Abstract
Vitamin D deficiency is a serious global health problem. Edible mushrooms are a good source of vitamin D for human health. The objective of this experiment was to investigate the efficiency of converting its precursor ergosterol to vitamin D2 in shiitake mushroom (Lentinus edodes) and oyster mushroom (Pleurotus ostreatus) powder in ethanol suspension under ultraviolet (UV) irradiation. UV irradiation conditions were optimized for several parameters, such as material form, wavelength, wavelength combination, and exposure time. Under the optimal conditions, UV irradiation increased the concentrations of vitamin D2 from undetectable to 40.59 ± 1.16 μg/g (dw) in dry shiitake mushroom powder and to 677.28 ± 40.42 μg/g (dw) (an approximately 16.69-fold increase) in ethanol suspension. The concentration of vitamin D2 increased from undetectable to 23.71 ± 5.72 μg/g (dw) in the dry oyster mushroom powder upon UV irradiation, whereas UV irradiation increased the concentration to 275.32 ± 48.45 μg/g (dw) (an approximately 11.61-fold increase) in the ethanol suspension. Comparing the effects of varying combinations of wavelengths showed that irradiation with UV-A, UV-C, or a combination of both is more effective than UV-B irradiation. In addition, the increase in vitamin D2 in shiitake mushrooms irradiated by UV-C was time-dependent, that is, dose-dependent. Nevertheless, the increase rates decreased with time. The concentration of ergosterol decreased with the increase in vitamin D2, but ergosterol was only partially converted to vitamin D2, whereas most of the ergosterol was probably UV-degraded. Exposure to ultraviolet light in ethanol suspension offers an effective way to increase the concentration of vitamin D2 and thus improve the nutritional value of edible mushrooms, as well as make them more functional as a source of vitamin D to improve the consumer health.
1. Introduction
Vitamin D deficiency is common all over the world, and the probability of this deficiency occurring is higher in Asian populations than in European populations.1 Currently, more than one billion people worldwide suffer from vitamin D deficiency due to changes in lifestyle, especially those resulting in limited sun exposure, enhanced use of sunscreens and skin protection, and due to people residing in northern latitudes with low ultraviolet (UV) irradiation, especially during winter.2
In nature, the amount of vitamin D2 is very small in wild mushrooms or is not detectable in fresh cultivated mushrooms. Although mushrooms are deficient in vitamin D2, earlier researchers have found them to be a rich source of ergosterol, provitamin D2, which can be converted to vitamin D2 by UV irradiation.3 UV light consists of three subregions of wavelengths: UV-C (190–290 nm), UV-B (290–320 nm), and UV-A (320–400 nm).4 Thus, mushrooms, which are considered a delicacy, are the only non-animal-based food containing vitamin D and ergosterol and are hence the only natural vitamin D source for vegetarians. Moreover, they are highly accepted by vegetarians as well as nonvegetarians.1b,5
Several studies have reported the formation of vitamin D2 in mushrooms following exposure to UV irradiation through conversion of the provitamin ergosterol, including different mushrooms,6 different UV wavelengths,7 different irradiation times,8 different irradiation intensities or doses,9 different irradiation parts,10 and different moisture contents.11
Previous studies have focused mainly on the direct UV irradiation of fresh or dried mushrooms by single-wavelength UV-A, UV-B, or UV-C irradiation. For example, the content of vitamin D2 in the shiitake mushroom was increased after exposure to UV-B at a dose of 25 kJ/m2. Due to the larger exposure area, irradiating slices was a more efficient way to increase the vitamin D2 content than irradiating the gill or pileus of the whole mushrooms.9a Exposure to UV-C also offers an effective means of increasing the content of vitamin D2 in mushrooms.12 The application of UV irradiation to improve the nutritional value of common edible mushrooms making them a readily available source of vitamin D is thus worth exploring.
Therefore, the objective of this study was to examine the effect of UV irradiation on the concentrations of vitamin D2 and ergosterol in shiitake mushroom (Lentinus edodes) and oyster mushroom (Pleurotus ostreatus) powder in ethanol suspension with different irradiation distances and times, different single wavelengths, various combinations of wavelengths, and different sequences of the combined wavelengths.
2. Materials and Methods
2.1. Materials
Fresh shiitake mushroom (L. edodes) and oyster mushroom (P. ostreatus) were purchased from a local supermarket supplied by Keming Co. (Lueyang, Shaanxi, China, 33.33°N and 106.15°E) and were cut off approximately 1 cm from the bottom of the stalks. The anhydrous alcohol was purchased from Aladdin Co (AR grade, Shanghai, China).
2.2. Preparation of Samples
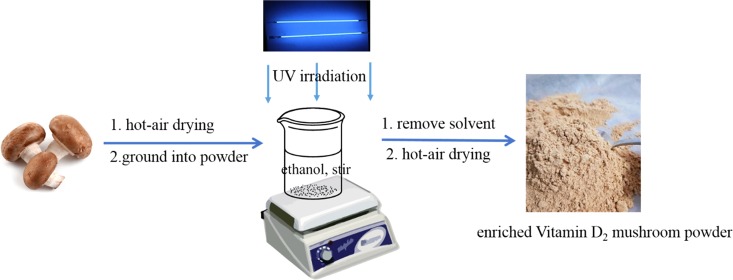
After removal of 1 cm from the bottom of the stalks, the mushrooms were dried by hot air (101-3A dry oven, Taisite Co., Tianjin, China) at 60 °C for about 12–16 h, ground into powder (FW177 mill, Taisite Co., Tianjin, China), and screened by a 40-mesh sieve. The mushrooms powders were then cooled to room temperature and stored at −20 °C.
2.3. UV Irradiation
2.3.1. Vitamin D2 and Ergosterol Concentrations in Shiitake or Oyster Mushroom Powder in Ethanol Solution Exposed to UV-C with Different Irradiation Material Forms
The mushroom dry powder (4.0 g) and anhydrous alcohol (80 mL) were mixed in a 250 mL glass beaker with a material–liquid ratio of 1:20 (g/mL). The mixtures were exposed to UV-C irradiation with an irradiation intensity of 3.29 mW/cm2 (UV Light Meter of ST 512-UVC, Sentry Optronics Co., Taipei, China) at a distance of 30 cm in a UV chamber (the length, width, and height are 40, 50, and 50 cm, respectively) with nine UV-C lamps (40 cm, Philips, TUV PL-L 36W/4p) for 2 h with magnetic stirring at 500 rpm (C-MAG HS7 digital magnetic stirrer, IKA Co., Staufen, Germany). The calculated irradiation dose after a 2 h irradiation period was 236.88 kJ/m2. Then, the solvent was removed entirely (RV 10 Rotary Evaporator, IKA Co., Staufen, Germany), and the obtained solid powder was further dried by hot air at 80 °C (101-3A dry oven, Taisite Co., Tianjin, China) for 2 h. The obtained irradiated solid named suspension samples were stored at −4 °C until analysis. Conversely, the same amounts of shiitake and oyster mushrooms dry powder were directly exposed to UV-C radiation in a beaker for 2 h with stirring every 10 min. The calculated irradiation dose after a 2 h irradiation period was also 236.88 kJ/m2. The obtained irradiated solid named powder samples were stored at −4 °C until analysis. Corresponding, the shiitake and oyster mushrooms powder free of any irradiation were named as the control samples.13
2.3.2. Vitamin D2 and Ergosterol Concentrations in Shiitake or Oyster Mushroom Powder in Ethanol Solution Exposed to UV-C with Different Irradiation Times and Distances
Shiitake or oyster mushroom powder and alcohol were mixed in a 250 mL glass beaker with a material–liquid ratio of 1:20 (g/mL). The mixtures were exposed to UV-C radiation at distances of 30 or 40 cm from UV lamps with irradiation intensities of 3.29 or 2.74 mW/cm2 (ST512-UVC UV Light Meter, Sentry Optronics Co., Taipei, China) in a UV chamber (the length, width, and height are 40, 50, and 50 cm, respectively) with nine UV-C lamps (40 cm, Philips, TUV PL-L 36W/4p) for 5, 10, 20, 30, 60, or 120 min with magnetic stirring at 500 rpm/min. The calculated irradiation doses after 5, 10, 20, 30, 60, and 120 min irradiation periods were 9.87, 19.74, 39.48, 59.22, 118.44, and 236.88 kJ/m2 for a distance of 30 cm and 8.22, 16.44, 32.88, 49.32, 98.64, and 197.28 kJ/m2 for a distance of 40 cm, respectively. Then, the solvent was removed entirely and dried at 80 °C for 2 h.13
2.3.3. Vitamin D2 and Ergosterol Concentrations in Shiitake or Oyster Mushroom Powder in Ethanol Solution Irradiated with Different Single Wavelengths
Shiitake or oyster mushroom powder and alcohol were mixed in a 250 mL glass beaker with a material–liquid ratio of 1:20 (g/mL). The mixtures were exposed to UV-C, UV-B, or UV-A irradiation at a distance of 10 cm from the UV lamps with irradiation intensities of 1.11, 1.67, or 4.81 mW/cm2, respectively (ST512-UVC or ST513-UVAB UV Light Meter, Sentry Optronics Co., Taipei, China) in a UV chamber (the length, width, and height are 30, 27, and 16 cm, respectively) with two UV-A lamps (40 cm, Philips, PL-C 36W, UVA), two UV-B lamps (14 cm, Philips, PL-S9W/01/2p), and two UV-C lamps (40 cm, Philips, TUV PL-L 36W/4p) for 2 h with magnetic stirring at 500 rpm. The calculated irradiation doses after a 2 h irradiation period were 79.92, 120.24, and 346.32 kJ/m2 under irradiation at UV-C, UV-B, and UV-A, respectively. Then, the solvent was removed entirely and dried at 80 °C for 2 h.13
2.3.4. Vitamin D2 and Ergosterol Concentrations in Shiitake or Oyster Mushroom Powder in Ethanol Solution Irradiated with Different Combinations of Wavelengths
Shiitake or oyster mushroom powder and alcohol were mixed in a 250 mL glass beaker with a material–liquid ratio of 1:20 (g/mL). The mixtures were exposed to UV-C + UV-B, UV-C + UV-A, UV-B + UV-A, or UV-C + UV-B + UV-A radiation at a distance of 10 cm from the UV lamps in a UV chamber (the length, width, and height are 30, 27, and 16 cm, respectively) with two UV-A lamps (40 cm, Philips, PL-C 36W, UVA), two UV-B lamps (14 cm, Philips, PL-S 9W/01/2p), and two UV-C lamps (40 cm, Philips, TUV PL-L 36W/4p) for 2 h with magnetic stirring at 500 rpm. Then, the solvent was removed entirely and dried at 80 °C for 2 h.13
2.3.5. Vitamin D2 and Ergosterol Concentrations in Shiitake or Oyster Mushroom Powder in Ethanol Solution Irradiated with Different Sequences of Combined Wavelengths
Shiitake or oyster mushroom powder and alcohol were mixed in a 250 mL glass beaker with a material–liquid ratio of 1:20 (g/mL). The mixtures were exposed to different sequences of combined wavelengths, such as UV-C (2 h) → UV-B (2 h), UV-C (2 h) → UV-A (2 h), UV-B (2 h) → UV-A (2 h), or UV-C (2 h) → UV-B (2 h) → UV-A (2 h), at a distance of 10 cm from the UV lamps in a UV chamber (the length, width, and height are 30, 27, and 16 cm, respectively) with two UV-A lamps (40 cm, Philips, PL-C 36W, UVA), two UV-B lamps (14 cm, Philips, PL-S/W/2 h), and two UV-C lamps (40 cm, Philips, TUV PL-L 36W/4p) for 2 h with magnetic stirring at 500 rpm/min. Then, the solvent was removed entirely and dried at 80 °C for 2 h.13
2.4. Sample Preparation
Extraction of native ergosterol and vitamin D2 without partitioning was conducted according to the simplified direct extraction method of Guan14 and Shao15 with minor modifications.
Samples of mushroom powder (0.2 g) were ultrasonically extracted with 3 mL of ethanol for 15 min at 50 °C with a constant power of 500 W and a fixed frequency of 40 kHz (KQ500E Ultrasonic Cleaner, Kunshan, China) and were centrifuged at 3500 rpm for 10 min (5810R Centrifuge, Eppendorf Co., Hamburger, Germany), and the supernatant (ethanol phase) was transferred into a vial. The mushroom residue was further extracted twice with 3 mL of ethanol. The ethanol phase was then collected and diluted with ethanol to 10 mL. The extract was filtered through a 0.45 μm filter before high-performance liquid chromatography (HPLC) analysis.
2.5. HPLC Analysis of Vitamin D2 and Ergosterol
A volume of 10 μL of filtered sample was injected into an HPLC system equipped with 1260 quat pump, 1290 vial sample, 1260 TCC column oven, and 1260 DAD VL detector (Agilent Co., Santa Clara) and was eluted through a reversed-phase C18 column (Zorbax 300 extend-C18, 4.6 × 100 mm2 (3.5 μm), Agilent Co., Santa Clara). The mobile phase was 0.1% formic acid/acetonitrile, 5:95, at a flow rate of 1 mL/min, and UV detection was performed at 264 and 282 nm. Vitamin D2 and ergosterol were determined by comparing the retention times to those of the standards, and quantification was conducted by using a calibration curve.
The two compounds vitamin D2 and ergosterol were well separated, with retention times of 5.8 and 7.8 min, respectively. Quantification of vitamin D2 and ergosterol was achieved by extrapolation from a standard curve. The eight-point calibration curves used for quantification, which were obtained using a linear fit, ranged from 1 to 200 μg/mL (y = 17.19x – 1.30) and from 10 to 100 μg/mL (y = 10.69x + 4.79) and had r2 values of 0.9999 and 0.9998 for vitamin D2 and ergosterol, respectively. The average recoveries (n = 9) of vitamin D2 and ergosterol were 98.69 and 100.31% with average relative standard deviations (RSDs) (n = 9) of 2.91 and 2.69%, respectively.
2.6. Statistical Analysis
The study was carried out using a completely randomized design. All experiments were repeated three times, and all data are expressed as the mean value ± standard deviation (SD). Data were analyzed using analysis of variance (ANOVA). Duncan’s HSD was used to determine significant differences between treatments. All statistical procedures were conducted using SPSS 16.0 for Windows. A significance level of p < 0.05 was used for all analyses.
3. Results and Discussion
3.1. Effect of UV-C Treatment of Different Material Forms on Vitamin D2 and Ergosterol Contents in Shiitake and Oyster Mushrooms
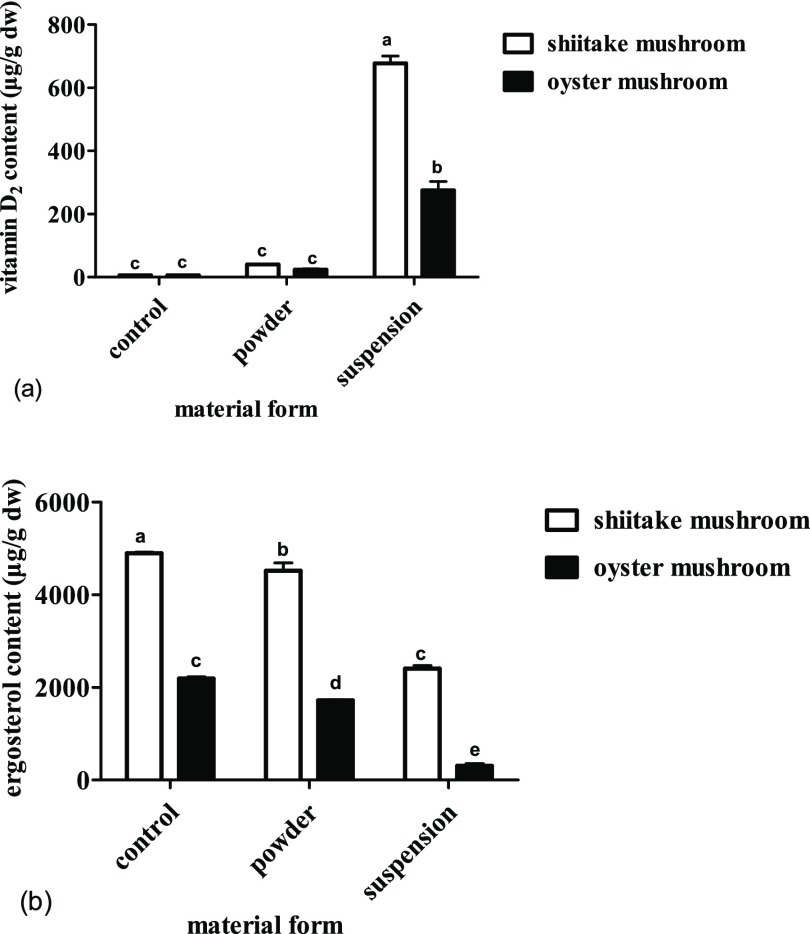
The amounts of vitamin D2 and ergosterol in mushroom powder exposed to UV-C with different material forms are shown in Figure 1. The concentration of vitamin D2 is influenced greatly by the material form, and for both shiitake and oyster mushrooms, irradiation of the dry powder resulted in much lower vitamin D2 content than irradiation of mushrooms in the ethanol suspension.
Figure 1.
Effect of UV irradiation of different material forms on the contents of vitamin D2 and ergosterol in shiitake and oyster mushrooms. (a) The contents of vitamin D2 in shiitake and oyster mushrooms by different treatments. (b) The contents of ergosterol in shiitake and oyster mushrooms by different treatments. The control is the unexposed mushrooms. Bars with different letters are significantly different (ANOVA and Duncan’s HSD, p < 0.05).
The results revealed that UV-C could increase the vitamin D2 contents in shiitake and oyster mushrooms in ethanol suspension, the vitamin D2 increase is about 11 103% in the shiitake mushrooms and about 4506% in oyster the mushrooms (p < 0.05), but induced substantial ergosterol loss, about 51% in the shiitake mushrooms and about 86% in the oyster mushrooms after 2 h of treatment.
For the shiitake mushrooms, the concentration of vitamin D2 in the dry powder form increased (p > 0.05) to 41 μg/g with an increase rate of 20 μg/h. In contrast, the concentration of vitamin D2 remarkably increased (p < 0.05) from undetectable for the unexposed control to 677 μg/g for the ethanol suspension with an increase rate of 338 μg/h. The vitamin D2 concentration in the ethanol suspension is about 17-fold higher than that in the dry powder. However, compared to the control, the ergosterol content decreased (p < 0.05) only 8% for the dry powder and 51% for the ethanol suspension (Figure 1b).
For the oyster mushroom, the concentration of vitamin D2 in the dry powder increased (p > 0.05) to 24 μg/g with an increase rate of 12 μg/h. However, these values were not statistically significant. In contrast, the concentration of vitamin D2 increased (p < 0.05) from undetectable for the unexposed control to 275 μg/g for the ethanol suspension with an increase rate of 138 μg/h. However, compared to the control, the ergosterol content decreased (p < 0.05) only 21% for the dry powder and 86% for the ethanol suspension (Figure 1b).
The optimum moisture content of mushrooms is approximately 70–80%.1b As the moisture level decreased, the specific surface area of the tissue increased, and consequently, the exposure to oxygen and oxidative atmosphere increased, resulting in the oxidation and photodegradation of vitamin D2.16 Our studies showed that irradiation of mushroom in the ethanol suspension resulted in a much higher vitamin D2 content than irradiation of the dry powder, which may be due to the enhanced UV penetration and exposure area, and avoid oxidation and photodegradation of vitamin D2 under stirring in ethanol suspension.
3.2. Effect of UV-C Irradiation Time and Distance on Vitamin D2 and Ergosterol Contents in Shiitake and Oyster Mushrooms
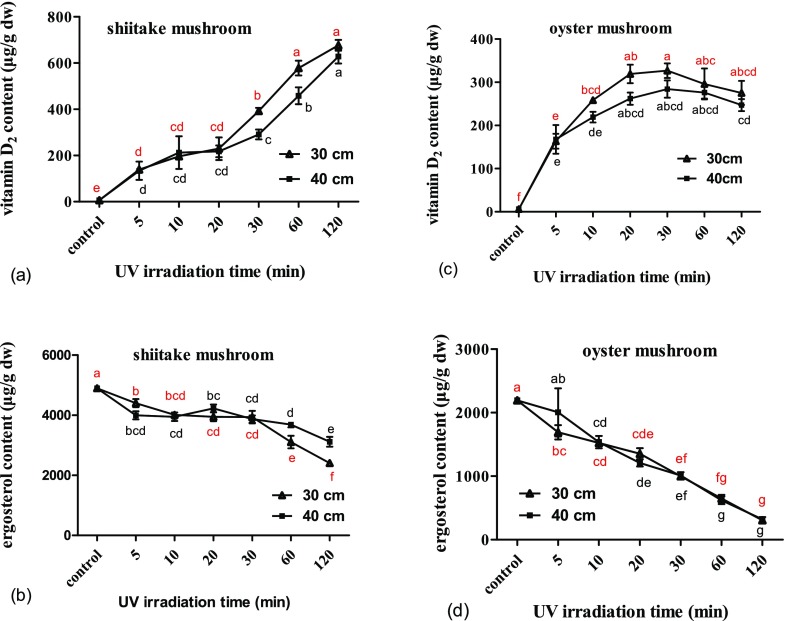
UV-C treatment led to an increased content of vitamin D2 in shiitake and oyster mushrooms (p < 0.05), and the increase is time-dependent, that is, dose-dependent. Nevertheless, the increase rates decreased with time. The ergosterol content in shiitake and oyster mushrooms was reduced (p < 0.05) by UV-C irradiation. Ergosterol content in shiitake mushrooms was less affected than that in oyster mushrooms. The ergosterol content decreased to 49% in shiitake mushrooms and 14% in oyster mushrooms after 2 h of treatment at a distance of 30 cm (p < 0.05) (Figure 2b,d). Irradiation from a distance of 30 cm produced a higher concentration of vitamin D2 in shiitake mushrooms after treatment for 30 and 60 min compared to those produced at a distance of 40 cm, and a lower concentration of ergosterol in shiitake mushrooms after treatment for 60 and 120 min compared to those produced at a distance of 40 cm (p < 0.05). Whereas there is no significant difference in the concentration of vitamin D2 or ergosterol in oyster mushrooms by irradiating from a distance of 30 cm for 5–120 min compared to those produced at a distance of 40 cm (Figure 2a–d).
Figure 2.
Effect of UV irradiation time and distance on vitamin D2 and ergosterol contents of shiitake and oyster mushrooms. (a) Contents of vitamin D2 in shiitake mushroom by different treatments. (b) Contents of ergosterol in shiitake mushroom by different treatments. (c) Contents of vitamin D2 in oyster mushroom by different treatments. (d) Contents of ergosterol in oyster mushroom by different treatments. The control is the unexposed mushrooms. Bars with different letters are significantly different (ANOVA and Duncan’s HSD, p < 0.05).
For the shiitake mushroom at a distance of 30 cm, the vitamin D2 content steadily increased (p < 0.05) from undetectable for the unexposed control to 139 μg/g for 5 min and 677 μg/g for 120 min of exposure, with rates of increase reduced (p < 0.05) gradually from 1671 to 338 μg/h (Figure 2a). Correspondingly, the ergosterol content decreased (p < 0.05) gradually from 90% for 5 min of exposure to 49% for 120 min (Figure 2b).
At a distance of 40 cm, the concentration of vitamin D2 in shiitake mushrooms increased (p < 0.05) from undetectable for the unexposed control to 134 μg/g for 5 min and 629 μg/g for 120 min of exposure, with increase rates steadily reduced (p < 0.05) from 1607 to 314 μg/h (Figure 2a). Correspondingly, the ergosterol content decreased (p < 0.05) gradually from 82% after 5 min of exposure to 64% after 120 min of exposure (Figure 2b).
For the oyster mushroom at a distance of 30 cm, the content of vitamin D2 increased (p < 0.05) from undetectable for the unexposed control to 163 μg/g for 5 min and 275 μg/g for 120 min, with rates of increase reduced (p < 0.05) from 1960 to 138 μg/h (Figure 2c). Correspondingly, the ergosterol content decreased (p < 0.05) gradually from 77% for 5 min of exposure to 14% for 120 min (Figure 2d).
At a distance of 40 cm, the concentration of vitamin D2 in oyster mushrooms increased (p < 0.05) from undetectable for the unexposed control to 168 μg/g for 5 min and 247 μg/g for 120 min, with increase rates steadily reduced (p < 0.05) from 2013 to 124 μg/h (Figure 2c). Correspondingly, the ergosterol content decreased (p < 0.05) from 91% for 5 min to 15% after 120 min of exposure (Figure 2d).
UV-C treatment tended to reduce the ergosterol contents in both shiitake and oyster mushrooms, and the longer the UV-C irradiation was, the more the ergosterol content was reduced. Furthermore, the trend in oyster mushrooms was more evident than that in shiitake mushrooms. For example, the ergosterol content in shiitake mushrooms decreased (p < 0.05) to 49 and 64% with UV-C irradiation at 30 and 40 cm for 120 min, respectively, whereas the ergosterol content in oyster mushrooms decreased (p < 0.05) to 15 and 14% under the same conditions (Figure 2b,d). The difference of ergosterol content between all of the treatments of shiitake mushrooms and oyster mushrooms either at 30 cm or at 40 cm to the unexposed control is significant (p < 0.05). However, there was no difference between the contents following 5 min UV-C treatments of oyster mushrooms at 40 cm to the control (Figure 2b,d). UV-C irradiation of fresh white and brown button mushrooms for 50, 100, and 200 s resulted in no significant decrease in the ergosterol content.14 UV-C irradiation of fresh white button mushrooms resulted in no significant reduction in ergosterol content.17
The ergosterol content in edible mushroom after UV irradiation differed for different strains. The ergosterol content in Agaricus bisporus sharply decreased as the UV-B or UV-C treatment period was prolonged, but the ergosterol content in A. bitorquis and Volvariella volvacea increased as the UV-B or UV-C treatment period was prolonged. For example, the ergosterol content in A. bitorquis, V. volvacea, and shiitake mushroom increased to 502% for 2 h, 126% for 1 h, and 125% for 1 h after UV-B or UV-C treatment compared to unexposed samples.4 An increased ergosterol content in UV-B-treated button mushroom powder and a significant decrease in ergosterol concentrations in all other types of UV-B-treated mushroom powder were also observed.6 In our study, the ergosterol contents in shiitake mushrooms decreased (p < 0.05) from 4898 μg/g for the control to 3997 μg/g for 5 min of exposure and 3949 μg/g for 10 min and then increased (p > 0.05) to 4227 μg/g for 20 min (Figure 2b). However, the 20 min exposure time was not statistically different from the 10 min exposure. Further investigation is needed to understand the change of ergosterol as a result of UV-C irradiation.
Although UV-C irradiation also induced vitamin D2 conversion in shiitake and oyster mushrooms, the increase rates in oyster mushrooms were not as high as those in shiitake mushrooms. However, the increase rates in both shiitake and oyster mushrooms are much higher than those reported by Mau et al.,4 which is due to the higher irradiation intensity and the suspension of ethanol. At irradiation distances of 30 or 40 cm, the increase in vitamin D2 in shiitake mushrooms irradiated by UV-C was time-dependent, that is, dose-dependent; nevertheless, the increase rates decreased with time (Figure 2a,c).
3.3. Effect of UV Irradiation by Different Single Wavelengths on Vitamin D2 and Ergosterol Contents in Shiitake and Oyster Mushrooms
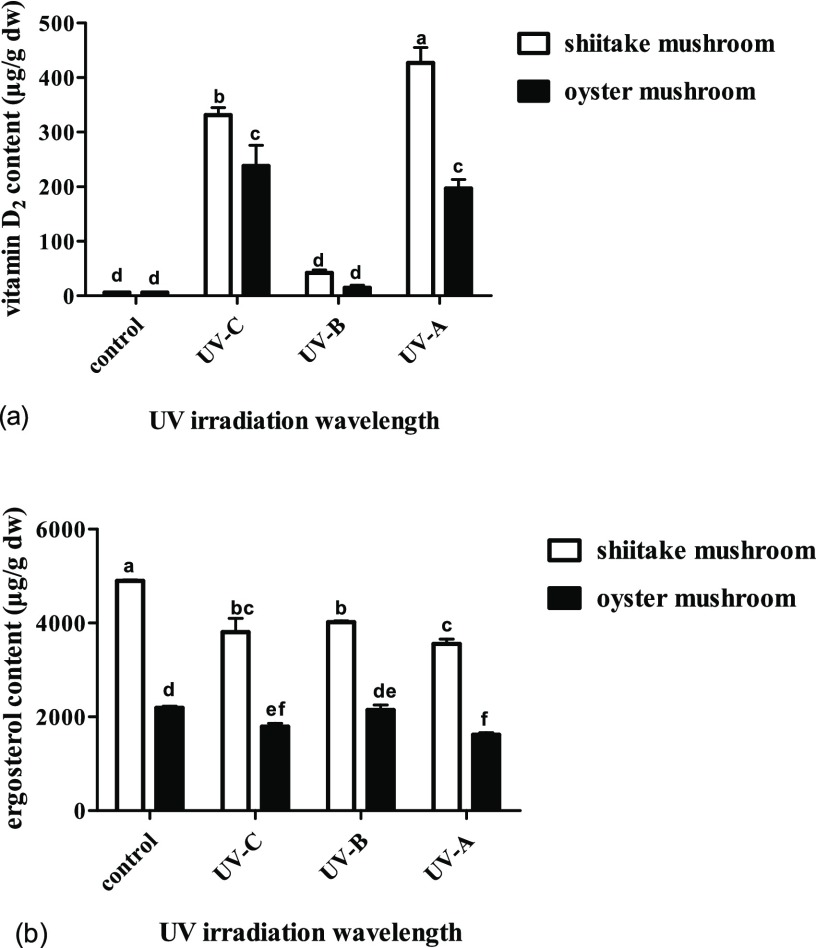
Different irradiation wavelengths have a greater effect on the vitamin D2 content, and the effects on different mushrooms vary. For the shiitake mushrooms, UV-A and UV-C irradiations are both more effective than UV-B irradiation, the content of vitamin D2 shows approximately 10.2- and 7.9-fold increase compared to UV-B, and there is a significant difference between UV-A and UV-C. The content of vitamin D2 increased from undetectable for the unexposed control to 427 μg/g for UV-A (p < 0.05), followed by 332 μg/g for UV-C (p < 0.05) and 42μg/g for UV-B (p > 0.05), with increase rates steadily reduced from 214 μg/h for UV-A to 166 μg/h for UV-C (p < 0.05) and to 21 μg/h for UV-B (p < 0.05) (Figure 3a). Correspondingly, the ergosterol content gradually decreased (p < 0.05) to 82% for UV-B, 78% for UV-C, and 73% for UV-A (Figure 3b).
Figure 3.
Effect of UV irradiation with different single wavelengths on the contents of vitamin D2 and ergosterol in shiitake and oyster mushrooms. (a) The contents of vitamin D2 in shiitake and oyster mushrooms by different treatments. (b) The contents of ergosterol in shiitake and oyster mushrooms by different treatments. The control is the unexposed mushrooms. Bars with different letters are significantly different (ANOVA and Duncan’s HSD, p < 0.05).
For the oyster mushrooms, UV-A and UV-C irradiation are both more effective than UV-B irradiation, the content of vitamin D2 increased approximately 16- and 13-fold compared to UV-B, but there is no significant difference between UV-A and UV-C. The content of vitamin D2 increased from undetectable for the unexposed control to 238 μg/g for UV-C (p < 0.05), followed by 197 μg/g for UV-A (p < 0.05) and the lowest value of 15 μg/g for UV-B (p > 0.05), with increase rates steadily reduced (p < 0.05) from 119 μg/h for UV-C, 99 μg/h for UV-A, and 8 μg/h for UV-B (Figure 3a). Correspondingly, the ergosterol content gradually decreased to 98% for UV-B (p > 0.05), 82% for UV-C (p < 0.05), and 74% for UV-A (p < 0.05) (Figure 3b).
The vitamin D2 content in edible mushrooms could be significantly increased by UV irradiation and that the irradiation wavelength is the most critical factor. Previous findings showed that UV-A, UV-B, or UV-C irradiation treatments all could increase the amount of vitamin D2 in different edible mushrooms. Treatment with shorter wavelengths of light had been shown to lead to the predominance of tachysterol, whereas longer wavelengths increase lumisterol contents.6
High levels of vitamin D2 could be obtained by UV-A irradiation of mushrooms with their gills facing the UV source for 2 h. After a 2 h irradiation period at a calculated dose of 25.2 kJ/m2, the highest value was observed for oyster mushrooms, while the lowest conversion to vitamin D2 was observed for button mushrooms among the four edible mushrooms tested.1b
Not only fresh mushrooms but also dried mushrooms can produce vitamin D2 when they are subjected to UV-B irradiation. Dried mushrooms can produce ergocalciferol under UV-B irradiation. Freeze-dried A. bisporus contained between 42 and 119 μg/g (dw), and hot-air-dried mushrooms contained between 22 and 81 μg/g (dw) vitamin D2.1818 UV-C treatment increased vitamin D2 levels in the caps and stems of both brown and white button mushrooms compared to the control samples.14
3.4. Effect of UV Irradiation by Different Combination Wavelengths on the Contents of Vitamin D2 and Ergosterol in Shiitake and Oyster Mushrooms
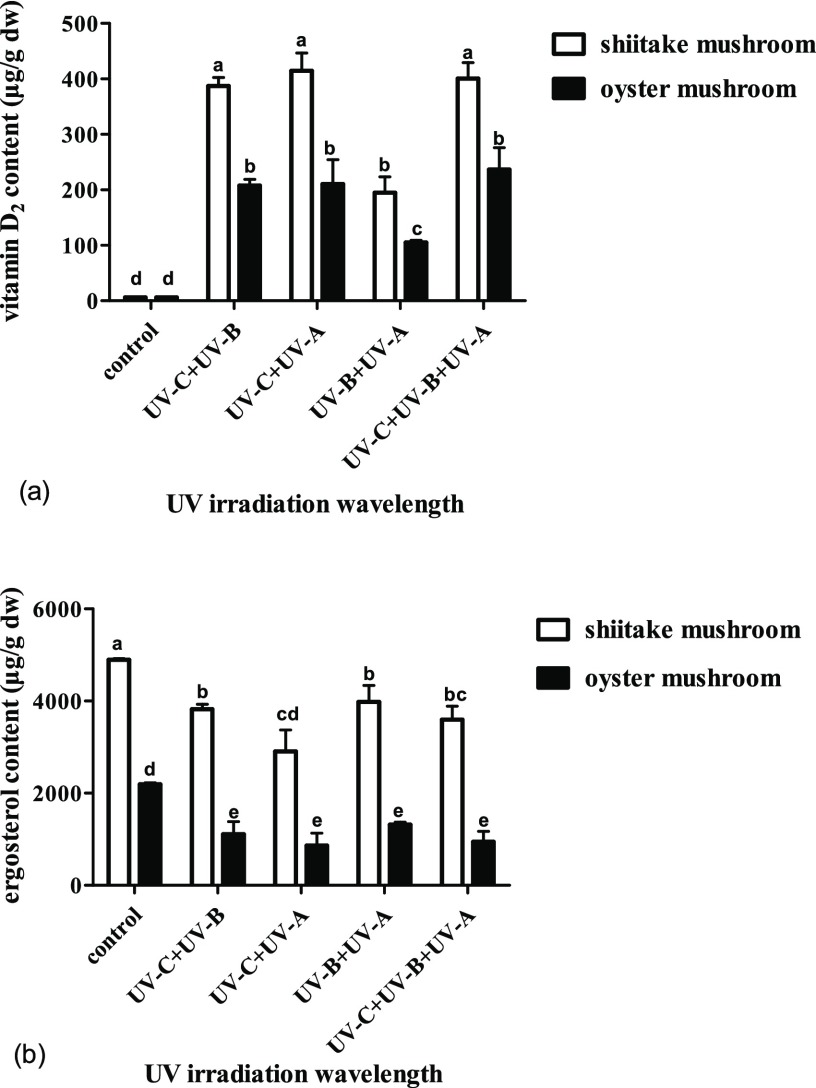
Different combinations of irradiation wavelengths have a great effect on vitamin D2 content, and the effects on varied mushrooms are different. The vitamin D2 content decreased (p < 0.05) without UV-C compared to the treatment with UV-C. For the shiitake mushrooms, the content of vitamin D2 increased (p < 0.05) from undetectable to the highest value of 414 μg/g for UV-C + UV-A, followed by 401 μg/g for UV-C + UV-B + UV-A, 387 μg/g for UV-C + UV-B, and the lowest value of 195 μg/g for UV-B + UV-A, with increase rates gradually decreased from 207 to 200 (p > 0.05), 194 (p > 0.05), and 97 μg/h (p < 0.05). There was no significant difference between UV-C + UV-A, UV-C + UV-B + UV-A, or UV-C + UV-B, but the difference between these three and UV-B + UV-A was significant (Figure 4a). Correspondingly, the ergosterol content steadily decreased (p < 0.05) to 81% for UV-B + UV-A, 78% for UV-C + UV-B, 73% for UV-C + UV-B + UV-A, and 59% for UV-C + UV-A. There was no significant difference for the ergosterol content between UV-B + UV-A, UV-C + UV-B + UV-A, and UV-C + UV-B, but the difference between UV-C + UV-A, UV-B + UV-A, and UV-C + UV-B was significant (Figure 4b).
Figure 4.
Effect of UV irradiation with different combinations of wavelengths on the contents of vitamin D2 and ergosterol in shiitake and oyster mushrooms. (a) Contents of vitamin D2 in shiitake and oyster mushrooms by different treatments. (b) Contents of ergosterol in shiitake and oyster mushrooms by different treatments. The control is the unexposed mushrooms. Bars with different letters are significantly different (ANOVA and Duncan’s HSD, p < 0.05).
For the oyster mushrooms, the content of vitamin D2 remarkably increased (p < 0.05) from undetectable to the highest value of 237 μg/g for UV-C + UV-B + UV-A, followed by 210 μg/g for UV-C + UV-A, 208 μg/g for UV-C + UV-B, and the lowest value of 105 μg/g for UV-B + UV-A, with increase rates gradually decreased from 118 to 105 (p > 0.05), 104 (p > 0.05), and 53 μg/h (p < 0.05). There was no significant difference between UV-C + UV-A, UV-C + UV-B + UV-A, or UV-C + UV-B, but the difference between these three and UV-B + UV-A was significant, which is similar to the shiitake mushrooms (Figure 4a). Correspondingly, the ergosterol content steadily decreased (p < 0.05) to 60% for UV-B + UV-A, 50% for UV-C + UV-B, 43% for UV-C + UV-B + UV-A, and 39% for UV-C + UV-A, and there was no significant difference in their ergosterol content between the four treatments (Figure 4b).
3.5. Effect of UV Irradiation by Different Sequence Combination Wavelengths on Contents of Vitamin D2 and Ergosterol in Shiitake and Oyster Mushrooms
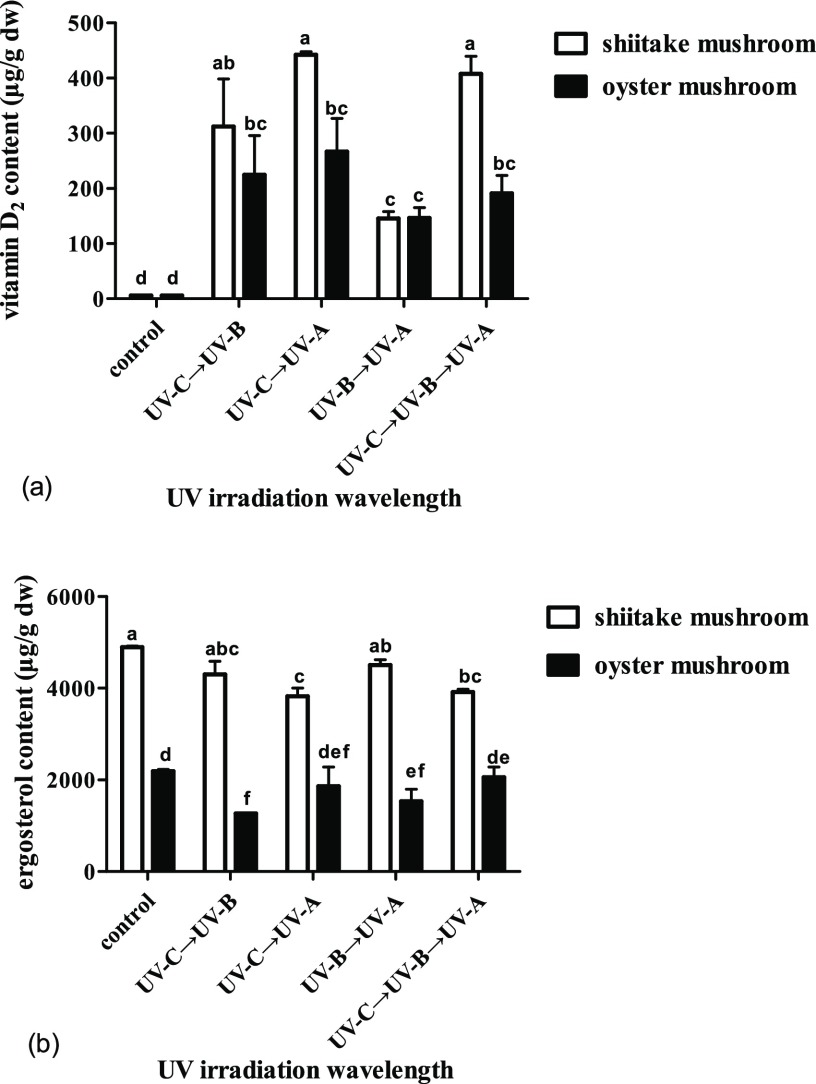
Different sequences of combined wavelengths have a great effect on vitamin D2 content, and the effect is different with varied mushrooms. For the shiitake mushroom, the content of vitamin D2 increased (p < 0.05) from undetectable of dry weight for the unexposed control to the highest of 442 μg/g for UV-C → UV-A, followed by 408 μg/g for UV-C → UV-B → UV-A, 312 μg/g for UV-C → UV-B, and the lowest of 146 μg/g for UV-B → UV-A, with increasing rates steadily decreased from 221 to 204 μg/h (p > 0.05), 156 μg/h (p > 0.05), and 73 μg/h (p < 0.05). There was no significant difference between UV-C → UV-A, UV-C → UV-B → UV-A, and UV-C → UV-B, but the difference between these three and UV-B → UV-A was significant (Figure 5a). Correspondingly, the ergosterol content gradually decreased to 92% for UV-B → UV-A (p > 0.05), 88% for UV-C → UV-B (p < 0.05), 80% for UV-C → UV-B → UV-A (p < 0.05), and 78% for UV-C → UV-A (p < 0.05) (Figure 5b).
Figure 5.
Effect of UV irradiation by different sequence combination wavelengths on contents of vitamin D2 and ergosterol in shiitake and oyster mushroom. (a) Contents of vitamin D2 in shiitake and oyster mushrooms by different treatments. (b) Contents of ergosterol in shiitake and oyster mushrooms by different treatments. The control is the unexposed mushrooms. Bars with different letters are significantly different (ANOVA and Duncan’s HSD, p < 0.05).
For the oyster mushrooms, the content of vitamin D2 increased (p < 0.05) from undetectable for the unexposed control to 267 μg/g for UV-C → UV-A, followed by 225 μg/g for UV-C → UV-B, 191 μg/g for UV-C → UV-B → UV-A, and 147 μg/g for UV-B → UV-A, with increase rates steadily reduced (p > 0.05) from 133 to 112, 96, and 73 μg/h. There was no significant difference between these four treatments (Figure 5a). Correspondingly, the ergosterol content gradually decreased to 94% for UV-C → UV-B → UV-A (p > 0.05), 85% for UV-C → UV-A (p > 0.05), 70% for UV-B → UV-A (p < 0.05), and 58% for UV-C → UV-B, but the difference between UV-C → UV-B and UV-C → UV-B → UV-A was significant. For both vitamin D2 and ergosterol contents, oyster mushrooms and shiitake mushrooms were different in their response to the different sequence combination wavelengths treatments (Figure 5b).
Provitamin D undergoes several reverse photoreactions when absorbing energy.
The transformation mechanism of ergosterol, previtamin D2, vitamin D2, and two byproducts, tachysterol and lumisterol, treated with different single wavelengths and combined wavelengths should be further studied.
4. Conclusions
The results obtained from the present study demonstrated that the concentration of vitamin D2 is enormously influenced by the irradiated form. For both shiitake and oyster mushrooms, the irradiation of samples in ethanol suspension resulted in much higher vitamin D2 content than the irradiation of dry powder samples. The increase in vitamin D2 by UV irradiation was time-dependent and dose-dependent; nevertheless, the increase rate decreased with time. UV-A or UV-C treatment or a combination of both were more effective than UV-B irradiation. The concentration of ergosterol decreased with increasing vitamin D2, but most likely that ergosterol was partially converted to vitamin D2, although the UV light might have degraded most ergosterol. Exposure to ultraviolet light in ethanol suspension offers an effective way to increase the concentration of vitamin D2 and thus improve the nutritional value of edible mushrooms, and make them more functional as a source of vitamin D to improve the consumer health.
This research was supported by the Key Project of Agricultural Science and Technology of Shaanxi Province (2018NY-154), Postdoctoral Program in Shaanxi University of Technology (SLGBH16-04), and Research Project of Shaanxi Provincial Education Department (20JS025 and 18JK0169).
The authors declare no competing financial interest.
References
- a Diamond T.; Levy S.; Smith A.; Day P. Vitamin D deficiency is common in Muslim women living in a Sydney urban community. Bone 2000, 27S, 27 10.1016/S8756-3282(00)80092-7. [DOI] [Google Scholar]; b Jasinghe V. J.; Perera C. O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem. 2005, 92, 541–546. 10.1016/j.foodchem.2004.08.022. [DOI] [Google Scholar]; c Goswami G.; Gupta N.; Goswami D.; Marwaha R. K.; Nikhil T.; Kochupillei N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am. J. Clin. Nutr. 2000, 72, 472–475. 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]; d Yan L.; Prentice A.; Zhang H.; Wang X.; String D. M.; Glden M. M. Vitamin D status and parathyroid hormone concentrations in Chinese women and men from north-east of the People’s Republic of China. Eur. J. Clin. Nutr. 2000, 54, 68–72. 10.1038/sj.ejcn.1600895. [DOI] [PubMed] [Google Scholar]; e Vieth R.; Cole D. E.; Hawker G. A.; Trang H. M.; Rubin L. A. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intakes do not prevent it. Eur. J. Clin. Nutr. 2001, 55, 1091–1097. 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- Holick M. F. The vitamin D deficiency pandemic:a forgotten hormone important for health. Public Health Reviews 2010, 32, 267–283. 10.1007/BF03391602. [DOI] [Google Scholar]
- Mattila P. H.; Piironen V. I.; Uusi-Rauva E. J.; Koivistoinent P. E. Vitamin D contents in edible mushrooms. J. Agric. Food Chem. 1994, 42, 2449–2453. 10.1021/jf00047a016. [DOI] [Google Scholar]
- Mau J. L.; Chen P. R.; Yang J. H. Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J. Agric. Food Chem. 1998, 46, 5269–5272. 10.1021/jf980602q. [DOI] [Google Scholar]
- Mattila P.; Suonpaa K.; Piironen V. Functional properties of edible mushrooms. Nutrition 2000, 16, 694–696. 10.1016/S0899-9007(00)00341-5. [DOI] [PubMed] [Google Scholar]
- Sapozhnikova Y.; Byrdwell W. C.; Lobato A.; Romig B. Effects of UV-B radiation levels on concentrations of phytosterols, ergothioneine, and polyphenolic compounds in mushroom powders used as dietary supplements. J. Agric. Food Chem. 2014, 62, 3034–3042. 10.1021/jf403852k. [DOI] [PubMed] [Google Scholar]
- Roberts J. S.; Teichert A.; H M. T. Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J. Agric. Food Chem. 2008, 56, 4541–4544. 10.1021/jf0732511. [DOI] [PubMed] [Google Scholar]
- Krings U.; Berger R. G. Dynamics of sterols and fatty acids during UV-B treatment of oyster mushroom. Food Chem. 2014, 149, 10–14. 10.1016/j.foodchem.2013.10.064. [DOI] [PubMed] [Google Scholar]
- a KO J. A.; LEE B. H.; LEE J. S.; PARK H. J. Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J. Agric. Food Chem. 2008, 56, 3671–3674. 10.1021/jf073398s. [DOI] [PubMed] [Google Scholar]; b Won D. J.; Kim S. Y.; Jang C. H.; Lee J. S.; Ko J. A.; Park H. J. Optimization of UV irradiation conditions for the vitamin D2-fortified shiitake mushroom (Lentinula edodes) using response surface methodology. Food Sci. Biotechnol. 2018, 27, 417–424. 10.1007/s10068-017-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain P.; Jakobsen J. Dose-response effect of sunlight on vitamin D2 production in Agaricus bisporus mushrooms. J. Agric. Food Chem. 2015, 63, 8156–8161. 10.1021/acs.jafc.5b02945. [DOI] [PubMed] [Google Scholar]
- Calvo M. S.; Babu U. S.; Garthoff L. H.; Woods T. O.; Dreher M.; Hill G.; Nagaraja S. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporosis Int. 2012, 24, 197–207. 10.1007/s00198-012-1934-9. [DOI] [PubMed] [Google Scholar]
- Koyyalamudi S. R.; Jeong S. C.; Song C. H.; Cho K. Y.; Pang G. Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J. Agric. Food Chem. 2009, 57, 3351–3355. 10.1021/jf803908q. [DOI] [PubMed] [Google Scholar]
- Dai-hua H. U.; Zhang J. X. Process optimization of increasing the vitamin D2 content in Flammulina velutipes sporocarp powder by UV irradiation. Chin. Food Sci.Technol. 2018, 43, 89–96. [Google Scholar]
- Guan W.; Zhang J.; Yan R.; Shao S.; Zhou T.; Lei J.; Wang Z. Effects of UV-C treatment and cold storage on ergosterol and vitamin D2 contents in different parts of white and brown mushroom (Agaricus bisporus). Food Chem. 2016, 210, 129–134. 10.1016/j.foodchem.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Shao S.; Hernandez M.; Kramer J. K. G.; Rinker D. L.; Tsao R. Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J. Agric. Food Chem. 2010, 58, 11616–11625. 10.1021/jf102285b. [DOI] [PubMed] [Google Scholar]
- Vayalil P. K.; Elmets C. A.; Katiyar S. K. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis 2003, 24, 927–936. 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- Teichmann A.; Dutta P. C.; Staffas A.; Jägerstad M. Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effects of UV irradiation. LWT - Food Sci. Technol. 2007, 40, 815–822. 10.1016/j.lwt.2006.04.003. [DOI] [Google Scholar]
- Sławińska A.; Fornal E.; Radzki W.; Skrzypczak K.; Zalewska-Korona M.; Michalak-Majewska M.; Parfieniuk E.; Stachniuk A. Study on vitamin D2 stability in dried mushrooms during drying and storage. Food Chem. 2016, 199, 203–209. 10.1016/j.foodchem.2015.11.131. [DOI] [PubMed] [Google Scholar]