Abstract
Droplet-based microfluidics have emerged as an important tool for diverse biomedical and biological applications including, but not limited to, drug screening, cellular analysis, and bottom-up synthetic biology. Each microfluidic water-in-oil droplet contains a well-defined biocontent that, following its manipulation/maturation, has to be released into a physiological environment toward possible end-user investigations. Despite the progress made in recent years, considerable challenges still loom at achieving a precise control over the content release with sufficient speed and sensitivity. Here, we present a quantitative study in which we compare the effectiveness and biocompatibility of chemical and physical microfluidic release methods. We show the advantages of electrocoalescence of water-in-oil droplets in terms of high-throughput release applications. Moreover, we apply programmable DNA nanotechnology to achieve a segregation of the biochemical content within the droplets for the controlled filtration of the encapsulated materials. We envision that the developed bifunctional microfluidic approach, capable of content segregation and selective release, will expand the microfluidic toolbox for cell biology, synthetic biology, and biomedical applications.
Introduction
High-throughput, robustness, well-defined compartmentalization, precise manipulability, and ease of use render droplet-based microfluidics an interesting tool for diverse applications, including synthetic biology,1,2 chemical synthesis,3 and biomedicine.4 Microfluidic water-in-oil droplets represent individual self-contained compartments with a defined content. The droplet size can be tuned precisely by the geometry of the microfluidic channels and the flow rates of the oil and the aqueous phases. During droplet production, different components can be encapsulated, ranging from nanoscopic molecules to micrometer-sized objects like beads and cells. Moreover, besides serving as passive containers for biocontent, water-in-oil droplets can additionally feature customized functionalities attached to their inner surface. For example, by using cholesterol-tagged DNA5 or gold-linked6 functional surfactants, the inner surface of the droplets can be functionalized with reactive groups and components.
Droplet-based microfluidic technology offers a high level of reproducibility, automation, and manipulability. By integrating electrodes into the microfluidic device, it becomes possible to apply an electric field on the passing droplets. The applied electric field can be used to actuate droplets for sorting based on dielectrophoresis.7,8 Additionally, the electric field can be employed to mix the content of several droplets by fusion based on electrocoalescence.9 Moreover, electrocoalescence of droplets is of particular importance for biomedical applications in which the release of cells or other biocontent from the droplets is necessary.
For the first time, electrocoalescence of droplets with a continuous aqueous phase has been introduced by Fidalgo et al.10 In this study, by applying an electric field across the microfluidic channel, the droplets were forced to coalesce in order to extract their content into a continuous aqueous flow. More recently, electrocoalescence of droplets with the continuous aqueous phase has been optimized in terms of applied voltage, influence of surfactant concentration, droplet diameter, and velocity.11 Moreover, numerical simulation, in combination with parameter optimization, has been implemented to derive rational design guidelines for optimized electrode configurations.12 Despite the progress made in recent years, considerable challenges still loom at many steps of realizing microfluidic release approaches suitable for end-user applications. This especially concerns the difficulty of achieving a precise control over the content release with sufficient speed and sensitivity.
In this study, we present a bifunctional microfluidic on-chip module for the controlled release of the droplet content. This approach is based on a combination of physical and chemical release based on (1) electrocoalescence and (2) programmable DNA nanotechnology that allows for an efficient segregation of the biochemical content within the droplets and enables chemical filtration of the released material into a continuous aqueous phase in a selective manner. To optimize the microfluidic release conditions in terms of release rate, we first performed a quantitative study in which we analyzed the effectiveness of the electric field-mediated release and showed its advantages in comparison to the chemical destabilization method. Moreover, we analyzed the biocompatibility of the electric field-mediated release method by releasing pre-encapsulated cells. Viability and proliferation assays on the released cells revealed that the electric field applied during the release process does not affect cellular functions. We are convinced that the developed microfluidic approach which combines DNA nanotechnology with the electric field-mediated release method can be used as a powerful and versatile tool for the controlled and selective release of the droplet content in droplet-based microfluidics.
Results and Discussion
Release of the Aqueous Content from Surfactant-Stabilized Water-in-Oil Droplets
Aiming to create a bifunctional microfluidic approach capable of content segregation and selective release, our first step was to assemble a microfluidic device and to optimize the release conditions. In the designed device (Figure 1A), the droplet-containing channel and the continuous aqueous phase meet in the release area and are separated again at a Y-junction at the end of the release area. Electrodes are installed on the aqueous phase-facing side of the microfluidic device. By applying a voltage difference between the active and shield electrodes, a nonuniform electric field in both the axial and transverse directions to the flow is created. Note that because of the electrical field an electrothermal flow can be induced; therefore, the temperature-dependent physicochemical properties of the aqueous and oil phases have to be considered.12,13
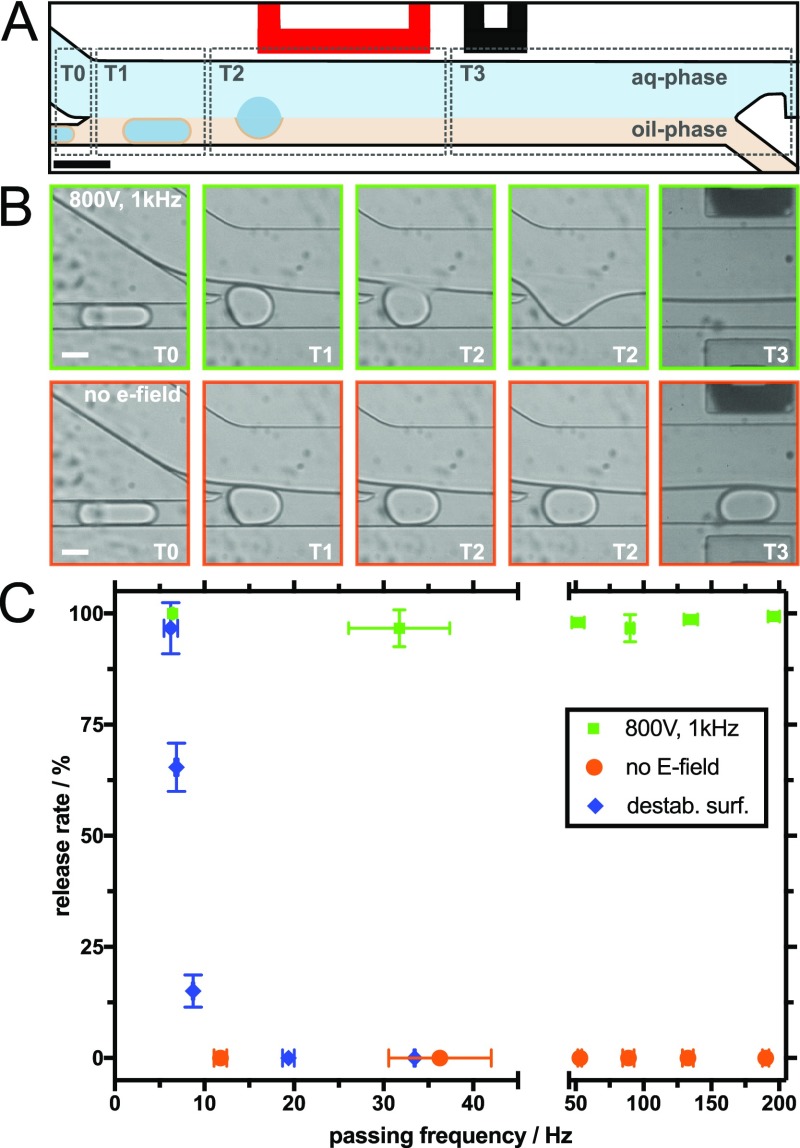
Figure 1.
Controlled release of the aqueous content from water-in-oil droplets. (A) Schematic illustration of the release area of the microfluidic device. T0–T3 indicate different time points during the release process of a single droplet when an electric field is applied. The red-colored electrode represents the active electrode. The scale bar is 80 μm. (B) Representative brightfield images obtained with a high-speed camera show different time points as the droplet passes the release area: electric field-mediated release (800 V, 1 kHz) is shown in the top row (green frames) and release without any intervention (no electric field and no added destabilizing chemicals) in the bottom row (orange frames). The white scale bars are 30 μm. (C) Release rate as a function of release frequency. Green data points mark release triggered by an electric field, orange data points show the same inlet pressures without the electric field, and blue data points depict release using a destabilizing surfactant (1H,1H,2H,2H-perfluoro-1-octanol). Error bars indicate the standard deviation of three independent experiments.
Figure 1A shows a schematic illustration of a sequential timeline (indicated by gray dotted lines outlining time points T0–T3) of droplet fusion with the continuous aqueous phase and the consequent release of the droplet content. At time point T0 (Figure 1B), the separated droplets are in the oil phase channel just before the entrance to the wide channel in which the two continuous phases meet. T1 is the time point where water-in-oil droplets enter the release area of the microfluidic device and come in contact with the continuous aqueous phase (Figure 1B). Because of the laminar flow conditions14,15 and the use of immiscible fluids, a stable fluid interface between the continuous aqueous phase and the droplet-containing oil phase is created. By applying an electric field (800 V, 1 kHz), the droplet fuses with the continuous aqueous phase because of electrocoalescence, causing the droplet contents to be released (T2 in Figure 1B; image with a green outline). In the absence of an electric field, the droplets remain stable and pass down the channel embedded in the oil phase (T2 in Figure 1B; image with an orange outline). It should be mentioned here that upon the contact of the surfactant-stabilized water-in-oil droplet with the continuous aqueous phase, the coalescence is prevented because of the combination of repulsive and attractive stresses, which is called disjoining pressure. A high electric field allows to overcome this pressure barrier and leads to coalescence between the droplet and the continuous aqueous phase.11,16 Final separation between the aqueous and oil phases occurs at the Y-junction (Figure 1A) of the microfluidic release device (T3).
A high release rate and efficiency are desirable for many biological and biomedical applications. Therefore, we set out to quantify the release efficiency (i.e., the proportion of successfully fused droplets) as a function of the release rate (i.e., the number of droplets released in a certain time frame). By counting the number of droplets during a particular passing time, we calculated the passing frequency of the device. Injecting liquids with different pressures into the microfluidic device changes the passing frequency and hence the release rate of the droplets.
Figure 1C shows the summary of the release efficiency at different passing frequencies (6–190 Hz) under different conditions: (a) application of an electric field (physically triggered release), (b) no physical or chemical intervention, and (c) addition of a destabilizing surfactant (chemically triggered release; see the following paragraph). Importantly, the release rate when applying an electric field remained above 95% (green data points), independent of how fast the droplets passed the channels. In contrast, droplet fusion did not occur if the electric field was turned off (orange data points). For detailed information regarding the experimental conditions, see Table S1 (Supporting Information) in which we summarized all inlet pressures and the corresponding results. In several previous studies, a chemical demulsifier has been employed to break the emulsion in order to release the droplet content into the continuous aqueous phase.8,17−19 Therefore, we used the microfluidic release device to compare the performances of the electric field- and chemical-mediated release methods. Toward this end, pure 1H,1H,2H,2H-perfluoro-1-octanol demulsifier was injected into the separation oil channel. Upon mixing with the droplet solution, it destabilizes the droplet water/oil interface by displacing the stabilizing polyethylene glycol (PEG)-based surfactants. It should be noted that because of the higher density of the demulsifier (1.65 g/mL) in comparison to the hydrofluoroether (HFE) 7500 oil (1.60 g/mL), the droplet passing frequency in the release area went down, despite the pressure applied to the inlet channels being identical. Therefore, to get comparable frequencies, the pressure of the aqueous phase inlet had to be adjusted. For example, at a droplet inlet pressure of 100 mbar, the passing frequency went down to 19.38 ± 0.02 Hz compared to 31.75 ± 5.65 Hz using pure HFE 7500 oil. As shown in Figure 1C (blue data points), chemical destabilization can lead to a successful fusion of the droplets at passing frequencies of around 5 Hz. However, at higher frequencies, the efficiency drops significantly. The reduction of efficiency at high frequencies can be attributed to the fact that the destabilizing surfactant needs longer time because of diffusion to displace efficiently the stabilizing surfactant at the droplet oil/water interface. As can be observed in Figure 1C, at frequencies above 5 Hz, the efficiency of electric field-mediated release is significantly higher than that of chemical-mediated release using a destabilizing surfactant. It is important to mention here that the high release efficiency of the field-mediated release is not limited to the maximum flow rate of 190 Hz. Similar efficiency values were observed at passing frequencies of up to 980 Hz. Other advantages of the electric field-mediated release method include: (a) the ability to work without added chemicals, thereby eliminating the risk of chemical contamination of the aqueous phase, a problem that can affect subsequent bioreactions and harm cells20 and (b) the ability to easily control and program the release trigger by switching the electric field on and off. Selective release is impossible using the chemical-mediated release method.
Biocompatibility Assessment: Electric Field-Mediated Release of Chinese Hamster Ovary Suspension Cells
Droplet-based microfluidics can be used for single-cell assays to study, for example, gene expression or to perform immunological assays.21 Encapsulated cells have been shown to be functional over several days in the resource-limited confinements of water-in-oil droplets.22 However, for further assessment and long-term culture of the cells, they must be released into the continuous aqueous phase. Therefore, prior to the development of the DNA nanotechnology-mediated bifunctional microfluidic approach for controlled filtration, we aimed to assess the biocompatibility of the electric field-mediated release. Toward this end, viability and proliferation assays on the released cells have been performed.
Figure 2A (green outline) shows the cell release process, which is highly similar to that of empty droplets: an applied electric field induces the coalescence of the cell-containing droplet with the continuous aqueous phase leading to the release of the encapsulated cells. In contrast, when no electric field is applied, the cell remains encapsulated and continues to travel within the oil phase toward the outlet channel (Figure 2A, orange outline).
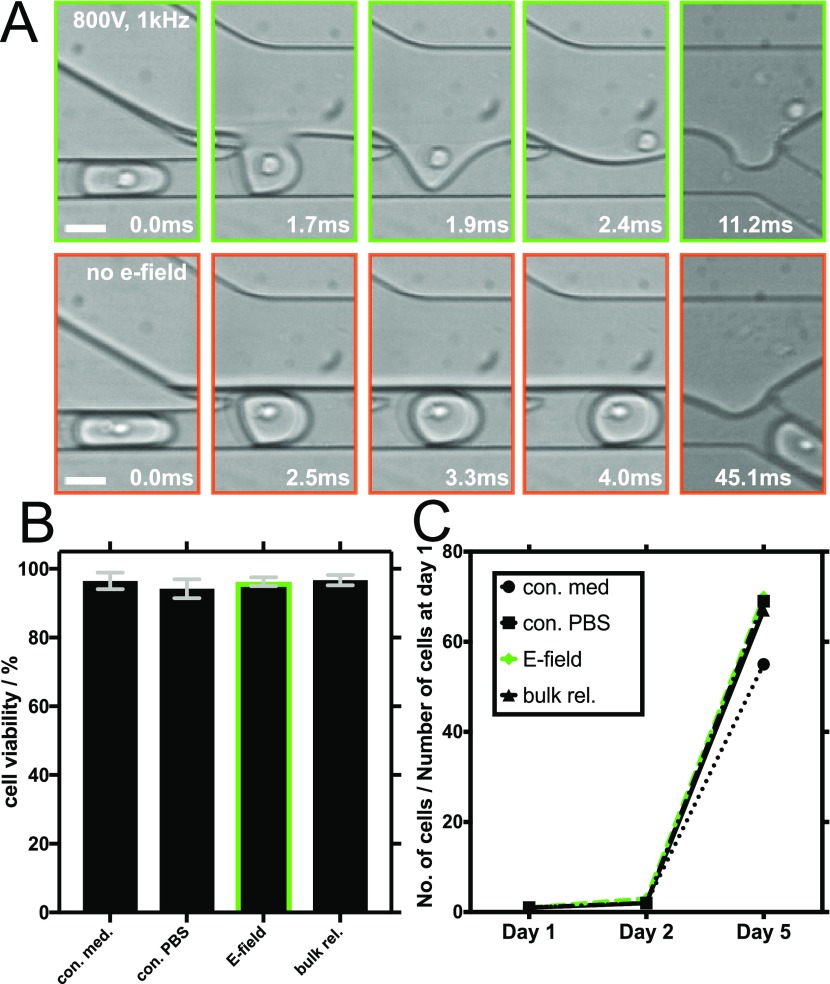
Figure 2.
Electric field-mediated release of encapsulated CHO suspension cells and an assessment of their long-term viability. (A) Representative brightfield images obtained with a high-speed camera showing different time points during cell release: electric field-mediated release (800 V, 1 kHz) is shown in the upper row (green frames) and release without an electric field in the lower row (orange frames). The white scale bars are 30 μm. (B) Results of trypan blue live/dead cell viability assays performed on CHO cells in PBS after their electric field-mediated release (E-field) and on control cells that were left untouched in PBS (con. PBS). Control experiments also included cells cultured in cell medium conditions without employment in microfluidic experiments (con. med) and cells cultured in PBS, encapsulated, and then released in bulk using a destabilizing surfactant (bulk.rel). The green bordered bar shows cell viability after the electric field-mediated release. Error bars indicate the standard deviation of three independent live/dead viability assays of each sample. (C) Summary of 5 day CHO cell proliferation assays following the electric field-mediated release and the control culturing conditions.
As shown in Figure 2B, the released Chinese hamster ovary (CHO) cells showed viability (96.27 ± 1.35%) similar to that of the CHO cells that were stored separately in the culture medium and left untouched during the duration of the encapsulation and release process (96.40 ± 2.56%). We decided to use phosphate-buffered saline (PBS) as a continuous aqueous phase because of its common implementation for short-term cell manipulations. To exclude any influence of buffer conditions on cell viability, we also stored control cells separately in PBS and left these untouched during the duration of the encapsulation and release process. The live/dead assays revealed that the untouched cells cultured in PBS showed viability (94.08 ± 2.64%) similar to that of the cells released by applying an electric field (96.27 ± 1.35%) and similar to that of the cells after chemical-mediated bulk release (96.67 ± 1.30%).
Moreover, to assess a possible long-term effect of the release process on cell viability, 1 × 105 of the released and 5 × 104 of the control cells were cultured in fresh media for 5 days (Figure 2C). The cell counts after 2 and 5 days revealed no difference in cell proliferation. We hence conclude that the electric field we applied during the release process affected neither the cellular viability nor the long-term proliferation of the CHO cells.
DNA-Mediated Segregation of the Biochemical Content within the Droplets for Selective Release
To enhance the specificity of the electric field-mediated release process, we derived a strategy for the chemical segregation of the droplet’s aqueous content. Toward this end, we functionalized the inner droplet surface with a cholesterol-tagged DNA. This serves as a programmable anchoring point for a complementary DNA strand carrying an arbitrary functional group. Note that cholesterol self-assembles at the droplet interface by hydrophobic interaction with the hydrophobic part of the polymer-stabilizing surfactant.5 To demonstrate and visualize the potential of this cholesterol-tagged DNA functionalization, we performed chemical filtration of the fluorescence-labeled DNA strands out of the inner droplet aqueous phase.
Figure 3A shows the representative fluorescence images obtained by confocal microscopy of DNA-functionalized and unfunctionalized water-in-oil droplets. The inner aqueous phase of the droplets contains Cy5-labeled DNA (λex = 649 nm) strands (for DNA sequences, see Materials and Methods). In the absence of the cholesterol-tagged DNA, the Cy5-labeled DNA is distributed homogeneously within the aqueous phase of the droplet (Figure 3A, left). Once the inner droplet interface is functionalized with cholesterol-tagged DNA, the complementary Cy5-labeled DNA binds to it and is visible as a fluorescent ring at the droplet interface (Figure 3A, right).
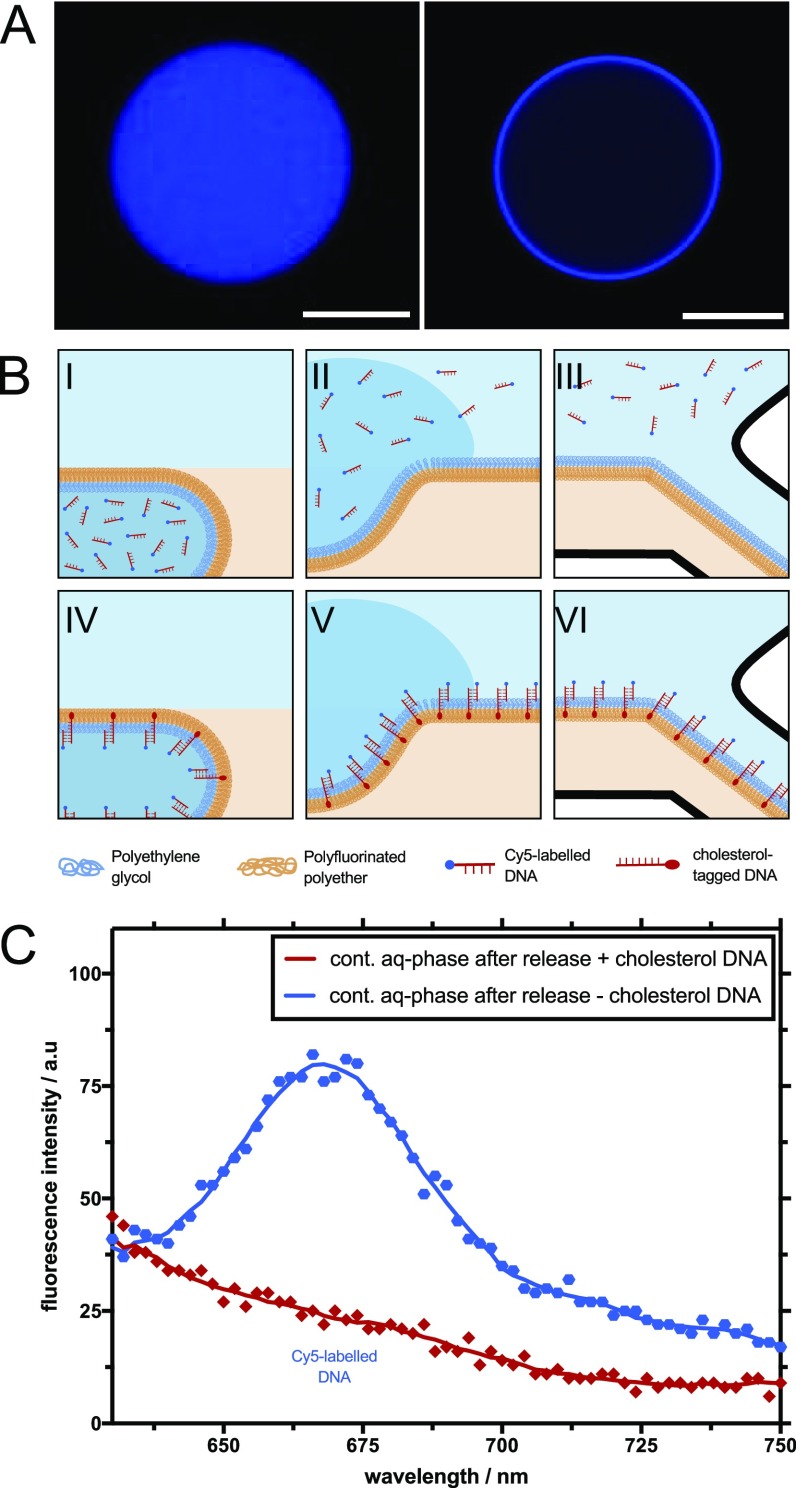
Figure 3.
Electric field-mediated content release from DNA-functionalized water-in-oil droplets. (A) Representative confocal fluorescence images of water-in-oil droplets containing randomly distributed Cy5-labeled DNA only (λex = 596 nm, left image) or Cy5-labeled DNA in the presence of complementary cholesterol-tagged DNA (right image). The scale bars are 20 μm. (B) Schematic illustration of different time points in the selective microfluidic release from unfunctionalized droplets (top row, I–III) and droplets functionalized with cholesterol-tagged DNA (bottom row, IV–VI). (C) Cy5 fluorescence emission spectrum of the continuous aqueous phase obtained after the release process. The blue line shows the continuous aqueous phase of the unfunctionalized droplets after the release, whereas the red line indicates the continuous aqueous phase of the DNA-functionalized droplets. The employment of cholesterol-tagged DNA inside the droplets effectively removed the Cy5-labeled DNA from the aqueous phase.
Figure 3B shows a schematic illustration of the DNA-mediated filtration process inside the microfluidic release device. In the top row, an unfunctionalized droplet containing homogeneously distributed Cy5-labeled DNA passes the oil/water interface in the release area of the microfluidic device (Figure 3B(I)). Because of the application of an electric field, the droplet fuses at the water/oil interface, leading to the release of the encapsulated DNA into the continuous aqueous phase of the device (Figure 3B(II)). Finally, the DNA is separated from the oil phase at the Y-junction of the outlet channels (Figure 3B(III)). In the case of droplets functionalized with cholesterol-tagged DNA (Figure 3B, bottom row), the Cy5-labeled DNA in the inner aqueous phase of the droplet is complementary to the associated DNA strand attached to the droplet interface, leading to the Watson–Crick base pairing of the two strands (Figure 3B(IV)). This means that during the electrocoalescence of the droplet, the inner aqueous phase of the droplet is released into the continuous aqueous phase, whereas the Cy5-labeled DNA remains bound to the cholesterol-tagged DNA at the oil/water interface (Figure 3B(V)). Note that to ensure the chemical separation of the Cy5-labeled DNA from the continuous aqueous phase, it is necessary to create a thin water film in the oil outlet channel (Figure 3B(VI)). Moreover, in order to accelerate the release of nonbound DNA into the continuous aqueous phase, we designed the ion concentration of the continuous aqueous phase to be lower in comparison to the ion concentration within the droplets. In these conditions, the ion concentration gradient might enhance the convection–diffusion of DNA toward the continuous aqueous phase. Moreover, upon the droplet electrocoalescence, spatial charge density would be induced across a conductivity gradient under an external alternating electric field. The electric field might lead to the acceleration of the DNA content release into the continuous aqueous phase.16
Figure 3C shows the fluorescence readout from the aqueous phase released under the same experimental conditions (i.e., same pressures, electric field strength, and volumes of the collected liquid at the outlet channel) from both the unfunctionalized and the cholesterol-tagged DNA-functionalized droplets. It should be noted that the content of the released aqueous phase is diluted by the continuous aqueous phase of the microfluidic device, thus leading to a weaker fluorescence signal in the case of both the functionalized and unfunctionalized droplets. The blue and red data points in Figure 3C indicate the fluorescence signal of the collected content after release from the unfunctionalized and DNA-functionalized droplets. Only in the released continuous aqueous phase of the unfunctionalized droplets, we observed the characteristic fluorescence emission spectrum of the Cy5-labeled DNA. In the released aqueous phase of the DNA-functionalized droplets, we were unable to detect any signal, thus indicating that most of the Cy5-labeled DNA strands were bound to the cholesterol-tagged DNA on the inner surface of the droplets and were sorted into the oil outlet channel. Moreover, to test the specificity of the DNA-mediated filtration, we performed release experiments of two different DNA strands in which only one is complementary to the cholesterol-tagged DNA (Figure S2, Supporting Information). Toward this end, 2 μM of complementary Cy5-labeled and noncomplementary 6-FAM-labeled DNA were encapsulated into the DNA-functionalized droplets and released with the microfluidic device. The fluorescence intensity measurements of the aqueous phase of the released droplet revealed a lower signal for the Cy5-labeled DNA in comparison to the 6-FAM-labeled DNA. It demonstrates the selective binding and filtration of the desired DNA strand.
The droplet-based microfluidic approach for selective release benefits from the large surface-to-volume ratio and short diffusion distances that molecules have to pass inside the picoliter volume droplets in comparison to bulk methods. Thus, it can be expected that DNA-based filtration using microfluidic droplets is more efficient than a similar approach in bulk. To verify this, we performed two control experiments where we compared the efficiency of DNA-based filtration in bulk conditions. In the first, the DNA-containing aqueous solution, consisting of 2 μM complementary Cy5-labeled DNA and noncomplementary 6-FAM-labeled DNA, was layered on top of a surfactant-containing oil phase for 10 min, and in the second, the same DNA-containing aqueous solution passes through the microfluidic release device (without encapsulation into droplets). The fluorescence analysis of the control samples revealed a minor reduction of Cy5 intensity in comparison to the same solution prior to filtration (Figure S3, Supporting Information). This is in strong contrast to the significant reduction of the Cy5 fluorescence intensity, as measured in the aqueous release phase after droplet-based separation (see Figure 3C). These experiments underscore that an enlarged surface-to-volume ratio and a lower diffusion distance within the droplets are essential for effective content filtration.
Summary and Conclusions
In this study, we present a new bifunctional microfluidic approach which is based on programmable DNA nanotechnology and electrocoalescence of droplets for the controlled and selective release of the aqueous phase from water-in-oil droplets. To achieve this, we first quantified the release efficiency of the electric field-mediated release method at different droplet passing frequencies and compared it with a chemical-mediated release method using a destabilizing surfactant. We observed that both of the release methods can work successfully at low passing rates (∼5 Hz). However, a high release efficiency (95%) at faster passing rates can be only achieved by employing the electric field-mediated release method. Cell viability and proliferation assays revealed that the electric field applied during the release process does not affect cellular functions. Finally, we combined the electric field-mediated release function of the microfluidic device with the method of programmable cholesterol-tagged DNA functionalization of the inner droplet surface for an efficient segregation of the biochemical content within the droplets. By doing so, we were able to chemically filter a complementary DNA strand (fluorescence labeled with Cy5) out of a DNA mixture that has been encapsulated within the water-in-oil droplets. We could also show that the large surface area of the microfluidic droplets leads to a high separation efficiency compared to bulk approaches. Relying on the sequence-specific and programmable function of the cholesterol-tagged DNA, a variety of components could be separated from the inner aqueous phase of the droplets. By linking proteins or aptamers to the DNA, the separated components could range from small molecules to macromolecular objects including living cells.5 This is groundbreaking for droplet-based microfluidics, as the secondary products of chemical reactions or contaminants could be easily removed from the aqueous phase. The presented bifunctional microfluidic separation and release method opens up new possibilities for greater control over content release from water-in-oil droplets. Its enormous flexibility and robustness make it a powerful tool for single-cell assays and the detection of immune responses as well as in the field of synthetic biology and drug screening.
Materials and Methods
Microfluidic Device Production
Microfluidic devices were designed using the computer-aided design (CAD) software QCAD-pro (RibbonSoft, Switzerland). For the photolithography process, 2 in. silicon wafers (MicroChemicals, Germany) were covered with a negative photoresist SU8-3025 (MicroChem, USA) and spin-coated (Laurell Technologies Corp., USA) at 2600 rpm to produce a uniform layer of 30 μm thickness. The wafers were then soft-baked on a hot plate at 95 °C for 15 min. Afterward, the CAD design was directly exposed to the photoresist by using the Tabletop Micro Pattern Generator μPG 101 (Heidelberg Instruments, Germany). Writing mode II with the exposure conditions of 50 mW for the output power of the laser, and 20% for the pixel pulse duration were used. For the postexposure bake, the wafer was placed on a hot plate for 1 min at 65 °C and then ramped and held at 95 °C for 5 min. The nonexposed parts of the resist were removed with mr-DEV 600 (MicroChemicals, Germany). The hard bake was carried out in an oven at 150 °C for 15 min. Subsequent soft lithography was performed as previously described.23,24 Briefly, polydimethylsiloxane (PDMS) (Sylgard 184, Dow Corning, USA) was prepared by mixing the oligomer with the polymerization catalyst at a 9:1 (w/w) ratio. The stirred PDMS was poured over the silicon wafer, degassed for several minutes in a desiccator, and cross-linked for 2 h at 65 °C in an oven. After hardening, the PDMS gel was peeled off the wafer, and a biopsy puncher was used (World Precision Instruments, USA) to punch the holes (0.5 mm) for the inlet and outlet polytetrafluoroethylene (PTFE) tubing connections (0.4 × 0.9 mm, Bola, Germany) and electrodes (1.0 mm). Following punching, the holes in PDMS were cleaned with ethanol and pressurized nitrogen gas to remove residual PDMS particles. Prior to the attachment of PDMS to a coverslip, the structured side of PDMS and the clean coverslip (#1, Carl Roth, Germany, 24 × 60 mm) were activated using an oxygen plasma (PVA TePla 100, PVA TePla, Germany; 0.45 mbar, 200 W, 20 s). After activation, PDMS was pressed on the coverslip and heated for at least 1 h at 65 °C. Sigmacote (Sigma-Aldrich, Germany) was applied to the channels of the device to render it hydrophobic. To insert electrodes into the microfluidic chip, the device was heated to 80 °C on a hot plate, and a low-melting-point alloy (51IN-32.5BI-16.5SN, Indium Corporation of America, USA) was melted inside the microchannels designed for the electrodes.25 Electric wires were connected to the melted solder.
Production of Surfactant-Stabilized Water-in-Oil Droplets
To form stable water-in-oil droplets at the flow-focusing T-junction (Figure S1A),26−28 we used an aqueous phase and an oil phase containing 3 wt % of perfluoropolyether–PEG block copolymer fluorosurfactants (PEG-based fluorosurfactants from Ran Biotechnologies, Inc., USA) dissolved in HFE 7500 oil (3M, USA). The flow rates were set to 400 μL/h for both phases. The liquids were injected with syringe pumps (11 PicoPlus Elite, Harvard Apparatus, USA) into the device in 1 mL syringes (Omnifix-F, B. Braun, Germany) connected by a cannula (Sterican, 0.4 × 20 mm2, BL/LB, B. Braun, Germany) and a PTFE tubing (0.4 × 0.9 mm, Bola, Germany). Under these settings, droplets with a diameter of 60 μm were produced at a rate of 1.3 kHz. A high-speed camera (Phantom 7.2, Vision Research, USA) was used to assess the quality of the produced droplets. The recorded videos were adjusted for brightness and contrast and analyzed with ImageJ (NIH, USA).
Release of the Content from Surfactant-Stabilized Water-in-Oil Droplets
To determine the efficiency of the pressure-dependent release rates by electric fields compared to the destabilizing surfactant (1H,1H,2H,2H-perfluoro-1-octanol, Sigma-Aldrich, Germany), the droplets were injected (pneumatic flow controller MFCS-EZ, Fluigent, Germany) at different pressure rates into the microfluidic release device (Figure S1B,C). The applied pressures in the experiments ranged from 50 to 300 mbar for the different inlet channels and were adjusted as follows (for tested conditions, see Table S1). First, to get nicely separated droplets, the inlets of the droplet channel and the separation channel were set to 50 and 45 mbar, respectively. Next, the continuous aqueous phase inlet pressure was adjusted to 70 mbar to get a stable-phase interface in the release channel. To release the content of the droplets into the continuous aqueous phase, an electric field of 800 V at 1 kHz was applied to the electrodes of the microfluidic device. Under these settings, the release of the droplets was very successful even for higher passing frequencies. For the release with a destabilizing surfactant, the separation liquid was exchanged from the pure HFE 7500 oil to a pure destabilizing surfactant solution. To obtain statistics on the droplet release rate and the release frequency, three high-speed camera videos of each condition were recorded and analyzed (Videos S1–S4).
Cell Encapsulation into Surfactant-Stabilized Water-in-Oil Droplets
CHO suspension cells (Public Health England) were cultured with a protein-free medium (EX-CELL ACF DHO Medium, Sigma-Aldrich, Germany) supplemented with 4 mM l-glutamine (Gibco l-glutamine, Thermo Fisher, USA); 4 × 106 cells in 200 μL medium were encapsulated into the water-in-oil droplets.
Biocompatibility Assessment: Electric Field-Mediated Release of CHO Suspension Cells
Previously encapsulated CHO suspension cells were released into the continuous aqueous phase (1× PBS, Gibco, Thermo Fisher, USA). The droplet inlet pressure was set to 400 mbar, and for the separation liquid (pure HFE 7500 oil) and the aqueous phase, we chose 395 and 255 mbar, respectively. The electric field was set to 800 V at 1 kHz. Under these settings, around 410 droplets per second were fused with the continuous aqueous phase to release cells (Video S5).
Cell Viability
To test the possible effects that the electric fields can have on the CHO cells, we prepared four different cell samples consisting of: (1) CHO suspension cells kept in a culture medium without encapsulation; (2) CHO cells stored in PBS buffer without encapsulation; (3) cells stored in a medium, which were encapsulated into droplets and released by the electric field into PBS in the microfluidic device; and (4) cells kept in a medium, which were encapsulated into droplets and released by adding a destabilizing surfactant to the droplets in bulk. The cell numbers in all samples were determined after centrifugation (1000 rpm for 2 min), followed by the resuspension of the pellets in 100 μL fresh PBS buffer. For the viability assay, 10 μL of cells was mixed with 10 μL of trypan blue and counted with a hemocytometer using a microscope (Axiovert 40 CFL, Zeiss, Germany). Triplicates of counts from the same vial were performed. To obtain live/dead percentages, the number of living cells was divided by the total cell number. The remaining cells were seeded in a T-75 flask (Greiner, Germany), and the total cell numbers were obtained 1, 2, and 5 days after seeding.
DNA Functionalization of Surfactant-Stabilized Water-in-Oil Droplets
All DNA sequences were generated and purchased from Integrated DNA Technologies, Inc. (HPLC-purified). The aqueous phase contained equimolar concentrations (2 μM) of the complementary DNA strands in PBS supplemented with 10 mM MgCl2. One strand had a 3′ cholesterol modification (DNA sequence: 5′ TGATGCATAGAAGGAA-CholTEG 3′), another one an added 5′ Cy5 (DNA sequence: 5′ Cy5-TTCCTTCTATGCATCA 3′), and the last one had an added 5′ 6-FAM (DNA sequence: 5′ 6-FAM-TTTTTTTTTTTTTTTTTTTT 3′). The oil phase contained 3 wt % of the fluorosurfactant. Both phases were injected as described for droplet production.
Confocal Fluorescence Microscopy
Confocal imaging was performed with a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems GmbH, Germany) equipped with a white light laser as well as an argon laser using a 40× water immersion objective (HC PL APO 40×/1.10 W, CORR CS2, Leica Microsystems GmbH, Germany). Droplets were collected from the microfluidic chip and sealed in a custom-made observation chamber. For image acquisition, the pinhole aperture was set to 1 Airy unit, and experiments were performed at room temperature. The recorded images were adjusted for optimal brightness and contrast and analyzed with ImageJ.
DNA-Mediated Segregation of the Biochemical Content within the Droplets for Selective Release
The previously generated DNA-functionalized droplets were released in the release area of the microfluidic chip. The droplet inlet pressure was set to 600 mbar, and the pressures for the separation liquid (pure HFE 7500) and the aqueous phase (PBS) were adjusted to 580 and 650 mbar, respectively. Under these settings, approximately 1000 droplets per second were released. The electric field was set to 800 V at 1 kHz.
Fluorescence Spectroscopy
Fluorescence spectroscopy was performed using a Tecan Infinite M200 plate reader (Tecan trading AG). The aqueous phase was collected after the droplets released their contents. Samples of 15 μL of each aqueous phase were deposited in a 384-well plate (Grainer Bio-One, black, flat bottom) for analysis. For the fluorescence intensity scan of the Cy5-labeled DNA, the excitation wavelength was set to 596 nm, and the fluorescence emission spectra were collected from 630 to 800 nm in steps of 2 nm. The excitation wavelength for measuring the 6-FAM-labeled DNA was set to 450 nm, and the fluorescence emission spectra were collected from 485 to 800 nm in steps of 2 nm.
Acknowledgments
The authors acknowledge funding from the Federal Ministry of Education and Research of Germany, grant agreement no. 13XP5073A, PolyAntiBak, and the MaxSynBio Consortium, which is jointly funded by the Federal Ministry of Education and Research of Germany and the Max Planck Society. They also acknowledge the support from SFB 1129 of the German Science Foundation and the VolkswagenStiftung (priority call “Life?”). J.P.S. is the Weston Visiting Professor at the Weizmann Institute of Science and part of the excellence cluster CellNetworks at the University of Heidelberg. K.G. received funding from the European Union Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 792270, by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy via the Excellence Cluster 3D Matter Made to Order (EXC-2082/1-390761711) and the Max Planck Society.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00344.
Passing frequencies and release rates of droplets subjected to different treatments, layout of different microfluidic devices, electric field-mediated selective content release from DNA-functionalized water-in-oil droplets, and fluorescence spectroscopy revealing the low release efficiency of bulk sorting (PDF)
release_droplet_content_destab._surf._high_rate (AVI)
release_droplet_content_destab._surf._low_rate (AVI)
release_droplet_content_800V_1kHz (AVI)
release_droplet_content_no_e-field (AVI)
release_cells (AVI)
The authors declare no competing financial interest.
Supplementary Material
References
- Tan Y.-C.; Hettiarachchi K.; Siu M.; Pan Y.-R.; Lee A. P. Controlled Microfluidic Encapsulation of Cells, Proteins, and Microbeads in Lipid Vesicles. J. Am. Chem. Soc. 2006, 128, 5656–5658. 10.1021/ja056641h. [DOI] [PubMed] [Google Scholar]
- Weiss M.; Frohnmayer J. P.; Benk L. T.; Haller B.; Janiesch J.-W.; Heitkamp T.; Börsch M.; Lira R. B.; Dimova R.; Lipowsky R.; Bodenschatz E.; Baret J.-C.; Vidakovic-Koch T.; Sundmacher K.; Platzman I.; Spatz J. P. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 2018, 17, 89–96. 10.1038/nmat5005. [DOI] [PubMed] [Google Scholar]
- Elvira K. S.; i Solvas X. C.; Wootton R. C. R.; deMello A. J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905–915. 10.1038/nchem.1753. [DOI] [PubMed] [Google Scholar]
- Vladisavljević G. T.; Khalid N.; Neves M. A.; Kuroiwa T.; Nakajima M.; Uemura K.; Ichikawa S.; Kobayashi I. Industrial lab-on-a-chip: design, applications and scale-up for drug discovery and delivery. Adv. Drug Delivery Rev. 2013, 65, 1626–1663. 10.1016/j.addr.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Jahnke K.; Weiss M.; Frey C.; Antona S.; Janiesch J. W.; Platzman I.; Göpfrich K.; Spatz J. P. Programmable Functionalization of Surfactant-Stabilized Microfluidic Droplets via DNA-Tags. Adv. Funct. Mater. 2019, 29, 1808647. 10.1002/adfm.201808647. [DOI] [Google Scholar]
- Platzman I.; Janiesch J.-W.; Spatz J. P. Synthesis of nanostructured and biofunctionalized water-in-oil droplets as tools for homing T cells. J. Am. Chem. Soc. 2013, 135, 3339–3342. 10.1021/ja311588c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baret J.-C.; Miller O. J.; Taly V.; Ryckelynck M.; El-Harrak A.; Frenz L.; Rick C.; Samuels M. L.; Hutchison J. B.; Agresti J. J.; Link D. R.; Weitz D. A.; Griffiths A. D. Fluorescence-activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 2009, 9, 1850–1858. 10.1039/b902504a. [DOI] [PubMed] [Google Scholar]
- Mazutis L.; Gilbert J.; Ung W. L.; Weitz D. A.; Griffiths A. D.; Heyman J. A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. 10.1038/nprot.2013.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagnoni M.; Le Lain G.; Cooper J. M. Electrocoalescence mechanisms of microdroplets using localized electric fields in microfluidic channels. Langmuir 2010, 26, 14443–14449. 10.1021/la101517t. [DOI] [PubMed] [Google Scholar]
- Fidalgo L. M.; Whyte G.; Bratton D.; Kaminski C. F.; Abell C.; Huck W. T. S. From microdroplets to microfluidics: selective emulsion separation in microfluidic devices. Angew. Chem., Int. Ed. 2008, 47, 2042–2045. 10.1002/anie.200704903. [DOI] [PubMed] [Google Scholar]
- Srivastava A.; Karthick S.; Jayaprakash K. S.; Sen A. K. Droplet Demulsification Using Ultralow Voltage-Based Electrocoalescence. Langmuir 2018, 34, 1520–1527. 10.1021/acs.langmuir.7b03323. [DOI] [PubMed] [Google Scholar]
- Schütz S. S.; Beneyton T.; Baret J.-C.; Schneider T. M. Rational design of a high-throughput droplet sorter. Lab Chip 2019, 19, 2220–2232. 10.1039/c9lc00149b. [DOI] [PubMed] [Google Scholar]
- Liu W.; Ren Y.; Chen F.; Song J.; Tao Y.; Du K.; Wu Q. A microscopic physical description of electrothermal-induced flow for control of ion current transport in microfluidics interfacing nanofluidics. Electrophoresis 2019, 40, 2683–2698. 10.1002/elps.201900105. [DOI] [PubMed] [Google Scholar]
- Kenis P. J. A.; Ismagilov R. F.; Whitesides G. M. Microfabrication Inside Capillaries Using Multiphase Laminar Flow Patterning. Science 1999, 285, 83–85. 10.1126/science.285.5424.83. [DOI] [PubMed] [Google Scholar]
- Matosevic S.; Paegel B. M. Stepwise synthesis of giant unilamellar vesicles on a microfluidic assembly line. J. Am. Chem. Soc. 2011, 133, 2798–2800. 10.1021/ja109137s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y.; Ren Y.; Hou L.; Liu W.; Jiang T.; Deng X.; Tao Y.; Jiang H. Electrically controlled rapid release of actives encapsulated in double-emulsion droplets. Lab Chip 2018, 18, 1121–1129. 10.1039/c7lc01387f. [DOI] [PubMed] [Google Scholar]
- Haller B.; Göpfrich K.; Schröter M.; Janiesch J.-W.; Platzman I.; Spatz J. P. Charge-controlled microfluidic formation of lipid-based single- and multicompartment systems. Lab Chip 2018, 18, 2665–2674. 10.1039/c8lc00582f. [DOI] [PubMed] [Google Scholar]
- Göpfrich K.; Haller B.; Staufer O.; Dreher Y.; Mersdorf U.; Platzman I.; Spatz J. P. One-Pot Assembly of Complex Giant Unilamellar Vesicle-Based Synthetic Cells. ACS Synth. Biol. 2019, 8, 937–947. 10.1021/acssynbio.9b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.; Thiele J.; Abdelmohsen L.; Xu J.; Huck W. T. S. Biocompatible macro-initiators controlling radical retention in microfluidic on-chip photo-polymerization of water-in-oil emulsions. Chem. Commun. 2014, 50, 112–114. 10.1039/c3cc46733c. [DOI] [PubMed] [Google Scholar]
- Roach L. S.; Song H.; Ismagilov R. F. Controlling Nonspecific Protein Adsorption in a Plug-Based Microfluidic System by Controlling Interfacial Chemistry Using Fluorous-Phase Surfactants. Anal. Chem. 2005, 77, 785–796. 10.1021/ac049061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausell-Tormos J.; Lieber D.; Baret J.-C.; El-Harrak A.; Miller O. J.; Frenz L.; Blouwolff J.; Humphry K. J.; Köster S.; Duan H.; Holtze C.; Weitz D. A.; Griffiths A. D.; Merten C. A. Droplet-based microfluidic platforms for the encapsulation and screening of Mammalian cells and multicellular organisms. Chem. Biol. 2008, 15, 427–437. 10.1016/j.chembiol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Martin K.; Henkel T.; Baier V.; Grodrian A.; Schön T.; Roth M.; Michael Köhler J.; Metze J. Generation of larger numbers of separated microbial populations by cultivation in segmented-flow microdevices. Lab Chip 2003, 3, 202–207. 10.1039/b301258c. [DOI] [PubMed] [Google Scholar]
- Duffy D. C.; McDonald J. C.; Schueller O. J. A.; Whitesides G. M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- Lisensky G. C.; Campbell D. J.; Beckman K. J.; Calderon C. E.; Doolan P. W.; Ottosen R. M.; Ellis A. B. Replication and Compression of Surface Structures with Polydimethylsiloxane Elastomer. J. Chem. Educ. 1999, 76, 537. 10.1021/ed076p537. [DOI] [Google Scholar]
- Siegel A. C.; Bruzewicz D. A.; Weibel D. B.; Whitesides G. M. Microsolidics: Fabrication of Three-Dimensional Metallic Microstructures in Poly(dimethylsiloxane). Adv. Mater. 2007, 19, 727–733. 10.1002/adma.200601787. [DOI] [Google Scholar]
- Thorsen T.; Roberts R. W.; Arnold F. H.; Quake S. R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. 10.1103/physrevlett.86.4163. [DOI] [PubMed] [Google Scholar]
- Anna S. L.; Bontoux N.; Stone H. A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. 10.1063/1.1537519. [DOI] [Google Scholar]
- Christopher G. F.; Anna S. L. Microfluidic methods for generating continuous droplet streams. J. Phys. D: Appl. Phys. 2007, 40, R319. 10.1088/0022-3727/40/19/r01. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.