Short abstract
This study was designed to determine whether plasma CXCL12 levels in postmenopausal osteoporosis (PMOP) patients are related to disease severity. A total of 91 PMOP females were recruited, and 88 postmenopausal non-osteoporotic (PMNOP) women and 90 healthy females were incorporated as controls. Dual-energy X-ray absorptiometry was utilised to explore bone-mineral density (BMD). The Genant semi-quantitative grading scale was used for vertebral fractures, and plasma CXCL12/SDF-1 levels were investigated by ELISA. Plasma TNF-α and C-telopeptide cross-linked collagen type 1 (CTX-1) were also tested. The Oswestry Disability Index (ODI) and a visual analogue scale (VAS) were completed in order to assess clinical severity. Plasma CXCL12 levels were considerably elevated in PMOP females compared to PMNOP women and healthy controls. Plasma CXCL12 concentrations were positively correlated with the Genant grading system. We observed significant and negative correlations of plasma CXCL12 levels with lumbar spine, femoral neck and total hip BMD. Moreover, plasma CXCL12 concentrations were positively correlated to VAS and ODI, as well as plasma TNF-α and CTX-1 levels. In conclusion, elevated plasma CXCL12 levels are correlated with disease severity in PMOP females.
Keywords: CXC chemokine ligand 12, stromal cell–derived factor 1, disease severity, postmenopausal osteoporosis
Introduction
Osteoporosis (OP) is a metabolic bone disorder described as low bone mass along with disturbance in bone microarchitecture, which causes augmented frequency of fractures as well as increasing the fragility of bones.1 As per the most recent International Osteoporosis Foundation report,2 compared to the 158 million cases in 2010, the number of osteoporotic patients (aged >50 yr) will double globally by 2040. Compared to the rest of the population, postmenopausal females are more susceptible to OP, partially because of the lack of estrogen and quick loss of calcium, which leads to extreme bone resorption.3,4
Currently, a diagnosis of OP is confirmed by complaints of increased back pain and functional impairment, sometimes with a sudden fracture and decreased bone-mineral density (BMD) determined by dual-energy X-ray absorptiometry (DXA).5 Even though DXA is regarded as the standard approach to diagnose OP, there are multiple limitations in predicting the risk of fracture.6 In addition, since OP starts long before the DXA can detect the disease, irreversible loss of bone volume has often already occurred by the time DXA diagnosis is established. Therefore, the search for markers with higher sensitivity is crucial and will have many benefits for assessing and treating OP patients, especially at the initial stages.
Chemokines play vital roles in the accumulation of leucocytes at the location of inflammation.7 Chemokines are categorised into C, CC, CXC and CX3C subfamilies, depending on the conserved cysteine motifs.8 Different families are involved in bone remodeling of chemokines, especially CC and CXC.8 In many inflammatory bone diseases, leucocyte recruitment driven by chemokines represents a momentous event in inflammation and bone metabolism. The leucocytes could generate bone resorptive factors, for example IL-I and TNF-α, further leading to a circuit of bone damage.9,10 Chemokines could also induce peripheral osteoclast progenitors (OCPs) to contribute to osteoresorption, homing of OCPs and their differentiation into mature osteoclasts.11
CXCL12 belongs to the subfamily of CXC chemokines, also known as as stromal cell–derived factor 1 (SDF-1). CXCL12 is illustrated by two conserved NH2-terminal cysteines and becomes intrinsically expressed by murine and human bone-marrow stromal cells, for example perivascular, endothelial and endosteal bone lining stromal cells in the stem-cell niche, and was also reported to be expressed by osteoclasts.12 CXCL12 acts as a local paracrine signalling factor and long-distance chemokine to assist in mobilising and targeting immune response and reparative cells to areas of injury and inflammation.13
The role of CXCL12 in regulating bone resorption and osteoclastic activity has been widely studied.14 During osteoclast differentiation, the expression of CXCL12 and its receptor CXCR4 increase, indicating an autocrine/paracrine mechanism of action of CXCL12 throughout the osteoclast differentiation process.15 CXCL12 has been related with pathological bone loss in many diseases, including osteoarthritis and rheumatoid arthritis.16,17 It has been also reported that higher levels of CXCL12 are correlated with lower total hip BMD.18 Furthermore, in a recent study, osteoclast protein levels and CXCL12 mRNA isolated from femoral cancellous bone of the OP group were suggestively greater than those in the control group.19 In another recent study, Shima found that LPS-induced osteoclastogenesis and bone resorption are enhanced by CXCL12 in vivo.20
All previous studies have indicated that CXCL12/SDF-1 might make a difference in the advancement of postmenopausal osteoporosis (PMOP). However, none of these studies have explored the correlation of CXCL12/SDF-1 levels with the progression of PMOP in patients. Therefore, the current study was carried out to investigate whether CXCL12/SDF-1 levels are raised in PMOP, as well as the relationship between plasma CXCL12/SDF-1 concentration and disease severity in PMOP females.
Patients and methods
Study patients
A total of consecutive 91 patients with PMOP were involved in this cross-sectional study. They were enrolled at our hospital between December 2017 and December 2018. Diagnosis of OP was confirmed as per the criteria of the World Health Organization.21 The inclusion criteria were: postmenopausal female patients who had been menopausal for >3 yr because of natural menopause, were capable of independent activities and were aged 45–80 yr. Participants also had a T score of –2.5 or lower for BMD at the lumbar spine or total hip, or had −2.5 < T score < −1.0 for BMD at the lumbar spine or total hip. Participants with secondary OP, osteoarthritis, asthma, rheumatoid arthritis, amyotrophic lateral sclerosis or other diseases, including malabsorption syndrome, inflammatory bowel disease, chronic liver disease or cirrhosis of the liver, were excluded, as these conditions could affect bone metabolism. Moreover, 88 age-matched postmenopausal non-osteoporotic (PMNOP) women and 90 healthy women of childbearing age were enrolled as controls. The ethics committee of our hospital approved the present study. All participants were aware of the study design and provided written informed consent.
Laboratory tests
Fasting venous blood was obtained from all participants at 8:00 a.m. following an overnight (>12 h) fast. A 30 ml peripheral blood sample was acquired from the side-arm of the sheath and anti-coagulated with sodium EDTA in vacutainer tubes on ice. Dipeptidyl peptidase-4 (DPP4) inhibitor (100 µM) Sitagliptin (SG; extracted from Januvia in our hospital) and 1× protease inhibitors (SIGMAFAST™ Protease Inhibitor Cocktail; Sigma–Aldrich, St Louis, MO) were added to avoid CXCL12 loss, as published previously.22 The samples were then quickly centrifuged using the two-stage method to avoid glycolysis at 4°C and 1600 g for 10 min first, and then the supernatant was extracted for further centrifugation at 4°C and 12,000 g for another 10 min. All plasma samples were preserved at −80°C prior to the investigation. The CXCL12 levels of patients and controls were blindly evaluated concurrently by using a quantitative sandwich ELISA kit (Quantikine; R&D Systems, Minneapolis, MN; cat. no. DSA00). Concisely, standards of recombinant human CXCL12 and plasma samples were added to 96-well microtiter plates pre-coated with rabbit polyclonal Ab against CXCL12 and incubated for 1 h at room temperature (RT; 25°C). Next, the wells were rinsed seven times with washing buffer and incubated for 30 min at 4°C with a HRP-labelled mouse mAb. After nine washes, substrate solution was added to each well, and the plate was incubated for 30 min at RT in the dark. Stop solution was added to stop the reaction. The absorbance was detected at 450 nm by an automated microtiter plate reader. The CXCL12 concentrations were computed using the standard curve. The inter- and intra-assay coefficients of variation for CXCL12 were 4.5–6.1% and 4.9–6.7%, respectively. The detection range was 156.0–10,000 pg/ml. In addition, other biomarkers such as inflammation factor TNF-α (R&D Systems) and bone resorption marker CTX-I were also tested. The inter- and intra-assay coefficients of TNF-α were 5.5% and 8.8%, respectively, whereas for CTX-1, the coefficients were 4.6% and 7.3%, respectively. All samples were tested at least three times.
BMD assessment
BMD (g/cm2) was evaluated by DXA (Hologic QDR-2000; Hologic Corp., Marlborough, MA). BMD at the lumbar spine (L1–L4), total hip and femoral neck of all participants was determined. To minimise inter-observer variations, all scans and analyses were performed by the same experienced physician.
Genant semi-quantitative grading scale for vertebral fractures
Lateral computed tomography (CT) scout views were used to identify vertebral fractures measured using the Genant semi-quantitative grading scale.23 Lumbar vertebrae from L1 to L4 were graded on visual examination and without direct vertebral measurement as normal (grade 0), mildly deformed (grade 1: decrease of 20–25% of height and 10–20% of projected vertebral area), moderately deformed (grade 2: decrease of 26–40% of height and 21–40% of projected vertebral area) and severely deformed (grade 3: decrease of >40% of height and projected vertebral area). In the present study, the enrolled patients were identified as vertebral fracture with a Genant grade ≥1. The evaluation of results was read by two experienced radiologists in our hospital. The κ-value was calculated to examine the consistency of the reading results.
Definition of symptomatic severity
The visual analogue scale (VAS) and Oswestry Disability Index (ODI) scores were recorded in order to assess the clinical severity in PMOP women. The VAS is a line 10 cm long marked with two lines anchoring each end of the scale. Patients were requested to put a mark on the scale based on the severity of their pain, where 0 cm was considered ‘no pain’ and 10 cm represented ‘pain as bad as it could be’.24 The ODI consists of 10 items on the degree of severity to which back (or leg) trouble had disturbed the capability to accomplish day-to-day living.25 The 10 sections covered pain and daily function (comprising pain intensity, personal hygiene, lifting, walking, sitting, standing, sleeping, sexual activity, social activity and travelling). Each item was graded on a six-point scale (0–5); a higher score indicated a greater level of disability.
Statistical analysis
GraphPad Prism v6.0 (GraphPad Software, Inc., San Diego, CA) was utilised for analysis. The Kolmogorov Smirnov test was utilised to assess the distribution of the quantitative data. Student’s t-test or Mann–Whitney U-test was used for comparisons between two groups. One-way ANOVA or Kruskal–Wallis tests was utilised for three or more groups, followed by Tukey or Tamhan post hoc analysis. Continuous data are presented as the mean ± SD. Categorical data are presented as the frequency and percentage. For normally distributed data, Pearson’s correlation coefficient was computed, and for distribution-free data, Spearman’s coefficient was computed. P-Values of < 0.05 signified statistical significance.
Statistical power analysis
The post hoc statistic power (1–β) was calculated using Power Analysis and Sample Size (PASS) 2008 Statistical Software (Kaysville, UT), determined by the obtained data of different mean CXCL12 concentrations, standard error and enrolled numbers of patients in each group. Statistical power was regarded as good at >0.8. The formula is given below:
Results
Basic data
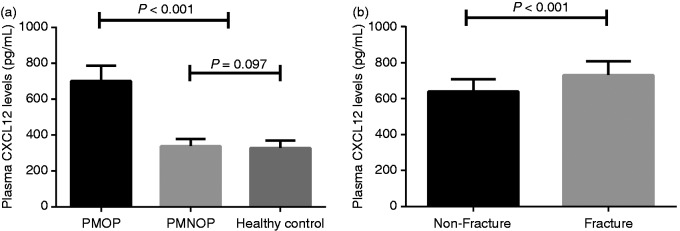
Statistical power was calculated to be 0.99. The basic features of all the enrolled patients are summarised in Table 1. There were no substantial changes with regards to age and years of menopause between the PMOP group and the PMNOP group. The mean body mass index (BMI) did not reach significance among the three groups. Plasma CXCL12 levels were suggestively greater in PMOP patients than in PMNOP women (701.3 ± 85.6 pg/ml vs. 338.9 ± 39.7 pg/ml, P < 0.001) and healthy controls (701.3 ± 85.6 pg/ml vs. 326.7 ± 39.4 pg/ml, P < 0.001; Figure 1a). No substantial difference was recorded between PMNOP and healthy controls (338.9 ± 39.7 pg/ml vs. 329.0 ±39.5 pg/ml, P = 0.097; Figure 1a).
Table 1.
Demographic statistics of all participants.
| PMOP (n = 91) | PMNOP (n = 88) | Control (n = 90) | P-Value | |
|---|---|---|---|---|
| Age | 64.7 ± 11.2** | 63.1 ± 10.5** | 35.6 ± 10.8 | < 0.001 |
| Yr of menopause | 10.1 ± 2.8 | 9.7 ± 2.2 | – | 0.079 |
| BMI (kg/m2) | 25.2 ± 2.3 | 25.7 ± 1.9 | 24.7 ± 2.0 | 0.092 |
| CXCL12 (pg/ml) | 701.3 ± 85.6*,# | 338.9 ± 39.7 | 326.7 ± 39.4 | < 0.001 |
Data are presented as the mean ± SD.
**P < 0.01 vs. control; *P < 0.05 vs. control; #P < 0.05 vs. the PMNOP group.
PMOP: postmenopausal osteoporosis; PMNOP: postmenopausal with no osteoporosis; BMI: body mass index.
Figure 1.
(a) Plasma CXCL12 levels among the postmenopausal osteoporosis (PMOP), postmenopausal with no osteoporosis (PMNOP) and healthy control groups. (b) Comparison of plasma CXCL12 levels between the vertebral fracture group and the non-fracture group.
Comparison of plasma CXCL12 levels between vertebral fracture group and non-fracture group
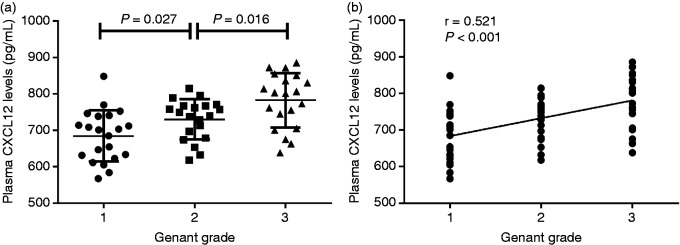
After examination, 61 patients were confirmed to have had vertebral fractures, with a κ-value of 0.91. Plasma CXCL12 levels were significantly greater in the lumbar vertebral fracture group compared to non-fracture in PMOP patients (731.3 ± 77.5 pg/ml vs. 640.3 ± 67.7 pg/ml, P < 0.001; Figure 1b). According to the Genant grading system, PMOP patients with lumbar vertebral fractures were classified into three subgroups. Twenty-one patients were classed as grade 1, 20 as grade 2 and 20 as grade 3. Plasma CXCL12 levels were suggestively greater in patients with Genant grade 3 compared to patients with Genant grade 2 (782.2 ± 74.7 pg/ml vs. 729.8 ± 55.0 pg/ml, P = 0.016). PMOP vertebral fracture patients classed as Genant grade 2 had distinctly elevated plasma CXCL12 levels compared to those classed as Genant grade 1 (729.8 ± 55.0 pg/ml vs 684.4 ± 70.2 pg/ml, P = 0.027; Figure 2a). Plasma CXCL12 concentrations were positively associated with Genant grade (r = 0.521; Figure 2b).
Figure 2.
(a) Plasma CXCL12 levels in the different Genant grade subgroups. (b) Relationship of plasma CXCL12 levels with Genant grade.
Correlation of plasma CXCL12 levels and BMDs
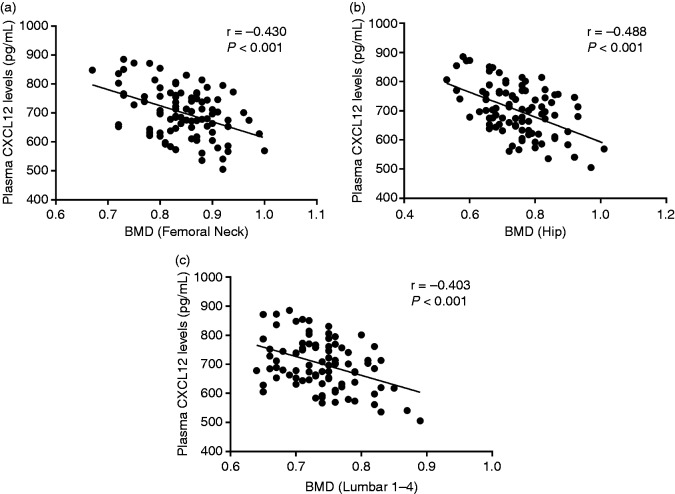
As depicted in Table 2, BMD at the femoral neck, total hip and lumbar spine (L1–L4) in PMOP was substantially lower compared to PMNOP women and controls. Furthermore, to examine the influence of CXCL12 on BMD, we inspected the association of plasma CXCL12 levels with BMD. On the basis of the correlation analysis, CXCL12 demonstrated a significant negative association with BMD at the femoral neck (r = –0.430; Figure 3a), total hip (r = –0.488; Figure 3b) and lumbar spine (L1–L4; r=−0.403; Figure 3c).
Table 2.
Comparisons of the BMD at the femoral neck, total hip, and L1–L4 lumbar spine among PMOP, PMNOP and healthy control groups.
| Control (n = 90) | PMNOP (n = 88) | PMOP (n = 91) | |
|---|---|---|---|
| BMD (neck) | 1.02 ± 0.13 | 0.99 ± 0.10 | 0.85 ± 0.07*,# |
| BMD (hip) | 1.05 ± 0.10 | 1.02 ± 0.11 | 0.77 ± 0.09*,# |
| BMD (L1–L4) | 1.10 ± 0.16 | 1.07 ± 0.13 | 0.74 ± 0.06*,# |
BMD (g/cm2) is presented as the mean ± SD.
*P < 0.05 vs. healthy control group; #P < 0.05 vs. the PMNOP group.
BMD: bone-mineral density.
Figure 3.
(a) Relationship of plasma CXCL12 levels with femoral neck bone-mineral density (BMD). (b) Relationship of plasma CXCL12 levels with total hip BMD. (c) Relationship of plasma CXCL12 levels with lumbar spine (L1–L4) BMD.
Correlation of plasma CXCL12 levels and clinical severity
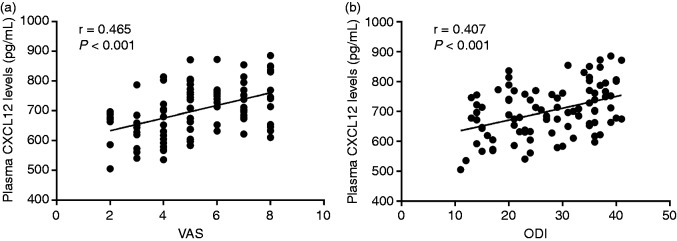
To explore further whether CXCL12 was related to symptomatic severity in PMOP patients, we examined the associations among plasma CXCL12 levels and VAS together with ODI. Interestingly, we found that plasma CXCL12 levels were positively correlated to clinical severity established by VAS (r = 0.465; Figure 4a) and ODI (r = 0.407; Figure 4b).
Figure 4.
(a) Relationship of plasma CXCL12 levels with visual analogue scale scores in PMOP patients. (b) Relationship of plasma CXCL12 levels with the Oswestry Disability Index in PMOP patients.
Correlation of plasma CXCL12 levels and biochemical indices
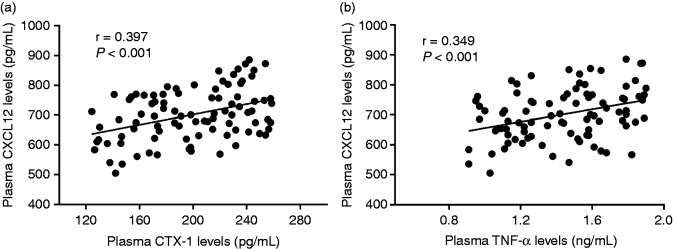
We ultimately explored the association of plasma CXCL12 concentrations with CTX-1 and TNF-α. We observed that plasma CXCL12 levels were positively related with plasma CTX-1 (r = 0.397; Figure 5a) and TNF-α concentrations (r = 0.349; Figure 5b).
Figure 5.
(a) Relationship of plasma CXCL12 levels with plasma CTX-1 levels. (b) Relationship of plasma CXCL12 levels with plasma TNF-α levels.
Discussion
In this study, we explored whether plasma CXCL12 is involved in the progression of primary PMOP. We established for the first time that plasma CXCL12 levels were negatively related to BMD at the lumbar spine (L1–L4), total hip and femoral neck in PMOP females. Elevated plasma CXCL12 levels were also correlated to severity established by VAS and ODI. In addition, increased CXCL12 levels were also positively associated with inflammatory marker TNF-α and bone resorption factor CTX-1. All the correlations continued to be significant after being adjusted for age and BMI (Table 3). These findings imply that increased plasma expression of CXCL12 may reflect disease severity and may act as a candidate marker in PMOP.
Table 3.
Correlation of plasma CXCL12 concentrations with BMD, clinical severity and biochemical indices in PMOP women after adjustment by age and BMI.
| Plasma CXCL12 concentration (pg/ml) |
Plasma CXCL12 concentration (pg/ml)a |
|||
|---|---|---|---|---|
| Variables | r | P | r | P |
| BMI | 0.024 | 0.567 | – | – |
| Age | 0.123 | 0.112 | – | – |
| Femoral neck BMD | –0.430 | < 0.001 | –0.386 | < 0.001 |
| Total hip BMD | –0.488 | < 0.001 | –0.434 | < 0.001 |
| Lumbar spine (L1–L4) BMD | –0.403 | < 0.001 | –0.357 | < 0.001 |
| VAS | 0.465 | < 0.001 | 0.412 | < 0.001 |
| ODI | 0.407 | < 0.001 | 0.360 | < 0.001 |
| Plasma CTX-1 levels | 0.397 | < 0.001 | 0.346 | < 0.001 |
| Plasma TNF-α levels | 0.349 | < 0.001 | 0.301 | 0.002 |
aAdjusted by age and BMI.
VAS: visual analogue scale; ODI: Oswestry Disability Index.
The interface of the immune and skeletal systems has suggested that there is a relationship between the two. Dysregulation of the immune system is associated with the beginning of various inflammatory autoimmune disorders, which cause antagonistic effects on bone integrity.26 Past decades have witnessed that OP is not simply an endocrine condition connected with decreased bone volume; but rather is much broader, with a substantial impact on the immune response.27 As one of the most important inflammatory factor families, chemokines are known to have important roles in osteoclast development and function.9,11 CXCL12 is the most widely studied CXC chemokine and has been related to osteoclast progenitor cell survival, differentiation, fusion and function.28
We first found that plasma CXCL12 levels were dramatically elevated in PMOP females compared to controls and were significantly related to decreased BMD and morbidity of vertebral fracture, indicating higher systemic CXCL12 levels may correlate with more global bone volume reduction. A previous study by Carbone et al. observed that circulating CXCL12 levels were negatively related with total hip and femoral neck BMD in an older adult cohort’s cardiovascular health study. However, a significant correlation with lumbar BMD could not be found.18 This disparity may be attributed to racial and sex differences. We also found CXCL12 levels were also positively related to the expression of TNF-α and CTX-1. TNF-α has been documented to prompt osteoclastogenesis in vitro29 and in vivo,30 and is regarded as a key factor in the progression of OP. In one study, CXCL12 was found to induce bone resorption via inflammatory factor, including TNF-α, and to promote the differentiation and activation of osteoclasts during collagen-induced arthritis.31 CTX-1 is a sensitive biochemical marker of bone turnover, and serum CTX reveals the rise of bone resorption in subjects with vertebral and hip fractures.32
Although processes of musculoskeletal pain in OP remain inadequately understood, osteoclast activity and peripheral and central sensitisation have been identified as several factors that lead to OP pain.33 In OP patients, high bone resorption results in pathological alterations of bone sensory nerve fibres, with an overexpression of nociceptors induced by low pH levels because of osteoclastic activity.34,35 Central sensitisation appears to have an essential involvement in developing and preserving chronicity of post-fracture pain in OP.36 The widespread release of inflammatory mediators, including IL-6 and TNF-α, leads to stimulation of glia that is inclined to self-renew and causes extreme activation of the spinal cord grey matter, resulting in sensory disruptions representative of neurological impairment.33 In our study, we found that increased plasma CXCL12 levels were strongly related to pain and functional ability defined by VAS and ODI. The CXCL12/CXCR4 axis is broadly dispersed on nociceptive structures in the peripheral nervous system and the CNS.37 Recently, studies have identified its crucial role in the advancement and continuance of pathological pain. Both neuronal and glial mechanisms have been involved in this CXCL12/CXCR4 axis-mediated pain processing.37 In a rat model of bone tumour pain, astrocytic mechanisms for the participation of the CXCL12/CXCR4 axis in tumor pain processing were illustrated.38 Shen et al. established that CXCL12 and CXCR4 were time-dependently augmented not only in the DRG of BCP rats but also in the spinal cord.38 In rat monoiodoacetate-induced OA, the effect of celastrol on pain relief was identified via suppression of the CXCL12/CXCR4 pathway.39
The current study has numerous probable limitations. First, it was carried out with a sample of Chinese postmenopausal females from our hospital, and the sample size was not sufficient enough to obtain definitive conclusions. Additional multicentre studies with larger sample size are necessary. Second, although we found that serum CXCL12 levels were significantly correlated with pain, the CXCL12 levels we tested were plasma levels of ‘total’ CXCL12 isoforms, which will not conclude decisively that higher CXCL12 levels may induce more pain. Third, the current study was cross-sectional in design, and the contributory relations need to be validated by longitudinal studies. Fourth, the levels of CXCL12 were only tested at the beginning. Considering no serial measurements of the circulating CXCL12 levels were taken, the current study did not generate data with regards to at what time and for how long biomarkers were raised in the patients. Furthermore, no other chemokines were tested that may also contribute to bone loss in PMOP patients. Lastly, we did not examine whether medication could change the CXCL12 levels in PMOP patients.
Conclusions
In conclusion, there is a positive association between CXCL12 levels in plasma and the disease severity of PMOP. Elevated CXCL12 levels in plasma were correlated with the disease severity of PMOP. Its role in medical applications requires additional investigations.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: this article was funded by the Project of Administration of traditional Chinese Medicine Guangdong Provincial of China (No. 20171143).
References
- 1.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol 2011; 6: 121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IOF Compendium of Osteoporosis. 1st ed., www.iofbonehealth.org (2017, accessed 2019).
- 3.Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the women's health initiative. Semin Reprod Med 2014; 32: 454–462. [DOI] [PubMed] [Google Scholar]
- 4.Watts NB. Postmenopausal osteoporosis: a clinical review. J Womens Health (Larchmt) 2018; 27: 1093–1096. [DOI] [PubMed] [Google Scholar]
- 5.Messina C, Maffi G, Vitale JA, et al. Diagnostic imaging of osteoporosis and sarcopenia: a narrative review. Quant Imaging Med Surg 2018; 8: 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick RK. Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev 2007; 12: 113–145. [PubMed] [Google Scholar]
- 7.Chen K, Bao Z, Tang P, et al. Chemokines in homeostasis and diseases. Cell Mol Immunol 2018; 15: 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legler DF, Thelen M. Chemokines: chemistry, biochemistry and biological function. Chimia (Aarau) 2016; 70: 856–859. [DOI] [PubMed] [Google Scholar]
- 9.Galliera E, Locati M, Mantovani A, et al. Chemokines and bone remodeling. Int J Immunopathol Pharmacol 2008; 21: 485–491. [DOI] [PubMed] [Google Scholar]
- 10.Danks L, Takayanagi H. Immunology and bone. J Biochem 2013; 154: 29–39. [DOI] [PubMed] [Google Scholar]
- 11.Sucur A, Jajic Z, Artukovic M, et al. Chemokine signals are crucial for enhanced homing and differentiation of circulating osteoclast progenitor cells. Arthritis Res Ther 2017; 19: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 2006; 12: 657–664. [DOI] [PubMed] [Google Scholar]
- 13.Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med (Berl) 2014; 92: 433–439. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama T, Hieshima K, Izawa D, et al. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol 2003; 170: 1136–1140. [DOI] [PubMed] [Google Scholar]
- 15.Chinni SR, Sivalogan S, Dong Z, et al. CXCL12/CXCR4 signaling activates Akt-I and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 2006; 66: 32–48. [DOI] [PubMed] [Google Scholar]
- 16.Lisignoli G, Toneguzzi S, Piacentini A, et al. CXCL2 (SDF-l) and CXCL13 (BCA-I) chemokines significantly induce proliferation and collagen type I expression in osteoblasts from osteoarthritis patients. J Cell Physiol 2006; 206: 78–85. [DOI] [PubMed] [Google Scholar]
- 17.Kanbe K, Chiba J, Inoue Y, et al. SDF-1 and CXCR4 in synovium are associated with disease activity and bone and joint destruction in patients with rheumatoid arthritis treated with golimumab. Mod Rheumatol 2016; 26: 46–50. [DOI] [PubMed] [Google Scholar]
- 18.Carbone LD, Bůžková P, Fink HA, et al. Association of plasma SDF-1 with bone mineral density, body composition, and hip fractures in older adults: the Cardiovascular Health Study. Calcif Tissue Int 2017; 100: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang T, Gong M, Han J, et al. Relationship between osteoporosis and expression of Bcl-2 and CXCL12. Exp Ther Med 2018; 15: 1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shima K, Kimura K, Ishida M, et al. C-X-C motif chemokine 12 enhances lipopolysaccharide-induced osteoclastogenesis and bone resorption in vivo. Calcif Tissue Int 2018; 103: 431–442. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. World Health Organ Tech Rep Ser 1994; 843: 1–129. [PubMed] [Google Scholar]
- 22.Baerts L, Waumans Y, Brandt I, et al. Circulating stromal cell–derived factor 1α levels in heart failure: a matter of proper sampling. PLoS One 2015; 10: e0141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1996; 11: 984–996. [DOI] [PubMed] [Google Scholar]
- 24.Chiarotto A, Maxwell LJ, Ostelo RW, et al. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the Brief Pain Inventory in patients with low back pain: a systematic review. J Pain 2018; 10: S1526–5900. [DOI] [PubMed] [Google Scholar]
- 25.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000; 25: 2940–2952. [DOI] [PubMed] [Google Scholar]
- 26.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 2007; 7: 429–442. [DOI] [PubMed] [Google Scholar]
- 27.Al-Daghri NM, Aziz I, Yakout S, et al. Inflammation as a contributing factor among postmenopausal Saudi women with osteoporosis. Medicine (Baltimore) 2017; 96: e5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo T, Liu H, Feng W, et al. Adipocytes enhance expression of osteoclast adhesion-related molecules through the CXCL12/CXCR4 signalling pathway. Cell Prolif 2017; 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller K, Murphy C, Kirstein B, et al. TNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 2002; 143: 1108–1118. [DOI] [PubMed] [Google Scholar]
- 30.Kitaura H, Sands MS, Aya K, et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J Immunol 2004; 173: 4838–4846. [DOI] [PubMed] [Google Scholar]
- 31.De Klerck B, Geboes L, Hatse S, et al. Pro-inflammatory properties of stromal cell–derived factor-1 (CXCL12) in collagen-induced arthritis. Arthritis Res Ther 2005; 7: R1208–R1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawana K, Takahashi M, Hoshino H, et al. Comparison of serum and urinary C-terminal telopeptide of type I collagen in aging, menopause and osteoporosis. Clin Chim Acta 2002; 316: 109–115. [DOI] [PubMed] [Google Scholar]
- 33.Catalano A, Martino G, Morabito N, et al. Pain in osteoporosis: from pathophysiology to therapeutic approach. Drugs Aging 2017; 34: 755–765. [DOI] [PubMed] [Google Scholar]
- 34.Julius D, Basbaum Al. Molecular mechanisms of nociception. Nature 2001; 413: 203–210. [DOI] [PubMed] [Google Scholar]
- 35.Mediati R, Vellucci R, Dodaro L. Pathogenesis and clinical aspects of pain in patients with osteoporosis. Clin Cases Miner Bone Metab 2014; 11: 169–172. [PMC free article] [PubMed] [Google Scholar]
- 36.Vellucci R, Terenzi R, Kanis JA. Understanding osteoporotic pain and its pharmacological treatment: supplementary presentation. Osteoporos Int 2018; 29: 2153–2154. [DOI] [PubMed] [Google Scholar]
- 37.Luo X, Wang X, Xia Z, et al. CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Rev Neurosci 2016; 27: 83–92. [DOI] [PubMed] [Google Scholar]
- 38.Shen W, Hu XM, Liu YN, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 2014; 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Ha C, Lin T, et al. Celastrol attenuates pain and cartilage damage via SDF-1/CXCR4 signalling pathway in osteoarthritis rats. J Pharm Pharmacol 2018; 7: 81–88. [DOI] [PubMed] [Google Scholar]