Abstract
Nanostructured gas sensors find diverse applications in environmental and agricultural monitoring. Herein, adsorption of phosgene (COCl2) on pure and copper-decorated B12N12 (Cu–BN) is analyzed through density functional theory (DFT) calculations. Adsorption of copper on B12N12 results in two optimized geometries, named Cu@b66 and Cu@b64, with adsorption energies of −193.81 and −198.45 kJ/mol, respectively. The adsorption/interaction energies of COCl2 on pure BN nanocages are −9.30, −6.90, and −3.70 kJ/mol in G1, G2, and G3 geometries, respectively, whereas the interaction energies of COCl2 on copper-decorated BN are −1.66 and −16.95 kJ/mol for B1 and B2, respectively. To examine the changes in the properties of pure and Cu–BN nanocages, geometric parameters, dipole moment, QNBO, frontier molecular orbitals, and partial density of states (PDOS) are analyzed to comprehensively illustrate the interaction mechanism. The results of these parameters reveal that COCl2 binds more strongly onto copper-doped BN nanocages. Moreover, a higher charge separation is observed in COCl2–Cu–BN geometries as compared to copper-decorated BN geometries. Therefore, these nanocages may be considered as potential candidates for application in phosgene sensors.
1. Introduction
The field of nanotechnology and nanoscience has developed rapidly. Discovery of nanotubes (carbon nanotubes) by Ijimia increased the demand for nanoscale materials and laid the foundation for the rapid development of the field of nanoscale materials (carbon nanoscale materials).1 Nanomaterials such as nanocages, nanoclusters, and nanotubes find vast applications in optical devices,2,3 catalysis,4 sensing materials (sensors),5 adsorption,6,7 and medical8 and electronic devices.9
Besides the use of carbon nanotubes and fullerene (C60) in advanced devices, extensive efforts have been made toward the synthesis of tubular or spherical fullerenes of diverse inorganic (non-carbon) materials over the last several years. Boron nitride (BN),10,11 silica,12 and aluminum nitride (AlN) nanotubes and co-axial cubic AlN–BN composites13 are the most important and interesting examples in this regard. Due to the closed electronic shells of nanoclusters, they are considered to be very stable and play a vital role in the development of materials used in advanced technologies. Therefore, they are the subject of several investigations.14 Studies illustrate that (XY)n clusters, X12Y12 (X = Al, B, Ga and Y = As, N, P), are the most stable (magic clusters).15,16 Toftlund and Jensen reported different geometries of BXNY nanoclusters in their reports and concluded that the B12N12 nanocluster is highly stable and important as compared to C24.15 These III–V clusters also find applications in light-emitting diodes and microelectronic devices.17,18 B12N12 and A12N12 (two semiconductor-like group III–V clusters) have excellent physiochemical properties.19,20
Boron nitrides (BN)x have drawn attention due to their large thermal conductivity, large highest occupied molecular orbital–lowest unoccupied molecular orbital (HOMO–LUMO) gap, high temperature stability, low dielectric constant, and high resistance to oxidation.21−23 Oku et al. synthesized B12N12 nanocages, which consist of eight hexagonal rings and six tetragonal rings.22 Moreover, the phenomenon of charge separation occurs between boron (B) and nitrogen (N), where the boron atom acts as an electron-deficient (Lewis acid) and the nitrogen atom acts as an electron-rich species (Lewis base). Due to this concept, the BN nanostructure is considered to behave as a noncatalyst (as it is a Lewis acid–Lewis base pair).
Recently, studies of the interaction of pure B12N12 nanoclusters with different systems, such as methylamine,24 CO2,25 CO,26 NO, hydrogen, N2, methane,27 mono-fluoromethane (MFM), thiophene,28 mono-chloromethane (MCM),29 SO2, and O3,30 using density functional theory (DFT) have been reported. Similar intermolecular interactions are also part of valuable literature in which interactions between different molecules were studied.31−33 Similarly, the noncovalent interaction among halide ion complexes in decaborane was also studied for elaborating different noncovalent interactions.34 The interaction and dissociative reactions of CH3–OH on B12N12 nanocages are also studied.35 Adsorption of biological molecules, such as cytosine, adenine, and uracil, on B12N12 nanoclusters was investigated.36 Further, biological molecules, such as guanine, are also adsorbed on X12Y12 nanocages.37 Adsorption of SCN– on pure and Mg–-, Al–-, and Si-doped BN (B12N12 and B16N16) nanocages was reported.38 Recently, adsorption of hydrogen molecules on nickel-decorated B12N1239 was reported. Moreover, adsorption of pyrrole on Al12N12, Al12P12, B12N12, and B12P12 was also reported previously.40 Other than adsorption, BN fullerenes find applications in storage materials,41−44 field-effect transistors,45 nonlinear optics,46,47 and magnetic nanoparticles.48 Recently, researchers disclosed the superatomic nature of BN nanocages.49 Moreover, diffusion of alkali metals has been studied on boron nitride fullerenes to evaluate their potential applications in batteries.50,51
Phosgene (COCl2), being the simplest acid chloride, is a colorless gas and is formally derived from carbonic acid. It is mainly used in industries (polyurethane industry) to produce polymeric isocyanates and in the preparation of carbamates, pharmaceuticals, and related pesticides. It is the building block of many organic compounds, dyes, and isocyanates and finds useful applications as a reagent in several industries. It is also called a chemical war weapon (highly toxic gas) used in World War I (as a chemical weapon). Exposure to phosgene may result in swelling of throat, change in voice, pulmonary irritation, or delayed pulmonary edema.52 For this reason, it is necessary to detect phosgene and remove it from air even when present at very small concentrations. The adsorption of phosgene gas with various surfaces, such as AlN nanotubes,53 charcoal surface,54 and TiO2,55 has been investigated. Phosgene detection by Sc-doped BN nanotubes was reported.56 Moreover, the interaction of phosgene gas (COCl2) with Ga-doped and Al-doped BN (B16N16 and B12N12) nanoclusters was reported by Shakerzadeh et al.57 Their study revealed that the interaction of phosgene gas with Al- and Ga-doped nanoclusters caused a reduction in the HOMO–LUMO gap. Using DFT calculations, the adsorption of phosgene on (XY)n (X = Al, B and Y = P, N) was also reported by Padash et al.58
Despite these advances, adsorption of phosgene on late transition metal (such as copper)-doped B12N12 fullerene has not yet been reported. Early transition metals bind too tightly to be detached from the surface at the end. On the other hand, late transition metals bind with these surfaces with reasonable affinity. A number of reports have already been published on nickel decoration in the literature, whereas copper-decorated surfaces are not well explored. A recent report in the literature illustrated that copper effectively binds with BN nanocages.59 Moreover, copper is generally not poisoned by analytes, whereas nickel is poisoned to some extent, particularly by sulfur-containing analytes.60−62 Copper also has good tendency to adsorb various oxygen-containing analytes. Therefore, all of these findings motivated us to design a system that efficiently binds the highly toxic COCl2 gas by structural modification (i.e., decoration of Cu metal on BN) and help in removing this dangerous gas from the environment. The decoration of nanostructures with metals is much preferred over doping (where an atom of the nanostructure is replaced with an external atom). For doping, a defect may be created, which can impart certain interesting properties but, at the same time, render the system unstable in terms of binding energies (low binding energies). On the other hand, decoration does not disturb the intrinsic stability of the systems.30,63,64 Moreover, the metal atoms can be easily detached from the nanostructure as and when required. These characteristics motivated us to study decoration rather than doping with metal atoms. In this study, we examined phosgene adsorption on pure BN and Cu-doped BN nanocages. First, we explored the electronic and geometric properties of the B12N12 surface upon adsorption of Cu metal through interaction energies, dipole moment, QNBO (charge), frontier molecular orbital analysis (HOMO and LUMO), and partial density of states (PDOS). Then, the interaction of phosgene gas with optimized structures of copper-decorated BN was investigated.
2. Computational Methodology
All calculations in this study were performed at the B3LYP/6-31G (d,p) level of theory using Gaussian 09.65 Geometry optimization, adsorption energies, dipole moment, charge transfer (QNBO), molecular electrostatic potential (MEP), frontier molecular orbitals (HOMO–LUMO distribution), and partial density of states (PDOS) were calculated to study the interaction mechanism. B3LYP/6-31G (d,p) is a reliable level of theory, which is frequently used for nanoclusters.39,46,66 Geometries are optimized without any symmetry constraints in different spin states with zero net charge on the complexes. The doublet spin state is the lowest energy spin state for copper-containing systems. Many different possible orientations of copper on the BN nanocage (M@b66, M@b64, M@R6, M@R4, M@Btop, and M@Ntop) were considered for optimization, but all above-mentioned input geometries converged into two optimized structures, which we named A1(M@b66) and A2 (M@b64).
Equation 1 is used to calculate the interaction or adsorption energy of Cu on the BN nanocage in three positions.
| 1 |
where ECu–BN stands for the energy of the Cu–BN nanocage complex. EBN stands for the total energy of the pure BN nanocage, and ECu describes the electronic energy of Cu.
Equations 2 and 3 are used to calculate the interaction or adsorption energies of COCl2 with the pure BN nanocage and copper-decorated BN nanocage.
| 2 |
| 3 |
Here, Eint(BN) and Eint(Cu–BN) represent the interaction/adsorption energy of phosgene with the BN nanocage and Cu-decorated BN, respectively. Ephosgene–BN and Ephosgene–Cu–BN represent the total electronic energies of the COCl2–BN nanocage complex and COCl2–copper-decorated BN nanocage. Ephosgene stands for the total energy of single COCl2.
Parr et al.67 in 1999 studied the chemical potential (μ) and expressed it by the following eq 4.
| 4 |
EHOMO represents the energy of the HOMO, and ELUMO represents the energy of the LUMO. Moreover, properties such as softness (S), hardness (η), and electrophilicity (ω) can be determined using the Koopmans theorem.68
| 5 |
| 6 |
| 7 |
Partial density of states (PDOS) diagrams for all systems are generated using MultiWFN software.69
3. Results and Discussion
3.1. Bond Length and Adsorption Energies
The BN optimized structure at the B3LYP/6-31G (d,p) basis set is shown in Figure 1. The BN cluster is quite stable as the nitrogen and boron sites in this cluster are equivalent. The cluster consists of six tetragonal (4-membered) and eight hexagonal (6-membered) rings. The B–N bond length varies depending on the position whether the bond is between a tetragonal and a hexagonal ring (b64) or between two hexagonal rings (b66). The B–N bond length shared between two hexagonal rings (b66) is 1.44 Å, whereas the B–N bond length shared between a tetragonal and a hexagonal ring (b64) is 1.49 Å.
Figure 1.
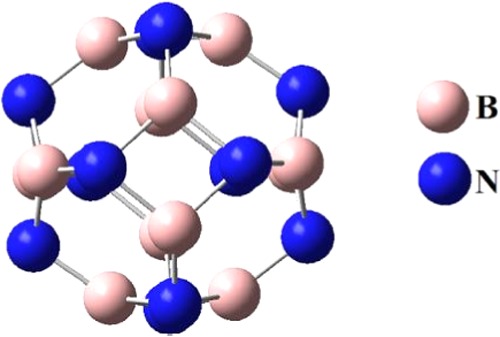
Optimized structure of B12N12.
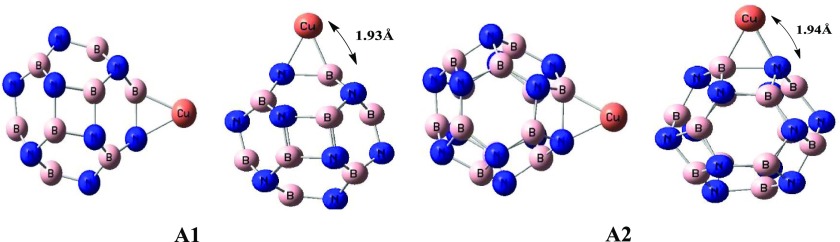
For decoration of copper on the BN nanocage, one finds several positions on which copper shows an interaction with rings. These positions may be M@Btop, M@Ntop, M@R6 (metal on the hexagonal ring), M@R4 (metal on the tetragonal ring), M@b64 (metal on the bond present between a 6- and a 4-membered ring), and M@b66 (metal on the bond present between two hexagonal rings). All possible input geometries were directed for optimization; however, only two geometries could be obtained, named A1 and A2, because all above-mentioned initial inputs converged merely into these two geometries after optimization. In these geometries, one is M@b66 named A1, whereas the other is M@b64 named A2.
In general, copper decoration on the BN cage causes changes in the geometry of the BN cage. When Cu (metal) is present on the bond present between two hexagonal rings (A1), the B–N bond length is elongated to 1.55 Å (as compared to 1.44 Å in the pure BN cage). The adsorption energy in this geometry (A1) is −193.81 kJ/mol. Moreover, the N–Cu and B–Cu bond lengths are 1.95 and 1.92 Å, respectively in the A1 geometry. In the same way, adsorption of Cu (metal) on the bond present between one hexagonal and one tetragonal ring (M@b64) in the A2 geometry causes an elongation of the B–N bond length to 1.66 Å (as compared to 1.49 Å in the pure cage). Furthermore, the B–Cu bond length increases to 1.94 Å as compared to 1.92 Å in A1, but the N–Cu bond length is shortened to 1.94 Å as compared to 1.95 Å in A1. The adsorption energy in the A2 geometry (198.45 kJ/mol) is higher than that in the A1 geometry. These two geometries are represented in Figure 2.
Figure 2.
Optimized structures of Cu-doped B12N12 nanocages.
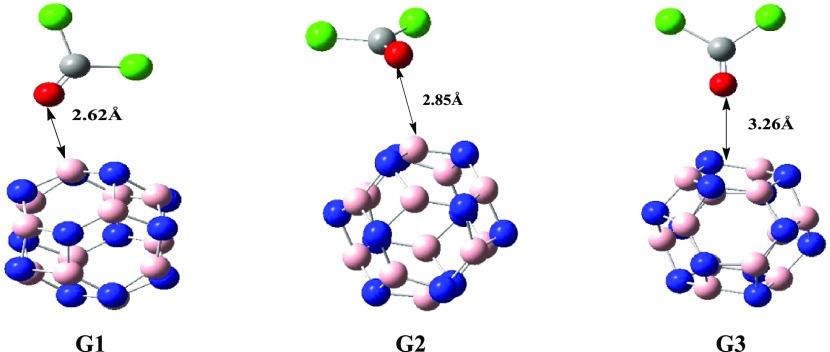
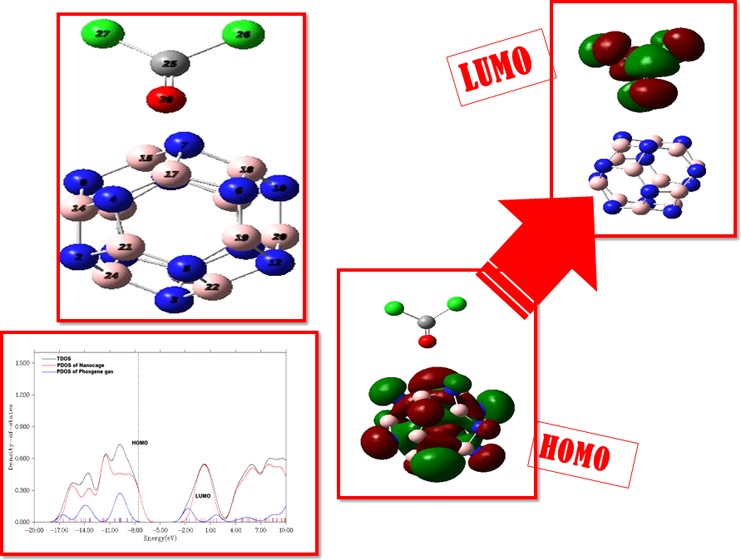
Then, we analyzed the interaction of phosgene (COCl2) gas with the pristine BN cage. Different orientations of COCl2 gas were studied on the pristine BN cage. As a result, we got three different geometries with respect to the orientation of COCl2 on the B12N12 nanocage. These geometries are named G1, G2, and G3 (Figure 3). In all orientations, carbonyl oxygen is oriented toward BN nanocages, but the orientation of COCl2 on B12N12 nanocages is different (Figure 3) in each case. In G1 and G2 orientations, COCl2 is oriented at distances of 2.62 and 2.85 Å, respectively, to the nearest atom on the cage with adsorption energies of −9.43 and −6.90 kJ/mol, respectively (Figure 3). In the G3 geometry, the value of bond length between COCl2 and the BN cage is 3.26 Å, as the gas is situated in the center of the ring in the relaxed structure, while adsorption energy in this case is −3.70 kJ/mol. This change in adsorption energies is due to the increase in the distance of oxygen (of the carbonyl group of phosgene gas) from the BN nanocage because strong adsorptions are observed at short distances (as in G1 and G2). From the data, it is obvious that COCl2 is not favorably adsorbed on the pristine BN cage due to the small value of the adsorption energy in all three cases. The most stable structure among the three was G1, which had a higher adsorption energy compared to G2 and G3, but the overall weak interactions of COCl2 with BN were observed.
Figure 3.
Different orientations of COCl2 on BN. In the G3 orientation, COCl2 is situated in the center of the ring, so the average distance is calculated.
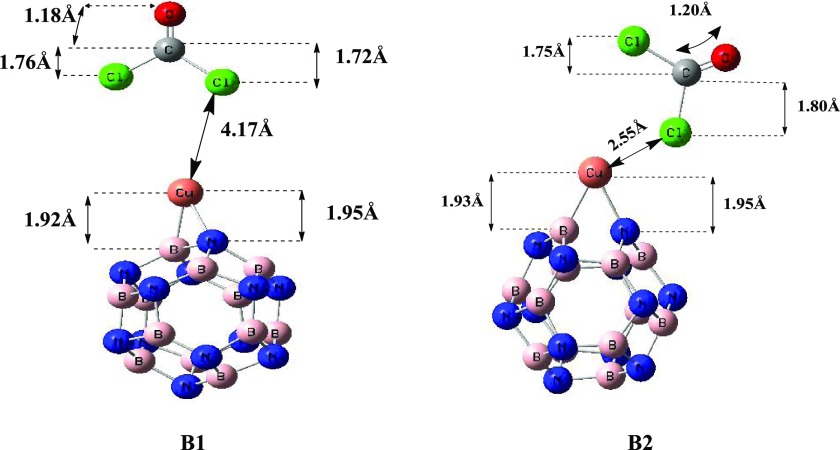
Furthermore, the adsorption of COCl2 on the copper-decorated BN nanocage was studied. Adsorption of phosgene on the A1 geometry resulted in the B1 geometry, where phosgene gas was physisorbed. The observed bond length between the nearest atom of COCl2 gas (“Cl” in B1) and the Cu–BN cage for B1 was 4.17 Å with an adsorption energy of −1.66 kJ/mol. Moreover, COCl2 does not elongate the B–N bond length (1.55 Å) in the B1 geometry mainly because it exhibits a weak physisorption.
Likewise, adsorption of COCl2 on A2 (Cu–BN) also resulted in a geometry named B2. The bond length between the nearest atom of gas (“Cl” in B2) and the Cu–BN cage was 2.55 Å with an adsorption energy of −16.95 kJ/mol. Comparing both orientations (B1 and B2), it is obvious that B2 is a more stable orientation than B1 because of its high adsorption energy. This quite strong interaction of COCl2 is also verified by the interaction distance of COCl2 from Cu. Adsorption of COCl2 on Cu–BN (A2) also causes an elongation of B–N bond lengths (1.67–1.70 Å in B2).
It is apparent that the adsorption of COCl2 on the BN nanocage not only changes the Cu–BN distances but also changes the B–N bond lengths of Cu–BN. Small variations in N–Cu and B–Cu bond lengths were noticed after adsorption of COCl2 on Cu–BN. So, from the above discussion, it is clear that COCl2 is favorably adsorbed on Cu-doped B12N12 (B2) as compared to pure B12N12 (G1, G2, and G3).
3.2. Dipole Moment
The change in the dipole moments of pure BN, Cu-decorated BN, and COCl2–Cu–BN was analyzed. The pure BN cage shows zero dipole moment as it is a symmetrical structure. Placement of Cu on this BN cage increases the dipole moment value from zero to 1.72 D in A1 and 1.49 D in A2. These changes are attributed to the placement of the Cu metal, which disturbs the charge separation in BN nanocages. In A1 and A2 geometries, the dipole moment vectors are pointed toward the BN nanocage. Moreover, the change in dipole moment was analyzed after adsorption of phosgene gas on BN. The dipole moment vectors in G1, G2, and G3 pointed toward COCl2, which evidenced the charge transfer from COCl2 to the nanocage, as shown in Figure S1 (Supporting Information). The values of dipole moment are 2.09, 1.36, and 1.54 D in G1, G2, and G3, respectively. The range of dipole moments is between 3.72 and 1.30 D for COCl2-adsorbed Cu@BN nanocages. After adsorption of COCl2 on Cu-decorated BN nanocages, the change in dipole moment originates due to charge transfer from the metal to phosgene gas. A comparison between B1 and B2 geometries illustrates a higher charge separation value in B2 due to higher charge transfer.
The highest change in dipole moment is observed when COCl2 complexes with Cu-B12N12 (B2), whereas the lowest dipole moment is calculated for B1 (COCl2–Cu–B12N12). In the B2 geometry, significant charge transfer occurs when COCl2 interacts with Cu-decorated BN (vide infra). On the hand, insignificant charge transfer is noticed in the B1 orientation. The dipole moment depends on the quantity of charges as well as their separation. Less intense charges on B1 are responsible for its low dipole moment among all geometries (vide infra). Moreover, the distance between COCl2 and Cu–BN is large in B1 as compared to other geometries, which is also another reason for its low dipole moment. The direction of the dipole moment vector is important in this regard; therefore, the dipole moment vector for all systems is given in the Supporting Information (Figure S1). The decreasing order of dipole moment is B2 > G1 > A1 > G3 > A2 > G2 > B1. This order specifies the maximum and minimum extremes of charge separation and dipole moment in all geometries Figure 4.
Figure 4.
Optimized structures of COCl2-adsorbed Cu–B12N12.
3.3. QNBO
QNBO was performed to rationalize the change in dipole moment after adsorption of Cu on the BN nanocage and that of COCl2 on pure and Cu-decorated BN nanocages. The values of QNBO on Cu in A1 and A2 are 0.517 and 0.540, respectively. The QNBO on the metal is further increased after the adsorption of COCl2 on Cu-decorated BN except for the B1 geometry. The values of QNBO for these geometries are 0.513 (B1) and 0.591 (B2). The QNBO on the metal after adsorption of COCl2 on Cu–BN varies among different geometries due to the interaction of COCl2. With the increase in dipole moment, the QNBO on the metal also increases except in the B1 geometry, in which the dipole moment decreases resulting in a decrease in QNBO. So, a regular change is observed in QNBO with the change in dipole moment for COCl2-adsorbed Cu–BN nanocages (B2), as shown in Table 1.
Table 1. Closest Distance of Cu to B12N12, COCl2 to Pure B12N12, and COCl2 to Cu–BN, QNBO on the Metal and Gas (COCl2), Dipole Moment, and Adsorption Energies of Different Systems.
| systems | dCu–BN (Å) | dCOCl2-Cu (Å) | QNBO on COCl2 (e) | QNBO on Cu (e) | μD (Debye) | Ead(kJ/mol) |
|---|---|---|---|---|---|---|
| Cu | 0.00 | 0.00 | ||||
| BN | 0.00 | |||||
| BN–COCl2 (G1) | 2.62 | 0.05 | 2.09 | –9.43 | ||
| BN–COCl2 (G2) | 2.85 | 0.02 | 1.36 | –6.90 | ||
| BN–COCl2 (G3) | 3.26 | 0.004 | 1.54 | –3.70 | ||
| Cu–BN (A1) | 1.93 | 0.517 | 1.72 | –193.81 | ||
| Cu–BN–COCl2 (B1) | 1.93 | 4.17 | 0.015 | 0.513 | 1.30 | –1.66 |
| Cu–BN (A2) | 1.94 | 0.540 | 1.49 | –198.45 | ||
| Cu–BN–COCl2 (B2) | 1.94 | 2.55 | 0.062 | 0.591 | 3.72 | –16.95 |
3.4. MEP Analysis
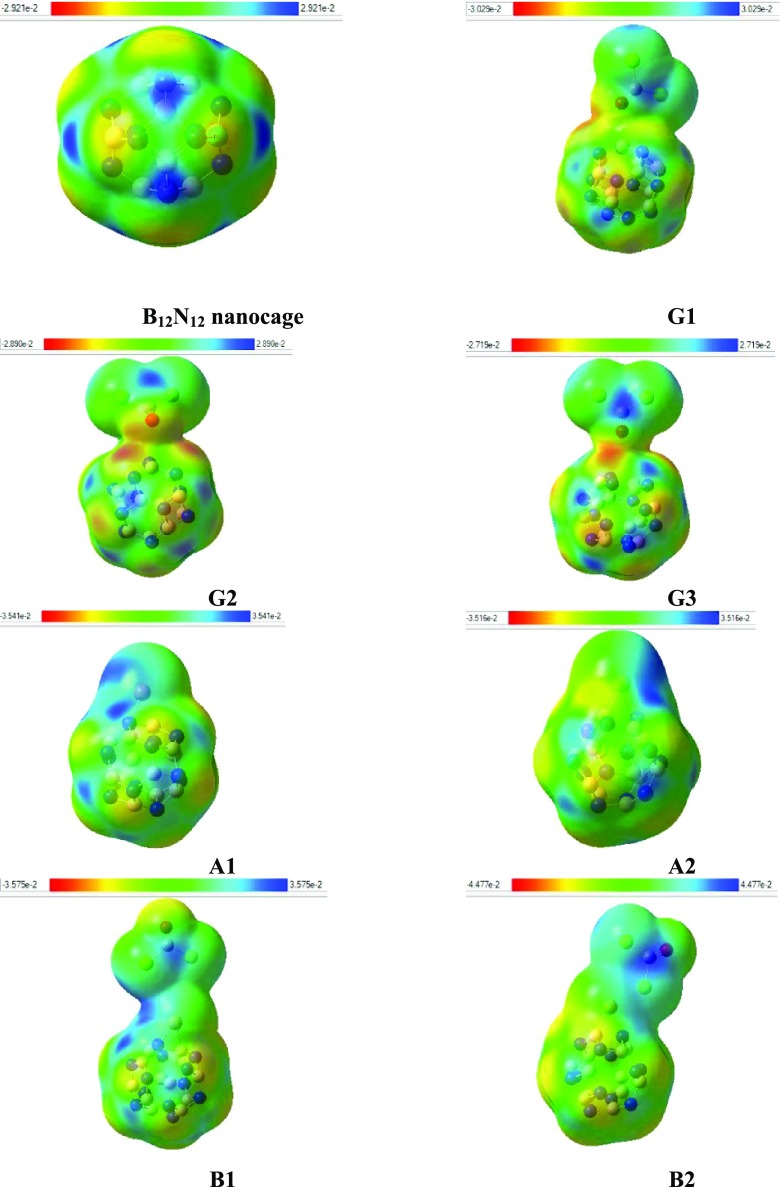
The molecular electrostatic potential (MEP) is used to comprehend the interaction between the components of Cu–BN, COCl2–BN, and Cu–BN–COCl2 nanocages. It signifies the extent of charge distribution in a molecule and correlates the molecular structure with physiochemical properties, i.e., chemical reactivity, dipole moment, and partial charges. In Figure 5, the electron deficient blue area (in the web version) specifies boron atoms, while electron rich yellow region represent nitrogen atoms. The pure BN cage, being symmetrical, shows both the charges to an equal extent, which vary slightly after the adsorption of COCl2 on the pure BN cage (G1, G2, and G3). The adsorption of COCl2 decreases the intensity of the blue region on the BN nanocage (shifting toward COCl2).
Figure 5.
MEP of different systems (for understanding the color in these figures, the reader must read the web version of this article). The isosurface value is 0.02 e/Å3.
However, after the decoration of Cu on the pure BN cage, the blue area is shifted on top of the Cu metal, whereas the yellow area in the cage becomes less intense. This change can be seen in A1 and A2. After the adsorption of COCl2 on Cu-decorated BN nanocages (B1 and B2), the blue area is shifted to the vicinity of the metal and the yellow color is regenerated on the cage (Figure 5). All this color shifting (charges) is attributed to an increase in dipole moment (D). For instance, pure BN has zero dipole moment, while Cu-decorated BN has some value of dipole moment.
3.5. Electronic Properties
Densities and electronic energy levels give a clear illustration of the effect of Cu decoration and COCl2 adsorption on pure and Cu-doped B12N12 nanocages. Some orbital parameters, such as energies of the HOMO and LUMO, Fermi level (EFL), and HOMO–LUMO band gap (Eg), are given in Table 2. The B12N12 nanocage is a semiconductor, which possesses a HOMO–LUMO gap (Eg) value of 6.84 eV. The HOMO and LUMO energies of the BN nanocage are −7.71 and −0.87 eV, respectively. The Fermi level, EFL, value is −4.29 eV. The Fermi level designates the midpoint of the HOMO–LUMO energy gap (in a molecule when the temperature is 0 K). The placement of Cu on the BN nanocage changes the HOMO–LUMO energies. For the A1 geometry, the energy of the HOMO is increased (−4.58 eV) but that of the LUMO is decreased (−1.72 eV). The HOMO–LUMO gap is also narrow (3.13 eV), and the Fermi level is positioned at −3.29 eV. For the A2 geometry, an increased value of HOMO energy is observed (−4.89 eV), while the energy of the LUMO is decreased (−1.83 eV), which results in a decrease in the HOMO–LUMO gap (3.05 eV). Moreover, the Fermi level is positioned at −3.36 eV. This change in energies of the HOMO and LUMO is attributed to the stabilization and destabilization of the LUMO and HOMO, respectively.
Table 2. Orbital Parameters: HOMO and LUMO Energies, Fermi Level, HOMO–LUMO Energy Gap for Different Systems.
| system | EHOMO (eV) | EFL (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|
| Cu | –5.98 | –3.32 | –0.67 | 5.30 |
| BN | –7.71 | –4.29 | –0.87 | 6.84 |
| BN–COCl2 (G1) | –7.53 | –4.81 | –2.09 | 5.43 |
| BN–COCl2 (G2) | –7.62 | –4.82 | –2.02 | 5.59 |
| BN–COCl2 (G3) | –7.58 | –4.72 | –1.87 | 5.72 |
| Cu–BN (A1) | –4.85 | –3.29 | –1.72 | 3.13 |
| Cu–BN–COCl2 (B1) | –4.87 | –3.41 | –1.97 | 2.87 |
| Cu–BN (A2) | –4.89 | –3.36 | –1.83 | 3.05 |
| Cu–BN–COCl2 (B2) | –4.78 | –3.64 | –2.50 | 2.28 |
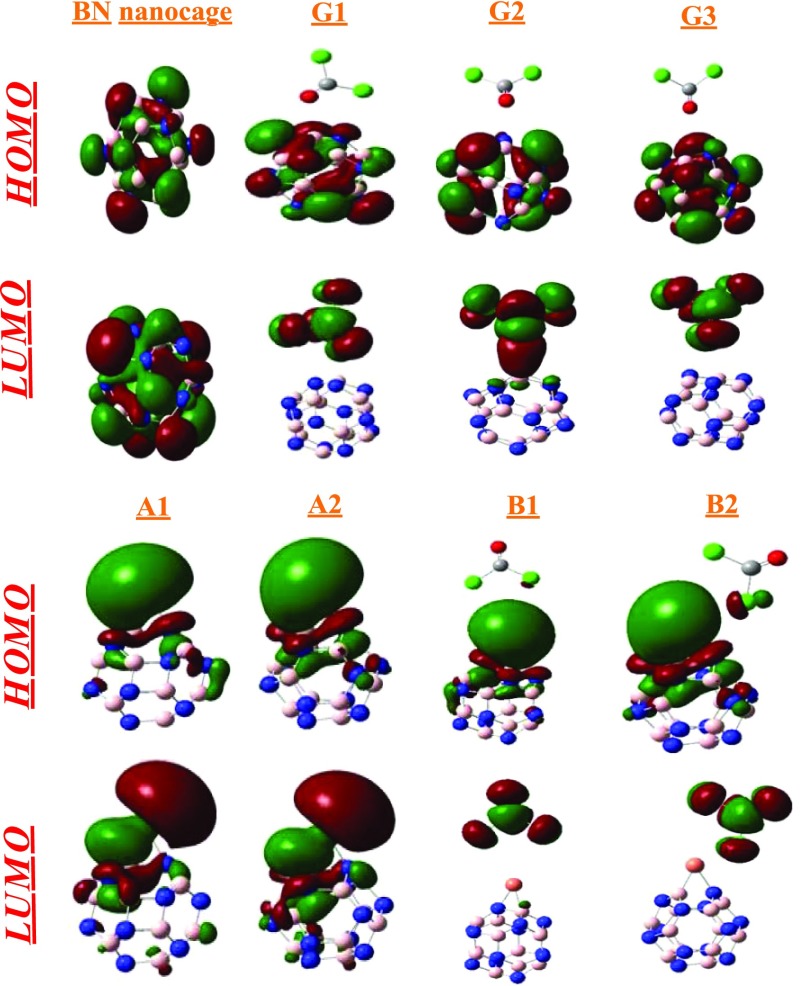
To justify these differences, the shapes of frontier orbitals are evaluated (Figure 6). In general, the adsorption of Cu on BN nanocages results in a shift of the HOMO to the copper metal. This happens due to the presence of many electronegative atoms that make the metal atom electron rich. The metal, being electropositive, cannot retain these electrons, and therefore, they are spread out as excess electrons.
Figure 6.
Side views of the HOMO and LUMO of different systems. The isosurface value is 0.02 e/Å3.
The HOMO–LUMO gap (Eg) is directly related to conductivity,70 and this relationship is given in the following equation causing a high energy level for the newly formed HOMO.46,47 According to calculations, the LUMO energies are decreased in A1 and A2 geometries as compared to that of the pure BN cage. A change is seen after the adsorption of gas (COCl2) on the Cu-doped BN nanocages. The adsorption of COCl2 on Cu-decorated BN slightly stabilizes the HOMO (except B2) and LUMO, where the HOMO–LUMO gaps are 2.87 eV (B1) and 2.28 eV (B2). The Fermi levels are −3.41 and −3.64 eV for B1 and B2 geometries, respectively. Adsorption of phosgene on Cu decreases the interaction of Cu with the BN nanocage. Due to the decrease in coordination to the metal center, the push of the outer d electrons also decreases. The HOMO of COCl2–Cu–BN has more density on the metal and some density on the cage (which is in diffuse form in the cage). The energies of the LUMO are also decreased upon adsorption of COCl2. The shifting of the LUMO on the nanocage causes stabilization of the LUMO by decrease in the energy of LUMO.
| 8 |
This equation indicates the significant increase in electrical conductivity by the decrease in the H–L energy gap (Eg). Based on our findings, it is clearly evident that the sensing ability of the Cu-decorated BN nanocage toward phosgene (COCl2) adsorption is significantly enhanced in comparison with the pristine BN nanocage.
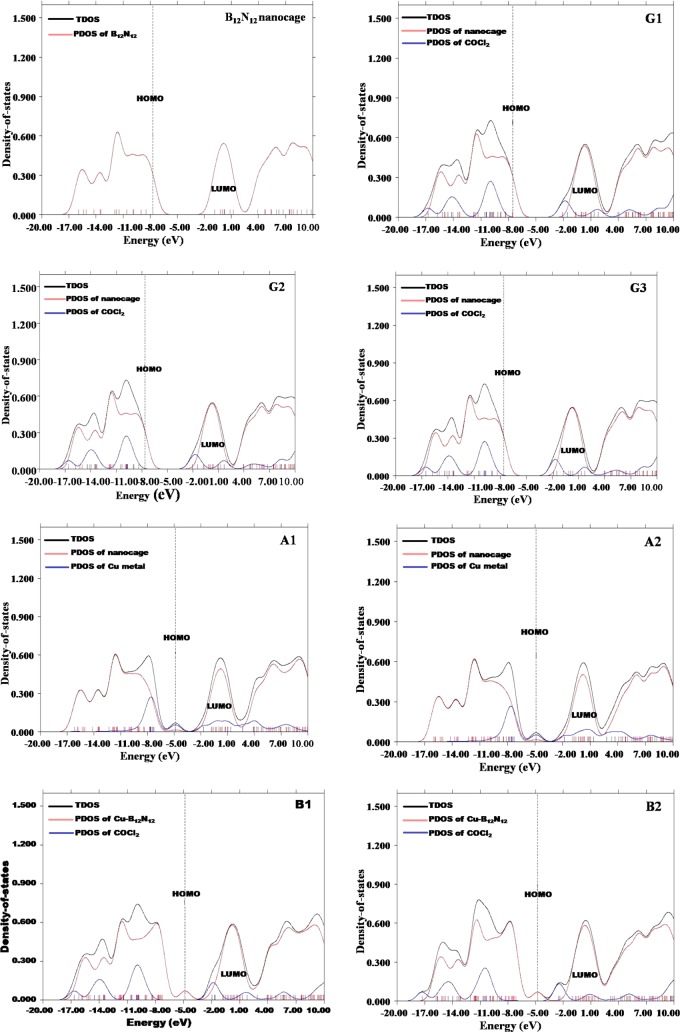
3.6. Partial Density of States
Partial density of states (PDOS) were analyzed to examine the structural changes and electronic properties of BN and Cu–BN nanocages after COCl2 adsorption (with the help of TDOS and PDOS). In the three different orientations (G1, G2, and G3) of COCl2 on the BN nanocage (Figure 7), the LUMO has density primarily localized on COCl2, whereas the HOMO is centered only on the BN nanocage. For Cu–BN systems, the LUMO is localized on the Cu metal, and the HOMO is merely located on the Cu metal. Upon adsorption of COCl2 on Cu–BN, slightly different results are obtained. In B1 and B2 geometries, the LUMO is distributed just on COCl2, whereas the HOMO is present only on Cu–BN. So, from the above discussion, it is clear that shifting of charge density takes place upon adsorption of COCl2 on Cu-doped B12N12.
Figure 7.
Different systems with their TDOS and PDOS graphs. The isosurface value is 0.02 e/Å3.
3.7. Global Indices of Reactivity
The effect of COCl2 adsorption on pure and copper-decorated BN cages is also calculated from global indices of reactivity. The properties such as ionization potential (I), electron affinity (A), chemical hardness (η), softness (S), chemical potential (μ), and electrophilicity (ω) are given in Table 3. Here, the ionization potential is the negative of the energy of the HOMO, whereas the electron affinity is the negative of the energy of the LUMO. Generally, the electron-accepting nature of a system is represented with positive values of electron affinity. All systems studied here exhibit good electron-accepting ability, which is a good feature for a system participating in charge-transfer reactions. Similarly, the ionization potential explains the electron-donating ability of a system. Pure B12N12 has a high ionization potential value (7.71 eV), which increases upon the interaction of COCl2 on the BN cage (G1, G2, and G3 orientations), but decreases comparatively for copper-decorated BN cages (A1 and A2 geometries). The ionization potential again increases after the adsorption of COCl2 on copper-decorated BN nanocages (B1 and B2). A similar trend is observed for electron affinity. Therefore, both properties suggest that our copper-decorated systems are efficient for COCl2 adsorption. Electrophilicity is related to the chemical reactivity of a compound. The pure B12N12 cage has an electrophilicity of 2.70 eV, and this property increases in copper-decorated BN nanocages (3.45 eV for A1 and 3.76 eV for A2). The highest value of electrophilicity is calculated for adsorption of COCl2 on copper-decorated B12N12 nanocages (4.04 eV for B1 and 5.81 eV for B2). So, the electrophilic index again suggests that our copper-decorated BN systems are best candidates for COCl2 adsorption. The chemical hardness and softness of a compound are directly related to the chemical stability (low reactivity). All our systems are hard in nature with a low value of global softness. The value of chemical potential μ shows a direct relation with the chemical stability and an inverse relation with the reactivity of the system. The chemical potential values suggest that COCl2-adsorbed copper-decorated BN nanocages are more stable with lower reactivity [B1 (μ = 3.41 eV) and B2 (μ = 3.64 eV)] as compared to A1 (μ = 3.29 eV) and A2 (μ = 3.36 eV). So, all above-mentioned findings suggest that our phosgene-adsorbed copper-decorated systems are stable, least reactive, and best candidates for sensing materials.
Table 3. Ionization Potential (I), Electron Affinity (A), Chemical Hardness (η), Chemical Potential (μ), Softness (S), and Electrophilicity (ω) of Different Systems.
| systems | I (eV) | A (eV) | η (eV) | μ (eV) | S (eV–1) | ω (eV) |
|---|---|---|---|---|---|---|
| Cu | 5.98 | 0.67 | 2.66 | –3.33 | 0.19 | 2.08 |
| BN | 7.71 | 0.87 | 3.42 | –4.29 | 0.15 | 2.70 |
| BN–COCl2 (G1) | 7.53 | 2.09 | 2.72 | –4.81 | 0.18 | 4.25 |
| BN–COCl2 (G2) | 7.62 | 2.02 | 2.80 | –4.82 | 0.18 | 4.15 |
| BN–COCl2 (G3) | 7.58 | 1.87 | 2.86 | –4.73 | 0.18 | 3.91 |
| Cu–BN (A1) | 4.85 | 1.72 | 1.57 | –3.29 | 0.32 | 3.45 |
| Cu–BN–COCl2 (B1) | 4.84 | 1.97 | 1.44 | –3.41 | 0.35 | 4.04 |
| Cu–BN (A2) | 4.89 | 1.83 | 1.53 | –3.36 | 0.33 | 3.76 |
| Cu–BN–COCl2 (B2) | 4.78 | 2.50 | 1.14 | –3.64 | 0.44 | 5.81 |
4. Conclusions
In this study, we applied the B3LYP/6-31G(d,p) basis set (DFT) to explore phosgene (COCl2) adsorption on pure and Cu-decorated B12N12 nanocages. All possible sites on the nanocages are investigated for copper interaction. Two optimized geometries, named Cu@b66 (A1) and Cu@b64 (A2), were observed after placing Cu on BN. The binding energy value of A2 is remarkably higher than that of A1, which suggests the enhanced adsorption capability of the BN nanocage toward the COCl2 molecule after Cu decoration. It is evident that COCl2 is more strongly adsorbed on Cu–B12N12 as compared to COCl2 when it is exclusively adsorbed on pure B12N12. Significant modifications in the electronic properties are observed in B12N12 by decoration of Cu and COCl2. QNBO and dipole moment for the selected systems are indicative of higher charge separation after adsorption of COCl2 on Cu–B12N12, as compared to Cu-doped B12N12. Dipole moment and QNBO trends correlate with each other for all systems. The HOMO–LUMO gap of pure BN is higher than those of all other geometries. COCl2 adsorption on pure B12N12 and Cu-decorated BN increases the electrophilicity of the B12N12 nanocage. Moreover, the partial density of states (PODs) and electronic energy level were calculated to show the effect of Cu and COCl2 decoration on B12N12 and Cu–B12N12 nanocages, respectively. The results propose the copper-decorated nanocage as an efficient sensor of phosgene and its industrial application in multiple areas.
Acknowledgments
The authors acknowledge the financial and technical support from the Shakarganj Limited Company, Jhang, Punjab, Pakistan.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00507.
Dipole moment vector representation in all systems; Optimized Cartesian coordinates of all geometries (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Iijima S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. 10.1038/354056a0. [DOI] [Google Scholar]
- Mias S.; Sudor J.; Camon H. PNIPAM: A Thermo-Activated Nano-Material for Use in Optical Devices. Microsyst. Technol. 2008, 14, 747–751. 10.1007/s00542-007-0457-3. [DOI] [Google Scholar]
- Gu M.; Zhang Q.; Lamon S. Nanomaterials for Optical Data Storage. Nat. Rev. Mater. 2016, 1, 16070 10.1038/natrevmats.2016.70. [DOI] [Google Scholar]
- Planeix J. M.; Coustel N.; Coq B.; Brotons V.; Kumbhar P. S.; Dutartre R.; Geneste P.; Bernier P.; Ajayan P. M. Application of Carbon Nanotubes as Supports in Heterogeneous Catalysis. J. Am. Chem. Soc. 1994, 116, 7935–7936. 10.1021/ja00096a076. [DOI] [Google Scholar]
- Varghese S. S.; Lonkar S.; Singh K. K.; Swaminathan S.; Abdala A. Recent Advances in Graphene Based Gas Sensors. Sensor. Actuators, B 2015, 218, 160–183. 10.1016/j.snb.2015.04.062. [DOI] [Google Scholar]
- Rad A. S.; Shabestari S. S.; Mohseni S.; Aghouzi S. A. Study on the Adsorption Properties of O 3, SO 2, and SO 3 on B-Doped Graphene Using DFT Calculations. J. Solid State Chem. 2016, 237, 204–210. 10.1016/j.jssc.2016.02.023. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Ni Adsorption on Al12P12 Nano-Cage: A DFT Study. J. Alloys Compd. 2016, 678, 317–324. 10.1016/j.jallcom.2016.03.175. [DOI] [Google Scholar]
- Qiang Y.; Antony J.; Sharma A.; Nutting J.; Sikes D.; Meyer D. Iron/Iron Oxide Core-Shell Nanoclusters for Biomedical Applications. J. Nanopart. Res. 2006, 8, 489–496. 10.1007/s11051-005-9011-3. [DOI] [Google Scholar]
- Talla J. A. Ab Initio Simulations of Doped Single-Walled Carbon Nanotube Sensors. Chem. Phys. 2012, 392, 71–77. 10.1016/j.chemphys.2011.10.014. [DOI] [Google Scholar]
- Chopra N. G.; Luyken R. J.; Cherrey K.; Crespi V. H.; Cohen M. L.; Louie S. G.; Zettl A. Boron Nitride Nanotubes. Science 1995, 269, 966–967. 10.1126/science.269.5226.966. [DOI] [PubMed] [Google Scholar]
- Han W.; Bando Y.; Kurashima K.; Sato T. Synthesis of Boron Nitride Nanotubes from Carbon Nanotubes by a Substitution Reaction. Appl. Phys. Lett. 1998, 73, 3085–3087. 10.1063/1.122680. [DOI] [Google Scholar]
- Sun X.-H.; Li C.-P.; Wong W.-K.; Wong N.-B.; Lee C.-S.; Lee S.-T.; Teo B.-K. Formation of Silicon Carbide Nanotubes and Nanowires via Reaction of Silicon (from Disproportionation of Silicon Monoxide) with Carbon Nanotubes. J. Am. Chem. Soc. 2002, 124, 14464–14471. 10.1021/ja0273997. [DOI] [PubMed] [Google Scholar]
- Wu Q.; Hu Z.; Wang X.; Lu Y.; Chen X.; Xu H.; Chen Y. Synthesis and Characterization of Faceted Hexagonal Aluminum Nitride Nanotubes. J. Am. Chem. Soc. 2003, 125, 10176–10177. 10.1021/ja0359963. [DOI] [PubMed] [Google Scholar]
- Haberland H.; Ludewigt C.; Richter T. Electron Attachment to Clusters Composed of Closed Shell, Hydrogen Containing Molecules. Z. Phys. D: At., Mol. Clusters 1989, 12, 289–290. 10.1007/BF01426958. [DOI] [Google Scholar]
- Jensen F.; Toftlund H. Structure and Stability of C24 and B12N12 Isomers. Chem. Phys. Lett. 1993, 201, 89–96. 10.1016/0009-2614(93)85039-Q. [DOI] [Google Scholar]
- Strout D. L. Structure and Stability of Boron Nitrides: Isomers of B 12 N 12. J. Phys. Chem. A 2000, 104, 3364–3366. 10.1021/jp994129a. [DOI] [Google Scholar]
- Kandalam A. K.; Blanco M. A.; Pandey R. Theoretical Study of AlnNn, GanNn, and InnNn (n = 4, 5, 6) Clusters. J. Phys. Chem. B 2002, 106, 1945–1953. 10.1021/jp0140062. [DOI] [Google Scholar]
- Costales A.; Kandalam A. K.; Franco R.; Pandey R. Theoretical Study of Structural and Vibrational Properties of (AlP) n, (AlAs) n, (GaP) n, (GaAs) n, (InP) n, and (InAs) n Clusters with n = 1, 2, 3. J. Phys. Chem. B 2002, 106, 1940–1944. 10.1021/jp013906f. [DOI] [Google Scholar]
- Paine R. T.; Narula C. K. Synthetic Routes to Boron Nitride. Chem. Rev. 1990, 90, 73–91. 10.1021/cr00099a004. [DOI] [Google Scholar]
- Maria; Iqbal J.; Ludwig R.; Ayub K. Phosphides or Nitrides for Better NLO Properties? A Detailed Comparative Study of Alkali Metal Doped Nano-Cages. Mater. Res. Bull. 2017, 92, 113–122. 10.1016/j.materresbull.2017.03.065. [DOI] [Google Scholar]
- Oku T.; Kuno M.; Kitahara H.; Narita I. Formation, Atomic Structures and Properties of Boron Nitride and Carbon Nanocage Fullerene Materials. Int. J. Inorg. Mater. 2001, 3, 597–612. 10.1016/S1466-6049(01)00169-6. [DOI] [Google Scholar]
- Oku T.; Nishiwaki A.; Narita I. Formation and Atomic Structure of B 12 N 12 Nanocage Clusters Studied by Mass Spectrometry and Cluster Calculation. Sci. Technol. Adv. Mater. 2004, 5, 635–638. 10.1016/j.stam.2004.03.017. [DOI] [Google Scholar]
- Oku T.; Hirano T.; Kuno M.; Kusunose T.; Niihara K.; Suganuma K. Synthesis, Atomic Structures and Properties of Carbon and Boron Nitride Fullerene Materials. Mater. Sci. Eng. B 2000, 74, 206–217. 10.1016/S0921-5107(99)00563-2. [DOI] [Google Scholar]
- Esrafili M. D.; Nurazar R. Methylamine Adsorption and Decomposition on B12N12 Nanocage: A Density Functional Theory Study. Surf. Sci. 2014, 626, 44–48. 10.1016/j.susc.2014.03.028. [DOI] [Google Scholar]
- Beheshtian J.; Baei M. T.; Peyghan A. A. Theoretical Study of CO Adsorption on the Surface of BN, AlN, BP and AlP Nanotubes. Surf. Sci. 2012, 606, 981–985. 10.1016/j.susc.2012.02.019. [DOI] [Google Scholar]
- Baei M. T.; Peyghan A. A.; Bagheri Z. A. DFT Study on CO 2 Interaction with a BN Nano-Cage. Bull. Korean Chem. Soc. 2012, 33, 3338–3342. 10.5012/bkcs.2012.33.10.3338. [DOI] [Google Scholar]
- Beheshtian J.; Peyghan A. A.; Bagheri Z.; Kamfiroozi M. Interaction of Small Molecules (NO, H2, N2, and CH4) with BN Nanocluster Surface. Struct. Chem. 2012, 23, 1567–1572. 10.1007/s11224-012-9970-9. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Adsorption of Thiophene on the Surfaces of X 12 Y 12 (X = Al, B, and Y = N,P) Nanoclusters; A DFT Study. J. Mol. Liq. 2017, 238, 303–309. 10.1016/j.molliq.2017.05.020. [DOI] [Google Scholar]
- Rad A. S. Application of B12N12 and B12P12 as Two Fullerene-like Semiconductors for Adsorption of Halomethane: Density Functional Theory Study. Semiconductors 2017, 51, 134–138. 10.1134/S1063782617010225. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. O 3 and SO 2 Sensing Concept on Extended Surface of B 12 N 12 Nanocages Modified by Nickel Decoration: A Comprehensive DFT Study. Solid State Sci. 2017, 69, 22–30. 10.1016/j.solidstatesciences.2017.05.007. [DOI] [Google Scholar]
- Muhammad S.; Janjua M. R. S. A.; Su Z. Investigation of Dibenzoboroles Having π-Electrons: Toward a New Type of Two-Dimensional NLO Molecular Switch?. J. Phys. Chem. C 2009, 113, 12551–12557. 10.1021/jp903075s. [DOI] [Google Scholar]
- Muhammad S.; Xu H.; Janjua M. R. S. A.; Su Z.; Nadeem M. Quantum Chemical Study of Benzimidazole Derivatives to Tune the Second-Order Nonlinear Optical Molecular Switching by Proton Abstraction. Phys. Chem. Chem. Phys. 2010, 12, 4791. 10.1039/b924241d. [DOI] [PubMed] [Google Scholar]
- Muhammad S.; Liu C.; Zhao L.; Wu S.; Su Z. A Theoretical Investigation of Intermolecular Interaction of a Phthalimide Based “On–off” Sensor with Different Halide Ions: Tuning Its Efficiency and Electro-Optical Properties. Theor. Chem. Acc. 2009, 122, 77–86. 10.1007/s00214-008-0486-8. [DOI] [Google Scholar]
- Muhammad S.; Minami T.; Fukui H.; Yoneda K.; Kishi R.; Shigeta Y.; Nakano M. Halide Ion Complexes of Decaborane (B 10 H 14) and Their Derivatives: Noncovalent Charge Transfer Effect on Second-Order Nonlinear Optical Properties. J. Phys. Chem. A 2012, 116, 1417–1424. 10.1021/jp209385b. [DOI] [PubMed] [Google Scholar]
- Esrafili M. D.; Nurazar R. A Density Functional Theory Study on the Adsorption and Decomposition of Methanol on B12N12 Fullerene-like Nanocage. Superlattices Microstruct. 2014, 67, 54–60. 10.1016/j.spmi.2013.12.020. [DOI] [Google Scholar]
- Baei M. T.; Taghartapeh M. R.; Lemeski E. T.; Soltani A. A Computational Study of Adenine, Uracil, and Cytosine Adsorption upon AlN and BN Nano-Cages. Phys. B 2014, 444, 6–13. 10.1016/j.physb.2014.03.013. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. A Comparative Density Functional Theory Study of Guanine Chemisorption on Al12N12, Al12P12, B12N12, and B12P12 Nano-Cages. J. Alloys Compd. 2016, 672, 161–169. 10.1016/j.jallcom.2016.02.139. [DOI] [Google Scholar]
- Soltani A.; Baei M. T.; Lemeski E. T.; Pahlevani A. A. The Study of SCN – Adsorption on B 12 N 12 and B 16 N 16 Nano-Cages. Superlattices Microstruct. 2014, 75, 716–724. 10.1016/j.spmi.2014.07.038. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Enhancement in Hydrogen Molecule Adsorption on B 12 N 12 Nano-Cluster by Decoration of Nickel. Int. J. Hydrogen Energy 2016, 41, 22182–22191. 10.1016/j.ijhydene.2016.08.158. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Adsorption of Pyrrole on Al12N12, Al12P12, B12N12, and B12P12 Fullerene-like Nano-Cages; a First Principles Study. Vacuum 2016, 131, 135–141. 10.1016/j.vacuum.2016.06.012. [DOI] [Google Scholar]
- Oku T.; Narita I. Calculation of H2 Gas Storage for Boron Nitride and Carbon Nanotubes Studied from the Cluster Calculation. Phys. B 2002, 323, 216–218. 10.1016/S0921-4526(02)00959-6. [DOI] [Google Scholar]
- Oku T.; Kuno M. Synthesis, Argon/Hydrogen Storage and Magnetic Properties of Boron Nitride Nanotubes and Nanocapsules. Diam. Relat. Mater. 2003, 12, 840–845. 10.1016/S0925-9635(02)00326-6. [DOI] [Google Scholar]
- Ayub K. Transportation of Hydrogen Atom and Molecule through X 12 Y 12 Nano-Cages. Int. J. Hydrogen Energy 2017, 42, 11439–11451. 10.1016/j.ijhydene.2017.02.202. [DOI] [Google Scholar]
- Ayub K. Binding Affinity and Permeation of X12Y12 Nanoclusters for Helium and Neon. J. Mol. Liq. 2017, 244, 124–134. 10.1016/j.molliq.2017.08.118. [DOI] [Google Scholar]
- Radosavljević M.; Appenzeller J.; Derycke V.; Martel R.; Avouris P.; Loiseau A.; Cochon J.-L.; Pigache D. Electrical Properties and Transport in Boron Nitride Nanotubes. Appl. Phys. Lett. 2003, 82, 4131–4133. 10.1063/1.1581370. [DOI] [Google Scholar]
- Ayub K. Are Phosphide Nano-Cages Better than Nitride Nano-Cages? A Kinetic, Thermodynamic and Non-Linear Optical Properties Study of Alkali Metal Encapsulated X 12 Y 12 Nano-Cages. J. Mater. Chem. C 2016, 4, 10919–10934. 10.1039/C6TC04456E. [DOI] [Google Scholar]
- Maria; Iqbal J.; Ayub K. Enhanced Electronic and Non-Linear Optical Properties of Alkali Metal (Li, Na, K) Doped Boron Nitride Nano-Cages. J. Alloys Compd. 2016, 687, 976–983. 10.1016/j.jallcom.2016.06.121. [DOI] [Google Scholar]
- Tokoro H.; Fujii S.; Oku T. Iron Nanoparticles Coated with Boron Nitride Nanolayers Synthesized by a Solid Phase Reaction. IEEE Trans. Magn. 2003, 39, 2761–2763. 10.1109/TMAG.2003.815591. [DOI] [Google Scholar]
- Xie W.; Wang J.; Wang J.; Wu X.; Wang Z.; Zhang R.-Q. High-Angular-Momentum Orbitals and Superatomic Characteristics of Boron-Nitrogen Cages. J. Phys. Chem. C 2020, 124, 3881–3885. 10.1021/acs.jpcc.9b11351. [DOI] [Google Scholar]
- Munsif S.; Ayub K. Diffusion of Alkali Metal Atoms (Li, Na, K) on Aluminum Nitride and Boron Nitride Nanocages; a Density Functional Theory Study. J. Mol. Liq. 2018, 259, 249–259. 10.1016/j.molliq.2018.03.009. [DOI] [Google Scholar]
- Munsif S.; Ayub K. Permeability and Storage Ability of Inorganic X 12 Y 12 Fullerenes for Lithium Atom and Ion. Chem. Phys. Lett. 2018, 698, 51–59. 10.1016/j.cplett.2018.02.065. [DOI] [Google Scholar]
- Grainge C.; Rice P. Management of Phosgene-Induced Acute Lung Injury. Clin. Toxicol. 2010, 48, 497–508. 10.3109/15563650.2010.506877. [DOI] [PubMed] [Google Scholar]
- Shahabi M.; Raissi H. Molecular Dynamics Simulation and Quantum Chemical Studies on the Investigation of Aluminum Nitride Nanotube as Phosgene Gas Sensor. J. Inclusion Phenom. Macrocyclic Chem. 2016, 86, 305–322. 10.1007/s10847-016-0664-6. [DOI] [Google Scholar]
- Dole M. The Rate of Adsorption of Phosgene and Chloropicrin on Charcoal. J. Chem. Phys. 1947, 15, 447–454. 10.1063/1.1746563. [DOI] [Google Scholar]
- Joung S.-K.; Amemiya T.; Murabayashi M.; Cai R.; Itoh K. Chemical Adsorption of Phosgene on TiO2 and Its Effect on the Photocatalytic Oxidation of Trichloroethylene. Surf. Sci. 2005, 598, 174–184. 10.1016/j.susc.2005.08.037. [DOI] [Google Scholar]
- Beheshtian J.; Peyghan A. A.; Bagheri Z. Detection of Phosgene by Sc-Doped BN Nanotubes: A DFT Study. Sens. Actuators, B 2012, 171–172, 846–852. 10.1016/j.snb.2012.05.082. [DOI] [Google Scholar]
- Shakerzadeh E.; Khodayar E.; Noorizadeh S. Theoretical Assessment of Phosgene Adsorption Behavior onto Pristine, Al- and Ga-Doped B 12 N 12 and B 16 N 16 Nanoclusters. Comput. Mater. Sci. 2016, 118, 155–171. 10.1016/j.commatsci.2016.03.016. [DOI] [Google Scholar]
- Padash R.; Rahimi-Nasrabadi M.; Rad A. S.; Sobhani-Nasab A.; Jesionowski T.; Ehrlich H. A Comparative Computational Investigation of Phosgene Adsorption on (XY)12 (X = Al, B and Y = N, P) Nanoclusters: DFT Investigations. J. Clust. Sci. 2018, 30, 203–218. 10.1007/s10876-018-1479-y. [DOI] [Google Scholar]
- Abbasi M.; Nemati-Kande E.; Mohammadi M. D. Doping of the First Row Transition Metals onto B 12 N 12 Nanocage: A DFT Study. Comput. Theor. Chem. 2018, 1132, 1–11. 10.1016/j.comptc.2018.04.003. [DOI] [Google Scholar]
- Wimmer E.; Fu C. L.; Freeman A. J. Catalytic Promotion and Poisoning: All-Electron Local-Density-Functional Theory of CO on Ni(001) Surfaces Coadsorbed with K or S. Phys. Rev. Lett. 1985, 55, 2618–2621. 10.1103/PhysRevLett.55.2618. [DOI] [PubMed] [Google Scholar]
- Yates J. T.; Garland C. W. Infrared Studies of Carbon Monoxide Chemisorbed on Nickel And on Mercury-poisoned Nickel Surfaces 1. J. Phys. Chem. A. 1961, 65, 617–624. 10.1021/j100822a007. [DOI] [Google Scholar]
- Imanaka T.; Nitta Y.; Teranishi S. Study of the Poisoning Effect on Nickel and Nickel Boride Catalysts by Means of a Flow Microcalorimeter. Bull. Chem. Soc. Jpn. 1973, 46, 1134–1136. 10.1246/bcsj.46.1134. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. How Can Nickel Decoration Affect H 2 Adsorption on B 12 P 12 Nano-Heterostructures?. J. Mol. Liq. 2018, 255, 168–175. 10.1016/j.molliq.2018.01.149. [DOI] [Google Scholar]
- Rad A. S.; Ayub K. Enhancement in Hydrogen Molecule Adsorption on B12N12 Nano-Cluster by Decoration of Nickel. Int. J. Hydrogen Energy 2016, 41, 22182–22191. 10.1016/j.ijhydene.2016.08.158. [DOI] [Google Scholar]
- Frisch M.; Trucks G.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.. et al. Gaussian 09, revision A. 02; Gaussian. Inc.: Wallingford, CT, 2009. 200 (11 june 2009).
- Hussain S.; Chatha S. A. S.; Hussain A. I.; Hussain R.; Mehboob M. Y.; Muhammad S.; Ahmad Z.; Ayub K. Zinc-Doped Boron Phosphide Nanocluster as Efficient Sensor for SO2. J. Chem. 2020, 1–12. 10.1155/2020/2629596. [DOI] [Google Scholar]
- Parr R. G.; Szentpály L.V.; Liu S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. 10.1021/ja983494x. [DOI] [Google Scholar]
- Pearson R. G. The Transition Metal-Carbon Monoxide Bond. Inorg. Chem. 1984, 23, 4675–4679. 10.1021/ic00194a051. [DOI] [Google Scholar]
- Lu T.; Chen F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Li S. S.Scattering Mechanisms and Carrier Mobilities in Semiconductors. In Semiconductor Physical Electronics; Springer: New York, NY, pp 211–245. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.