2985(11)80455-9. [DOI] [PubMed] [Google Scholar]
- 240.Pie S., Truffa-Bachi P., Pla M., Nauciel C. Th1 response in Salmonella typhimurium-infected mice with a high or low rate of bacterial clearance. Infect. Immun. 1997;65:4509–4514. doi: 10.1128/IAI.65.11.4509-4514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.MacFarlane A.S., Schwacha M.G., Eisenstein T.K. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect. Immun. 1999;67:891–898. doi: 10.1128/IAI.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J. Immunol. 1990;145:1265–1269. [PubMed] [Google Scholar]
- 243.Mittrucker H.W., Kohler A., Mak T.W., Kaufmann S.H. Critical role of CD28 in protective immunity against Salmonella typhimurium. J. Immunol. 1999;163:6769–6776. [PubMed] [Google Scholar]
- 244.Mei Y., Zhao L., Liu Y., Gong H., Song Y., Lei L., Zhu Y. Combining DNA Vaccine and AIDA-1 in Attenuated Salmonella Activates Tumor-Specific CD4(+) and CD8(+) T-cell Responses. Cancer Immunol. Res. 2017;5:503–514. doi: 10.1158/2326-6066.CIR-16-0240-T. [DOI] [PubMed] [Google Scholar]
- 245.Wahid R., Fresnay S., Levine M.M., Sztein M.B. Cross-reactive multifunctional CD4+ T cell responses against Salmonella enterica serovars Typhi, Paratyphi A and Paratyphi B in humans following immunization with live oral typhoid vaccine Ty21a. Clin. Immunol. 2016;173:87–95. doi: 10.1016/j.clim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mastroeni P., Villarreal-Ramos B., Hormaeche C.E. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro-Salmonella vaccines. Microb. Pathog. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-F. [DOI] [PubMed] [Google Scholar]
- 247.Mittrucker H.W., Raupach B., Kohler A., Kaufmann S.H. Cutting edge: Role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J. Immunol. 2000;164:1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 248.Perez-Shibayama C., Gil-Cruz C., Pastelin-Palacios R., Cervantes-Barragan L., Hisaki E., Chai Q., Onder L. IFN-gamma-producing CD4+ T cells promote generation of protective germinal center-derived IgM + B cell memory against Salmonella Typhi. J. Immunol. 2014;192:5192–5200. doi: 10.4049/jimmunol.1302526. [DOI] [PubMed] [Google Scholar]
- 249.Kantele A., Arvilommi H., Jokinen I. Specific immunoglobulin-secreting human blood cells after peroral vaccination against Salmonella typhi. J. Infect. Dis. 1986;153:1126–1131. doi: 10.1093/infdis/153.6.1126. [DOI] [PubMed] [Google Scholar]
- 250.Tang Y., Foster N., Jones M.A., Barrow P.A. A model of persistent Salmonella infection: Salmonella Pullorum modulates the immune response of the chicken from a Th17 towards a Th2-type response. Infect. Immun. 2018 doi: 10.1128/IAI.00307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Belkaid Y., Harrison O.J. Homeostatic Immunity and the Microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]