Short abstract
Human umbilical-cord mesenchymal stem cells (hUCMSCs) are a safe and convenient source of MSCs and have shown beneficial effects in neonatal infection and sepsis animal models. However, the factors leading to improved outcomes are still unclear. The aim of this study was to investigate the antibacterial effect and regulation of antimicrobial resistance of hUCMSCs. We separated imipenem-resistant Pseudomonas aeruginosa (PA) from neonates and incubated it with hUCMSCs as well as their culture medium. Assessment of direct inhibition of bacterial growth was done by counting CFUs. The concentration of antibacterial peptides in the culture medium of hUCMSCs was measured. Standard PA was inoculated with a sub-inhibitory concentration of imipenem with and without hUCMSC conditioned medium and antimicrobial peptides. The sensitivity to imipenem was detected until PA showed resistance to imipenem. Outer membrane protein (OprD2) mRNA expression in PA before and after the induction of imipenem resistance was analysed. We found that HUCMSCs possessed direct antimicrobial properties against bacteria and could alleviate antibiotic resistance via reserving OprD2 expression in PA.
Keywords: Human umbilical-cord mesenchymal stem cells, imipenem-resistant Pseudomonas aeruginosa, antimicrobial peptides, antibiotic resistance, outer membrane protein D2
Introduction
Infection is listed as the primary cause of neonatal death.1 Neonates in a neonatal intensive care unit (NICU) are vulnerable to infection because of their immunocompromised condition and frequent exposure to invasive medical devices. Empirical antimicrobial therapy is promptly initiated to prevent adverse outcomes.2 However, antibiotic exposure in neonates not only increases the incidence of super-infection, necrotizing enterocolitis and death, but also leads to the development of bacterial antibiotic resistance, which has already become a serious challenge worldwide.3 It has been reported that Gram-negative bacteria account for 70.8% of neonatal infection in the NICU, among which Pseudomonas aeruginosa (PA) accounts for 11.3% and is one of the most common antibiotic-resistant bacteria associated with greater mortality.4,5
Cathelicidin/LL-37 and HBD-2 belong to two of the most important human antimicrobial peptides families and play a significant role in the human immune defence reaction, including the activation of inflammatory cells and regulation of adaptive immunity.6,7 For neonates, particularly those born extremely prematurely, antimicrobial peptides are key aspects of the neonatal immune defence system.4
PA possesses intrinsic antimicrobial resistance mainly via deficiency of the outer membrane protein OprD.8 It is thought to be the most prevalent mechanism for carbapenem resistance. Imipenem, as the last defence of PA invasion, is most frequently used to treat PA infections. Unfortunately, in recent yr, its use has resulted in an increasing resistance rate, which has been as high as 21.1%.8–10
Further effort in the discovery of new antibiotics may still induce antimicrobial resistance.11 Therefore, the development of innovative treatments is both warranted and essential to reduce the use of antibiotics and improve antimicrobial susceptibility.
Mesenchymal stem cells (MSCs) play an important role in immune regulation and thus improve the outcomes of patients with severe infection or sepsis.12 Compared to bone-marrow MSCs (BMSCs), human umbilical-cord MSCs (hUCMSCs) not only have stronger proliferation and differentiation ability, but also are relatively naive and have fewer ethics-associated controversies, and therefore may have a wider application in neonatal infectious diseases.13,14
The aim of this study was to investigate the antibacterial effect and regulation of antimicrobial resistance of hUCMSCs, with the hope that this may provide a possible innovative therapy for neonatal infection and alleviate antibiotic resistance.
Materials and methods
Chemicals and reagents
Standard PA (ATCC27853) strains were offered by the Clinical Laboratory Centre of China’s Ministry of Health. Imipenem-resistant PA (IRPA) was obtained from a bacteriological laboratory in our hospital. It was tested using the Kirby–Bauer method and found to be resistant to imipenem according to CLSI M100-S22-2012.15 hUCMSCs were purchased from American Type Culture Collection (ATCC; Manassas, VA). Mouse mAb to human β-defensin-2 Ab (1 µg/ml), human CAP/LL37 Ab (0.1 µg/ml) and mouse isotype IgG1 Ab were purchased from R&D Systems (Minneapolis, MN). FBS was from HyClone Laboratories, Inc. (San Angelo, TX). The LL-37 ELISA kit was purchased from Hycult Biotechnology (Uden, The Netherlands). The human β-defensin-2 ELISA kit was purchased from Phoenix Pharmaceuticals, Inc. (Burlingame, CA). Synthetic human LL-37 was from AnaSpec (Fremont, CA).
Cell culture
hUCMSCs were purchased from ATCC. The cells met all the criteria for the classification as MSCs as defined by the International Society of Cellular Therapy. On arrival, cells were thawed and expanded in tissue culture flasks (BD Falcon™; BD Biosciences, San Jose, CA) at a density of 500,000 cells/150 cm2. Cells were passaged every 3–4 d by trypsinization when they reached 70–80% confluency and were used for the experiments between passages 5 and 10. Between each passage, viability was measured with trypan blue exclusion. hUCMSCs were cultured in α-minimum essential medium without ribonucleosides or deoxyribonucleosides containing 2 mM L-glutamine and 16.5% FBS, without antibiotics. Cells were cultured in a humidified incubator at 5% CO2 and 37°C under sterile conditions. Before each experiment, cells were trypsinized, counted, washed with PBS and re-suspended in appropriate medium (RPMI-1640 medium (RPMI)) + 5% FBS.
Bacterial culture and antimicrobial assays
IRPA obtained from samples of neonates tested by the Kirby–Bauer method to be resistant to imipenem in our bacteriological laboratory according to CLSI M100-S22-201215 and standard PA ATCC 27853 were used for these experiments. IRPA were seeded on a selective agar plate kept at 4°C and grown overnight (16 h) at 37°C in liquid Luria-Bertani (LB) medium (Difco™; BD Biosciences) with slight agitation. Before each experiment, the bacteria were washed once and re-suspended in PBS, and the OD (at λ = 600 nm) of the suspension was measured. The number of CFU was calculated according to the following equation: OD600 = 0.5 corresponds to 5 × 108 CFU/ml, OD600 = 1.8 corresponds to 2 × 109 CFU/ml for IRPA.16
Assessment of direct inhibition of bacterial growth by hUCMSCs or their conditioned medium (CM) was done by counting CFUs. Briefly, hUCMSCs in six-well plates (1 × 106 cells/ml, 2 ml) in RPMI supplemented with 5% FBS were infected with IRPA (1500 CFU) and incubated for 6 h in a humidified CO2 incubator. Aliquots of culture medium were taken from each well, serially diluted with sterile PBS and plated on selective Peudomonas isolation agar plates (Difco™; BD Biosciences). Colonies were counted after overnight incubation at 37°C.
Antimicrobial activity of hUCMSC CM with or without IRPA stimulation was tested by a micro-dilution susceptibility test according to Andrä et al. with slight modifications.17 Briefly, hUCMSC CM was collected from the wells, centrifuged at 15,000 g for 10 min and frozen at –20°C (to eliminate any residual bacterial organisms). Prior to the experiments, samples were thawed on ice, transferred to a six-well plate (2 ml) and incubated with IRPA (1500 CFU) for 6 h at 37°C. Then, CFUs were counted as described earlier.
Protein assays for cathelicidin/LL-37 and HBD-2
Concentrations of HBD-2 and cathelicidin/LL-37 in culture medium of hUCMSC with or without PA stimulation were measured previously by ELISA kit from Phoenix Pharmaceuticals and Hycult Biotechnology, respectively.
Determination of sub-inhibitory concentration of imipenem and IRPA induction
Imipenem resistance were tested using the Kirby–Bauer method by measuring the inhibition zone, with ≥19 mm as sensitive, 16–18 mm as intermediate and ≤15 mm as resistant. The minimal inhibitory concentration (MIC) of imipenem was determined using the double dilution method, with values of 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5 and 0.25 μg/ml referring to CLSI M100-S22-2012.15 The imipenem resistance induction method was employed based on the sub-inhibitory concentration of imipenem. Standard PA was inoculated into a broth tube with 2 ml of brain heart infusion (BHI) and then cultured at 35°C overnight. PA suspension (50 μl) was then inoculated into 5 ml of BHI containing 1/4 MIC imipenem and passaged every 24 h. During each passage, 200 µl CM of hUCMSCs stimulated with and without PA previously and synthetic human LL-37 (8 ng/ml) were added. The sensitivity to imipenem was detected by Kirby–Bauer method until PA showed resistance to imipenem according to the criteria described earlier. During each passage, PA was transferred to a blood agar plate to eliminate potential contamination because of improper operation.
RNA isolation and RT-PCR
mRNA was isolated from PA using a bacterial RNA kit (Omega Bio-Tek, Inc., Norcross, GA). After isolation, RNA samples were treated with DNase I for 60 min at room temperature to remove contaminating DNA. The quality of the RNA was assessed with the NanoDrop ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE) according to the manufacturer’s instructions. Absorbance ratios (260/280 and 260/230 nm) of 1.8–2.0 indicated a pure RNA sample. Primers for OprD2 and 16sRNA were synthesized by Guangzhou Jige Biotechnology (Guangzhou, China). The sequences were as follows: OprD2 (forward 5-ACCTAGCCTCCTATGGCGTTCCC-3; reverse 5-CGAGGTTGGTTTCGTGGTGCTT-3), 16sRNA (forward 5-TAGGTGGTTCAGCAAGTTGGATGTGAAATC-3, reverse 5-TGTCAGTATCAGTCCAGGTGGTCGC-3). First-strand cDNA was synthesized from 600 ng of total RNA using a RevertAid™ First Strand cDNA Synthesis Kit protocol from Fermentas (Waltham, MA) according to the manufacturer’s instructions in a reaction volume of 20 µl. Quantitative RT-PCR was performed using Maxima® SYBR Green/ROX qPCR Master Mix for each sample using an ABI StepOnePlus (Applied Biosystems, Foster City, CA).
The cycle conditions for both were as follows: denaturing step (95°C for 10 min) followed by 40 cycles of denaturing (95°C for 15 s), annealing (60°C for 30 s) and extending (72°C for 15 s), 95°C for 15 s, 60°C for 1 min and 95°C for 15 s. All PCR reactions were repeated three times at different time points. Relative gene expression was determined by normalizing with 16s RNA using the 2–ΔΔCt method.
Statistical analyses
All experiments were carried out at least three times. Results are expressed as the mean ± SD if the data were normally distributed. Statistical analyses were performed using an unpaired two-tailed Student’s t-test or one way ANOVA. A P-value of < 0.05 was considered statistically significant. All statistical analyses were done using IBM SPSS Statistics for Windows v21.0 (IBM Corp., Armonk, NY).
Results
hUCMSCs inhibit PA growth
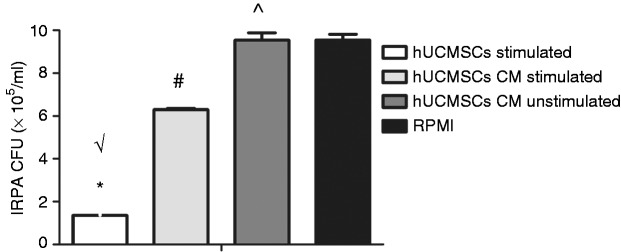
To test the effect of hUCMSCs on IRPA growth and to determine if the observed antibacterial effect was associated with soluble secreted factors, we infected hUCMSCs with IRPA for 6 h. Then we assessed the ability of the CM to inhibit IRPA growth by incubation with IRPA. It was found that the CM of hUCMSCs stimulated with IRPA significantly inhibited IRPA growth compared to the control medium (RPMI). Compared to the CM of hUCMSCs stimulated previously, hUCMSCs showed a stronger antibacterial ability. This suggested that the mechanism of hUCMSCs’ antimicrobial activity against IRPA was associated with secreted products induced with previous bacterial challenge and a possible direct cell–bacteria contact effect (Figure 1).
Figure 1.
hUCMSCs or their culture medium has antimicrobial activity against IRPA. Bacterial growth was assessed by CFU counts. Data are the mean ± SD. *P < 0.0001 vs. RPMI; #P < 0.01 vs. RPMI; ^P > 0.05 vs. RPMI; √P < 0.01 vs. hUCMSCs CM-stimulated by ANOVA (n = 21).
hUCMSCs secrete human cathelicidin antimicrobial peptide/LL-37 and human β defensin-2
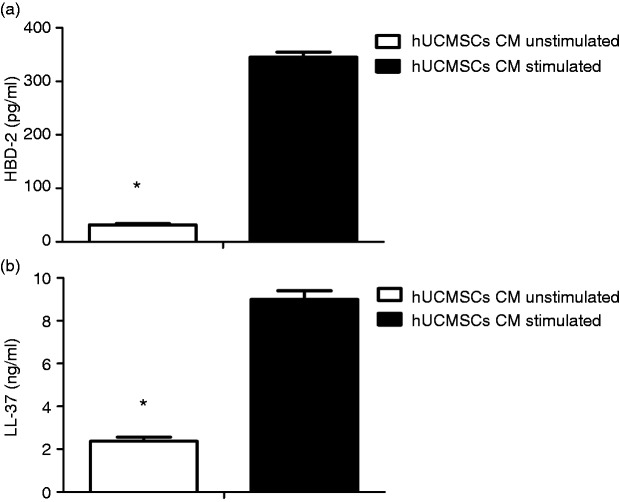
To investigate potential candidates responsible for the observed antimicrobial effect, we analysed the CM of hUCMSCs with or without stimulation for the presence of peptides with known antimicrobial activity. Protein levels measured by ELISA for human β defensin-2 and human cathelicidin antimicrobial peptide hCAP-18/LL-37 were significantly higher in hUCMSC CM compared to control CM (Figure 2a and b).
Figure 2.
hUCMSCs HBD-2 and LL-37 expression are up-regulated by IRPA stimulation. Data are the mean ± SD. *P < 0.001 (n = 21).
hUCMSCs delay formation of IRPA
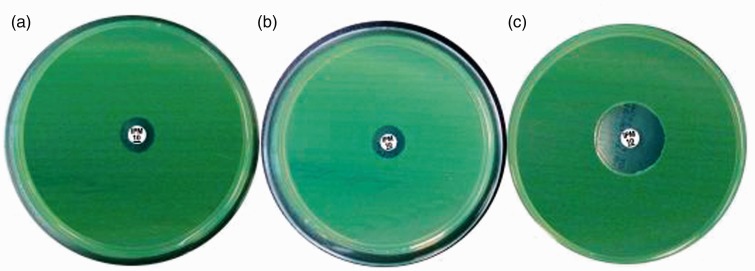
During the induction of imipenem resistance at a sub-inhibitory concentration (1/4 MIC), imipenem-resistant PA appeared in P17 in control medium and LL-37 groups, while in the stimulated CM group, imipenem resistance occurred in P19. Inhibition zones of PA for imipenem in every passage in the three groups were measured. The diameters of inhibition zone were 12 mm in control medium and LL-37 groups and 20 mm in the stimulated CM group in P17 (Figure 3). This indicated that hUCMSCs delayed the formation of IRPA induced at a sub-inhibitory concentration, and soluble factors other than LL-37 may account for this beneficial effect.
Figure 3.
hUCMSC delayed formation of IRPA induced at a sub-inhibitory concentration (diameters of inhibition zone in P 17: (a) control CM, 12 mm; (b) LL-37, 12 mm; (c) stimulated CM, 20 mm). Images are representative for each condition run in triplicate.
Imipenem sensitivity was also measured in IRPA separated from neonatal samples before and after co-culture with hUCMSC by the Kirby–Bauer method. We found that PA in these clinical samples was less resistant to imipenem after co-culture with hUCMSCs (Table 1). This suggested hUCMSCs could improve imipenem sensitivity in PA.
Table 1.
Number of IRPA samples from neonates before and after incubation with hUCMSCs.
| Samples from different sites | Number of IRPA samples before incubation with hUCMSCs | Number of IRPA samples after incubation with hUCMSCs |
|---|---|---|
| Sputum | 16 | 9 |
| Tracheal incubation | 1 | 0 |
| Eye discharge | 1 | 1 |
| Gastric contents | 1 | 0 |
| Skin pus | 1 | 1 |
| BALF | 1 | 1 |
| Total | 21 | 12 |
IRPA: imipenem-resistant Pseudomonas aeruginosa; hUCMSCs: human umbilical-cord mesenchymal stem cells; BALF: bronchoalveolar lavage fluid.
OprD2 mRNA expression in PA before and after the induction of imipenem resistance
OprD2 mRNA expression after induction in PA, which showed imipenem resistance in P17, decreased significantly in control CM and LL-37 groups. However, this was reversed by adding CM of hUCMSCs stimulated with PA previously, which still retained 13.24% OprD2 mRNA expression compared to standard PA before induction. This result proved hUCMSCs may improve antibiotic resistance by restoring OprD2 expression (Figure 4).
Figure 4.
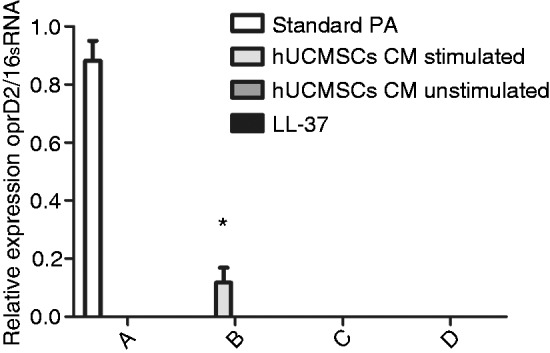
hUCMSCs restored OprD2 mRNA expression after 1/4 minimal inhibitory concentration imipenem induction in P17. (a) Standard PA; (b) CM-stimulated hUCMSCs; (c) CM-unstimulated hUCMSCs; (d) LL-37. *P < 0.05 vs. standard PA before induction (n = 3). OprD2 mRNA expression was not detected in (c) or (d).
Discussion
The high morbidity and mortality caused by infectious diseases are a main threat to neonates admitted to NICU, accounting for 36% of neonatal deaths.18 The abuse and misuse of broad-spectrum antibiotics results in serious antibiotic-resistant strains, therefore adding to the challenge of treating neonatal infectious diseases.19 It has been reported that antibiotic exposure is associated with significant mortality and neurodevelopmental impairment among survivors.3 PA is among the most common antibiotic-resistant strains, and imipenem was thought to be an effective medication for PA infection. However, in recent yr, IRPA has become a significant concern in medical institutions around the world.8 Innovative and effective treatment is necessary and warranted.
In our study, we found that the growth of IRPA was inhibited by hUCMSCs. The mechanisms were associated with secreted products induced with previous bacterial challenge, including LL-37, HBD-2 and a direct cell–bacteria contact effect. Previous studies have demonstrated that human antimicrobial peptides are effective agents against bacteria, fungi and viruses. They obtained a broad-spectrum effect, and could activate innate and adaptive immune responses, and thus play an important role in the neonatal defence response to bacterial infection. In our study, we found that the antimicrobial peptides cathelicidin/LL-37 and human β-defensin-2 were increased significantly in the CM of hUCMSCs stimulated with IRPA via ELISA compared to control CM of hUCMSCs without stimulation. These results suggest that the antibacterial activity of hUCMSCs is mediated in part by the secretion of cathelicidin/LL-37 and human β-defensin-2. Krasnodembskaya et al. reported that BMSCs can secrete antimicrobial peptides (LL-37) and that adding Ab to LL-37 in the CM could abrogate its antibacterial activity completely.15 We confirmed that hUCMSCs also possessed an antibacterial effect mediated in part by antimicrobial peptides secretion. In addition, we demonstrated that compared to MSCs from BM, hUCMSCs showed a direct potent antimicrobial effect when co-cultured with PA, which could not be explained by the paracrine effect of antibacterial peptides only. This indicated hUCMSCs may possess different functions compared to MSCs from BM.
Hospital-acquired pneumonia caused by PA is difficult to cure because of its intrinsic resistance to cephalosporin.8,20 Considering the severe outcomes of PA infection among newborns, there is an urgent need to seek new therapies to reduce antibiotic use and improve bacterial sensitivity to current meditation. Our research revealed that formation of IRPA was delayed with the supplement of CM from hUCMSCs stimulated with PA previously compared to the LL-37 group and the control CM group. Although LL-37 mediated part of the antibacterial effect, it did not improve antibiotic sensitivity in PA. Clinically, we also revealed that after incubation with hUCMSCs, the IRPA separated from some samples of neonates became sensitive to imipenem. It was interesting that although antimicrobial peptides produced by hUCMSCs could defend against bacterial infection, it did not alter their intrinsic sensitivity to antibiotics.
To explore further the underlying mechanism responsible for the improvement of antibiotic sensitivity by hUCMSCs, we also determined OprD2 mRNA expression in PA before and after induction. OprD is a well-characterized channel for imipenem influx. Its deficiency is thought to be the most important mechanism for carbapenem resistance. Among the loops in OprD, loop L2 was regarded as the most critical factor in maintaining admission of substrates in the lumen, especially antibiotics.9 Tamber et al. reported that it is the deletion or decreasing of OprD2 expression that results in PA’s resistance to imipenem.21 Ochs et al. injected plasmids encoding OprD2 into IRPA, and found that PA regained sensitivity to imipenem.22 Our results showed that OprD2 expression in PA declined after induction and even disappeared compared to standard strains. This could be reversed by adding CM of hUCMSCs stimulated with PA, which still retained 13.24% OprD2 mRNA expression. These results showed that hUCMSCs might delay PA’s imipenem resistance by up-regulation of OprD2 mRNA, which was not mediated by LL-37. We suspect that except for LL-37, hUCMSCs may also secret some other cytokines which are important in improving antibiotic sensitivity. Therefore, hUCMSCs may work as a whole in inhibiting bacterial growth and alleviating antibiotic resistance. We emphasize the importance of cell therapy in infectious diseases other than a single factor secreted by cells.
However, our study also has some limitations. First, the experiment was only limited to PA and without tests on other bacteria. Therefore, the antibacterial effect of hUCMSCs remains to be further confirmed in other strains. Second, although LL-37 is the likely inhibitory factor, further research is needed to confirm this. Third, in vivo experiments are needed to test the beneficial effect further before applying it clinically.
In conclusion, we showed that hUCMSCs can inhibit bacterial growth and alleviate antibiotic resistance which is mediated partly by secretion of cathelicidin LL-37 and HBD-2 and up-regulation of OprD2. These studies provide insights into a possible innovative and invaluable therapy for neonatal infection and antibiotic resistance.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet 2005; 365: 891–900. [DOI] [PubMed] [Google Scholar]
- 2.Tzialla C, Borghesi A, Pozzi M, et al. Neonatal infections due to multi-resistant strains: Epidemiology, current treatment, emerging therapeutic approaches and prevention. Clin Chim Acta 2015; 451: 71–77. [DOI] [PubMed] [Google Scholar]
- 3.Cotten CM. Adverse consequences of neonatal antibiotic exposure. Curr Opin Pediatr 2016; 28: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SJ, Saiman L. Antibiotic resistance in neonatal intensive care unit pathogens: mechanisms, clinical impact, and prevention including antibiotic stewardship. Clin Perinatol 2010; 37: 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bas AY, Demirel N, Zenciroglu A, et al. Nosocomial blood stream infections in a neonatal intensive care unit in Ankara, Turkey. Turk J Pediatr 2010; 52: 464–470. [PubMed] [Google Scholar]
- 6.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med 2002; 165: 992–995. [DOI] [PubMed] [Google Scholar]
- 7.Tecle T, Tripathi S, Hartshorn KL. Review: defensins and cathelicidins in lung immunity. Innate Immun 2010; 16: 151–159. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Luo YF, Williams BJ, et al. Structure and function of OprD protein in Pseudomonas aeruginosa: from antibiotic resistance to novel therapies. Int J Med Microbiol 2012; 302: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samanta S, Scorciapino MA, Ceccarelli M. Molecular basis of substrate translocation through the outer membrane channel OprD of Pseudomonas aeruginosa. Phys Chem Chem Phys 2015; 17: 23867–23876. [DOI] [PubMed] [Google Scholar]
- 10.Cohen R, Babushkin F, Cohen S, et al. A prospective survey of Pseudomonas aeruginosa colonization and infection in the intensive care unit. Antimicrob Resist Infect Control 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel SJ, Saiman L. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol 2012; 36: 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med 2014; 2: 1016–1026. [DOI] [PubMed] [Google Scholar]
- 13.Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells 2014; 6: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishige I, Nagamura-Inoue T, Honda MJ, et al. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int J Hematol 2009; 90: 261–269. [DOI] [PubMed] [Google Scholar]
- 15.Franklin R, Matthew A, Alder Jeff, et al. Performance standards for antimicrobial susceptibility testing: twenty-second informational supplement, [M]. Approved standard M100-S22. Clinical and Laboratory Standards Institute, 2010.
- 16.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010; 28: 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andra J, Berninghausen O, Wulfken J, et al. Shortened amoebapore analogs with enhanced antibacterial and cytolytic activity. FEBS Lett 1996; 385: 96–100. [DOI] [PubMed] [Google Scholar]
- 18.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet 2005; 365: 891–900. [DOI] [PubMed] [Google Scholar]
- 19.Worldwide country situation analysis: response to antimicrobial resistance, https://who.int/drugresistance/documents/situationanalysis/en/ (accessed 29 April 2015).
- 20.El SA, Alhajhusain A. Update on the treatment of Pseudomonas aeruginosa pneumonia. J Antimicrob Chemother 2009; 64: 229–238. [DOI] [PubMed] [Google Scholar]
- 21.Tamber S, Ochs MM, Hancock RE. Role of the novel OprD family of porins in nutrient uptake in Pseudomonas aeruginosa. J Bacteriol 2006; 188: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochs MM, McCusker MP, Bains M, et al. Negative regulation of the Pseudomonas aeruginosa outer membrane porin OprD2 selective for imipenem and basic amino acids. Antimicrob Agents Chemother 1999; 43: 1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]