Abstract

The integration of light-converting media and microflow chemistry renders new opportunities for high-efficient utilization of solar energy to drive chemical reactions. Recently, we proposed a design of fluorescent fluid photochemical microreactor (FFPM) with a separate light channel and reaction channel, which displays excellent advantages in energy efficiency, flexibility, and general use. However, the limitations of the scalability of the microchannel reactor are still a big challenge to be overcome. Herein, we illustrate the scalability of such an FFPM via a 2n numbering-up strategy by 3D printing technology. Channel shape, number, and interchannel spacing have been optimized, and the serpentine FFPM shows the best scalability with an excellent conversion rate and massive throughput. Reactors with up to eight channels have been fabricated and displayed conversions comparable to that obtained in a single-channel reactor, which provides a feasible strategy and an optimized structure model for batch production of fine chemicals.
Introduction
The photochemical reaction has been widely used in the synthesis of vitamins, small-molecule drugs, spices, and other fine chemicals due to its green, efficient, and sustainable energy supply.1−3 In the recent decade, visible-light catalysis has ushered a significant resuscitation due to the vigorous development of photoredox catalysis and very mild reaction conditions.4−6 As the peak region in the solar spectrum, the visible-light range accounts for about 50% of the total solar radiation energy.7,8 The direct use of solar energy to drive chemical reactions is expected to reduce the dependence on fossil fuels and achieve a world with green, clean, and sustainable energy supply.
As is known, the solar irradiance has always suffered from the shortages of energy fluctuation and low energy density (about 6.6 mol·m–2·h–1), which directly affects the rate of photochemical transformation.7,8 To improve the light irradiation intensity to the reaction medium, many novel materials and devices have been developed, such as photonic crystal,9,10 antireflection film,11−14 parabolic concentrator,15−17 Fresnel lens, and so on.18−20 Except for increasing the light intensity, the emergence of microreactors provides a new strategy to improve the conversion rate of the reaction.21 The small-size microchannel has the characteristics of high-mass and heat-transfer efficiency, high mixing efficiency, uniform illuminance throughout the whole system, and easy integration with different solar concentrators, which makes it the most efficient solution for solar photochemistry.22,23 Noel’s group first developed a kind of luminescent solar concentrator-photomicroreactor (LSC-PM), which integrates the luminescent solar concentrator with microflow chemistry.24,25 The complementary advantages enable visible improvement of photon energy density and monochromaticity received by the reaction medium, which renders high conversion and product purity. Moreover, a diverse set of photon-driven transformations powered by solar irradiation with the use of the improved LSC-PM were demonstrated.26 Recently, based on 3D printing technology, our group developed a novel fluorescent fluid photochemical microreactor (FFPM), which has a separate light channel and a reaction channel.27 This FFPM displays all the advantages of LSC-PM in terms of enhancing the photochemical reaction process and improving the conversion rate. The fluorescent fluid in the light channel can be flexibly controlled and easily replaced, which significantly improves the universality of the photochemical microreactor. Moreover, the recycling of fluorescent dyes also makes the FFPM more efficient, environmentally friendly, and low-cost effective, which is conducive to its widespread application.
However, like all other microreactor technologies, the output of a single reactor is limited, and it is a great challenge to meet the actual requirements for industrial production. Therefore, the scalability of such FFPMs is in high demand. For the photochemical reaction, to avoid the light attenuation effect caused by the Lambert–Beer law and maintain the uniformity of the reaction conditions as much as possible, the numbering-up strategy has been widely applied and proven to be very useful to increase the overall production gradually.28−31 Numbering-up is to drive multiple parallel microchannels by a single pumping system. In this process, the uniformity of flow distribution and light distribution over different channels are vital factors to be considered. Both theoretical simulation and experiments showed that the symmetrical 2n numbering-up strategy has proper flow distribution with the standard deviation of single-phase flow <5% for the multicapillary photomicroreactor system.32 Zhao et al. recently developed a bifurcation/chamber design using 3D printing mold and proved that the deviation of flow distribution was less than 3%, which has been considered as an ideal design for the numbering-up strategy.33
In this work, we mainly reported on the scale-up of FFPMs with the plane sandwich structure via a 2n numbering-up method by the combination of digital design and 3D printing technology (Figure 1). The performance of three kinds of single-channel FFPMs with different shapes (line, semi-arc, and serpentine) was first studied; the serpentine FFPM displays the highest illuminant intensity, the best conversion rate, and the fastest reaction kinetics. It also shows an excellent light distribution with standard deviations less than 10% during its numbering-up to 16 channels. The uniformity of flow distribution and light distribution ensure that the conversion rate of the numbered-up reactor is similar to that of a single-channel reactor, which provides a feasible strategy and an optimized structure model for batch production of fine chemicals.
Figure 1.
Schematic diagram showing the fabrication process of the FFPM device (photograph courtesy of Zhigang Zhu, Copyright 2020).
Results and Discussion
Effect of Channel Width on Light Distribution and Conversion
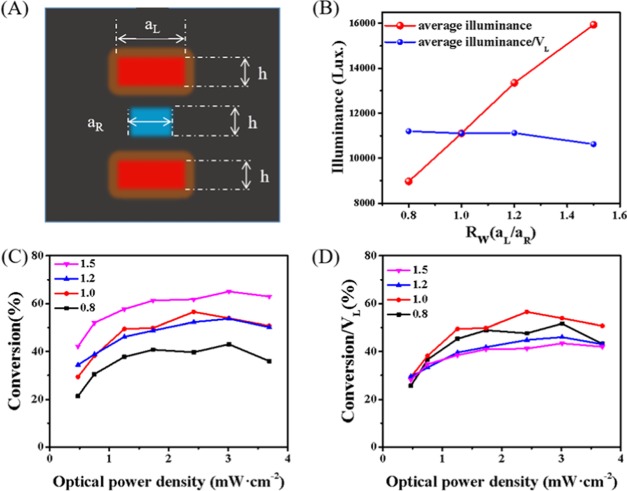
To maximize the photon flux reaching the reaction channel, the structural parameters of the sandwich FFPM are optimized, especially the width ratio (Rw) of the light channel and the reaction channel (Figure 2A). The average illuminance received by the reaction channel at different Rw values is analyzed based on the Monte Carlo ray-tracing method.34−36 As shown in Figure 2B, the average illuminance increases with the increase of Rw, while the illuminance of the unit fluorescent fluid volume is almost unchanged in the range of Rw = 0.8–1.2 and slightly decreases when Rw is greater than 1.2. Subsequently, the effects of different Rw values on the conversion rate are further discussed (Figure 2C). With the Rw value rising from 0.8 to 1.5, the conversion rate increases correspondingly, which is mainly due to the increased photon density caused by the increased volume of the fluorescent fluid. However, to balance the conversion and the cost of fluorescent dyes, we further investigate the effect of per unit volume fluorescent fluid on the conversion rate (Figure 2D). It can be seen that when Rw is 1.0, the reaction conversion rate achieved by per unit volume dye is the highest. Therefore, the Rw values of subsequent reactor designs are all kept at 1.0.
Figure 2.
(A) Schematic representation of the sandwich structure of our FFPM with two light channels and one reaction channel. Rw is defined as the width ratio of the light channel and the reaction channel. (B) Results of illuminance varying with Rw. (C) Effects of Rw on the conversion of DPA at different applied voltages. (D) Effects of Rw on the conversion of DPA under the irradiation of per unit fluorescent fluid volume (VL).
Effect of Channel Shape on Light Distribution and Conversion
Previous studies have shown that the shape and structure of the FFPM remarkably influence the illuminance received by reaction channels and the mass transfer process.27 Here, we designed three kinds of FFPMs with different shapes, i.e., line, semi-arc, and serpentine. The specific structural parameters of each FFPM are listed in Table 1.
Table 1. Specific Structural Parameters of Three FFPMs.
Figure 3 shows the illuminance distribution on the central cross-section of the reaction channel illuminated by fluorescent fluid sources of different shapes. Compared with the line FFPM, the semi-arc and serpentine reactors display significant enhancement in illuminance intensity, which may be due to the larger volume of the fluorescent fluid and the interaction enhancement effect between adjacent parts. The serpentine reactor shows the highest illuminance, which is mainly due to the much closer alignment.
Figure 3.
Illuminance distribution on the central cross-section of the reaction channel illuminated by fluorescent fluid sources of different shapes. The cross-section is 1.5, 5, and 5 mm × 60 mm for the line channel (A), semi-arc channel (B), and serpentine channel (C), respectively.
The performance of the three FFPMs on DPA conversion was evaluated, which is a result of the comprehensive effect of the light field and flow field. Here, a blue LED strip with a peak emission of 440 nm is selected as the irradiation light source, and the flow rate varies from 400 to 50 μL/min (Figure 4A–C). It can be seen that in all reactors, the conversion rate gradually increases with the decrease of flow rate (i.e., the extension of residence time) and the increase of dye concentration. Compared with the linear FFPM, the performance of the semi-arc and serpentine reactors is improved, which is mainly due to their enhanced illuminance (Figure 3) and longer residence time caused by a longer path (Table 1, Figure S1).
Figure 4.
Effect of the three FFPMs on the reaction conversion rate at different flow rates: (A) line, (B) semi-arc, and (C) serpentine (condition: blue LED 440 nm, 22 V, 200 ppm LR305).
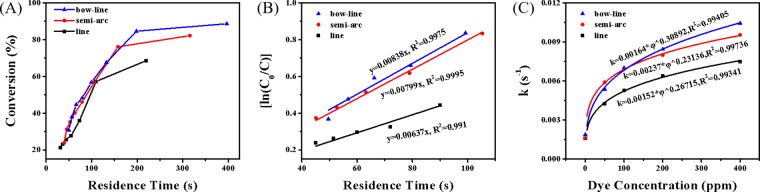
To compare the performance of the three FFPMs more intuitively, Figure 5A shows the curves of conversion versus flow rate under the 400 ppm LR305 irradiation. The serpentine FFPM presents the highest conversion of 94% at 50 μL/min, while the value is 73% and 87% for the linear and semi-arc reactors, respectively. It is worth noting that, when the conversion is fixed, the serpentine reactor allows the highest flow rate, about 2–3 times that of the linear reactor and 1.2–1.3 times that of the semi-arc reactor, which enables much larger throughput. For example, when the conversion is 73%, the flow rate for the serpentine reactor is 150 μL/min, equivalent to the total flow of three linear reactors (each one is 50 μL/min). Furthermore, compared with the 3-line channel reactor, the 1-serpentine reactor can save about 30% of the space and 24% of the fluorescent dye. The serpentine design is more economical, compact, and flexible (Figure 5B).
Figure 5.
(A) Conversion versus flow rates of the three FFPMs under the 400 ppm LR305 irradiation and (B) comparision of 3-line with 1-serpentine on area and space.
Reaction Kinetic Analysis of FFPMs of Different Shapes
The illuminance and fluid velocity will eventually affect the photochemical reaction process in the reaction channel, which is ultimately manifested as the change of apparent reaction dynamics. Figure 6A shows the relationship between the residence time and conversion rates of the three FFPMs under the 200 ppm LR305 irradiation. It can be seen that at the same residence time, the conversion of the serpentine FFPM is much higher than those of the linear and semi-arc reactors, which means it has faster reaction kinetics. The photocatalytic oxidation of DPA has been proven to be a first-order reaction with respect to DPA.37,38 The apparent reaction rate constant k can be directly obtained from the slope of the ln(C0/C)–t plot in Figure 6B. Obviously, the serpentine reactor shows the highest k value and renders much faster transformation. The dye concentration and reactor shape are the key factors that affect the photon flux in the reaction channel and also affect the value of k. In our previous work, an empirical formula of k = a*φb were inferred to quantitatively evaluate the performance of the FFPM, where a and b were defined as structural factors.27Figure 6C shows the variation of k with the dye concentration for the three FFPMs. It can be seen that the serpentine reactor can more effectively play the role of collecting and transmitting light of the fluorescent fluid and speed up the reaction process more directly.
Figure 6.
(A) Relationship between the residence time and conversion rates of the three FFPMs under the 200 ppm LR305 irradiation. (B) First-order dynamics fitting of the three FFPMs. (C) Variation of k with the dye concentration for the three FFPMs.
Reaction Performance of the Serpentine FFPM under a Solar Simulator
To demonstrate the effectiveness of the serpentine FFPM in natural sunlight, the performance of this reactor working under a solar simulator (CEL-HXF300) of 1.0 sun illuminance was further investigated (Figure 7). Under the irradiation of simulated sunlight, the serpentine FFPMs with LR305 all demonstrated excellent performance for the significantly increased fluorescent intensity, which is caused by the ultrahigh intensity of the solar simulator and the broad absorption spectrum of LR305 with respect to the solar light. When the residence time is short, the DPA conversion shows a corresponding increase with the increase of the LR305 concentration from 50 to 200 ppm. The FFPM with 200 ppm LR305 shows the highest conversion of 97% at 80 s, resulting in about 30% improvement compared with 0 ppm. However, an obvious decline in conversion is observed when the LR305 concentration reaches 400 ppm, which may be explained by aggregation-caused quenching.39 With the extension of the residence time, the difference in the conversion rate caused by LR305 concentration gradually disappeared, and there was still an increase of about 8% compared with 0 ppm.
Figure 7.

The performance of the serpentine FFPM under the solar simulator.
Most notably, the FFPM effectively shortened the reaction time to equilibrium, about 80 s, at least 60% shorter than the microreactor without LR305. The short residence time ensures the optimal conversion at high flow rates (about 300 μL/min). These experiments display enormous potential of our designed serpentine FFPM in harnessing sunlight to drive photochemical reactions and enable large throughput to high conversion.
The Numbering-up Design of the Serpentine FFPM
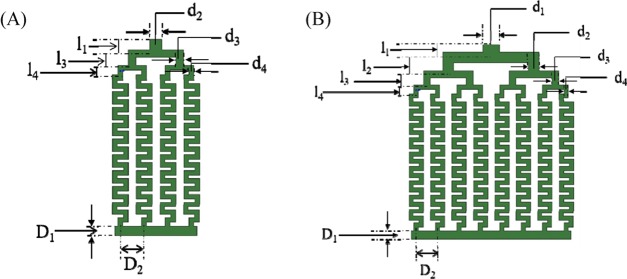
For the numbered-up reactor, the uniformity of the flow distribution is the key factor affecting the final yield and product quality. Here, a design of the bifurcation/chamber was selected in the process of reactor’s numbering-up. Moreover, to ensure the uniformity of each channel and reduce the influence of the distributing zone as much as possible, the length of the reaction channel is kept the same, and the channel diameter and length at different levels in the distributing zone follow Murray’s law40−42 to achieve the best flow distribution. Here we designed 4-channel and 8-channel numbered-up reactors. The schematic diagram of reactor models and the specific parameters are shown in Figure 8 and Table 2, respectively.
Figure 8.
The schematic diagram of numbered-up reactors. (A) 4-channel and (B) 8-channel.
Table 2. Specific Parameters for 4-Channel and 8-Channel Reactorsa.
| device | distributing level 1 | distributing level 2 | distributing level 3 |
|---|---|---|---|
| 8-channel | D1 = k1D2 | D2 = k1D3 | D3 = k1D4 |
| 4-channel | l2 = k2l3 | l3 = k2l4 |
l1 =
2.63 mm, l4 = 3 mm, d4 = 1.5 mm, k1 =  , k2 =
, k2 = 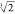 , D1 = 3 mm, D2 = 7.5 mm.
, D1 = 3 mm, D2 = 7.5 mm.
Simulation Study of Light Distribution for the Numbered-up Reactor
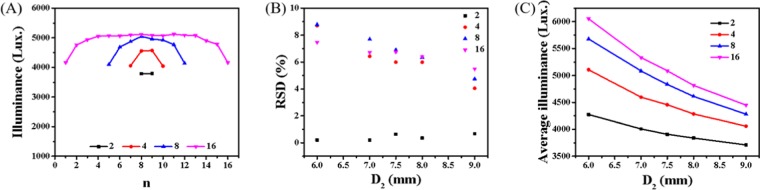
The most unique design of our FFPM is that the dye of LR305 is introduced into the light channel as fluid. One light channel can be regarded as a volume light source, and its emission light is waveguided toward the reaction channels. Consequently, the interchannel spacing and number of channels are the key factors that affect the photon flux received by reaction channels. Here we investigated the light distribution of the numbered-up reactor via Monte Carlo ray-tracing modeling.34−36Figure 9A shows the illuminance intensity received by each reaction channel in the numbered-up reactor. For the interactional enhancement effect between the adjacent light channels, the light intensity fluctuates with the position of the reaction channel and almost shows symmetrical distribution with illuminance gradually decreasing from the center to edges. The two channels at the edge were weaker for the mutual enhancement from only one side. To evaluate the uniformity of the light distribution, a relative standard deviation (RSD) of the light illuminance in n parallel reaction channels was adopted, which is defined as:
where
Figure 9B shows the RSD values of 2-, 4-, 8-, and 16-channel reactor with different interchannel spacings (D2). It can be seen that excellent light uniformity with the RSD value <10% is obtained, which in general is regarded as a reference value. That is to say, the slight difference in illuminance caused by the different positions of the reaction channel can be ignored. Figure 9C shows the average illuminance of the numbered-up reactor, which decreases with the increase of interchannel spacing and increases with the number of channels. Therefore, appropriate channel numbers and interchannel spacing are the key factors to be considered in the numbering process.
Figure 9.
(A) Illuminance intensity received by each reaction channel in the numbered-up reactor (D2 = 7.5 mm). (B) RSD values of 2-, 4-, 8-, and 16-channel reactors with different interchannel spaces. (C) Relationship between the average illuminance of the numbered-up reactors and interchannel spacings.
Reaction Performance of the Numbered-up Reactor
Using 3D printing technology, we fabricated five 4-channel reactors with different D2 values (D2 = 6, 7, 7.5, 8, and 9 mm), respectively. The digital photos of a 4-channel reactor with D2 of 7.5 mm are shown in Figure S2. Figure 10A,B displays the actual reaction performance of these 4-channel reactors without and with LR305, respectively. For the reactors without LR305, the optimal D2 is 7 mm, as a result of the balance between interface scattering and direct irradiation, while for the reactors with 200 ppm LR305, the smaller the interchannel spacing, the stronger the adjacent enhancement effect, so the conversion is generally higher than that of 0 ppm. Furthermore, the conversion increases with the increase of D2 until 7.5 mm and then decreases at 8 mm dramatically. The decline may be caused by the weak illuminance intensity; the sudden recovery at 9 mm may be attributed to the increase of the direct exposure of the light to the side of the reaction channel. Then the best D2 value is 7.5 mm.
Figure 10.
(A) Actual reaction performance of the 4-channel reactors without LR305. (B) The actual reaction performance of the 4-channel reactors with 200 ppm LR305; and (C) conversion–time curves for the numbered-up reactors with different reaction channels.
Finally, numbered-up reactors of 4- and 8-channel reactors were prepared with D2 = 7.5 mm. The conversion rate for numbered-up reactors with 4- and 8-reaction channels showed very good stability, and the conversion rate was almost equal to that of the single-channel reactor (Figure 10C), which demonstrates that the numbered-up reactor designed by us has excellent scalability and can be used for the batch synthesis of fine chemicals such as drugs, spices, and pesticide.
Conclusions
In summary, we proposed a simple and fast strategy to scale up the sandwich-type FFPM by 3D printing technology. As an emerging additive manufacturing method, 3D printing can directly print the designed numbered-up reactors in one process, which greatly simplifies the preparation process and shortens the preparation time. In this work, three single-channel reactors with different shapes were first compared in terms of light intensity, reactant throughput, and reaction kinetics, and the serpentine reactor showed the best conversion rate and largest throughput. Moreover, the effects of the channel number and interchannel spacing on the light intensity and light distribution during the numbering-up were studied, and the optimal parameters were obtained. The final bifurcation/chamber design with up to eight parallel channels with D2 = 7.5 mm shows good conversions similar to those gained in a single-channel device. This work provides a feasible strategy and an optimized structure model for batch production of chemicals. Further scaling can be reached by placing several numbered-up reactors in parallel, and the productivity demands of large-scale chemical processes can be achieved gradually.
Experimental Sections
Materials
Ethyl alcohol (EtOH, >99.7%), acetonitrile (ACN, >99.5%), 1,9-diphenylanthracene (DPA, >98%), methylene blue (MB, >98.5%), and lumogen F red 305 (LR305, >95.0%) were purchased from Aladdin. All the reagents listed above were used without further purification for the 3D printing of the FFPM. The clear photosensitive resin was purchased from Formlabs Tech Co., Ltd., which has excellent transparency and a moderate refractive index (1.51).
Device Design and Fabrication
In this work, all the FFPMs were fabricated directly by the 3D printing technology.43 The computer-aided design of the FFPM with specific parameters was first designed using 3D drawing software and converted to a G-code file via the PreForm 2.5.0 software. Then the structure was printed by a Formlabs Form 2 3D printer (Formlabs Tech Co., Ltd.). After printing, the reactor was immediately transferred into a wash tank for cleaning. The residual resin inside the channels was extracted with airflow and copious ethanol to ensure that all channels were unblocked. Subsequently, the reactor was placed into the FORM Cure for photocurable treatment. Then the fluorescent fluid of LR305 with different concentrations was injected into the light channel for light harvesting and wavelength conversion, and thus resulting in the final FFPM. The designed numbered-up reactors can be formed in one process, which greatly simplifies the preparation process and shortens the preparation time.
Performance Evaluation of Microreactors
To maximize the use of the broad spectrum of sunlight, a combination of fluorescent dye LR305 and photocatalyst MB was selected.24 Because the broad absorption spectrum of LR305 matched perfectly with the solar spectrum and can effectively harvest and convert sunlight into a narrow wavelength emission light, which just covers the maximum absorption of MB (Figure S3). The combination of LR305 and MB can significantly increase the photon flux received by the reactant molecules. The cycloaddition of DPA to the corresponding endoperoxide catalyzed by MB was selected as a model reaction to evaluate the performance of the FFPM devices (Scheme 1).44
Scheme 1. The Singlet Oxygen-Mediated Cycloaddition of 9,10-Diphenylanthracene; the Photocatalyst Is Methylene Blue.

In the experiment, the solutions of 0.1 mM DPA and 0.2 mM MB in acetonitrile were first mixed and then loaded into a 50 mL syringe. The mixture was injected into the reactor system by a syringe pump (Longer Pump Co. Ltd.) at a certain flow rate. The syringe and PFA tubing with a solution outside the reactor were protected with aluminum foil to avoid conversion occurrence outside the reactor. The product was collected with a 4 mL centrifuge tube, and the conversion can be measured with a UV–visible spectrometer (PERSEE, TU1900) by measuring the absorption peak variation of DPA at 372 nm. The conversion η can be calculated according to the concentration change of the DPA with the following equation:
where C0 and C are the initial and residual concentrations of DPA, respectively.
Experiments on the reactor parameter optimization and shape design were carried out using a blue LED strip (5050 model, 24 V); the intensity of the light filed can be controlled by adjusting the working voltage of the LED strip. The relationship between the working voltage and the optical power density is shown in Figure S4. The FFPM was placed in the center of the reactor holder with LED strips wrapped around the inner wall of the cylinder holder so that the reactor can receive uniform light illumination (Figure S5A, in the Supporting information). In the experiment of using simulated sunlight as the light source, the height of the light source was adjusted to ensure 1.0 solar illuminance. The FFPM was placed just under the irradiation center of the light source, on a table covered with aluminum foil to make full use of direct light and reflected light to ensure that both the upper and lower fluorescent fluid could play a role in collecting and converting the light energy (Figure S5B, in the Supporting information).
Acknowledgments
The authors are thankful for the financial support from the National Natural Science Foundation of China (Nos. 51703017 and 21872018), the Financial Grant from the China Postdoctoral Science Foundation (No. 2019T120203).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00511.
Relationship between the residence time and flow rate of the three FFPMs; digital photos of a 4-channel reactor with and without LR305; absorption and emission spectra for the LR305 and MB; relationship between the working voltage of the blue LED strip and the optical power density; experimental devices under blue LED irradiation and solar simulator; specific kinetic experimental data for the three FFPMs; results of illuminance simulation for each reaction channel at different location of a 4-channel reactor (PDF)
Author Present Address
Department of Chemistry, Dalian University of Technology, Dalian 116024, P. R. China.
The authors declare no competing financial interest.
Supplementary Material
References
- Hoffmann N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 2008, 108, 1052–1103. 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]
- Bach T.; Hehn J. P. Photochemical reactions as key steps in natural product synthesis. Angew. Chem., Int. Ed. 2011, 50, 1000–1045. 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]
- Chatani S.; Kloxin C. J.; Bowman C. N. The power of light in polymer science: photochemical processes to manipulate polymer formation, structure, and properties. Polym. Chem. 2014, 5, 2187–2201. 10.1039/C3PY01334K. [DOI] [Google Scholar]
- Cambié D.; Bottecchia C.; Straathof N. J.; Hessel V.; Noel T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 2016, 116, 10276–10341. 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Esser P.; Pohlmann B.; Scharf H. D. The photochemical synthesis of fine chemicals with sunlight. Angew. Chem., Int. Ed. 1994, 33, 2009–2023. 10.1002/anie.199420091. [DOI] [Google Scholar]
- Cambié D.; Noel T. Solar photochemistry in flow. Top. Curr. Chem. 2018, 376, 45. 10.1007/s41061-018-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierer J. J.; David A.; Megens M. M. III-nitride photonic-crystal light-emitting diodes with high extraction efficiency. Nat. Photonics 2009, 3, 163–169. 10.1038/nphoton.2009.21. [DOI] [Google Scholar]
- Wu S.; Xia H.; Xu J.; Sun X.; Liu X. Manipulating luminescence of light emitters by photonic crystals. Adv. Mater. 2018, 30, 1803362 10.1002/adma.201803362. [DOI] [PubMed] [Google Scholar]
- Iwahashi T.; Morishima M.; Fujibayashi T.; Yang R.; Lin J.; Matsunaga D. Silicon nitride anti-reflection coating on the glass and transparent conductive oxide interface for thin film solar cells and modules. J. Appl. Phys. 2015, 118, 145302 10.1063/1.4932639. [DOI] [Google Scholar]
- Kanda H.; Uzum A.; Harano N.; Yoshinaga S.; Ishikawa Y.; Uraoka Y.; Fukui H.; Harada T.; Ito S. Al2O3/TiO2 double layer anti-reflection coating film for crystalline silicon solar cells formed by spray pyrolysis. Energy Sci. Eng. 2016, 4, 269–276. 10.1002/ese3.123. [DOI] [Google Scholar]
- Yao Y.; Lee K. T.; Sheng X.; Batara N. A.; Hong N.; He J.; Xu L.; Hussain M. M.; Atwater H. A.; Lewis N. S.; Nuzzo R. G.; Rogers J. A. Porous nanomaterials for ultrabroadband omnidirectional anti-reflection surfaces with applications in high concentration photovoltaics. Adv. Energy Mater. 2017, 7, 1601992 10.1002/aenm.201601992. [DOI] [Google Scholar]
- Zhang L.; Xiong Z.; Shan L.; Zheng L.; Wei T.; Yan Q. Layer-by-layer approach to (2+1) D photonic crystal superlattice with enhanced crystalline integrity. Small 2015, 11, 4910–4921. 10.1002/smll.201501026. [DOI] [PubMed] [Google Scholar]
- Li G.; Pei G.; Ji J.; Su Y. Outdoor overall performance of a novel air-gap-lens-walled compound parabolic concentrator (ALCPC) incorporated with photovoltaic/thermal system. Appl. Energy 2015, 144, 214–223. 10.1016/j.apenergy.2015.01.112. [DOI] [Google Scholar]
- Skouri S.; Ali A. B. J.; Bouadila S.; Salah M. B.; Nasrallah S. B. Design and construction of sun tracking systems for solar parabolic concentrator displacement. Renewable Sustainable Energy Rev. 2016, 60, 1419–1429. 10.1016/j.rser.2016.03.006. [DOI] [Google Scholar]
- Tian M.; Su Y.; Zheng H.; Pei G.; Li G.; Riffat S. A review on the recent research progress in the compound parabolic concentrator (CPC) for solar energy applications. Renewable Sustainable Energy Rev. 2018, 82, 1272–1296. 10.1016/j.rser.2017.09.050. [DOI] [Google Scholar]
- Clement C. E.; Thio S. K.; Park S. Y. An optofluidic tunable Fresnel lens for spatial focal control based onelectrowetting-on-dielectric (EWOD). Sens. Actuators, B 2017, 240, 909–915. 10.1016/j.snb.2016.08.125. [DOI] [Google Scholar]
- Kuiper S.; Hendriks B. H. W. Variable-focus liquid lens for miniature cameras. Appl. Phys. Lett. 2004, 85, 1128–1130. 10.1063/1.1779954. [DOI] [Google Scholar]
- Ryu K.; Rhee J. G.; Park K. M.; Kim J. Concept and design of modular Fresnel lenses for concentration solar PV system. Sol. Energy 2006, 80, 1580–1587. 10.1016/j.solener.2005.12.006. [DOI] [Google Scholar]
- Neumann M.; Zeitler K. Application of microflow conditions to visible light photoredox catalysis. Org. Lett. 2012, 14, 2658–2661. 10.1021/ol3005529. [DOI] [PubMed] [Google Scholar]
- Colmenares J. C.; Varma R. S.; Nair V. Selective photocatalysis of lignin-inspired chemicals by integrating hybrid nanocatalysis in microfluidic reactors. Chem. Soc. Rev. 2017, 46, 6675–6686. 10.1039/C7CS00257B. [DOI] [PubMed] [Google Scholar]
- Su Y.; Hessel V.; Noël T. A compact photomicroreactor design for kinetic studies of gas-liquid photocatalytic transformations. AIChE J. 2015, 61, 2215–2227. 10.1002/aic.14813. [DOI] [Google Scholar]
- Cambié D.; Zhao F.; Hessel V.; Debije M. G.; Noël T. A leaf-inspired luminescent solar concentrator for energy-efficient continuous-flow photochemistry. Angew. Chem., Int. Ed. 2017, 56, 1050–1054. 10.1002/anie.201611101. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Cambie D.; Hessel V.; Debije M. G.; Noel T. Real-time reaction control for solar production of chemicals under fluctuating irradiance. Green Chem. 2018, 20, 2459. 10.1039/C8GC00613J. [DOI] [Google Scholar]
- Cambié D.; Dobbelaar J.; Riente P.; Vanderspikken J.; Shen C.; Seeberger P. H.; Gilmore K.; Debije M. G.; Noel T. Energy-efficient solar photochemistry with luminescent solar concentrator based photomicroreactors. Angew. Chem., Int. Ed. 2019, 58, 14374–14378. 10.1002/anie.201908553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Zhu Z.; Liu B.; Li C.; Yu Y.; Tao S.; Li T. Fluorescent fluid in 3D-printed microreactors for the acceleration of photocatalytic reactions. Adv. Sci. 2019, 6, 1900583 10.1002/advs.201900583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rawashdeh M.; Yu F.; Nijhuis T. A.; Rebrov E. V.; Hessel V.; Schouten J. C. Numbered-up gas-liquid micro/milli channels reactor with modular flow distributor. Chem. Eng. J. 2012, 207–208, 645–655. 10.1016/j.cej.2012.07.028. [DOI] [Google Scholar]
- Iwasaki T.; Kawano N.; Yoshida J. Radical polymerization using microflow system: Numbering-up of microreactors and continuous operation. Org. Process Res. Dev. 2006, 10, 1126–1131. 10.1021/op060127u. [DOI] [Google Scholar]
- Bula W. P.; Verboom W.; Reinhoudt D. N.; Gardeniers H. J. Multichannel quench-flow microreactor chip for parallel reaction Monitoring. Lab Chip 2007, 7, 1717–1722. 10.1039/b710680g. [DOI] [PubMed] [Google Scholar]
- Nagaki A.; Hirose K.; Tonomura O.; Taniguchi S.; Taga T.; Hasebe S.; Ishizuka N.; Yoshida J. Design of a numbering-up system of monolithic microreactors and its application to synthesis of a key intermediate of valsartan. Org. Process Res. Dev. 2016, 20, 687–691. 10.1021/acs.oprd.5b00414. [DOI] [Google Scholar]
- Su Y.; Kuijpers K.; Hessel V.; Noël T. A convenient numbering-up strategy for the scale-up of gas–liquid photoredox catalysis in flow. React. Chem. Eng. 2016, 1, 73–81. 10.1039/C5RE00021A. [DOI] [Google Scholar]
- Zhao F.; Cambie D.; Janse J.; Wieland E. W.; Kuijpers K. P. L.; Hessel V.; Debije M. G.; Noel T. Scale up of a luminescent solar concentrator based photomicroreactor via numbering-up. ACS Sustainable Chem. Eng. 2018, 6, 422–429. 10.1021/acssuschemeng.7b02687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z. D.; He Y. L.; Cui F. Q. A new modelling method and unified code with MCRT for concentrating solar collectors and its applications. Appl. Energy 2013, 101, 686–698. 10.1016/j.apenergy.2012.07.048. [DOI] [Google Scholar]
- Cheng Z. D.; He Y. L.; Cui F. Q.; Du B. C.; Zheng Z. J.; Xu Y. Comparative and sensitive analysis for parabolic trough solar collectors with a detailed Monte Carlo ray-tracing optical model. Appl. Energy 2014, 115, 559–572. 10.1016/j.apenergy.2013.11.001. [DOI] [Google Scholar]
- Fan M.; You S.; Xia J.; Zheng W.; Zhang H.; Liang H.; Li X.; Li B. An optimized Monte Carlo ray tracing optical simulation model and its applications to line-focus concentrating solar collectors. Appl. Energy 2018, 225, 769–781. 10.1016/j.apenergy.2018.05.067. [DOI] [Google Scholar]
- Donkers R. L.; Mark S. W. Elucidation of the electron transfer reduction mechanism of anthracene endoperoxides. J. Am. Chem. Soc. 2004, 126, 1688–1698. 10.1021/ja035828a. [DOI] [PubMed] [Google Scholar]
- Ciscato L. F. M. L.; Bartoloni F. H.; Bastos E. L.; Baader W. J. Direct kinetic observation of the chemiexcitation wtep in peroxyoxalate chemiluminescence. J. Org. Chem. 2009, 74, 8974–8979. 10.1021/jo901402k. [DOI] [PubMed] [Google Scholar]
- Förster T.; Kasper K. Ein Konzentrationsumschlag der Fluoreszenz. Z. Phys. Chem. 1954, 1, 275–277. 10.1524/zpch.1954.1.5_6.275. [DOI] [Google Scholar]
- Murray C. D. The physiological principle of minimun work. I. The vascular system and the cost of blood volume. Proc. Acad. Natl. Sci. USA 1926, 12, 207–214. 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejan A.; Lorente S. Constructal tree-shaped flow structures. Appl. Therm. Eng. 2007, 27, 755–761. 10.1016/j.applthermaleng.2006.10.008. [DOI] [Google Scholar]
- Stephenson D.; Patronis A.; Holland D. M.; Lockerby D. A. Generalizing Murray’s law: An optimization principle for fluidic networks of arbitrary shape and scale. J. Appl. Phys. 2015, 118, 174302 10.1063/1.4935288. [DOI] [Google Scholar]
- Au A. K.; Huynh W.; Horowitz L. F.; Folch A. 3D-printed microfluidics. Angew. Chem., Int. Ed. 2016, 55, 3862–3881. 10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitre S. P.; McTiernan C. D.; Vine W.; DiPucchio R.; Grenier M.; Scaiano J. C. Visible-light actinometry and intermittent illumination as convenient tools to study Ru(bpy)3Cl2 mediated photoredox transformations. Sci. Rep. 2015, 5, 16397 10.1038/srep16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.