Abstract
Introduction:
Engaging patients to make informed choices is paramount but difficult in busy practices. This study sought to engage patients outside the clinical setting to better understand how they approach cancer screening decisions, including their primary concerns and their preferences for finalizing their decision.
Methods:
Twelve primary care practices offering patients an online personal health record invited eligible patients to complete a 17-item online interactive module. Among 11,458 registered users, invitations to complete the module were sent to adults aged 50–74 years who were overdue for colorectal cancer screening and to women aged 40–49 years and men aged 55–69 who had not undergone a recent mammogram or prostate-specific antigen test, respectively.
Results:
The module was started by 2,355 patients and completed by 903 patients. Most respondents (76.8%) knew they were eligible for screening. Preferred next steps were talking to the clinician (76.6%), reading/research (28.6%), and consulting trusted friends/family (16.4%). Priority topics included how much screening improves life expectancy, comparative test performance, and the prevalence/health risks of the cancer. Leading fears were getting cancer/delayed detection (79.2%), abnormal results (40.5%), and testing complications (39.1%), the last referring to false test results, medical complications, or unnecessary treatments. Men eligible for prostate-specific antigen screening were more likely than women eligible for mammography to express concerns about testing complications and to prioritize weighing pros and cons over gut feelings (p<0.05).
Conclusions:
Although this sample was predisposed to screening, most patients wanted help in finalizing their decision. Many wanted to weigh the pros and cons and expressed fears of potential harms from screening. Understanding how patients approach decisions may help design more effective engagement strategies.
INTRODUCTION
How patients approach preference-sensitive decisions—and the healthcare system’s role in helping them make informed choices—is important to care delivery1-5 but poorly understood.6 Clinicians are expected to educate patients by supplying factual information about the benefits, harms, and uncertainties associated with available options.7-11 Decision aids often assume that patients are willing to engage in a cognitive assessment of tradeoffs, but how patients actually make decisions and the role of scientific information versus intuition are only partially understood.12-16
Guidelines often recommend that clinicians help patients clarify their values and preferences after considering the benefits, harms, and scientific uncertainties for preference-sensitive decisions about cancer screening, such as whether women should begin mammography screening at age 40 years,17 whether men should undergo prostate-specific antigen (PSA) screening,18-20 and which test to choose for colorectal cancer (CRC) screening.21,22 Understanding the journey patients take in making these decisions—the path they take from first considering the decision through the deliberation and consultation that leads to a final choice—is important when designing interventions to support the process. Relatively little is known about the informational preferences, motivations, fears, and beliefs that patients bring to these decisions.23
Research on these issues has often occurred in the clinical setting, where patients’ choices can be observed and they can more easily be recruited for surveys.24 These methods, although expedient, may be inadequate to fully understand the decision-making process. The decision journey for most patients likely begins before they reach the clinical setting and can continue afterward. Engaging people at the outset of decisions could provide important evidence about the resources they use and the extent to which they rely on clinicians for support across the continuum of encounters that follow.
The expanding use of portals, which patients can access outside the clinical encounter, provides a mechanism for studying the path patients take in their decision journey; it can reach them at various stages of the process, even before they first discuss the matter with their clinician.25,26 This study took advantage of a portal for managing preventive services27 to explore how people approach decisions in the three cancer screening scenarios discussed above—where the right choice depends on personal preferences. This article presents data regarding patients’ awareness of cancer screening, primary concerns, and anticipated next steps to finalize a decision. The results were examined by screening test because the surrounding issues vary considerably by test. This is a proof of concept, intended to demonstrate whether the portal tool could be used at the individual level and yield insights about how patients approach decisions.
METHODS
Patient recruitment occurred at 12 primary care practices in Virginia that use MyPreventiveCare (MPC),28 an interactive online patient portal, linked to the practices’ electronic health record, which issues personally tailored recommendations for prevention and chronic care.28-30 The practices belong to a suburban, private practice group that shares the same portal.
Study Sample
The combined MPC/electronic health record database was used to identify three groups of patients with MPC accounts: (1) women aged 40–49 years who had not had a mammogram within 2 years, (2) men aged 55–69 years who had not had a PSA test within 2 years, and (3) adults aged 50–74 years who were not up-to-date with CRC screening based on recommendations of the U.S. Preventive Services Task Force in effect at the time (a colonoscopy or sigmoidoscopy within the past 10 or 5 years, respectively, or a stool blood test within the past year).21,22 From January to August 2014, patients meeting these eligibility criteria were invited to complete an online instrument, the Informed Decision Making (IDM) module. Patients received prompts to do so when they logged onto MPC (Phase 1), or email invitations when they had an upcoming appointment (Phase 2), or all eligible patients were invited regardless of their appointment status (Phase 3). Patients eligible for more than one screening test chose which module they would complete.
Measures
As documented elsewhere,31 the IDM module was developed in 2013 through an iterative year-long process of intensive stakeholder engagement involving focus groups, advisory boards, cognitive testing, and usability testing. Patients, clinicians, content experts, and health systems helped review the module’s wording, content, and usability. The resulting module included 17 questions; Table 1 summarizes the questions and Appendix Table 1 (available online) provides the response options. Module questions were derived from both validated questions in other instruments and those developed specifically for this project based on stakeholder input.32-38
Table 1.
Items in the Informed Decision-Making (IDM) Module
| Questions | Item number |
|---|---|
| Items analyzed in this report | |
| Awareness of screening | |
| Have you previously heard that you should or should not have [cancer screening test]? | 1 |
| Plans for screening | |
| How far along are you with making a decision about [cancer screening test]? | 2 |
| If you had to make a choice, what would you do next to decide whether to get [cancer screening test]? | 3 |
| What steps do you want to take next? | 10 |
| Which of the following would you like to do? | 11 |
| Priority topics | |
| For the items below, select how important each is to you. | 4 |
| How important are the following fears or worries to your decision about [cancer screening test]? | 9 |
| Preferred sources of reading and research | |
| When you do your own reading and research for a decision like cancer screening, how helpful are…? Role of intuition |
6 |
| Role of intuition | |
| How important to you are your gut feelings or instincts about cancer screening compared with weighing the pros and cons? | 8 |
| Confidence going forward | |
| Please click the button that best describes how you feel about your decision. | 12 |
| Items not analyzed in this report | |
| Choose the option that shows how you would want the topic explained and then adjust the slider to the level of detail you desire for the topic.a | 5 |
| What is the best way for you to use statistics, like numbers and percentages, to learn what to expect from different screening options?a | 7 |
| Would you like to discuss [cancer screening decision] at your next appointment? MyPreventiveCare can notify your clinician.a | 13 |
| What about [cancer screening test] would you like to discuss with your clinician?a | 14 |
| For the [cancer screening test] decision, slide the slider to the phrase that reflects the role you would prefer with your clinician.a | 15 |
| [consent form for audiorecording of visit]a | 16 |
| Please give us feedback on MyQuestions by indicating how much you agree or disagree.a | 17 |
Note: Appendix Table 1 (available online) provides full list of response options.
Data from responses to these questions not reported in this article.
The IDM module also served as a customized educational resource that delivered information interactively based on patients’ responses. An Action Page featured four tiles addressing the topics patients identified previously as very important; patients who clicked on the tiles were presented with information on the desired topic in the format they preferred (i.e., words, numbers, pictures, or stories) and with a full library of information resources drawn from reputable evidence-based sources (e.g., National Cancer Institute; Appendix Figure 1, available online).
Statistical Analysis
MPC provided data on the selected cancer screening topic and IDM responses. The practice electronic health record provided data on patient characteristics. Categorical and continuous variables were summarized in aggregate and by cancer type. SAS/STAT, version 9.4 was used to conduct chi-square tests to compare IDM responses across the three cancer types.
RESULTS
Of 11,458 eligible patients who visited the 12 practices during the three recruitment periods, 11,094 were contacted or received prompts. Of these, 2,355 (21.2%) patients started the IDM module. The last question was answered by 903 (38.3%) of these patients (8.1% of eligible patients). The attrition pattern is discussed in detail elsewhere.39 The greatest attrition occurred early in the module; most (68.3%) respondents who answered question 6 finished the module. Participation differed significantly by type of screening (p<0.001): the IDM was started by 17.6% of women aged 40–49 years who were eligible for mammography screening, 20.4% of patients overdue for CRC screening, and 34.3% of men eligible for PSA screening; the corresponding completion rates also differed significantly by screening type (5.3%, 8.0%, and 16.4%, respectively, p<0.0001). Patients eligible for breast cancer, CRC, and PSA screening differed by age and gender (Table 2). As reported elsewhere,27 use of the IDM module was lower among women, patients with no prior screening, Hispanics, Asians, patients who preferred a language other than English, and uninsured patients. As reported elsewhere, portal users were older, more likely to have comorbidities, and less likely to be African American or Hispanic.28
Table 2.
Demographic Characteristics of Patients Who Started the Informed Decision-Making Module
| Screening subgroups | ||||
|---|---|---|---|---|
| Characteristics | Total | Breast cancer | Colorectal cancer | Prostate cancer |
| Mean age, years (SD, N)a | 54.0 (8.27, 2,355) | 43.8 (3.01, 638) | 56.8 (6.24, 1,249) | 60.7 (4.72, 468) |
| Gendera | ||||
| Male | 1,067 (45.3) | 0 (0.0) | 601 (48.1) | 466 (99.6) |
| Female | 1,288 (54.7) | 638 (100.0) | 648 (51.9) | 2 (0.4)b |
| Racea | ||||
| Asian | 192 (9.0) | 83 (14.3) | 85 (7.5) | 24 (5.7) |
| African American | 125 (5.9) | 40 (6.9) | 65 (5.8) | 20 (4.8) |
| White | 1,637 (76.9) | 405 (69.8) | 885 (78.5) | 347 (82.4) |
| Other | 110 (5.2) | 40 (6.9) | 47 (4.2) | 23 (5.5) |
| Unreported | 65 (3.1) | 12 (2.1) | 46 (4.1) | 7 (1.7) |
| Ethnicity | ||||
| Hispanic/Latino | 81 (4.0) | 24 (4.3) | 42 (3.9) | 15 (3.7) |
| Non-Hispanic/Latino | 1,723 (84.3) | 479 (85.5) | 897 (83.3) | 347 (85.3) |
| Unknown | 240 (11.7) | 57 (10.2) | 138 (12.8) | 45 (11.1) |
| Language | ||||
| English | 1,856 (85.6) | 509 (87.5) | 977 (84.5) | 370 (86.1) |
| Other | 312 (14.4) | 73 (12.5) | 179 (15.5) | 60 (13.9) |
| Insurance type | ||||
| Private | 2,014 (92.9) | 582 (100.0) | 1,052 (91.0) | 380 (88.4) |
| Public (Medicare/Medicaid) | 154 (7.1) | 0 (0) | 104 (9.0) | 50 (11.6) |
Note: Total respondents (N) was 2,355 for questions on age and gender, 2,129 for race, 2,044 for ethnicity, and 2,168 for language and insurance type. Data are shown as n (%) unless otherwise noted.
p<0.001 across screening subgroups.
Programming error in which system misclassified women as eligible for prostate cancer screening.
Most patients (n=1,810, 76.9%) had previously heard they “should or should not have screening.” Awareness was greater among those overdue for CRC screening (83.5%) than those eligible for mammography (71.3%) or PSA (66.7%) screening (p<0.001). Of those who specified how they heard about screening (n=1,791), the most commonly noted source (85.7%) was the clinician. A sizable minority of respondents reported having read or heard about screening from the media (48.2%) or other individuals (e.g., family/friends; 38.7%). Men eligible for PSA screening were less likely to have heard about PSA screening from others (25.4%) than were patients eligible for breast or CRC screening (40.9% and 41.8%, respectively, p<0.001).
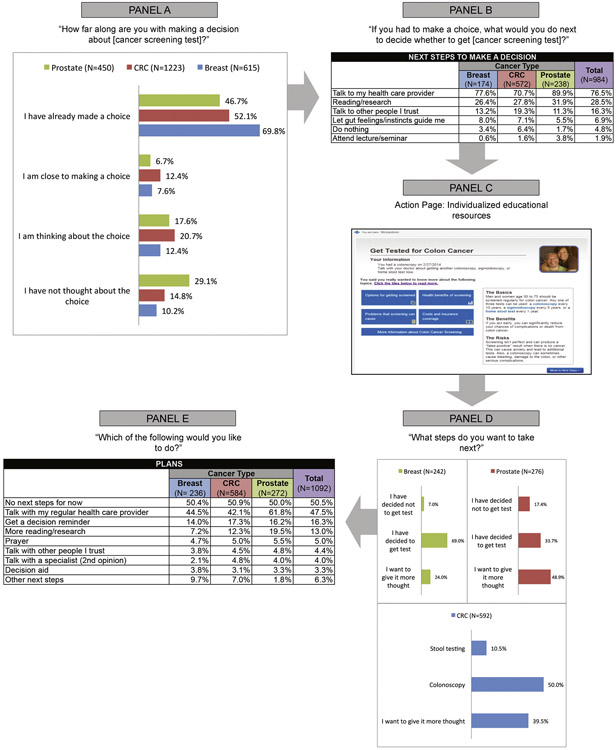
Figure 1 charts the evolution of patients’ screening intentions before and after exposure to the personalized educational material on the Action Page. At the start of the module, approximately half the respondents (55.8%) reported that they had “already made a choice” about screening; 16.4% reported that they had not yet thought about it (Figure 1, Panel A). Patients eligible for mammography were more likely to have made a decision than men in the PSA group (p<0.001). When asked what they would do next to make a decision, most patients (76.5%) chose talking with their clinician, a more common response among men eligible for PSA screening than among the two other groups (p<0.001). Patients in the CRC group were more likely than other patients to want to consult others they trust (p=0.014; Figure 1, Panel B). After seeing the personalized educational material on the Action Page (Figure 1, Panel C), plans to get screened were more common among patients eligible for mammography (69.0%) and CRC screening (60.5%) than those eligible for PSA screening (33.7%, p=0.001; Figure 1, Panel D). Men in the PSA group were more likely than other patients to say they wanted to give it more thought or to have decided against screening (p<0.001). Half (50.5%) of all respondents planned no immediate next steps (Figure 1, Panel E). Among this subgroup planning no next steps, 25.5% of respondents (and 39.0% of men eligible for PSA screening) indicated they still planned to talk with their clinician.
Figure 1.
The decision journey: screening plans before and after exposure to action page. CRC, colorectal cancer.
Reading and research were selected more often by men in the PSA group (19.5%) than by those eligible for mammography (7.2%) or CRC (12.3%) screening (p<0.001). Across all three groups, patients were as likely to seek spiritual guidance as to talk with family, friends, or specialists.
When asked to choose questions that were very important to their screening decision, the leading choice for all groups was the survival benefits of screening (78.0%), followed by the comparative performance of tests (75.6%; Table 3; Appendix Table 2 [available online]). The potential harms of screening were very important to 68.2% of patients, a greater concern among patients eligible for mammography (72.8%) and PSA (70.6%) screening than among the CRC group (65.3%) (p=0.010). Topics least often selected as very important were practice guidelines (58.4%) and questions about insurance and costs (56.5%). Insurance and costs were less important to men in the PSA group (44.6%) than to patients eligible for mammography (66.4%) or CRC (56.4%) screening (p<0.001). Compared with other groups, women eligible for mammography assigned greater importance to survival benefits, comparative test performance, and the frequency and danger of cancer (p≤0.013). Information about how the tests are performed was significantly more important to patients eligible for CRC screening than to those in the other groups (p<0.001).
Table 3.
Topics Identified by Respondents as “Very Important”
| Screening subgroups | ||||
|---|---|---|---|---|
| Topic | Total | Breast cancer |
Colorectal cancer |
Prostate cancer |
| Questions | ||||
| Total, N | 1,801 | 441 | 999 | 361 |
| How much would screening improve my chances of living longer? | 1,404 (78.0) | 381 (86.4)a | 749 (75.0) | 274 (75.9) |
| Does one kind of screening test work better than another? | 1,362 (75.6) | 358 (81.2)b | 731 (73.2) | 273 (75.6) |
| How common or how dangerous is the cancer? | 1,320 (73.3) | 347 (78.7)c | 712 (71.3)d | 261 (72.3) |
| What are my options (choices) for getting screened or not? | 1,263 (70.1) | 328 (74.4) | 688 (68.9) | 247 (68.4) |
| Are there problems that screening might cause? | 1,228 (68.2) | 321 (72.8)e | 652 (65.3)a | 255 (70.6) |
| How is the screening test performed? | 1,113 (61.8) | 272 (61.7) | 652 (65.3)a,d | 189 (52.4)a |
| What are the screening guidelines of expert organizations? | 1,052 (58.4) | 272 (61.7) | 570 (57.1)d | 210 (58.2) |
| What are the costs and what will my insurance cover? | 1,017 (56.5) | 293 (66.4) | 563 (56.4)d | 161 (44.6)a |
| Please specify what else you would like to know.f | 354 (24.7) | 99 (28.3) | 194 (24.4) | 61 (21.3) |
| Fears or worries | ||||
| Total, N | 1,166 | 247 | 639 | 280 |
| Getting cancer or not catching cancer early enough | 927 (79.5) | 212 (85.8)a | 499 (78.1) | 216 (77.1) |
| Getting bad news when the results come back | 478 (41.0) | 123 (49.8)a | 262 (41.0) | 93 (33.2) |
| Having complications from the screening test | 456 (39.1) | 85 (34.4) | 254 (39.7) | 117 (41.8) |
| Costs of screening | 254 (21.8) | 64 (25.9) | 144 (22.5) | 46 (16.4) |
| Going to clinicians | 225 (19.3) | 54 (21.9) | 118 (18.5) | 53 (18.9) |
| Regretting my decision | 184 (15.8) | 44 (17.8) | 107 (16.7) | 33 (11.8) |
| Pain/embarrassment of the test | 141 (12.1) | 24 (9.7) | 100 (15.6)a | 17 (6.1) |
| Specify what else you are worried aboutg | 73 (9.1) | 13 (7.6) | 38 (8.6) | 22 (11.5) |
Note: AppendixTable 2 (available online) provides data on topics identified as “somewhat important” or “not that important.” Data are shown as n (%) unless otherwise noted.
p≤0.001 in comparison with the two other screening groups.
p≤0.005 in comparison with the two other screening groups.
p≤0.013 in comparison with the two other screening groups.
Denominator=998.
p=0.010 in comparison with the two other screening groups.
Smaller denominators answered this question: breast (n=350), colorectal (n=794), prostate (n=287) screening.
Smaller denominators answered this question: breast (n=172), colorectal (n=440), prostate (n=192) screening.
When asked to choose fears or worries about screening that were very important, almost twice as many patients chose getting cancer or not catching cancer early enough (79.5%) over the next greatest fear (getting abnormal results, 41.0%; Table 3). Almost as many patients (39.1%) were fearful of complications from screening. Among men eligible for PSA screening, fear of complications outranked the fear of getting abnormal results (41.8% vs 33.2%). For the women eligible for mammography, the fear of getting cancer or delayed detection (85.8%) and the fear of abnormal results (49.8%) exceeded corresponding values in the other groups (p<0.001). Patients eligible for CRC screening assigned greater importance to fear of pain or embarrassment from the test (15.6%) than did patients in the mammography (9.7%) or PSA (6.1%) groups (p<0.001).
More patients (55.7%) chose Internet research or other online information as most helpful compared with educational materials from a doctor’s office (45.5%; Appendix Table 3, available online). Only 21.3% chose print publications in magazines or newspapers. TV and commercial videos were chosen more often by women eligible for mammography (15.2%) than by patients in the CRC and PSA groups (8.1% and 6.6%, respectively, p<0.001).
When given a choice between weighing pros and cons and relying on “gut feelings” and instincts to reach a decision, 60.4% of patients said weighing pros and cons was more important, whereas 16.2% of patients said that gut feelings/instincts were more important. The role of intuition varied across types of screening. As shown in Appendix Figure 2 (available online), men eligible for PSA screening assigned greater importance to weighing pros and cons than did patients in the two other groups, whereas patients eligible for mammography were more likely than others to say they would rely on gut feelings/instincts (p<0.001).
Most patients expressed confidence in their knowledge and decision (Appendix Figure 3, available online). Men eligible for PSA screening were less likely than others to strongly agree about their knowledge of benefits and risks, which benefits and risks were important to them, having enough support, and feeling sure about the best choice (p<0.001).
DISCUSSION
This proof of concept tested whether the IDM module could elucidate the decision-making preferences of patients facing complex decisions. As in other studies,40-43 it showed that most patients were predisposed to screening. Most expressed confidence in their decisions and cited fears of getting cancer or delayed detection as very important to their decision. Nonetheless, most patients—even those planning to be screened—also wanted help in finalizing their decision. They particularly wanted help from their clinician but also from research and personal contacts. The information needs they cited most frequently as very important to their decision were survival benefits, comparative effectiveness of tests, and dangers posed by the target cancer.
Despite the common concern that patients overlook the potential harms of screening,40,44 patients in this study expressed interest in weighing pros and cons, and two of three patients cited potential harms as very important to their decision. Widely discussed cancer screening determinants seemed less important to these respondents, such as concerns about coverage and costs, perhaps reflecting recent expansions in coverage for preventive services or that 92.9% of the sample had private insurance. In this sample, the often controversial screening guidelines issued by expert organizations were less important than other concerns. Pain and embarrassment from screening ranked last as a fear or worry, even among patients facing CRC screening, a population that normally places greater emphasis on these concerns.45,46
In this study, as in other research,47,48 patients expressed varied decision-making preferences. The psychology literature speaks of a dual process49-51 for assessing risk: (1) rational deliberation based on logic and evidence (classical decision theory, consumerist model); and (2) affective, experiential heuristics that entail instinct, intuition, and feelings.52-62 In this study, these tendencies varied by screening test. For example, candidates for mammography were more likely than those considering PSA testing to rely on gut feelings and instincts rather than weighing pros and cons. Patients considering CRC screening were more fearful about pain or embarrassment from the test.
Attitudes toward screening and expressed needs depend on where patients are in their decision journey.27 Although 55.8% of patients in this sample had made a decision, 16.4% had not yet thought about it. Accordingly, point-in-time (e.g., waiting room) surveys that offer snapshots at any given moment in that journey may paint an incomplete picture of how patients’ needs evolve over time.63
Most patients chose consultation with their clinician as the preferred next step, but many clinicians lack the time, reimbursement, medicolegal protections, and proficiency for the in-depth discussions required to meet the needs identified in this study.64-69 Studies show that clinicians often fail to address questions, discuss risks, and accommodate personal preferences,70-82 and patients are often reluctant to press for more information.83,84 Decision aids can supply objective information on options,85 but their integration into clinical practice has been challenging.86,87
Information technology can expand the reach of decision support while better determining where patients are in their decision journey, clarifying individual needs, and engaging in a shared assessment of pros and cons.25,88 Applications like the IDM module offer patients a new platform to stimulate thinking about key questions and next steps, obtain relevant educational materials that are vetted for quality and communicate details that clinicians often lack at their fingertips during appointments, and forward preferences and information to clinicians ahead of appointments. For clinicians, outsourcing such tasks to an interactive online environment allows limited encounter time to focus on core relationship issues that a virtual interface cannot address.89-91 The online medium also allows clinicians to communicate with patients and offer the guidance that this study indicates is highly valued in guiding decisions.
Currently, such resources cater preferentially to more activated patients and early adopters of new technology. Time is required before other patients, and their clinicians, can adjust to unfamiliar models of care. In this study, only 21.0% of eligible patients began the IDM module and only 8.1% completed it. A previously published examination of the attrition observed in this study suggests that the instrument’s length and certain problematic questions may have dampened the response rate.39 Whether a shortened module or other refinements to ease completion would improve uptake remains to be tested. Secular trends in the expanding use of portals could make such decision support platforms especially important as patients grow more accustomed to this interface.92 Long-term acceptance and how use of such tools will differ by age, race/ethnicity, and other demographics are not known.
The population in this study consisted largely of white, English-speaking patients with private health insurance coverage. These demographics and the low completion rate may affect the generalizability and interpretation of the results. For example, patients from other backgrounds might express different preferences for using online educational resources or for weighing pros and cons rather than relying on gut feelings. This study neither can claim that the preferences exhibited by this particular patient sample are generalizable to all patients in all health systems, nor was it expected to. This proof of concept was intended to demonstrate whether the IDM module can capture these types of data. Future applications in diverse populations can provide more generalizable, as well as longitudinal, evidence about the decision-making preferences of patients facing different types of complex decisions.
Limitations
This pilot study, like others, encountered other limitations that future research can address. First, the IDM module examined the decision journey but did so only at the moments in time when patients completed the instruments. Second, self-reported responses (e.g., the way patients professed to make decisions) may mischaracterize actual cognitive processes or subsequent behavior,93 which direct observation could capture. Third, several factors may have limited generalizability. For example, the size and composition of the denominator differed, sometimes substantially, across questions in the IDM module. This report does not document important differences in responses by age, gender, race/ethnicity, and readiness for screening.
CONCLUSIONS
Nonetheless, these findings convey important messages to clinicians and public health leaders. First, a sizable minority of patients (23.1%) had not heard about screening, let alone a need to make a decision. Better efforts to educate patients about options are warranted. Second, even patients who claimed to have reached a decision wanted to consult their clinician, so health systems must be equipped to meet their needs. Third, patients’ preference for online resources makes patient portals a potentially attractive technology for offering ongoing decision support between office visits. The module that this study piloted was completed by only a minority of eligible patients, but with greater familiarity and refinement such tools could offer a new model for assisting patients with a variety of clinical conditions. Patients are navigating increasingly complex and consequential choices.94 Advances in behavioral science and information technology offer intriguing new solutions to support their decisions.
Supplementary Material
ACKNOWLEDGMENTS
The study was approved by the Virginia Commonwealth University IRB. The study was funded by contract PI-12-001 from the Patient Centered Outcomes Research Institute and the National Center for Advancing Translational Sciences (Clinical Translational Science Award Grant Number ULTR00058).
The authors thank the project coordinator (Paulette L. Kashiri, MPH), other staff (Steven W. Mitchell, BS; Kristin L. Schmidt), and the co-investigators who did not coauthor this report but helped complete this study: Ronald Epstein, MD; Rebecca Etz, PhD; Frank Franzak, PhD; Suzanne C. Makarem, PhD; Annette M. O’Connor, MScN, PhD; Mary C. Politi, PhD; and Stephen F. Rothemich, MD, MS. The authors thank the Clinician Working Group for advice on the design and implementation of this study and the 12 participating practices at Privia Medical Group and Fairfax Family Practice Centers: Broadlands Family Practice–Ashburn, Broadlands Family Practice–Brambleton, Fairfax Family Practice, Family Medicine of Clifton/Centreville, Fox Mill Family Practice, Herndon Family Medicine, Lorton Station Family Medicine, Prince William Family Medicine–Gainesville, Prince William Family Medicine–Manassas, South Riding Family Medicine, Town Center Family Medicine, and Vienna Family Medicine. The authors are indebted to the patients who participated in the Patient Working Group and the MyPreventiveCare Advisory Board. The authors thank Ghalib Bello, PhD for early biostatistical analysis of the data while completing graduate studies and Eric Peele for development of the web interface for this project.
Footnotes
No financial disclosures were reported by the authors of this paper.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2017.10.027.
REFERENCES
- 1.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 3.Blanc X, Collet TH, Auer R, et al. Publication trends of shared decision making in 15 high impact medical journals: a full-text review with bibliometric analysis. BMC Med Inform Decis Mak. 2014;14:71 10.1186/1472-6947-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9–18. 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alston C, Paget L, Halvorson G, et al. Communicating with patients on health care evidence Discussion paper. Washington, DC: National Academy of Medicine, 2012. [Google Scholar]
- 6.Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2014;9:CD006732 10.1002/14651858.CD006732.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282(24):2313–2320. 10.1001/jama.282.24.2313. [DOI] [PubMed] [Google Scholar]
- 8.Woolf SH. Shared decision-making: the case for letting patients decide which choice is best. J Fam Pract. 1997;45(3):205–208. [PubMed] [Google Scholar]
- 9.Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101(5 Suppl): 1214–1228. 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan SL, Harris RP, Woolf SH, Shared Decision-Making Workgroup of the U.S. Preventive Services Task Force. Shared decision making about screening and chemoprevention. A suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26(1):56–66. 10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301–312. 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman RM, Elmore JG, Fairfield KM, Gerstein BS, Levin CA, Pignone MP. Lack of shared decision making in cancer screening discussions: results from a national survey. Am J Prev Med. 2014;47(3):251–259. 10.1016/j.amepre.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Peters E, Dieckmann N, Dixon A, Hibbard JH, Mertz CK. Less is more in presenting quality information to consumers. Med Care Res Rev. 2007;64(2):169–190. 10.1177/10775587070640020301. [DOI] [PubMed] [Google Scholar]
- 14.Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):1145–1151. 10.1093/annonc/mdp534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz PH. Questioning the quantitative imperative: decision aids, prevention, and the ethics of disclosure. Hastings Cent Rep. 2011;41(2):30–39. 10.1353/hcr.2011.0029. [DOI] [PubMed] [Google Scholar]
- 16.Epstein RM. Whole mind and shared mind in clinical decision-making. Patient Educ Couns. 2013;90(2):200–206. 10.1016/j.pec.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Preventive Services Task Force. Breast cancer recommendation statement from the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164(6):448 10.7326/L16-0404. [DOI] [PubMed] [Google Scholar]
- 18.American Urological Association. Early detection of prostate cancer. www.auanet.org/guidelines/early-detection-of-prostate-cancer-(2013-reviewed-and-validity-confirmed-2015). Accessed October 17, 2017.
- 19.American Cancer Society. Testing for Prostate Cancer: “Should I Be Tested? Is it the Right Choice for Me?”. Atlanta, GA: American Cancer Society, 2010. [Google Scholar]
- 20.Moyer VA, U.S.Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 21.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the U.S. Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Final Recommendation Statement: Colorectal Cancer: Screening. U.S. Preventive Services Task Force; https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening. October 2014. Accessed December 6, 2017. [Google Scholar]
- 23.Longo DR, Woolf SH. Rethinking the information priorities of patients. JAMA. 2014;311(18):1857–1858. 10.1001/jama.2014.3038. [DOI] [PubMed] [Google Scholar]
- 24.Frank SH, Stange KC, Langa D, Workings M. Direct observation of community-based ambulatory encounters involving medical students. JAMA. 1997;278(9):712–716. 10.1001/jama.1997.03550090036029. [DOI] [PubMed] [Google Scholar]
- 25.Krist AH, Woolf SH. A vision for patient-centered health information systems. JAMA. 2011;305(3):300–301. 10.1001/jama.2010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agoritsas T, Heen AF, Brandt L, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ. 2015;350:g7624 10.1136/bmj.g7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krist AH, Woolf SW, Hochheimer C, et al. Harnessing information technology to inform patients facing routine decisions. Ann Fam Med. 2017;15(3):217–224. 10.1370/afm.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krist AH, Woolf SH, Bello G, et al. Engaging primary care patients to use a patient-centered personal health record. Ann Fam Med. 2014;12(5):418–426. 10.1370/afm.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krist AH, Peele E, Woolf SH, et al. Designing a patient-centered personal health record to promote preventive care. BMC Med Inform Decis Mak. 2011;11(1):73 10.1186/1472-6947-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krist AH, Woolf SH, Rothemich SF, et al. Interactive preventive health record to enhance delivery of recommended care: a randomized trial. Ann Fam Med. 2012;10(4):312–319. 10.1370/afm.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woolf SH, Zimmerman E, Haley A, Krist AH. Authentic engagement of patients and communities can transform research, practice, and policy. Health Aff (Millwood). 2016;35(4):590–594. 10.1377/hlthaff.2015.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor AM, Stacey D, Jacobsen M. Ottawa Personal Decision Guide. http://decisionaid.ohri.ca/decguide.html. Accessed August 5, 2011. [Google Scholar]
- 33.Légaré F, Kearing S, Clay K, et al. Are you SURE? Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56(8):e308–e314. [PMC free article] [PubMed] [Google Scholar]
- 34.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 35.O’Connor AM. Validation of a decisional conflict scale. Med Decis Mak. 1995;15(1):25–30. 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor AM. Ottawa Decision Support Framework to Address Decisional Conflict. Ottawa, ON: Ottawa Hospital Research Institute; 2006. [Google Scholar]
- 37.Longo DR. Understanding health information, communication, and information seeking of patients and consumers: a comprehensive and integrated model. Health Expect. 2005;8(3):189–194. 10.1111/j.1369-7625.2005.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.International Patient Decision Aids Standards (IPDAS) Collaboration. IPDAS 2005: Criteria for judging the quality of patient decision aids. www.ipdas.ohri.ca/IPDAS_checklist.pdf. Accessed October 17, 2017. [Google Scholar]
- 39.Hochheimer CJ, Sabo RT, Krist AH, Day T, Cyrus J, Woolf SH. Methods for evaluating respondent attrition in web-based surveys. J Med Internet Res. 2016;18(11):e301 10.2196/jmir.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz LM, Woloshin S, Fowler Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 41.Torke AM, Schwartz PH, Holtz LR, Montz K, Sachs GA. Older adults and forgoing cancer screening: “I think it would be strange.” JAMA Intern Med. 2013;173(7):526–531. 10.1001/jamainternmed.2013.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aronowitz RA. Do not delay: breast cancer and time, 1900–1970. Milbank Q. 2001;79(3):355–386. 10.1111/1468-0009.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Royce TJ, Hendrix LH, Stokes WA, Allen IM, Chen RC. Cancer screening rates in individuals with different life expectancies. JAMA Intern Med. 2014;174(10):1558–1565. 10.1001/jamainternmed.2014.3895. [DOI] [PubMed] [Google Scholar]
- 44.Jones RM, Woolf SH, Cunningham TD, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010;38(5):499–507. 10.1016/j.amepre.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38(5):508–516. 10.1016/j.amepre.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medina GG, McQueen A, Greisinger AJ, Bartholomew LK, Vernon SW. What would make getting colorectal cancer screening easier? Perspectives from screeners and nonscreeners. Gastroenterol Res Pract. 2012;2012:895807 10.1155/2012/895807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zikmund-Fisher BJ, Couper MP, Fagerlin A. Disparities in patient reports of communications to inform decision making in the DECISIONS survey. Patient Educ Couns. 2012;87(2):198–205. 10.1016/j.pec.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Protière C, Moumjid N, Bouhnik AD, Le Corroller Soriano AG, Moatti JP. Heterogeneity of cancer patient information-seeking behaviors. Med Decis Making. 2012;32(2):362–375. 10.1177/0272989X11415114. [DOI] [PubMed] [Google Scholar]
- 49.Chaiken S, Trope Y. Dual-Process Theories in Social Psychology. New York, NY: Guilford Press, 1999. [Google Scholar]
- 50.Reyna VF. How people make decisions that involve risk: a dualprocesses approach. Curr Dir Psychol Sci. 2004;13(2):60–66. 10.1111/j.0963-7214.2004.00275.x. [DOI] [Google Scholar]
- 51.Sloman SA. The empirical case for two systems of reasoning. Psychol Bull. 1996;119(1):3–22. 10.1037/0033-2909.119.1.3. [DOI] [Google Scholar]
- 52.Epstein S Integration of the cognitive and the psychodynamic unconscious. Am Psychol. 1994;49(8):709–724. 10.1037/0003-066X.49.8.709. [DOI] [PubMed] [Google Scholar]
- 53.Slovic P, Peters E, Finucane ML, Macgregor DG. Affect, risk, and decision making. Health Psychol. 2005;24(4 Suppl):S35–S40. 10.1037/0278-6133.24.4.S35. [DOI] [PubMed] [Google Scholar]
- 54.Zajonc RB. Feeling and thinking: preferences need no inferences. Am Psychol. 1980;35(2):151–175. 10.1037/0003-066X.35.2.151. [DOI] [Google Scholar]
- 55.Cameron LD, Leventhal H. The Self-Regulation of Health and Illness Behaviour. New York: Routledge; 2003. [Google Scholar]
- 56.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127(2):267–286. 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 57.Gasper K, Clore GL. The persistent use of negative affect by anxious individuals to estimate risk. J Pers Soc Psychol. 1998;74(5):1350–1363. 10.1037/0022-3514.74.5.1350. [DOI] [PubMed] [Google Scholar]
- 58.Peters E, Slovic P. The springs of action: Affective and analytical information processing in choice. Person Soc Psychol Bull. 2000;26(12):1465–1475. 10.1177/01461672002612002. [DOI] [Google Scholar]
- 59.Finucane ML, Alhakami A, Slovic P, Johnson SM. The affect heuristic in judgments of risks and benefits. J Behav Decis Making. 2000;13(1):1–17 . [DOI] [Google Scholar]
- 60.Schwartz B The Paradox of Choice: Why More is Less. London: Harper Collins; 2004. [Google Scholar]
- 61.Epstein RM, Peters E. Beyond information: exploring patients’ preferences. JAMA. 2009;302(2):195–197. 10.1001/jama.2009.984. [DOI] [PubMed] [Google Scholar]
- 62.Baruch F Chapter 18: Cognitive processes in stated preference methods In: Mäler K, Vincent JR, eds. Handbook of Environmental Economics. Vol 2 - Valuing Environmental Changes. Amsterdam: Elsevier; 2005:937–968. [Google Scholar]
- 63.Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295–301. 10.1016/j.pec.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Legare F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526–535. 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Torrey WC, Drake RE. Practicing shared decision making in the outpatient psychiatric care of adults with severe mental illnesses: redesigning care for the future. Community Ment Health J. 2010;46 (5):433–440. 10.1007/s10597-009-9265-9. [DOI] [PubMed] [Google Scholar]
- 66.Wunderlich T, Cooper G, Divine G, et al. Inconsistencies in patient perceptions and observer ratings of shared decision making: the case of colorectal cancer screening. Patient Educ Couns. 2010;80(3):358–363. 10.1016/j.pec.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woolf SH, Krist A. The liability of giving patients a choice: shared decision making and prostate cancer. Am Fam Physician. 2005;71(10):1871–1872. [PubMed] [Google Scholar]
- 68.Edwards M, Davies M, Edwards A. What are the external influences on information exchange and shared decision-making in healthcare consultations: a meta-synthesis of the literature. Patient Educ Couns. 2009;75(1):37–52. 10.1016/j.pec.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 69.Levinson W Patient-centred communication: a sophisticated procedure. BMJ Qual Saf. 2011;20(10):823–825. 10.1136/bmjqs-2011-000323. [DOI] [PubMed] [Google Scholar]
- 70.Fagerlin A, Sepucha KR, Couper MP, Levin CA, Singer E, Zikmund-Fisher BJ. Patients’ knowledge about 9 common health conditions: the DECISIONS survey. Med Decis Making. 2010;30(5 Suppl):35S–52S. 10.1177/0272989X10378700. [DOI] [PubMed] [Google Scholar]
- 71.Jr Street. Aiding medical decision making: a communication perspective. Med Decis Making. 2007;27(5):550–553. 10.1177/0272989X07307581. [DOI] [PubMed] [Google Scholar]
- 72.Ling BS, Trauth JM, Fine MJ, et al. Informed decision-making and colorectal cancer screening: is it occurring in primary care? Med Care. 2008;46(9 Suppl 1):S23–S29. 10.1097/MLR.0b013e31817dc496. [DOI] [PubMed] [Google Scholar]
- 73.Gourlay ML, Lewis CL, Preisser JS, Mitchell CM, Sloane PD. Perceptions of informed decision making about cancer screening in a diverse primary care population. Fam Med. 2010;42(6):421–427. [PMC free article] [PubMed] [Google Scholar]
- 74.Lafata JE, Divine G, Moon C, Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med. 2006;31(3):202–209. 10.1016/j.amepre.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffman RM, Couper MP, Zikmund-Fisher BJ, et al. Prostate cancer screening decisions: results from the National Survey of Medical Decisions (DECISIONS study). Arch Intern Med. 2009;169(17):1611–1618. 10.1001/archinternmed.2009.262. [DOI] [PubMed] [Google Scholar]
- 76.Flocke SA, Stange KC, Cooper GS, et al. Patient-rated importance and receipt of information for colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2168–2173. 10.1158/1055-9965.EPI-11-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woolf SH, Krist AH, Johnson RE, Stenborg PS. Unwanted control: how patients in the primary care setting decide about screening for prostate cancer. Patient Educ Couns. 2005;56(1):116–124. 10.1016/j.pec.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Guerra CE, Jacobs SE, Holmes JH, Shea JA. Are physicians discussing prostate cancer screening with their patients and why or why not? A pilot study. J Gen Intern Med. 2007;22(7):901–907. 10.1007/s11606-007-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nekhlyudov L, Li R, Fletcher SW. Informed decision making before initiating screening mammography: Does it occur and does it make a difference? Health Expect. 2008;11(4):366–375. 10.1111/j.1369-7625.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lafata JE, Cooper GS, Divine G, et al. Patient-physician colorectal cancer screening discussions: delivery of the 5A’s in practice. Am J Prev Med. 2011;41(5):480–486. 10.1016/j.amepre.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwartz PH, Edenberg E, Barrett PR, Perkins SM, Meslin EM, Imperiale TF. Patient understanding of benefits, risks, and alternatives to screening colonoscopy. Fam Med. 2013;45(2):83–89. [PubMed] [Google Scholar]
- 82.Zikmund-Fisher BJ, Couper MP, Singer E, et al. Deficits and variations in patients’ experience with making 9 common medical decisions: the DECISIONS survey. Med Decis Making. 2010;30(5 Suppl):85S–95S. 10.1177/0272989X10380466. [DOI] [PubMed] [Google Scholar]
- 83.Frosch DL, May SG, Rendle KA, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled ‘difficult’ among key obstacles to shared decision making. Health Aff (Millwood). 2012; 31(5):1030–1038. 10.1377/hlthaff.2011.0576. [DOI] [PubMed] [Google Scholar]
- 84.Joseph-Williams N, Edwards A, Elwyn G. Power imbalance prevents shared decision making. BMJ. 2014;348:g3178 10.1136/bmj.g3178. [DOI] [PubMed] [Google Scholar]
- 85.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10:CD001431 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 86.Lewis CL, Adams J, Tai-Seale M, et al. A randomized controlled effectiveness trial for PSA screening decision support interventions in two primary care settings. J Gen Intern Med. 2015;30(6):810–816. 10.1007/s11606-015-3214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jimbo M, Rana GK, Hawley S, et al. What is lacking in current decision aids on cancer screening? CA Cancer J Clin. 2013;63(3):193–214. 10.3322/caac.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Politi MC, Adsul P, Kuzemchak MD, Zeuner R, Frosch DL. Clinicians’ perceptions of digital vs. paper-based decision support interventions. J Eval Clin Pract. 2015;21(2):175–179. 10.1111/jep.12269. [DOI] [PubMed] [Google Scholar]
- 89.Politi MC, Street Jr. The importance of communication in collaborative decision making: facilitating shared mind and the management of uncertainty. J Eval Clin Pract. 2011;17(4):579–584. 10.1111/j.1365-2753.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- 90.Epstein RM, Street RL Jr. Shared mind: communication, decision making, and autonomy in serious illness. Ann Fam Med. 2011;9(5):454–461. 10.1370/afm.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quill TE, Brody H. Physician recommendations and patient autonomy: finding a balance between physician power and patient choice. Ann Intern Med. 1996;125(9):763–769. 10.7326/0003-4819-125-9-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 92.Kruse CS, Bolton K, Freriks GJ. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. Med Internet Res. 2015;17(2):e44 10.2196/jmir.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eberth JM, Vernon SW, White A, Abotchie PN, Coan SP. Accuracy of self-reported reason for colorectal cancer testing. Cancer Epidemiol Biomarkers Prev. 2010;19(1):196–200. 10.1158/1055-9965.EPI-09-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zikmund-Fisher BJ, Couper MP, Singer E, et al. The DECISIONS study: a nationwide survey of United States adults regarding 9 common medical decisions. Med Decis Making. 2010;30(5 Suppl): 20S–34S. 10.1177/0272989X09353792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.