Abstract
Sex identification of ancient animal biological remains can benefit our understanding of historical population structure, demography and social behavior. Traditional methods for sex identification (e.g., osteological and morphometric comparisons) may be ineffective when animal remains are not well preserved, when sex distinguishing characteristics have not yet developed, or where organisms do not exhibit sex-associated phenotypic dimorphisms. Here we adapt a method developed for human sex determination so that it can be used to identify the sex of ancient and modern animal taxa. The method identifies sex by calculating the ratio of DNA reads aligning to the X chromosome to DNA reads aligning to autosomes (termed the Rx ratio). We tested the accuracy of this method using low coverage genomes from 15 modern elephants (Loxodonta africana) for which sex was known. We then applied this method to ancient elephant ivory samples for which sex was unknown, and describe how this method can be further adapted to the genomes of other taxa. This method may be especially useful when only low-coverage genomic data are obtainable. Furthermore, because this method relies on only the X and not the Y chromosome, it can be used to determine the sex of organisms for which a reference genome was obtained from a female or for which only the X chromosome is reported. Such taxa include the domestic cat, sheep, goat, and horse; and non-domesticated animals such as the Sumatran orangutan, western lowland gorilla and meerkat.
Keywords: XY karyotype, Rx ratio, Loxodonta, low-coverage, sex assessment, molecular sexing
Identifying the sex of animals can yield insights into population structure (Schoener and Schoener 1980; Bodkin et al. 2000), demographic histories (Heyer et al. 2012) and social interactions (Pedersen et al. 1990; Lonsdorf et al. 2014). It can add to knowledge of extinct and extant animal populations and reveal how they have changed across time. Sex identification can aid our understanding of extinct animal biology (Allentoft et al. 2010), past hunting practices and domestication (Collier and White 1976; D’Errico and Vanhaeren 2002). For many ancient or historical samples, however, the sex of specimens is unknown. Sex identification may be hindered when remains are very degraded or only partially preserved, when remains are from young individuals where sex distinguishing characteristics have yet to develop, or when remains are from taxa that do not exhibit phenotypic sexual dimorphism (Hamilton et al. 1986). Such factors may preclude sex identification through traditional methods such as osteological or morphometric comparison (measurements of skeletal ratios/aspects) (Safont et al. 2000; Rogers 2005; Bruzek and Murail 2006). Molecular sex identification circumvents these issues, requiring only a small sample for DNA analysis. For ancient samples with a low quantity and quality of DNA (Quincey et al. 2013), molecular methods test for DNA authenticity by determining whether amplified DNA exhibits damage patterns typical of ancient DNA (Jónsson et al. 2013). Molecular methods therefore permit sex identification of degraded or partial specimens, from young and from sexually monomorphic taxa. Molecular sex identification methods have involved the analysis of genes associated with male and female sex chromosomes in birds (Griffiths et al. 1998; Fridolfsson and Ellegren 1999), reptiles (Quinn et al. 2009), mammals (Sullivan et al. 1993; Gibbon et al. 2009), and fish (Chen et al. 2007). For example, in some mammals, molecular sex identification involves differentiating between amelogenin gametologues on the X and Y chromosomes (Sullivan et al. 1993; Gibbon et al. 2009).
For sex identification of archeological human remains, Mittnik et al. (2016) developed a method that uses low coverage whole genome data to calculate the Rx ratio, which compares DNA sequence reads that align to the X chromosome to DNA sequence reads that align to autosomal chromosomes. The Rx ratio is different for females and males, since they have two or one X-chromosomes, respectively. The Rx ratio would be expected to be ca. 1.0 for females and 0.5 for males. Mittnik et al. (2016) identified individuals as female if the Rx 95% CI lower bound was higher than 0.80, and as male if the 95% confidence interval (CI) upper bound for Rx was lower than 0.60.
Here, we present an extension and expansion of the method of Mittnik et al. (2016) to permit sex identification of ancient and modern samples of non-human taxa. We adjust the Rx equation to mathematically account for different chromosome numbers across animal taxa. Our method, in principle, allows for accurate sex determination of any organism with XY sex determination for which a reference genome is available with chromosome-level resolution. We verify the method using low-coverage genomes from 15 modern elephants for which sex is known, and apply this method successfully to low-coverage genomes of ten ancient elephant ivory samples for which sex was unknown. These ancient ivory samples are from a 16th Century shipwreck uncovered in Namibia and believed to be the Bom Jesus, a Portuguese trading ship lost in 1533 en route to India (Werz 2010; Alves 2011).
Materials and Methods
DNA extraction and shotgun sequencing
DNA was extracted from skin biopsy samples from 15 African elephants for which sex was recorded in the field when the samples were collected. The modern elephant samples were from nine females and six males (Table 1). Genomic libraries were constructed for the 15 modern elephants at the UIUC Core Sequencing Facility using TruSeq DNA library preparation. To generate low-coverage genomes for the modern elephant samples, we sequenced the 15 samples as part of a larger pool of samples in a single HiSeq 4000 lane (150bp paired-end). DNA from the ancient ivory was extracted following methods described in Cui et al. (2013). Cui et al. (2013) provide details, for example, on starting template amounts (0.20g per ancient sample) and treatment protocols. Ancient DNA work (extractions and genomic library preparation) was conducted in the Malhi Ancient DNA Laboratory, which is dedicated exclusively to studies involving ancient DNA, at the Carl R. Woese Institute for Genomic Biology, University of Illinois at Urbana-Champaign (UIUC). All rounds of DNA extraction included a negative control to verify that reagents and equipment were not contaminated and that there was no cross-contamination between samples, and not more than eight samples were processed at any one time. Libraries for the ten ancient ivory samples were constructed using the NEBNext Ultra II DNA Library Prep kit and NEBNext Multiplex Oligos (Unique Dual Indexes) for Illumina. Because ancient DNA are prone to have cytosine to uracil nucleotide base changes (Hofreiter et al. 2001), the extracted ancient DNA was pre-treated with USER (Uracil-Specific Excision Reagent) enzyme. The modern and ancient libraries were pooled separately, and each pool was shotgun sequenced on a HiSeq 4000 platform at the UIUC Core Sequencing Facility.
Table 1. Known sex of modern elephants, and predicted sex using the Rx ratio.
| Sample ID | Rx ratioa | 95% CIb | Known sex | Predicted sex |
|---|---|---|---|---|
| DS1531 | 0.9348111 | 0.9241519 | Female | Female |
| 0.9454702 | ||||
| DS1548 | 0.4844121 | 0.4781392 | Male | Male |
| 0.4906849 | ||||
| DS1514 | 0.9300965 | 0.9194486 | Female | Female |
| 0.9407445 | ||||
| DS1543 | 0.4918646 | 0.4866054 | Male | Male |
| 0.4971237 | ||||
| DS1506 | 0.4878141 | 0.4826157 | Male | Male |
| 0.4930124 | ||||
| LO3503 | 0.9321284 | 0.9215853 | Female | Female |
| 0.9426715 | ||||
| LO3509 | 0.9415045 | 0.9308473 | Female | Female |
| 0.9521617 | ||||
| LO3511 | 0.9287396 | 0.917744 | Female | Female |
| 0.9397352 | ||||
| LO3521 | 0.8712463 | 0.8574852 | Female | Female |
| 0.8850075 |
The Rx ratio compares DNA sequence reads that align to the X chromosome to DNA sequence reads that align to autosomal chromosomes, and would be expected to be ca. 1.0 for females and 0.5 for males.
The top value represents lower bound of the 95% Confidence Intervals (CI) and the lower value represents the upper bound of the 95% CI.
Bioinformatic analyses and Rx based sex identification
Sample reads were de-multiplexed and trimmed using the program FastP v.0.19.6 (Chen et al. 2018) to have a minimum sequence length of 25bp. Reads were aligned to the chromosome-level assembly of the African savanna elephant genome (Loxodonta africana assembly Loxafr4.0, Broad Institute (Palkopoulou et al. 2018)) using bowtie2 (Langmead and Salzberg 2012) with the local alignment option, and capping fragment length at 1000bp. Aligned sequences were transformed to BAM format in SAMtools v. 1.1 (Li et al. 2009). Using SAMtools, BAM files were filtered to remove unmapped reads and reads with a quality score less than 30, then sorted and indexed, with PCR duplicates marked and removed with the Picard Toolkit v. 2.10.1. Index statistics for BAM files were generated using “idxstats” in SAMtools (Li et al. 2009).
The Rx_identifier.r script of Mittnik et al. (2016) was modified to accommodate the number of chromosome pairs found in elephants, which is different from the number in humans, for which the script was originally developed (see Supplementary Appendix 1 for a stepwise protocol of how to modify this script for any organism that has a chromosome-level reference genome and XY sex determinism). We verified that the row numbers in the Rx_identifier script corresponded to the correct chromosome identities in our sorted idxstat files. The modified Rx_identifier.r script was then implemented using the program R v. 3.3.3 (R-Development-Core-Team 2017) and the indxstat files as input. Output statistics for each sample included the Rx ratio, and sex identification based on the data ranges of Mittnik et al. (2016), where a sample was identified as male if its 95% confidence interval (CI) upper bound for Rx was lower than 0.60 and identified as female if its Rx 95% CI lower bound was higher than 0.80. The 95% CI was computed as Rx± 1.96 SE (standard error), where the SE measures the amount of variability in the Rx mean compared to autosomes (22 for humans, 27 for elephants). We determined whether sequence coverage was sufficient by performing a linear regression of the number of sequenced and mapped reads on each chromosome against the number of reference reads. Output statistics were visualized by plotting individual Rx ratios (Figure 1) using R v. 3.3.3 (R-Development-Core-Team 2017). The bioinformatic analyses were repeated using BWA (Li and Durbin 2010) to check for inconsistencies that could be associated with sequence aligner choice, but no inconsistencies were observed and sex identification was completely consistent between the two analyses. Ancient DNA damage patterns were verified by aligning trimmed reads to the African savannah elephant genome (LoxAfr 4.0) using BWA (Li and Durbin 2010) and quantifying damage in mapDamage2 (Jónsson et al. 2013) using a fragment size of 70bp.
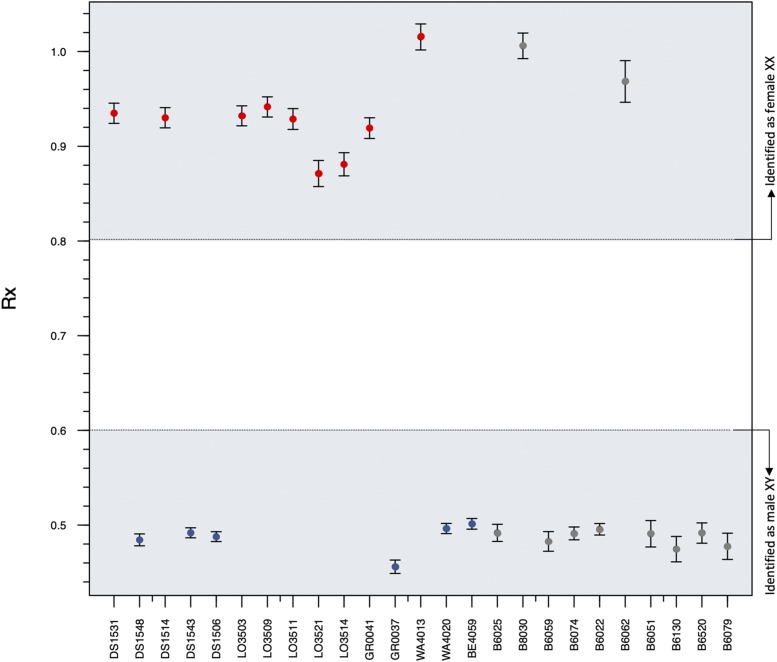
Figure 1.
Rx values for modern elephants of known sex (red and blue) and ancient elephant samples of unknown sex (gray). Rx is the ratio of sequence read alignments to the X chromosome compared to sequence read alignments to all autosomes. Rx values are shown for low coverage modern elephant genomes of known males (blue) and females (red), and for ancient elephant genomes for ivory samples of previously unknown sex (gray). An Rx ratio with an upper 95% CI of less than 0.6 indicates male sex, and an Rx ratio with a lower 95% CI that is greater than 0.8 indicates female sex.
To determine how effective the Rx method is for determining the sex of samples with even lower coverages that ours, we subsampled the existing ancient ivory data to include datasets of approximately 10 000 and 1 000 reads. We used Sambamba (Tarasov et al. 2015) to subsample datasets, and reanalyzed the subsampled datasets using the Rx method.
Data availability
All idxstats files for modern and ancient elephant genomes, and all Rx ratio result files for the 10 000- and 1000-read subsampled files are available from GSA Journals figshare portal. The most recent update of the savanna elephant reference genome (LoxAfr4) is available at ftp://ftp.broadinstitute.org/pub/assemblies/mammals/elephant/loxAfr4/. R-scripts and a step-by-step description of how to adapt the R-script to any species chromosome-level genome can be found at https://github.com/adeflamingh/de_Flamingh_et_al_2020_G3.git or as Appendix 1 and 2, or as part of the supplementary material on GSA Journals figshare. The study was conducted under the University of Illinois Institutional Animal Care and Use Committee approved protocol number 18042. Samples were imported through a CITES permit. Supplemental material available at figshare: https://doi.org/10.25387/g3.11837157.
Results
Although all ancient samples were pre-treated with USER enzyme, which may potentially mask damage patterns (see Methods), DNA damage patterns in the ancient ivory were still evident and typical of ancient DNA (Supplementary Figure 1). Each of the ancient samples showed increased rates of C to T and G to A mismatches relative to the reference genome, as would be expected in authentic ancient DNA.
The adapted Rx_identifier.R script (Supplementary Appendix 2) was able to identify sex for all 15 modern individuals (nine females, six males) with 100% accuracy (Figure 1; Table 1). For the ancient DNA remains, the adapted script identified eight individuals as male, and two individuals as female (Figure 1; Table 2). Linear regressions of the number of reference genome reads with the number of mapped reads resulted in significant F-statistic values (P < 0.001) for both modern and ancient remains, indicating that the sequence coverage for all genomes was sufficient for accurate sex determination.
Table 2. Predicted sex of ancient elephant samples using the Rx ratio.
| Ancient sample ID | Read count | Rx ratioa | 95% CIb | Predicted sex |
|---|---|---|---|---|
| B6025a | 991,719 | 0.4917869 | 0.4827309 | Male |
| 0.5008429 | ||||
| B8030 | 6,186,805 | 1.00599 | 0.9925127 | Female |
| 1.019467 | ||||
| B6059 | 3,672,375 | 0.4827319 | 0.4723486 | Male |
| 0.4931151 | ||||
| B6074 | 864,649 | 0.4912222 | 0.4844642 | Male |
| 0.4979802 | ||||
| B6022 | 1,848,409 | 0.4955726 | 0.4894958 | Male |
| 0.5016494 | ||||
| B6062 | 919,574 | 0.9684064 | 0.9464218 | Female |
| 0.9903909 | ||||
| B6051 | 460,668 | 0.4908337 | 0.476899 | Male |
| 0.5047683 | ||||
| B6130 | 17,797,452 | 0.4745888 | 0.4611754 | Male |
| 0.4880023 | ||||
| B6520 | 141,855 | 0.4915878 | 0.4808738 | Male |
| 0.5023018 | ||||
| BB6079 | 5,729,007 | 0.477581 | 0.4637086 | Male |
| 0.4914535 |
The Rx ratio compares DNA sequence reads that align to the X chromosome to DNA sequence reads that align to autosomal chromosomes, and would be expected to be ca. 1.0 for females and 0.5 for males.
The top value represents lower bound of the 95% Confidence Intervals (CI) and the lower value represents the upper bound of the 95% CI.
The Rx ratio method effectively identified the sex when using data files with > 100 000 reads (all 95% CI are within the specified Rx cut-off values; Supplementary figure 2). The method was mostly effective when using subsampled ancient ivory data files with 10 000 reads (only sample B6079 had 95% CI outside of Rx cut-off values), but proved less effective when using subsampled datasets with 1000 reads (the span of the 95% CI increased for all samples and four samples had 95% CI outside of Rx cut-off values; Supplementary figure 2).
Discussion
We adapted a method previously developed Mittnik et al. (2016) for sex identification of human remains for use with non-human taxa, and successfully identified the sex of modern and ancient elephants from low coverage genome data. Because the Rx ratio sex identification method presented in this study relies only on the X and not the Y chromosome, it can be used to identify the sex of organisms in which the reference genome was obtained from a female animal or where only the X and not the Y chromosome is reported in the reference genome assembly for the taxon. Such taxa would include (but not be limited to) the domestic cat, sheep, goat, horse, dromedary camel, European rabbit; and also include many wild animals such as the Sumatran orangutan, western lowland gorilla, gelada and meerkat (Supplementary Table 1). Being able to identify the sex of samples could benefit agricultural studies on domesticated animals, and could inform conservation initiatives that focus on non-domestic wildlife. Because this method is amenable to low coverage data from low quantity DNA (e.g., ancient or degraded DNA), it can be employed as a non-invasive approach to identifying sex of endangered or rare species, for example, through the analysis of DNA from hair tufts (McKelvey et al. 2006; Stanton et al. 2016) or herbivore scat (Huber et al. 2002). By requiring only minute quantities of DNA as a starting template, the method could be extended to other types of degraded DNA such as archival samples from museum collections (Wandeler et al. 2003; Bi et al. 2013) or forensic samples (Jobling and Gill 2004; Alaeddini et al. 2010).
Sex identification using the Rx ratio could be adapted to any taxa that exhibit XY sex determination for which a chromosome-level genome assembly is available. It should be possible to further extend the method to taxa that have a ZW sex determination system, in which males are the homogametic sex ZZ, and females have Z and W chromosomes. Such taxa include birds (Chue and Smith 2011), amphibians (Nakamura 2009) and crustaceans (Cui et al. 2015). For ZW sex determination systems, individuals should be identified as male (ZZ) if the lower bound of their 95% Rx ratio CI is approximately 0.8 or higher, and female (ZW) if the upper bound of the 95% Rx ratio CI is approximately 0.6 or less. For ZW sex determination the script should be adapted so that the Z chromosome replaces the X chromosome in the Rx_identifier.R script, and the W chromosome replaces the Y chromosome (if it is present in the reference genome). Again, since the script could rely only on the Z chromosome and not the W chromosome, this method may be used on any individual, male or female, with ZW sex determination if there is a chromosome-level reference genome assembly available for that species. Future studies would be needed to validate the use of the adapted script on animals other than elephants or humans.
We investigated whether the Rx method can effectively identify the sex of individuals when using genome coverage even lower than that of the ancient ivory samples. We found that there is a substantial broadening of the 95% CI as the read count of the data file decreases (Supplementary figure 2). We suggest that the Rx cut-off values presented by Mittnik et al. (2016), and in this paper, may be useful indicators of the ability of Rx script to accurately and precisely identify individual sex, and caution users to be less confident in sex identification if the confidence intervals extend beyond these cut-off values.
The Rx ratio method was successfully used here on low coverage genomic data from both modern and ancient (Supplementary Table 2) elephants. The ability to accurately identify sex based on low coverage data may be especially useful with ancient samples with DNA of low quantity and quality (Quincey et al. 2013), and for studies that index and pool a large number of individuals for sequencing (e.g., PoolSeq studies). Such studies may have low coverage per individual, but many individuals may be indexed and pooled to represent a population. The limited requisites and ease of adaptation and implementation of this method would allow for convenient and effective identification of the sex of modern and ancient animal remains.
Acknowledgments
This project was supported by US Fish and Wildlife Service African Elephant Conservation Grant AFE-1816-F18AS00055, and by a Program in Ecology, Evolution and Conservation Biology Research Award to AdF through the Cooperative State Research, Education, and Extension Service, US Department of Agriculture, under project numbers ILLU 875–952 and ILLU-538-939. We thank the CORE sequencing facility for their help with constructing the genomic DNA TruSeq libraries. For technical and other assistance, we thank T. Perrin-Stowe and Y. Ishida. We thank Judith Sealy and Shadreck Chirikure at the University of Cape Town, South Africa, and David Reich at Harvard Medical School, Boston, USA, for respectively providing access to the ancient ivory samples and modern elephant genomic data.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25387/g3.11837157.
Communicating editor: T. Pyhäjärvi
Literature Cited
- Alaeddini R., Walsh S. J., and Abbas A., 2010. Forensic implications of genetic analyses from degraded DNA—a review. Forensic Sci. Int. Genet. 4: 148–157. 10.1016/j.fsigen.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Allentoft M. E., Bunce M., Scofield R. P., Hale M. L., and Holdaway R. N., 2010. Highly skewed sex ratios and biased fossil deposition of moa: ancient DNA provides new insight on New Zealand’s extinct megafauna. Quat. Sci. Rev. 29: 753–762. 10.1016/j.quascirev.2009.11.022 [DOI] [Google Scholar]
- Alves, F. J. S., 2011 The 16th century Portuguese shipwreck of Oranjemund, Namibia. Report on the Missions Carried out by the Portuguese Team in 2008 and 2009.
- Bi K., Linderoth T., Vanderpool D., Good J. M., Nielsen R. et al. , 2013. Unlocking the vault: next–generation museum population genomics. Mol. Ecol. 22: 6018–6032. 10.1111/mec.12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin J. L., Burdin A. M., and Ryazanov D. A., 2000. Age-and sex-specific mortality and population structure in sea otters. Mar. Mamm. Sci. 16: 201–219. 10.1111/j.1748-7692.2000.tb00913.x [DOI] [Google Scholar]
- Bruzek J., and Murail P., 2006. Methodology and reliability of sex determination from the skeleton, pp. 225–242 in Forensic anthropology and medicine, Springer; 10.1007/978-1-59745-099-7_9 [DOI] [Google Scholar]
- Chen S., Zhou Y., Chen Y., and Gu J., 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34: i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-L., Li J., Deng S.-P., Tian Y.-S., Wang Q.-Y. et al. , 2007. Isolation of female-specific AFLP markers and molecular identification of genetic sex in half-smooth tongue sole (Cynoglossus semilaevis. Mar. Biotechnol. (NY) 9: 273–280. 10.1007/s10126-006-6081-x [DOI] [PubMed] [Google Scholar]
- Chue J., and Smith C. A., 2011. Sex determination and sexual differentiation in the avian model. FEBS J. 278: 1027–1034. 10.1111/j.1742-4658.2011.08032.x [DOI] [PubMed] [Google Scholar]
- Collier S., and White J. P., 1976. Get them young? Age and sex inferences on animal domestication in archaeology. Am. Antiq. 41: 96–102. 10.2307/279046 [DOI] [Google Scholar]
- Cui Y., Lindo J., Hughes C. E., Johnson J. W., Hernandez A. G. et al. , 2013. Ancient DNA analysis of mid-holocene individuals from the Northwest Coast of North America reveals different evolutionary paths for mitogenomes. PLoS One 8: e66948 10.1371/journal.pone.0066948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Hui M., Liu Y., Song C., Li X. et al. , 2015. High-density linkage mapping aided by transcriptomics documents ZW sex determination system in the Chinese mitten crab Eriocheir sinensis. Heredity 115: 206–215. 10.1038/hdy.2015.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Errico, F., and M. Vanhaeren, 2002 Criteria for identifying red deer age and sex from their canines. Application to Upper Palaeolithic and Mesolithic ornaments.
- Fridolfsson A.-K., and Ellegren H., 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30: 116–121. 10.2307/3677252 [DOI] [Google Scholar]
- Gibbon V., Paximadis M., Štrkalj G., Ruff P., and Penny C., 2009. Novel methods of molecular sex identification from skeletal tissue using the amelogenin gene. Forensic Sci. Int. Genet. 3: 74–79. 10.1016/j.fsigen.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Griffiths R., Double M. C., Orr K., and Dawson R. J. G., 1998. A DNA test to sex most birds. Mol. Ecol. 7: 1071–1075. 10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Hamilton, J., L. Tilson, and G. Frank, 1986 Sexual Monomorphism in Spotted Hyenas, Crocuta crocuta 73: 63–73.
- Heyer E., Chaix R., Pavard S., and Austerlitz F., 2012. Sex-specific demographic behaviours that shape human genomic variation. Mol. Ecol. 21: 597–612. 10.1111/j.1365-294X.2011.05406.x [DOI] [PubMed] [Google Scholar]
- Hofreiter M., Jaenicke V., Serre D., von Haeseler A., and Pääbo S., 2001. DNA sequences from multiple amplifications reveal artifacts induced by cytosine deamination in ancient DNA. Nucleic Acids Res. 29: 4793–4799. 10.1093/nar/29.23.4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S., Bruns U., and Arnold W., 2002. Sex determination of red deer using polymerase chain reaction of DNA from feces. Wildl. Soc. Bull. 30: 208–212. [Google Scholar]
- Jobling M. A., and Gill P., 2004. Encoded evidence: DNA in forensic analysis. Nat. Rev. Genet. 5: 739–751. 10.1038/nrg1455 [DOI] [PubMed] [Google Scholar]
- Jónsson H., Ginolhac A., Schubert M., Johnson P. L. F., and Orlando L., 2013. mapDamage2. 0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29: 1682–1684. 10.1093/bioinformatics/btt193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., and Salzberg S. L., 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., and Durbin R., 2010. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26: 589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J. et al. , 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf E. V., Anderson K. E., Stanton M. A., Shender M., Heintz M. R. et al. , 2014. Boys will be boys: sex differences in wild infant chimpanzee social interactions. Anim. Behav. 88: 79–83. 10.1016/j.anbehav.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey K. S., von Kienast J., Aubry K. B., Koehler G. M., Maletzke B. T. et al. , 2006. DNA analysis of hair and scat collected along snow tracks to document the presence of Canada lynx. Wildl. Soc. Bull. 34: 451–455. 10.2193/0091-7648(2006)34[451:DAOHAS]2.0.CO;2 [DOI] [Google Scholar]
- Mittnik A., Wang C. C., Svoboda J., and Krause J., 2016. A molecular approach to the sexing of the triple burial at the upper paleolithic site of Dolní Vêstonice. PLoS One 11: e0163019 10.1371/journal.pone.0163019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., 2009. Sex determination in amphibians. Semin. Cell Dev. Biol. 20: 271–282 Elsevier. 10.1016/j.semcdb.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Palkopoulou E., Lipson M., Mallick S., Nielsen S., Rohland N. et al. , 2018. A comprehensive genomic history of extinct and living elephants. Proc. Natl. Acad. Sci. USA 115: E2566–E2574. 10.1073/pnas.1720554115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J. M., Glickman S. E., Frank L. G., and Beach F. A., 1990. Sex differences in the play behavior of immature spotted hyenas, Crocuta crocuta. Horm. Behav. 24: 403–420. 10.1016/0018-506X(90)90018-S [DOI] [PubMed] [Google Scholar]
- Quincey D., Carle G., Alunni V., and Quatrehomme G., 2013. Difficulties of sex determination from forensic bone degraded DNA: A comparison of three methods. Sci. Justice 53: 253–260. 10.1016/j.scijus.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Quinn A. E., Radder R. S., Sarre S. D., Georges A., Ezaz T. et al. , 2009. Isolation and development of a molecular sex marker for Bassiana duperreyi, a lizard with XX/XY sex chromosomes and temperature-induced sex reversal. Mol. Genet. Genomics 281: 665–672. 10.1007/s00438-009-0437-7 [DOI] [PubMed] [Google Scholar]
- R-Development-Core-Team , 2017. R: A language and environment for statistical computing.
- Rogers T. L., 2005. Determining the sex of human remains through cranial morphology. J. Forensic Sci. 50: 1–8. [PubMed] [Google Scholar]
- Safont S., Malgosa A., and Subirà M. E., 2000. Sex assessment on the basis of long bone circumference. American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists 113: 317–328. 10.1002/1096-8644(200011)113:3<317::AID-AJPA4>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- Schoener T. W., and Schoener A., 1980. Densities, sex ratios, and population structure in four species of Bahamian Anolis lizards. J. Anim. Ecol. 49: 19–53. 10.2307/4276 [DOI] [Google Scholar]
- Stanton D. W. G., Hart J., Vosper A., Kümpel N. F., Wang J. et al. , 2016. Non-invasive genetic identification confirms the presence of the Endangered okapi Okapia johnstoni south-west of the Congo River. Oryx 50: 134–137. 10.1017/S0030605314000593 [DOI] [Google Scholar]
- Sullivan K. M., Mannucci A., Kimpton C. P., and Gill P., 1993. A rapid and quantitative DNA sex test: fluorescence-based PCR analysis of XY homologous gene amelogenin. Biotechniques 15: 636–638. [PubMed] [Google Scholar]
- Tarasov A., Vilella A. J., Cuppen E., Nijman I. J., and Prins P., 2015. Sambamba: fast processing of ngs alignment formats. Bioinformatics 31: 2032–2034. 10.1093/bioinformatics/btv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandeler P., Smith S., Morin P. A., Pettifor R. A., and Funk S. M., 2003. Patterns of nuclear DNA degeneration over time—a case study in historic teeth samples. Mol. Ecol. 12: 1087–1093. 10.1046/j.1365-294X.2003.01807.x [DOI] [PubMed] [Google Scholar]
- Werz B. E. J. S., 2010. Sub-saharan Africa’s oldest shipwreck: historical-archeological research of an early modern-era Portuguese merchantman on the Namibian coast. Mar. Mirror 96: 430–442. 10.1080/00253359.2010.10657159 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All idxstats files for modern and ancient elephant genomes, and all Rx ratio result files for the 10 000- and 1000-read subsampled files are available from GSA Journals figshare portal. The most recent update of the savanna elephant reference genome (LoxAfr4) is available at ftp://ftp.broadinstitute.org/pub/assemblies/mammals/elephant/loxAfr4/. R-scripts and a step-by-step description of how to adapt the R-script to any species chromosome-level genome can be found at https://github.com/adeflamingh/de_Flamingh_et_al_2020_G3.git or as Appendix 1 and 2, or as part of the supplementary material on GSA Journals figshare. The study was conducted under the University of Illinois Institutional Animal Care and Use Committee approved protocol number 18042. Samples were imported through a CITES permit. Supplemental material available at figshare: https://doi.org/10.25387/g3.11837157.