Abstract

The hydrophobic adsorption is an alternative to traditional organic solvent extraction for the recovery and purification of Penicillin G (PenG). However, there is a lack of information concerning the effect of process variables and technical feasibility while balancing product degradation. After assessing the integrity of PenG under different conditions, Amberlite XAD-4 was selected from among three different adsorbents. During the batch process using only 0.05 gXAD-4/mLmedium, the adsorption yield increased from 36% at pH 6 to 44% at pH 4. More than 90% of the antibiotic was captured from the fermentation broth using 0.083 gXAD-4/mLmedium in a 45 min batch performed at pH 4 and 4 °C. Moreover, there was no PenG degradation. The desorption conditions were evaluated, and 95% of the antibiotic could be recovered in only one batch using water–ethanol, which is an unexplored PenG desorption process. The results showed selective adsorption, indicating that the process can also be useful for purification purposes. Hydrophobic adsorption with ethanol desorption is efficient, scalable, and green and could be used in place of traditional methods or in extractive fermentation.
Introduction
Penicillin G (PenG) is one of the most important industrial pharmaceutical products. This antibiotic is not only used directly to fight bacterial infections but also as a raw material to produce other β-lactam antibiotics.1Penicillium chrysogenum is the usual fungus employed for PenG production under strictly aerobic cultivation. The recovery process is based on liquid extraction from the fermentation broth,2 commonly using solvents such as butyl acetate. Despite being a well-established process with important historic developments,1 there is still much room for improvement of the process, especially considering the downstream operations.
The liquid extraction method has a number of drawbacks, including high product losses,3 toxicity, the risk of product contamination, and volatility/flammability of the solvent. Furthermore, the product should ideally be continuously removed from the fermentation process (extractive fermentation), as a way to improve productivity and yield;4,5 although in practice, this is not done, largely due to the volumes of solvent required and regulation restrictions. Despite these problems, this method is still employed in the industry since it is cheap and efficient.
Recent research efforts have been made to find alternatives to organic solvents for the separation process. Liu et al.6 proposed extraction with a hydrophobic ionic liquid instead of organic solvents, achieving an extraction efficiency of 91% at pH 1.5–2. Other proposed methods for extraction include polymers in aqueous two-phase systems,7−9 solvent sublation,10 and filtration using a membrane or microfiltration.11−13 However, there are no reports of practical industrial applications of the latter methods.
Hydrophobic adsorption is a relatively simple process that is extensively used industrially and in environmental cleanup procedures.5,14,15 In this technique, desorption of the antibiotic may be performed with an environmentally friendly solvent. The process can be adapted and scaled-up for running in a cyclic batch column system, in parallel with the production of PenG, hence enabling simultaneous production and extraction. Furthermore, the process can be automated for continuous removal of PenG from the fermentation broth. In order to favor the hydrophobic extraction of PenG, the pH of the broth must be decreased to 2–4 so that the hydrophobicity of the antibiotic is increased.
Grzegorczyk and Carta16 investigated the adsorption of PenG and amino acids using different polymeric hydrophobic adsorbents. However, the effects of pH, temperature, and fermentation impurities on the adsorption of PenG were not evaluated. Lee et al.17 obtained isotherms for the adsorption of PenG by XAD-4 and XAD-16 but did not evaluate the influence of temperature, solvent, or pH on PenG adsorption. Nonetheless, their results showed that the adsorption of PenG was more favorable compared to other compounds including cephalosporin C.
The study of adsorption applied to PenG is a complex task due to the chemical nature of the molecule, which requires finding a balance between adsorption and stability. The pKa of the acid group in the Penicillin G molecule is around 2.7,18 so its average affinity for hydrophobic adsorbents increases when the pH decreases since there is a greater quantity of neutral species present in the solution. However, the stability of this penicillin is much higher at neutral pH than at low pH.19−22 Therefore, the efficiency of the adsorption process is expected to be a trade-off between the yield and the integrity of the antibiotic.
Most of the reported studies have focused on theoretical and general aspects of the adsorption of PenG by non-ionic polymeric sorbents. However, the chemical complexity of the fermentation broth may negatively affect the adsorption capacity. For this reason, assays using the real medium are essential as a strategy for ensuring a more reliable approach to the problem. Moreover, the effects of process variables were not evaluated, the degradation of PenG was not taken into consideration, and results with changes of pH during the process were not reported.
In the present work, the aim was to investigate the use of hydrophobic resins to separate Penicillin G from a typical fermentation broth, examining its unexplored potential and evaluating the most important variables by means of batch processes. Assessment was first made of the influence of temperature and pH on the integrity of the antibiotic. Having selected appropriate operational conditions, evaluation was made of the performances of different resins and the effects of pH and temperature on the adsorption efficiency and the integrity of the molecule. In view of the results obtained, several possible approaches for performing the adsorption were identified and evaluated.
Finally, assessment was made of the use of ethanol–water to desorb the PenG in order to recover the product and regenerate the adsorption resin. This desorption process possibility has not previously been explored, to the best of our knowledge. Ethanol is a relatively cheap solvent that can be considered environmentally friendly due to its global production using bioprocesses (“biosolvent”) and offers good safety and health aspects.23,24 Furthermore, it presents low toxicity25,26 and should not cause any deleterious health consequences if present in trace amounts in products.
Results and Discussion
Stability of PenG in Unbuffered Aqueous Solutions at Different pH Values and Temperatures
Preliminary assays were performed to evaluate the stability of PenG in aqueous solution, varying the pH from 2 to 7.0. The PenG processing usually occurs at the lowest possible temperature. Typical values are in the range of 0–5 °C.3 For the sake of comparison, the stability was assessed at 4 and 12 °C. The initial and final (after 60 min of incubation) PenG concentrations were determined. The results (Table 1) showed that at 4 °C, there were no losses of PenG at pH from 3.0 to 7.0, while losses of around 5 and 40% occurred at pH 2.5 and 2, respectively. At 12 °C, there were losses of around 10, 30, and 60% at pH 3.0, 2.5, and 2, respectively.
Table 1. Percentage of PenG Remaining in the Medium after 60 min of Incubation at pH Values from 2 to 7, at 4 and 12 °C.
| temp. | pH 2.0 | pH 2.5 | pH 3.0 | pH 4.0 | pH 5.0 | pH 7.0 |
|---|---|---|---|---|---|---|
| 4 °C | 62.5 ± 2.8 | 95.8 ± 2.0 | 100.0 ± 3.4 | 100.0 ± 3.0 | 100.0 ± 5.4 | 100.0 ± 8.1 |
| 12 °C | 39.0 ± 1.6 | 71.4 ± 1.5 | 90.2 ± 3.9 | 100.0 ± 4.5 | 100.0 ± 7.8 | 100.0 ± 6.5 |
These results, which showed that the stability of PenG decreased with decrease of pH and increase of temperature, were in agreement with previous data reported in the literature.19,20
The degradation of PenG in aqueous solution is reported to begin with opening of the β-lactam ring and formation of penicillenic acid. The molecule formed is an intermediate for a broad range of products but does not present antibacterial activity.19,21 It has also been reported that PenG is more stable in buffered solutions due to the pH control.20,21 In the present work, the unbuffered condition was chosen to represent the worst-case scenario. The results obtained showed that pH 4.0 was an important point marking increased stability. For each condition, determination was then made of the longest adsorption time that could be used in batch runs, without PenG degradation. The results are shown in Table 2.
Table 2. Longest Time before any Detectable PenG Degradation in a Solution under Different pH Values and Temperaturesa.
| temp. | pH 2.0 | pH 2.5 | pH 3.0 | pH 4.0 | pH 5.0 | pH 7.0 |
|---|---|---|---|---|---|---|
| 4 °C | 2 min | 15 min | 1 h | 1 h | 1 h | 1 h |
| 12 °C | 2 min | 2 min | 4 min | 1 h | 1 h | 1 h |
Maximum of 60 min assay.
Influence of the Adsorbent and pH
Considering the results shown in Table 2, the influence of pH and the adsorbent was evaluated performing replicate PenG adsorption assays using different adsorbents, during 1 h, at pH 4 to 7. All discussions about these materials should take into account that the applied resins have low surface functionality, different polarities, and low net surface charge for a wide range of pH.27−29
Related to the pH influence, the results (Table 3) indicated that the highest efficiencies were obtained at pH 4. This result was expected since the carboxyl pKa value for PenG (∼2.7) implied the presence of a higher number of neutral PenG molecules with greater affinity for the hydrophobic resins.
Table 3. Percentage of PenG Adsorbed in Different Adsorbents at Different pH Values during 1 h Batch Assay at 4 °C.
| adsorbent | pH 4 | pH 5 | pH 6 |
|---|---|---|---|
| XAD-4 | (44.0 ± 2.3)% | (39.9 ± 1.5)% | (36.2 ± 2.6)% |
| XAD-7 | (39.3 ± 1.9)% | (35.8 ± 2.2)% | (32.1 ± 1.2)% |
| XAD-761 | (31.1 ± 2.1)% | (28.5 ± 3.1)% | (25.2 ± 2.1)% |
XAD-4 is the most hydrophobic and has the highest surface area among the three evaluated resins.30−43 Consequently, at all pH values, the highest yields were obtained with XAD-4 followed by XAD-7 (Table 3). XAD-4 is frequently used to adsorb organic compounds due to the aromatic nature of its surface, mainly from polar solvents.34,36,40,41 Therefore, it was expected that the presence of the aromatic phenyl groups in PenG and XAD-4 would lead to the best adsorption results. The aliphatic nature of XAD-7 allows it to adsorb non-polar compounds from aqueous systems and polar compounds from non-polar solvents.37,40,41,43,44 XAD-761 has a partial hydrophilic nature due to the phenolic hydroxyl and methoxyl groups in its surface.34,35,38,40,41 The surface area may account for the difference between XAD-7 and XAD-761 (Table 8). Under these tested pH conditions, Amberlite XAD-4 was the best adsorbent for PenG among the solids evaluated and was then selected to be used in the next assays.
Table 8. Textural Properties of a Typical Sample of the Amberlite Resins.
| properties | XAD-4 | XAD-7 | XAD-761 |
|---|---|---|---|
| specific surface area (m2/g) | 725 | 450 | 150–250 |
| porosity | 0.5 | 0.5 | 0.45a |
| pore mean diameter (nm) | 5 | 9 | 22.5a |
| pore volume (mL/g) | 0.98 | 1.14 | 0.42a |
Measured by mercury porosimetry by Bautista et al.42
Other characteristics should be taken into account in future application tests: first, the cost of the adsorbent, which could be selected from among many others commercially available and second, the trade-off between the capacity and selectivity. As a consequence of its hydrophobicity, there could be other adsorbents that present better selectivity for the PenG adsorption while taking into account the contaminants presented in the fermentation broth. Otherwise, the capacity has impacts on the process cost and productivity. A compromise may be necessary in order to reach the best industrial configuration. In this case, it is important to consider if the priority is the extractive fermentation or the purification process.
Adsorption Kinetics for XAD-4
In order to confirm that the adsorption time used to evaluate the adsorbents was sufficiently long for equilibrium to be reached, kinetic adsorption assays were performed using XAD-4, with the PenG concentration in the broth being followed during 1 h, at the two temperatures and the three pH values. The results obtained during the adsorption process are presented in Figure 1 from which it can be clearly seen that equilibrium was reached within 1 h, under all the conditions used.
Figure 1.
PenG adsorption kinetics in XAD-4 using cultivation broth at pH 4.0, 5.0, and 7.0, at (A) 4 and (B) 12 °C.
At this point, it is pertinent to introduce some discussions about the observed adsorption kinetics. First of all, the decrease of pH leads to an increase of neutral PenG chemical species (pK 2.7). As a consequence, the lower the pH, the more efficient should be the hydrophobic adsorption. This behavior can be observed from the kinetic data (Figure 1), and it is confirmed in the equilibrium data (Table 4 and Figure 2). For this reason, PenG concentration in the final stages of the process is lower for pH 4 and higher for pH 7. It is interesting to note that the overall rate of adsorption is controlled by the transport phenomena due to the high rate of physical adsorption at the surface.45,46 Consequently, the higher adsorption rate is promoted by the higher overall driving force of the diffusion during the process. The increment in the driving force is due to the lower pH while favoring the adsorption step process in the surface.
Table 4. Model Parameters and Correlation Coefficients for the Langmuir Isotherm Models Fitted with Equilibrium Data of PenG/XAD-4 Adsorption.
| Langmuir
model |
||||
|---|---|---|---|---|
| pH | T (°C) | qm (mg/g) | KL (mg/mL) | R2 |
| 4 | 4 | 595.06 ± 51.54 | 21.7 ± 3.6 | 0.9942 |
| 7 | 4 | 534.68 ± 56.36 | 18.6 ± 4.1 | 0.9885 |
| 4 | 12 | 439.84 ± 14.82 | 6.17 ± 0.7 | 0.9958 |
| 7 | 12 | 431.72 ± 11.34 | 9.19 ± 0.7 | 0.9981 |
Figure 2.
Fitting of the Langmuir model to equilibrium data obtained for adsorption of PenG from cultivation broth, in XAD-4 resin, at 4 and 12 °C, pH 4.0 and 7.0.
The temperature effect is easier to observe with the adsorption isotherms due to the proximity of kinetic data in different temperatures. The adsorption process is generally exothermic.45,46 As a consequence, decreasing the system temperature favors the adsorption.
Adsorption Isotherm for XAD-4
If it is intended to apply an adsorption method to recover a product, it is important to identify the adsorption isotherm model that provides the best representation of the system studied. The isotherm model provides informative data, and its predictive capacity enables the analysis and design of an adsorption process. The adsorption capacity of the resin will depend on the product properties, as well as the effects of temperature, solution pH, and impurities.
The experimental equilibrium data for the adsorption of PenG from the broth onto the XAD-4 were obtained under different conditions (4 and 12 °C, pH 4.0 and 7.0). The data were then fitted using the Langmuir, Freundlich, and linear isotherm models.
Table 4 shows the estimated model parameters and the correlation coefficients (R2) for the Langmuir isotherms. Parameters for the other models tested and other statistical analyses are provided in the Supporting Information. The calculated value of F for the Langmuir model was the highest among all models and a lot more than the tabulated value, meaning that the regression was statistically significant. Under all the conditions, the highest R2 value was obtained for the Langmuir model. The fitting of this model to the equilibrium data is presented in Figure 2 for all the conditions tested. The maximum capacity of the XAD-4 for PenG adsorption could then be estimated for each experimental condition (qm in Table 4).
The results shown in Table 4 confirmed the expected positive influence of a lower temperature on the exothermic adsorption process. The pH had less influence in the equilibrium data analysis for the range studied. Taking into account the pKa of the acid group, at pH 7, the percentage ratio between the ionic and neutral forms of the molecule is around 99.99/0.01, while at pH 4.0, it changes slightly to approximately 94.79/5.21. The ratio only reaches 50/50 at pH 2.74. Nevertheless, it is surprising that the charge on the other side of the PenG molecule did not prevent its adsorption onto the resin by means of the hydrophobic ring. Considering a PenG concentration of 50 mg mL–1 in the medium, at 12 °C and pH 7, the PenG could be recovered using a ratio of resin mass/medium volume below 15%, even in a batch process.
The medium composition is another important variable whose influence should be investigated since other molecules present in the broth may compete for the resin sites. Therefore, in order to evaluate this influence, as well as to enable comparison with other data reported in the literature, equilibrium data were obtained with PenG dissolved in water. Figure 2 shows the fitting of the Langmuir model to the experimental values for the adsorption of PenG from the cultivation broth, while Figure 3 presents the results for adsorption in the absence of contaminants at pH 4.0 and 4 °C. The estimated parameter values were very close to those obtained previously: qm = 644.0 ± 57.0 mg PenG/g XAD-4 and KL = 20.0 ± 1.9 mg PenG/mL.
Figure 3.
Adsorption isotherm of purified PenG in XAD-4 from solutions in water, at pH 4.0 and 4 °C. The line shows the fitted Langmuir model.
The proximity between the equilibrium data for PenG adsorption in the presence and absence of broth impurities indicated that the resin was selective for adsorption of PenG, so the adsorption process could be performed directly in the cultivation broth. The maximum capacity obtained here was similar to the values reported elsewhere for the adsorption of Penicillin G by hydrophobic resins.16,17 However, the results should not be directly compared because they were obtained under different operational conditions.
Adsorption of PenG by XAD-4 and pH Gradient
In the experiments described so far, the adsorption process was studied only at pH 4, during 1 h, and with a relatively low ratio of resin mass/medium volume. However, it is important to study the effect of these variables. The lower the pH, the higher is the ratio between the neutral and ionic forms of the antibiotic, which might improve the adsorption process. On the other hand, this condition might also cause degradation of the molecule, even before completion of 1 h of operation. Faster degradation rates were observed at pH less than 3.0 (Table 1). With the aim of finding another viable operational condition with higher adsorption efficiency, investigation was made of the performance of the process at pH lower than 4. Figure 4 presents the results obtained in the first assay in which most of the process was carried out at pH 4.0.
Figure 4.
Concentration of PenG in the supernatant along the adsorption process in XAD-4, at pH 4 and 3.5, 4 °C, and a resin mass (g)/medium volume (mL) ratio of 10%. The percentage values represent the yield of the process at each instant.
As shown in Figure 4, 91% of the PenG had already been adsorbed after only 1 h of the process at pH 4.0, indicative of an absence of degradation (see Table 1), with productivity of 0.55 gPenG/(gXAD-4 h). After 1 h, a low adsorption rate was expected due to the low concentration of PenG in the bulk liquid and consequent proximity to the equilibrium condition. The pH was then decreased to 3.5, which resulted in the adsorption capacity increasing to 0.58 gPenG/gXAD-4, while the productivity decreased to 0.38 gPenG/(gXAD-4 h).
With the aim of achieving 99% PenG adsorption, another assay (the second one described in the Methods section) was designed, with adjustment of the pH and the resin mass/medium volume ratio during the process. The adsorption procedure involved five steps. During the process, the resin mass/medium volume ratio was increased from 2.78 to 11.1%, while the pH varied according to a gradient from pH 7 to 3.2 (Experimental Section, Table 9). The resin was added at each pH change in order not only to increase the total adsorption capacity of the process but also to make the kinetics faster while changing the pH. In order to preserve the PenG integrity, the adsorption time interval at each pH was the longest possible, as shown in Table 2. As a consequence of the strategy, the adsorption process at low pH took place in shorter time intervals and when the concentration of PenG in solution was relatively low. The goal here was to benefit from the low pH but maintaining the antibiotic integrity. The assay is an illustration of the pH role in the adsorption process. The process approximately emulated the conditions expected when PenG at low concentration in a bulk liquid system reaches a section of the non-saturated adsorbent in a continuous column at lower pH. This adsorption process allowed removal of PenG from the bulk liquid at each stage until 99% yield was reached at the end (Figure 5).
Table 9. PenG Adsorption Assay 2, Operational Conditions, and Throw-Out Time.
| step | pH | time (min) | total mass (g) |
|---|---|---|---|
| step 1 | 7.0 | 45 | 0.5 |
| step 2 | 5.0 | 45 | 1.0 |
| step 3 | 4.0 | 45 | 1.5 |
| step 4 | 3.5 | 30 | 2.0 |
| step 5 | 3.2 | 30 | 2.0 |
Figure 5.
Batch adsorption process of PenG in XAD-4 from a fermentation broth with pH gradient and increasing of the resin mass/medium volume ratio at 4 °C. The percentage values represent the yield of the process at each instant.
As expected, the use of pH lower than 4.0 increased the adsorption efficiency but could potentially damage the penicillin and might not be necessary. The results (Figure 5) showed that it was possible to adsorb 99% of the PenG while preserving the integrity of the molecule. On the other hand, the yield obtained in a simple 45 min batch process at pH 4.0 was impressive when compared to the typical PenG loss in a traditional liquid extraction system where typically between 10 and 15% is lost.3
The batch column adsorption could enable high productivity and recovery efficiency to be achieved, but the studies required to determine the breakthrough curve, column capacity, and economic feasibility would be better performed under specific industrial operational conditions for PenG production. The results point out that the use of a pH gradient in a column would extend the column operation time and capacity (breakthrough point). The pH could be controlled at 4.0 during the feeding. Alternatively, the column could be operated using a spatial or temporal pH gradient, reaching pH 4.0 or even lower pH at the end of the process. This operation mode might lead to even higher productivity and a longer cycle of operation, maintaining product loss in a tolerable range. In this case, specific studies would be required in order to determine the column parameters and to evaluate product degradation. Nevertheless, it is important to point out that the objective of this work was to establish the technical feasibility of this method for recovering PenG directly from the cultivation medium. Optimization of the adsorption process could be performed under specific operational conditions in the industry where the method is intended to be applied. In the next step, the desorption process was investigated.
Evaluation of the Desorption Process with Ethanol–Water
In order to optimize the ethanol–water desorption process and obtain a representative yield for evaluation of the technical feasibility of the process, an experimental design was used to investigate the desorption conditions. Table 5 shows the experimental conditions and the desorption results obtained.
Table 5. Percentage of PenG Desorbed in Various Conditions for the First Experimental Design.
| T (°C) | pH | [EtOH] | % desorbed |
|---|---|---|---|
| –1 | –1 | –1 | 29.62 |
| +1 | –1 | –1 | 34.02 |
| –1 | +1 | –1 | 32.90 |
| +1 | +1 | –1 | 31.09 |
| –1 | –1 | +1 | 64.09 |
| +1 | –1 | +1 | 62.85 |
| –1 | +1 | +1 | 74.39 |
| +1 | +1 | +1 | 75.34 |
| –1.68 | 0 | 0 | 66.07 |
| +1.68 | 0 | 0 | 68.97 |
| 0 | –1.68 | 0 | 65.60 |
| 0 | +1.68 | 0 | 63.47 |
| 0 | 0 | –1.68 | 31.45 |
| 0 | 0 | +1.68 | 79.58 |
| 0 | 0 | 0 | 70.36 |
| 0 | 0 | 0 | 73.15 |
| 0 | 0 | 0 | 70.53 |
| 0 | 0 | 0 | 72.36 |
The desorption percentage varied from 29.6 to 79.6% during the assays (Table 5). The center point replicates were used to confirm the reproducibility of the method. The data could be described using a second-degree polynomial model, as follows (with coded variables)
| 1 |
The coefficients shown in bold type are statistically significant. The main purpose of the experimental design was to map the effects and find regions with high desorption yield. Nonetheless, further evaluation of the model using analysis of variance (ANOVA) is provided in the Supporting Information. The model satisfactorily represented the process, with a coefficient of determination (R2) of 0.90. Application of the F-test showed significant fitting at the 95% confidence level.
The results were evaluated visually in the form of a response surface plot (Supporting Information). The screening showed that it was necessary to operate at pH between 5.2 and 6.8, using an ethanol concentration between 65 and 100%. After the screening already described, the process was further investigated using a face-centered central composite design (FCC), with two levels, six replicates at the central point, and axial points. Many center point replicates were obtained in order to ensure reproducibility in the most promising region. The temperature was fixed at 8 °C since it was not statistically significant, and the conditions for the other variables are shown in Table 6.
Table 6. Conditions for the Second Experimental Design.
| variables | –1 | 0 | 1 |
|---|---|---|---|
| pH | 5.2 | 6.0 | 6.8 |
| [EtOH] | 65.0 | 82.5 | 100.0 |
The percentage of PenG desorbed varied between 74.1 and 97.7% (Table 7). The ANOVA results are included as Supporting Information. The R2 was 0.74 and the F-test showed significant fitting at the 95% confidence level. Although the R2 was lower the replicates at the center point indicated good reproducibility, and their results assured that it is possible to reach more than 90% desorption, estimating the yield as (95.0 ± 1.9)%.
Table 7. Desorbed Percentage for the Second Experimental Design.
| pH | [EtOH] | % desorbed |
|---|---|---|
| –1 | –1 | 76.64 |
| –1 | +1 | 79.29 |
| 0 | 0 | 94.93 |
| 0 | 0 | 97.74 |
| 0 | 0 | 96.25 |
| 0 | 0 | 94.48 |
| 0 | 0 | 91.93 |
| 0 | 0 | 94.56 |
| 0 | –1 | 74.06 |
| –1 | 0 | 74.28 |
| 1 | 0 | 80.33 |
| 0 | +1 | 79.34 |
| +1 | –1 | 78.33 |
| +1 | +1 | 77.01 |
The desorption process was influenced by both ethanol concentration and pH and could be described by a second-degree polynomial model, as follows
| 2 |
In order to maximize desorption in the batch process and reach more than 90% yield, it was necessary to maintain the pH between 5.6 and 6.5, with a concentration of ethanol between 75 and 90% (Figure 6). This enabled a high degree of antibiotic recovery to be achieved using only one batch desorption. Similar ranges of these parameters would also be recommended for a column process. Finally, the purity of the desorbed penicillin was investigated.
Figure 6.
Contour plot of the second-degree polynomial model for the percentage of desorbed PenG of the second design as a response of pH and ethanol concentration (% mass).
Separation and Selectivity
The selectivity toward PenG purified by hydrophobic adsorption from a complex medium onto XAD-4 followed by desorption with ethanol was assessed using HPLC analysis. Figure 7 presents chromatograms obtained before and after the purification process. Peaks 1, 2, and 3 are impurities, while peak 4 corresponds to PenG. There were significant decreases of the impurity peaks after only one adsorption–desorption batch cycle, indicating high selectivity of the hydrophobic adsorption at pH 4 and 4 °C. The Abstract graphic illustrates visually the solution before (broth medium) and after the process.
Figure 7.
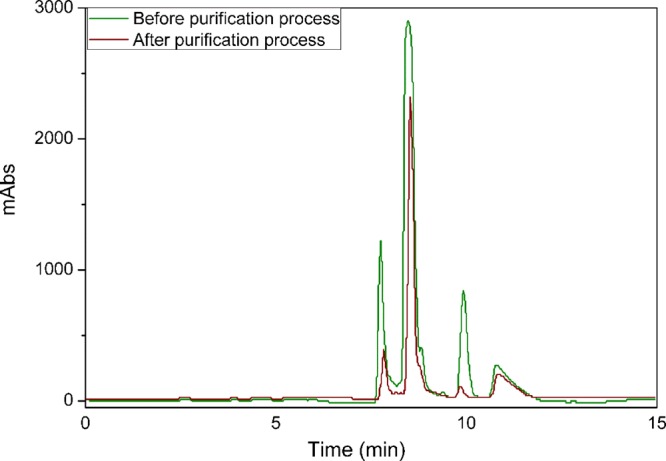
Chromatograms before and after a batch purification process (adsorption followed by desorption) from the fermentation broth.
The area of the PenG peak after the purification process was 85% of the previous one. Peaks 1, 2, and 3 presented 28, 49, and 20% of the areas before the purification process, respectively. The second peak had a lesser reduction. However, if the main purpose is the purification process, then it is possible that less hydrophobic materials could offer a better compromise between selectivity and capacity. An investigation in this scope was not the goal of this work but would be a good topic for future studies in the area.
Conclusions
In terms of capacity, the best hydrophobic adsorbent for PenG adsorption was Amberlite XAD-4. Kinetic and thermodynamic studies of the adsorption of PenG from fermentation broth indicated that lower pH and temperature led to a higher adsorption yield. At pH 4.0 and 4 °C, an adsorption yield of 91% was achieved in 45 min, with no degradation of the antibiotic. The batch assay conducted using a gradient to reach pH lower than 4 indicated that it was possible to achieve 99% adsorption although with some risk of PenG degradation. The best desorption results with ethanol–water were with ethanol concentration between 75 and 90%, at pH between 5.6 and 6.5. More than 90% of the antibiotic could be recovered in only one batch.
The process was selective, which highlights it also as an alternative to purify the product, besides the use in extractive fermentation. Adsorption using a column would certainly lead to high productivity and efficiency. The results indicated that it would be possible to achieve high productivity during the operational cycle while maintaining product loss within a tolerable range. Taken together, the present findings represent advancement toward the development of an alternative and greener process for the recovery of PenG and toward a practical extractive fermentation process.
Experimental Section
Materials
Penicillin G potassium was obtained from Prodotti S/A. Amberlite XAD-4, XAD-7, and XAD-761 resins were from Rohm and Haas. Amberlite is a trade name of Rohm and Haas Company (Philadelphia, PA). The common application and chemical structure of these commercial resins are well-known from the literature and established by Rohm and Haas Company.30−43 XAD-4 is a macroreticular cross-linked aromatic polymer. XAD-761 constitutes a cross-linked phenol–formaldehyde polycondensate matrix, while XAD-7 is an acrylic ester resin. XAD-7 and XAD-761 have moderate polarity due to their chemical structure. The physical properties are compiled in Table 8. Most of them are supplied by the manufacturer (Rohm and Haas Company).36−42
Other reactants used were of analytical grade and water was double-distilled. Penicillium chrysogenum was donated by the Fundação Andre Tosello, located in Campinas (SP, Brazil), that keeps one of the biggest tropical culture collections in the world (cct@fat.org.br). The stock medium was prepared in a tube with 20 g L–1 barley extract, 20 g L–1 peptone, 1 g L–1 glucose, and 20 g L–1 agar, following directions from Fundação Andre Tosello. The stock medium was changed every four months.
Fermentation Process
Inocula from the stock culture were transferred to Erlenmeyer flasks containing 100 mL of medium and maintained for 24 h at 25 °C and 250 rpm agitation. The medium was produced with water (800 mL L–1), corn steep liquor (CSL, 28 mL L–1), sodium phosphate monobasic (5.2 g L–1), (NH4)2SO4 (5.2 g L–1), Ca(OH)2 (1.2 g L–1), CaCO3 (0.8 g L–1), polypropylene 2000 antifoaming agent (2.6 mL L–1), and saccharose (10 g L–1). In the next step, the medium was transferred to Erlenmeyer flasks containing fresh fermentation broth followed by addition of 2.56 g L–1 potassium phenylacetate (PenG precursor). The fermentation was allowed to proceed for 24 h after which the fermentation broth was filtered and the filtrate was kept at −20 °C for conservation. The medium and procedure cultivation, as well as corn steep liquor, potassium phenylacetate, and penicillin G, were gently provided by Prodotti Farmacêutica Ltda. (Brazil).
HPLC Analysis
Analyses were performed using a Shimadzu LC-10 high-performance liquid chromatography (HPLC) system equipped with a C-18 column (μBondPack, 3.9 × 300 mm). The eluent consisted of 20% acetonitrile, 80% phosphate buffer (10 mmol L–1, pH 6.5), and sodium thiosulfate (10 mmol L–1). The eluent flow rate was 1 mL min–1 and the UV detector wavelength was 254 nm. The method is common for the determination of antibiotic concentration.47,48
Penicillin Stability
Batch assays were performed in unbuffered aqueous solutions. Purified PenG was diluted (3 g L–1) in a stirred glass reactor with temperature control. In each case, the pH was adjusted in the range 2–7.0. Samples were collected and diluted (1:1 v/v) in phosphate buffer solution (100 mM, pH 7.0) in order to stabilize the molecule. The PenG concentration was determined by HPLC. The experiments were performed in triplicate.
Treatment of Adsorbents
Before use, the XAD resins were treated by triple washing with methanol (2 mL per g), each time for 30 min. The resins were then abundantly washed with distilled water and dried for 24 h at 50 °C under vacuum. Before use, the resin was hydrated with ethanol and washed 10 times with water. The procedure had the purpose to wash out the storage solution. The hydration procedure with ethanol was due to the hydrophobic character of the adsorbents, making the permeation easier.43
Selection of the Adsorbent (Adsorption Yield)
Different adsorbents were evaluated. The pretreated adsorbent (0.25 g) was added to 5 mL of fermentation broth containing 50 g L–1 of PenG at 4 °C under agitation, with adjustment of the pH (6.0, 5.0, or 4.0). Samples were collected at the beginning and after 45 min with filtration for determination of the PenG concentration, as described previously. The yield of the process (adsorbed percentage) was calculated using eq 3
| 3 |
where C0 is the initial concentration and C* is the concentration in the supernatant after 45 min.
Adsorption Kinetics
Assays were performed using 0.1 g of pretreated XAD-4 added to 2 mL of fermentation broth. Different pH values (4.0, 5.0, and 7.0) and temperatures (4 and 12 °C) were applied. Broth samples were collected at 0, 2, 4, 6, 10, 20, 30, 45, and 60 min for determination of the PenG concentration.
Adsorption Isotherms
Different initial concentrations of PenG in solution were used to obtain adsorption equilibrium data for XAD-4 at pH 4.0, 5.0, and 7.0 at two temperatures (4 and 12 °C). The assays employed 0.1 g of the pretreated adsorbent in 2 mL of fermentation broth or aqueous PenG solution. The PenG concentration was determined as described previously. Under equilibrium conditions, the solid concentration could be obtained using the supernatant concentration data as follows
| 4 |
where q* is the equilibrium concentration in the solid phase (mg/g), C* is the equilibrium concentration in the liquid phase (mg/mL), C0 is the initial concentration in the liquid (mg/mL), VL is the adsorption liquid volume (mL), and w is the mass of the adsorbent (g). The concentration in the supernatant was determined in samples following filtration. Equilibrium isotherm models (linear, Langmuir, and Freundlich) were fitted using the experimental values of q* and C*. The best fitting was presented for the Langmuir model (as described in results)
| 5 |
where qm is the resin maximum capacity or saturation concentration (mg/g) and KL is the Langmuir parameter that was adjusted (mg/mL). It corresponds to 1/Kad, and Kad is the adsorption constant.
Batch Adsorption with and without a pH Gradient
The pH of the fermentation broth was adjusted to 7.0 in a stirred glass reactor kept at 4 °C. The pH was then adjusted to the desired values using an automatic system (718 STAT Titrino, Metrohm). The PenG concentration was determined as described previously.
Assay 1
The pH of the initial solution (18 mL of fermentation broth containing 50 g L–1 of PenG) at pH 7.0 was slowly decreased to pH 4.0. At this point, 1.5 g of XAD-4 was added and the concentration of PenG was monitored during 1 h. The pH was then decreased to 3.5 and the adsorption process was monitored during 30 min.
Assay 2
XAD-4 (0.5 g) was added to 18 mL of fermentation broth containing 50 g L–1 PenG at pH 7.0, and the process was monitored during 45 min. After that, the pH was altered step by step, and 0.5 g of the adsorbent was added at each process step until pH 3.5 (step 4). Table 9 shows the assay conditions with pH, solid mass, and monitored time of each step. The pH was decreased for step 5, but no adsorbent was added.
Desorption Process
Masses of XAD-4 (0.1 g) with adsorbed PenG were added to 2 mL volumes of solutions of ethanol–water at different proportions. Desorption was performed during 1 h in an incubator under agitation at 250 rpm. The percentage of PenG desorbed was then quantified.
The main goal was to show the feasibility of using ethanol–water in the process. For this, the response surface methodology was used to evaluate the effects of temperature, pH, and ethanol concentration on desorption. A central composite design (23, with axial points and four replicates at the center point) was applied to construct the model. The levels are presented in Table 10. After that, a new experimental design was applied in order to obtain a short range of variables to be used during the desorption. For the purpose of clarity, the second design will be described in the Results section.
Table 10. Levels for the First Experimental Design to the Analysis of the Desorption Processa.
| variables | –1.68 | –1 | 0 | 1 | 1.68 |
|---|---|---|---|---|---|
| T (°C) | 4.0 | 5.6 | 8.0 | 10.4 | 12.0 |
| pH | 4.0 | 4.6 | 5.5 | 6.4 | 7.0 |
| [EtOH] (%) | 0.0 | 20.0 | 50.0 | 80.0 | 100.0 |
[EtOH] is the percentage of ethanol (mass/mass).
Acknowledgments
We gratefully acknowledge the financial support from FAPESP (Fundação de Amparo á Pesquisa do Estado de São Paulo, grant 06/53595-8), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), FINEP (Financiadora de Inovação e Pesquisa), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). We also thank Prodotti Farmacêutica Ltda. for donations and Fundação Andre Tosello for the Penicillium chrysogenum donation.
Glossary
Nomenclature
- C0
initial PenG concentration in the liquid phase (mg/mL)
- C*
final or equilibrium PenG concentration in the liquid phase (mg/mL)
- q*
concentration of PenG in the solid phase at equilibrium (mg/g)
- VL
adsorption liquid volume (mL)
- Y (%)
adsorption yield (in %)
- w
mass of adsorbent (g)
- qm
resin maximum capacity or saturation concentration (mg/g)
- KL
Langmuir model parameter (mg/mL)
- Kad
adsorption constant (Langmuir, mL/ mg)
- [EtOH]
percentage of ethanol (in mass/mass)
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04175.
Linear and Freundlich parameters and ANOVA and surface response for desorption optimization (PDF)
Author Present Address
§ Embrapa Agroenergia, Empresa Brasileira de Pesquisa Agropecuária, Brasília, DF, Brazil.
Author Contributions
∥ R.L.C.G. and T.F.d.P. contributed equally to this work.
Author Contributions
A.N.C.d.B. and D.S.R. performed and planned assays. A.N.C.d.B. and E.F.Q.S. performed data analysis and considerations. T.F.d.P. and R.L.C.G. contributed equally with discussions and analysis and wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ozcengiz G.; Demain A. L. Recent advances in the biosynthesis of penicillins, cephalosporins and clavams and its regulation. Biotechnol. Adv. 2013, 31, 287–311. 10.1016/j.biotechadv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Elander R. P. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- Kheirolomoom A.; Kazemi-Vaysari A.; Ardjmand M.; Baradar-Khoshfetrat A. The combined effects of pH and temperature on penicillin G decomposition and its stability modeling. Process Biochem. 1999, 35, 205–211. 10.1016/S0032-9592(99)00052-7. [DOI] [Google Scholar]
- Gutiérrez L. F.; Sánchez Ó. J.; Cardona C. A. Analysis and design of extractive fermentation processes using a novel short-cut method. Ind. Eng. Chem. Res. 2013, 52, 12915–12926. 10.1021/ie301297h. [DOI] [Google Scholar]
- Oliveira L. M.; Brites L. M.; Bustamante M. C. C.; Parpot P.; Teixeira J. A.; Mussatto S. I.; Barboza M. Fixed-Bed Column Process as a Strategy for Separation and Purification of Cephamycin C from Fermented Broth. Ind. Eng. Chem. Res. 2015, 54, 3018–3026. 10.1021/ie504499z. [DOI] [Google Scholar]
- Liu Q.; Li Y.; Li W.; Liang X.; Zhang C.; Liu H. Efficient Recovery of Penicillin G by a Hydrophobic Ionic Liquid. ACS Sustainable Chem. Eng. 2015, 4, 609–615. 10.1021/acssuschemeng.5b00975. [DOI] [Google Scholar]
- Rabieenezhad A.; Roosta A. Experimental study and thermodynamic modelling of penicillin-G extraction using PEG 6000 and K2HPO4 aqueous two-phase system. J. Chem. Thermodyn. 2018, 120, 54–59. 10.1016/j.jct.2018.01.010. [DOI] [Google Scholar]
- Roosta A.; Jafari F.; Javanmardi J. Liquid-liquid equilibrium in an aqueous two-phase system of polyethylene glycol 6000, sodium sulfate, water, glucose, and penicillin-G: experimental and thermodynamic modeling. J. Chem. Eng. Data 2015, 61, 565–570. 10.1021/acs.jced.5b00715. [DOI] [Google Scholar]
- Pazuki G.; Vossoughi M.; Taghikhani V. Partitioning of penicillin G acylase in aqueous two-phase systems of poly(ethylene glycol) 20000 or 35000 and potassium dihydrogen phosphate or sodium citrate. J. Chem. Eng. Data 2010, 55, 243–248. 10.1021/je900319s. [DOI] [Google Scholar]
- Bi P. Y.; Dong H. R.; Guo Q. Z. Separation and Purification of Penicillin G from fermentation broth by solvent sublation. Sep. Purif. Technol. 2009, 65, 228–231. 10.1016/j.seppur.2008.10.028. [DOI] [Google Scholar]
- He L.; Li L.; Sun W.; Zhang W.; Zhou Z.; Ren Z. Extraction and recovery of penicillin G from solution by cascade process of hollow fiber renewal liquid membrane. Biochem. Eng. J. 2016, 110, 8–16. 10.1016/j.bej.2016.02.002. [DOI] [Google Scholar]
- Adikane H. V.; Singh R. K.; Nene S. N. Recovery of penicillin G from fermentation broth by microfiltration. J. Membr. Sci. 1999, 162, 119–123. 10.1016/S0376-7388(99)00129-5. [DOI] [Google Scholar]
- Lee C. J.; Yeh H. J.; Yang W. J.; Kan C. R. Extractive separation of penicillin G by facilitated transport via carrier supported liquid membranes. Biotechnol. Bioeng. 1993, 42, 527–534. 10.1002/bit.260420417. [DOI] [PubMed] [Google Scholar]
- Silva M.; Castellanos L.; Ottens M. Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. Ind. Eng. Chem. Res. 2018, 57, 5359–5369. 10.1021/acs.iecr.7b05071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likozar B.; Senica D.; Pavko A. Interpretation of Experimental Results for Vancomycin Adsorption on Polymeric Resins in a Fixed Bed Column by Mathematical Modeling with Independently Estimated Parameters. Ind. Eng. Chem. Res. 2013, 52, 9247–9258. 10.1021/ie400832p. [DOI] [Google Scholar]
- Grzegorczyk D. S.; Carta G. Adsorption of amino acids on porous polymeric adsorbents--ii. intraparticle mass transfer. Chem. Eng. Sci. 1996, 51, 819–826. 10.1016/0009-2509(95)00290-1. [DOI] [Google Scholar]
- Lee J. W.; Shim W. G.; Yang W. C.; Moon H. Adsorption equilibrium of amino acids and antibiotics on non-ionic polymeric sorbents. J. Chem. Technol. Biotechnol. 2004, 79, 413–420. 10.1002/jctb.1006. [DOI] [Google Scholar]
- Rapson H. D. C.; Bird A. E. Ionisation constants of some penicillins and of their alkaline and penicillinase hydrolysis products. J. Pharm. Pharmacol. 1963, 15, 222T–231T. 10.1111/j.2042-7158.1963.tb11216.x. [DOI] [PubMed] [Google Scholar]
- Herschbach G. J. M.; Van Der Beek C.P.; Van Dijk P.W.M.. The penicillins: properties, biosynthesis, and fermentation. In Biotechnology of Industrial Antibiotics; Vandamme E. J., editor. Marcel Dekker: New York: 1984, 45–104. [Google Scholar]
- Macek T. J.; Hanus E. J.; Feller B. A. The stability of Penicillin G sodium in aqueous solution. J. Am. Pharm. Assoc. 1948, 37, 322–327. 10.1002/jps.3030370809. [DOI] [PubMed] [Google Scholar]
- Lu X.; Xing H.; Su B.; Ren Q. Effect of buffer solution and temperature on the stability of Penicillin G. J. Chem. Eng. Data 2008, 53, 543–547. 10.1021/je7006378. [DOI] [Google Scholar]
- Qi J.; Liu Q.; Liu H. Stability of penicillin G in ionic liquid [Bmim]PF6. Chin. J. Chem. Eng. 2018, 26, 1430–1434. 10.1016/j.cjche.2018.02.030. [DOI] [Google Scholar]
- Capello C.; Fischer U.; Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. 10.1039/b617536h. [DOI] [Google Scholar]
- DeVierno Kreuder A.; House-Knight T.; Whitford J.; Ponnusamy E.; Miller P.; Jesse N.; Rodenborn R.; Sayag S.; Gebel M.; Aped I.; Sharfstein I.; Manaster E.; Ergaz I.; Harris A.; Grice L. N. A method for assessing greener alternatives between chemical products following the 12 principles of green chemistry. ACS Sustainable Chem. Eng. 2017, 5, 2927–2935. 10.1021/acssuschemeng.6b02399. [DOI] [Google Scholar]
- Bakalinsky A. T., Penner M. H., Alcohol properties and determination . In Encyclopedia of food sciences and nutrition (secondedition); Academic Press, 2003, 107–111. [Google Scholar]
- Gupta P. K.Solvent, vapor, and gases. In Illustrated Toxicology; Academic Press, 2018, 247–263. [Google Scholar]
- Geng X.; Ren P.; Pi G.; Shi R.; Yuan Z.; Wang C. High selective purification of flavonoids from natural plants based on polymeric adsorbent with hydrogen-bonding interaction. J. Chromatogr. A 2009, 1216, 8331–8338. 10.1016/j.chroma.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Qiu Y.; Ling F. Role of surface functionality in the adsorption of anionic dyes on modified polymeric sorbents. Chemosphere 2006, 64, 963–971. 10.1016/j.chemosphere.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Domínguez J. R.; González T.; Palo P.; Cuerda-Correa E. M. Removal of common pharmaceuticals present in surface Waters by Amberlite XAD-7 acrylic-ester-resin: Influence of pH and presence of other drugs. Desalination 2011, 269, 231–238. 10.1016/j.desal.2010.10.065. [DOI] [Google Scholar]
- Daignault S. A.; Noot D. K.; Williams D. T.; Huck P. M. A review of the use of XAD resins to concentrate organic compounds in water. Water Res. 1988, 22, 803–813. 10.1016/0043-1354(88)90017-6. [DOI] [Google Scholar]
- Juang R.-S.; Shiau J.-Y.; Shao H.-J. Effect of Temperature on Equilibrium Adsorption of Phenols onto Nonionic Polymeric Resins. Sep. Sci. Technol. 1999, 34, 1819–1831. 10.1081/SS-100100740. [DOI] [Google Scholar]
- Kyriakopoulos G.; Doulia D.; Hourdakis A. Effect of ionic strength and pH on the adsorption of selected herbicides on Amberlite. Int. J. Environ. Anal. Chem. 2006, 86, 207–214. 10.1080/03067310500247678. [DOI] [Google Scholar]
- Corrêa R. A.; Calçada L. A.; Peçanha R. P. Development of a fluidized bed system for adsorption of phenol from aqueous solutions with commercial macroporous resins. Braz. J. Chem. Eng. 2007, 24, 15–28. 10.1590/S0104-66322007000100002. [DOI] [Google Scholar]
- Sousa A. D.; de Brito E. S. Effect of treatment with adsorbent resin on the volatile profile and physicochemical characteristics of clarified cashew apple juice. Food Sci. Technol. 2013, 33, 619–623. 10.1590/S0101-20612013000400004. [DOI] [Google Scholar]
- Sandhu A. K.; Gu L. Adsorption/Desorption Characteristics and Separation of Anthocyanins from Muscadine (Vitis rotundifolia) Juice Pomace by Use of Macroporous Adsorbent Resins. J. Agric. Food Chem. 2013, 61, 1441–1448. 10.1021/jf3036148. [DOI] [PubMed] [Google Scholar]
- Product Information; technical bulletin, Amberlite XAD-4; ROHM AND HAAS COMPANY: Philadelphia, PA, 2003.
- Product Information; technical bulletin, Amberlite XAD-7; ROHM AND HAAS COMPANY: Philadelphia, PA, 2003.
- Product Information; technical bulletin, Amberlite XAD-761; ROHM AND HAAS COMPANY: Philadelphia, PA, 2004.
- Kunin R. The use of macroreticular polymeric adsorbents for the treatment of waste effluents. Pure Appl. Chem. 1976, 46, 205–211. 10.1351/pac197646020205. [DOI] [Google Scholar]
- Product selection of Sigma-Aldrich/Merck. [Online] <https://www.sigmaaldrich.com/technical-documents/articles/analytical/purification/polymeric-resin-selection.html> (accessed January 20, 2020).
- Amberlite data sheet information. Sigma-Aldrich Company. [Online] <https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/1/xad4pis.pdf> (accessed January 20, 2020).
- Bautista L. F.; Martínez M.; Aracil J. Adsorption Equilibrium of α-amylase in aqueous solutions. AIChE J. 1999, 45, 761–768. 10.1002/aic.690450411. [DOI] [Google Scholar]
- Sapers G. M. Deodorization of a colorant prepared from Red Cabbage. J. Food Science 1982, 47, 972–976. 10.1111/j.1365-2621.1982.tb12758.x. [DOI] [Google Scholar]
- Juang R.-S.; Shiau J.-Y. Adsorption isotherms of phenols from water onto macroreticular resins. J. Hazard. Mater. 1999, 70, 171–183. 10.1016/S0304-3894(99)00152-1. [DOI] [PubMed] [Google Scholar]
- Ruthven D. M.Principles of adsorption and adsorption processes; John Wiley & sons, 1984. [Google Scholar]
- Guiochon G.; Felinger A.; Shirazi D. G.; Katti A. M.. Fundamentals of preparative and nonlinear chromatography; Elsevier, Academic press, second edition, 2006. [Google Scholar]
- Ribeiro M. P. A.; Ferreira A. L. O.; Giordano R. L. C.; Giordano R. C. Selectivity of the enzymatic synthesis of ampicillin by E. Coli PGA in the presence of high concentrations of substrates. J. Mol. Catal. 2005, 33, 81–86. 10.1016/j.molcatb.2005.03.003. [DOI] [Google Scholar]
- Ribeiro M. P. A.; Pádua T. F.; Leite O. D.; Giordano R. L. C.; Giordano R. C. Multivariate calibration methods applied to the monitoring of the enzymatic synthesis of amipicilin. Chemom. Intell. Lab. Syst. 2008, 90, 169–177. 10.1016/j.chemolab.2007.09.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








