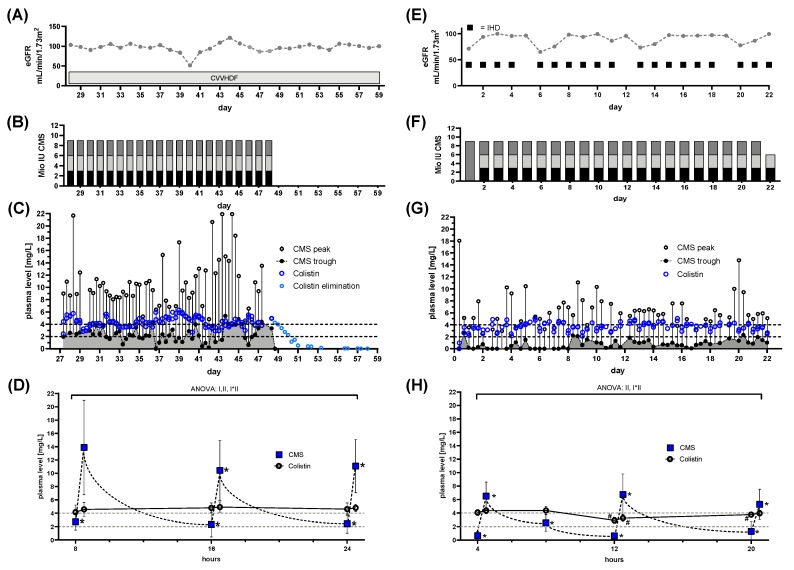
Figure 8.
Renal function, dose regimen and drug plasma levels of patient 6: a total of 236 samples were analyzed. First treatment cycle (A)-(D): (colisthimethate sodium (CMS) peak n = 59, CMS trough n = 55, Colistin peak n = 62, Colistin trough n = 59). (A) estimated glomerular filtration rate (eGFR) derived from serum creatinine levels and renal replacement therapy (RRT) via continuous venovenous hemodiafiltration (CVVHDF). (B) Dose regimen used. Shaded blocks depict the particular individual doses each day. (C) Time course of CMS trough and peak levels along with Colistin levels. The CMS reservoir is indicated by the area gray shaded portion of panel (C). Colistin elimination was measured by continuing monitoring after the end of treatment (light blue dots) and the individual fitted elimination is plotted in Figure 1A. Target range for Colistin plasma levels is indicated by dashed lines. (D) Pooled data of daily administrations of CMS or Colistin levels plasma levels during all days with RRT during the first treatment cycle. (E–H): CMS peak n = 56, CMS trough n = 59, Colistin peak n = 59, Colistin trough n = 56. (A) eGFR derived from serum creatinine levels and RRT via intermittent hemodialysis (IHD). (F) Dose regimen used. Shaded blocks depict the particular individual doses each day. (G) Time course of CMS trough and peak levels along with Colistin levels. The CMS reservoir is indicated by the area gray shaded portion of panel (G). Target range for Colistin plasma levels is indicated by dashed lines. (H) Pooled data of daily administrations of CMS or Colistin levels plasma levels during all days with RRT during the second treatment cycle. Statistics Panel (D): mean ± SD; repeated measures ANOVA. Effect I: Differences between CMS and Colistin levels, p = 0.0095. Effect II: time effect, p < 0.0001. I*II: interaction of effects I&II, p < 0.0001. Post-hoc tests: * p < 0.05 vs. previous CMS plasma level. Panel (H): mean ± SD; repeated measures ANOVA. Effect I: Differences between CMS and Colistin levels, n.s. Effect II: time effect, p < 0.0001. I*II: interaction of effects I&II, p < 0.0001. Post-hoc tests: # p < 0.05 vs. previous Colistin plasma level, * p < 0.05 vs. previous CMS plasma level.