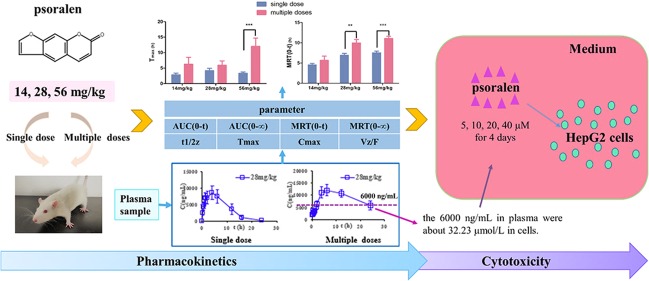
Abstract
Psoralen is a furanocoumarin compound found in many herb medicines and is claimed to contribute to the hepatotoxicity caused by lots of traditional Chinese medicine. So far, there has been no research on the differences in pharmacokinetics of single and repeated dosing of psoralen. Moreover, the research on the cumulative toxicity of low concentration and long-term administration on cells has not been reported. Therefore, this study investigated the pharmacokinetic differences and the accumulated cytotoxicity of psoralen from repeated administration. The study found that after single or repeated administration of psoralen for 3 months at various dosages (14, 28, and 56 mg/kg), the pharmacokinetic parameters of female rats between single dose and repeated dose administration are totally different. Compared with a single administration, multiple administrations increased psoralen’s in vivo exposure, prolonged the peak time, prolonged the half-life of the drug, reduced the drug clearance rate, and prolonged the drug’s stay in the body. HepG2 cells were exposed to low doses (5, 10, 20, or 40 μM) of psoralen for 1, 2, 3, or 4 days. A 20 and 40 μM dose of psoralen did not induced cell death in the 1st day but significantly decreased the cell viability at the 3rd and 4th day of repeated administration, respectively. In addition, multiple administrations of psoralen decreased cell viability due to G2 arrest.
1. Introduction
Fructus Psoraleae, which is known as “Buguzhi” in traditional Chinese medicine, is the dried ripe seeds of Psoralea corylifolia Linn. Its diverse biological activities have been identified, such as antitumor effects,1 estrogen-like activity,2 anti-osteoporosis,3 and antibacterial activity,4,5 etc. It was commonly used to treat various skin diseases, such as psoriasis, vitiligo, and so on. Despite its clinical effects, an increasing number of reports regarding liver damage were published.6,7
A number of components have been isolated and studied from this plant, including coumarins,8−10 flavonoids,11,12 and monoterpene phenols.13 Psoralen is a coumarin compound and the main active ingredient extracted from Psoralea corylifolia L. It was reported that psoralen stimulates osteoblast differentiation and increases its activity via BMP signaling. Psoralen was also demonstrated with anticancer activity and is able to prevent bone metastasis of breast cancer.14−18 In addition, psoralen was exhibited to be able to inhibit CYP2E1 and to induce CYP3A4, which are used for its own metabolism, therefore delaying its clearance from the body.19 Now, psoralen has been commonly used to treat psoriasis and osteoporosis.20−22 Developmental toxicity was also revealed in psoralen in zebrafish embryos or larvae,23 and disturbance of amino acid metabolism was also shown in Sprague Dawley rats with psoralen administration.24,25 Furthermore, psoralen impairs liver regeneration and function compensation in mice, while hepatic toxicity was shown in in HepG2 cells through PERK and ATF6-related ER stress pathways.26 However, the mechanism of liver damage caused by psoralen is still to be elucidated.
The accumulation of a drug should be concerned after long-term repetitive administration.27 Nevertheless, current studies on psoralen focus on its acute toxicity without exploring its long-term pharmacokinetics. In this paper, we investigated the pharmacokinetic and toxicity of psoralen in different doses (14, 28, and 56 mg/kg) and different durations of administration. Meanwhile, the cytotoxicity of psoralen to HepG2 cells was also investigated at low concentrations but long-term exposure.
2. Results
2.1. Method Validation
2.1.1. Specificity
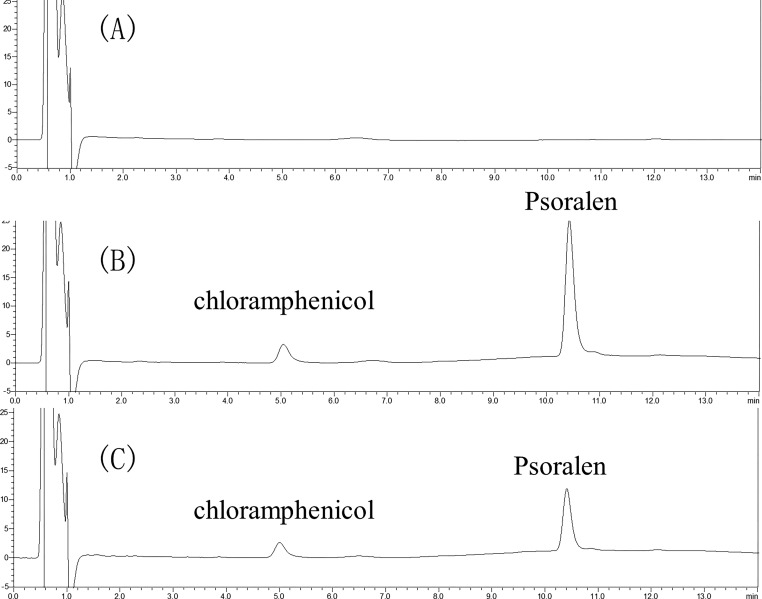
Blank plasma, blank loading, and plasma sample results are shown in Figure 1. The results showed that no interfering peaks were observed in the samples. The IS retention time was 5.03 min, and the psoralen retention time was 10.41 min.
Figure 1.
Chromatograms of the quantification of psoralen in rat plasma samples: (A) blank plasma, (B) blank plasma sample added psoralen, and (C) plasma sample from a rat after the oral administration of psoralen.
2.1.2. Linearity and Sensitivity
The calibration curve of psoralen was linear at concentrations from 0.05 to 20 μg/mL plasma. The linear regression equation for calibration curve is y = 0.604x + 0.098 (r2 = 0.9994) in rat plasma. The LLOD in rat plasma was 20 ng/mL. The LLOQ in rat plasma was 50 ng/mL.
2.1.3. Precision and Accuracy
Psoralen plasma samples at three concentrations (0.2, 2, and 8 μg/mL) were analyzed for their accuracy and precision. The data are shown in Table 1. The precision (RSD) was less than 10%. The intra-day and inter-day accuracies for psoralen were 87.10–106.65 and 87.03–106.47%, respectively. These results indicated that the present method had good precision and accuracy.
Table 1. Intra- and Inter-day Accuracy and Precision of Psoralen in Rat Plasma (n = 5).
| concentration (μg/mL) |
||||
|---|---|---|---|---|
| category | added | founded (mean ± SD) | precision (%) | accuracy (%) |
| intra-day | 0.2 | 0.174 ± 0.006 | 3.68 | 87.10 |
| 2.0 | 2.040 ± 0.031 | 1.50 | 101.98 | |
| 8.0 | 8.532 ± 0.080 | 0.94 | 106.65 | |
| inter-day | 0.2 | 0.174 ± 0.006 | 3.30 | 87.03 |
| 2.0 | 2.009 ± 0.037 | 1.84 | 100.43 | |
| 8.0 | 8.518 ± 0.094 | 1.10 | 106.47 | |
2.1.4. Extraction Recovery
The extraction recoveries of psoralen that were administrated to 0.2, 2, and 8 μg/mL in rat plasma were found to be 75.45, 75.21, and 73.78%, respectively.
2.1.5. Stability
Analyte stability was assessed under various conditions, and all RSD were less than 5%. The results indicated that psoralen, under these conditions, was stable in plasma samples (Table 2).
Table 2. Stability of Psoralen in Rat Plasma (n = 5).
| stability (%, RSD) |
||||
|---|---|---|---|---|
| concentration (μg/mL) | concentration measured (μg/mL) | room temperature (24 h) | long-term (30 days at –20 °C) | three freeze–thaw cycles at –20 °C |
| 0.2 | 0.21 ± 0.04 | 0.08 | 3.62 | 2.83 |
| 2.0 | 1.97 ± 0.02 | 1.63 | 3.25 | 1.17 |
| 8.0 | 8.01 ± 0.17 | 0.17 | 1.84 | 2.17 |
2.2. Pharmacokinetic Study
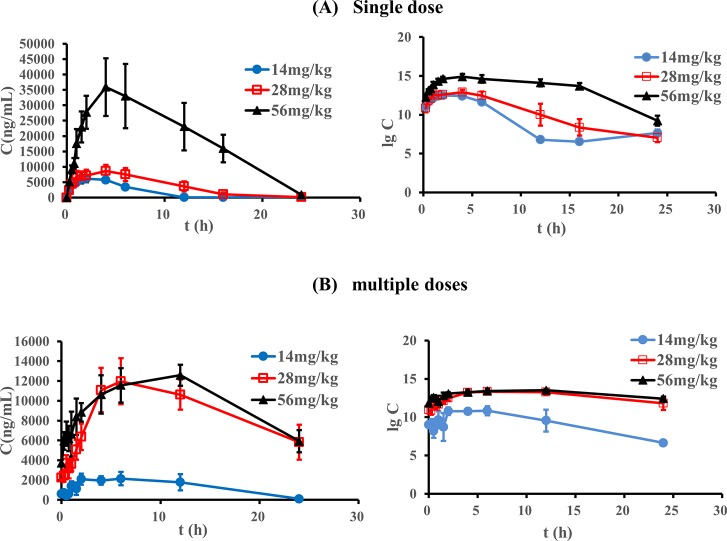
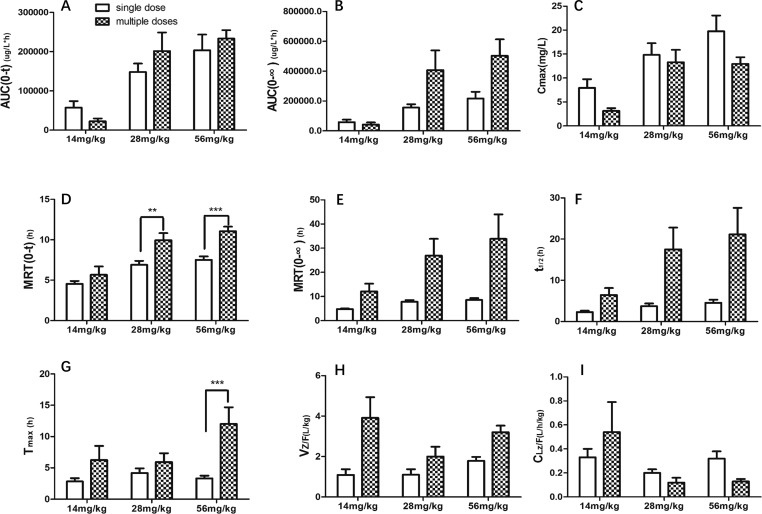
The plasma concentrations of psoralen were determined by the above described UPLC method. The mean plasma concentration–time profiles after oral administration of different doses of psoralen (n = 6) are shown in Figure 2. The main pharmacokinetic parameters in rats are summarized in Tables 3 and 4. The comparison of pharmacokinetic parameters between single and multiple doses of psoralen is shown in Figure 3. The results of two-way ANOVA analysis showed that AUC(0-∞), MRT(0-t), and Cmax are significantly related to the administration period and dose. On the other side, AUC(0-t) and CLZ/F are only significantly associated with the dose, while MRT(0-∞), t1/2, Tmax, and VZ/F are only significantly linked to the administration.
Figure 2.
Average plasma concentration–time curves of psoralen in rats and semi-logarithmic plots (n = 6): (A) single dose groups and (B) multiple doses groups.
Table 3. Pharmacokinetic Parameters of a Single Dose of Psoralen in Rats (n = 6)a.
| psoralen |
||||
|---|---|---|---|---|
| parameter | unit | 14 mg/kg | 28 mg/kg | 56 mg/kg |
| AUC(0-t) | mg/Lah | 57.35 ± 40.48 | 148.33 ± 51.99 | 203.55 ± 97.42 |
| AUC(0-∞) | mg/Lah | 58.31 ± 40.65 | 156.62 ± 53.51 | 216.27 ± 111.91 |
| MRT(0-t) | h | 4.54 ± 0.88 | 6.90 ± 1.20 * | 7.51 ± 1.05 ** |
| MRT(0-∞) | h | 4.76 ± 0.77 | 7.82 ± 1.66 * | 8.58 ± 1.93 * |
| t1/2 | h | 2.29 ± 0.81 | 3.70 ± 1.62 | 4.51 ± 1.88 |
| Tmax | h | 2.83 ± 1.29 | 4.17 ± 1.83 | 3.33 ± 1.03 |
| VZ/F | L/kg | 1.09 ± 0.69 | 1.10 ± 0.66 | 1.79 ± 0.48 |
| CLZ/F | L/h/kg | 0.33 ± 0.17 | 0.20 ± 0.08 | 0.32 ± 0.15 |
| Cmax | mg/L | 7.96 ± 4.39 | 14.82 ± 5.97 | 19.77 ± 7.98 |
*p < 0.05, **p < 0.01 vs 14 mg/kg group.
Table 4. Pharmacokinetic Parameters of Multiple Doses of Psoralen in Rats (n = 6)a.
| psoralen |
||||
|---|---|---|---|---|
| parameter | unit | 14 mg/kg | 28 mg/kg | 56 mg/kg |
| AUC(0-t) | mg/Lah | 22.75 ± 1.40 | 201.61 ± 115.68 | 233.40 ± 51.75 ** |
| AUC(0-∞) | mg/Lah | 42.53 ± 27.50 | 406.77 ± 32.47 | 502.90 ± 269.59 |
| MRT(0-t) | h | 5.67 ± 2.05 | 9.94 ± 2.15 *** | 11.06 ± 1.36 *** |
| MRT(0-∞) | h | 12.09 ± 6.41 | 26.93 ± 16.96 | 33.84 ± 24.87 |
| t1/2 | h | 6.44 ± 3.37 | 17.52 ± 12.91 | 21.15 ± 15.79 |
| Tmax | h | 6.25 ± 4.50 | 5.92 ± 3.47 | 12.00 ± 6.57 *** |
| VZ/F | L/kg | 3.91 ± 2.04 | 2.00 ± 1.17 | 3.20 ± 0.80 |
| CLZ/F | L/h/kg | 0.54 ± 0.51 | 0.12 ± 0.11 | 0.14 ± 0.059 |
| Cmax | mg/L | 3.15 ± 1.15 | 13.30 ± 6.35 | 12.98 ± 3.38 ** |
*p < 0.05, **p < 0.01 vs 14 mg/kg group, ***p < 0.001 vs 28 mg/kg group.
Figure 3.
Comparison of pharmacokinetic parameters between single dose and multiple doses of different doses: (A) AUC(0-t); (B) AUC(0-∞); (C) Cmax; (D) MRT(0-t); (E) MRT(0-∞); (F) t1/2; (G) Tmax ; (H) VZ/F; (I) CLZ/F; **p < 0.01,***p < 0.001.
After a single administration of 14 mg/kg psoralen, the Cmax in blood reached 7,960 ng/mL. With the doses increased to 28 and 56 mg/kg, AUC, MRT, and t1/2 also increased, but only MRT increased significantly. In the multiple doses groups, as the dose increased, AUC(0-t), MRT(0-t), Tmax, and Cmax all increased significantly; more importantly, in the multiple doses groups, the plasma concentrations were more than 6000 ng/mL at 24 h after the last administration of 28 and 56 mg/kg psoralen, while the plasma concentrations of the other groups were less than that in the LLOQ.
The AUC(0-t) values of the 14, 28, and 56 mg/kg psoralen groups after single administration were 57.35 ± 40.48, 148.33 ± 51.99, and 203.55 ± 97.42 mg/L·h, respectively. The AUC(0-t) values after multiple administrations were 22.75 ± 1.40, 201.61 ± 115.68, and 233.40 ± 51.75, respectively. After multiple administrations, the AUC(0-t) of the psoralen increased significantly in the 56 mg/kg administration group compared with the 14 mg/kg administration group, indicating the plasma level in a dose-dependent manner with multiple administrations.
The MRT(0-t) values of the 14, 28, and 56 mg/kg psoralen groups after single administration were 4.54 ± 0.88, 6.90 ± 1.20, and 7.51 ± 1.05, respectively, and the MRT(0-∞) values were 4.76 ± 0.77 , 7.82 ± 1.66, and 8.58 ± 1.93; MRT(0-t) and MRT(0-∞) are both dose-dependent. The MRT(0-t) values after multiple administrations were 5.67 ± 2.05, 9.94 ± 2.15, and 11.06 ± 1.36, respectively. Compared with the 14 mg/kg administration group, there was a significant difference in the MRT(0-t) of 28 and 56 mg/kg administration groups (p < 0.05), indicating that the drug’s residence time in the body after both single administration and multiple administrations extends.
The t1/2 values of the 14, 28 and 56 mg/kg psoralen groups after single administration were 2.29 ± 0.81, 3.70 ± 1.62, and 4.51 ± 1.88 (h), respectively. Meanwhile, the t1/2 values were 6.44 ± 3.37, 17.52 ± 12.91, and 21.15 ± 15.79 (h) after multiple administrations. Comparing the data, it can be seen that after single administration and multiple administrations, the elimination half-life values of psoralen are both dose-independent. It shows that with an increase in the administered dose, the time for the drug to clear in the body has a tendency to prolong.
The Tmax values of 14, 28, and 56 mg/kg psoralen groups after single administration were 2.83 ± 1.29, 4.17 ± 1.83, and 3.33 ± 1.03 (h), respectively. The Tmax values of multiple doses were 6.25 ± 4.50, 5.92 ± 3.47, and 12.00 ± 6.57 (h), respectively. The absorption rates of psoralen in rats after multiple administrations were much slower than those of single administrations; in the meantime, the peak time is prolonged as the dose is increased.
The Cmax values of 14, 28, and 56 mg/kg psoralen groups after single administration were 7.96 ± 4.39, 14.82 ± 5.97, and 19.77 ± 7.98 (mg/L), respectively. The Cmax values after multiple administrations were 3.15 ± 1.15, 13.30 ± 6.35, and 12.98 ± 3.38 (mg/L), respectively. Compared with the 14 mg/kg administration group, the maximum blood concentration of 56 mg/kg in the multiple administrations group increased significantly with the increase in the dosage of psoralen.
2.3. Effects of Psoralen on HepG2 Cells
To investigate the cytotoxicity effects of psoralen in human hepatocytes, the viability of HepG2 cells was detected using MTT assay after being exposed to low doses of psoralen for 1, 2, 3, and 4 days. As shown in Figure 4, in the first two days, 5–40 μM psoralen did not significantly induce cell death but rather showed a tendency to encourage cell proliferation. At the 3rd day, 5, 10, and 40 μM psoralen significantly decreased the cell viability (p < 0.05). At the 4th day, 5, 10, 20, and 40 μM psoralen significantly decreased the cell viability to 82.2 ± 1.2%, 75.7 ± 1.7%, 81.8 ± 2.6%, and 69.1 ± 4.7% of the control (all p < 0.001), respectively.
Figure 4.
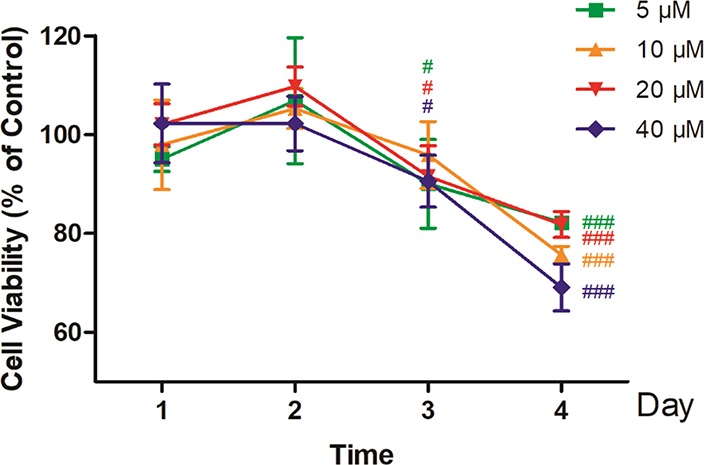
Time-related cytotoxicity of psoralen on HepG2 cells detected by MTT assay. HepG2 cells were treated with 5, 10, 20, or 40 μM psoralen for 1, 2, 3, or 4 days, respectively. Cell viability was detected by MTT assay. Values represent the mean ± SD (n = 4), #p < 0.05 vs control, ###p < 0.001 vs control.
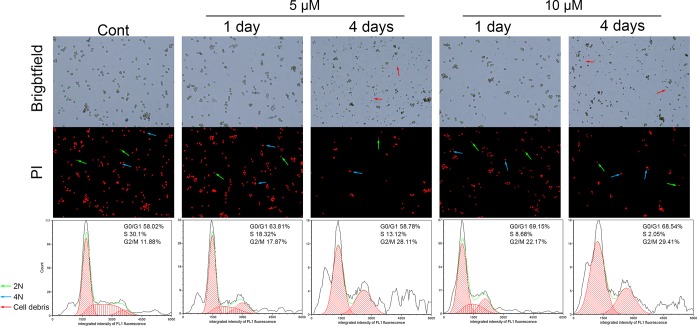
As displayed in Figure 5, after 24 h of psoralen exposure, a higher proportion of cells were in the G2/M phase (11.88% in control group to 17.87% in the 5 μM group and 22.17% in the 10 μM group). On the other hand, the percentage of cells in the S phase decreased from 30.1% in the control group to 18.32% in the 5 μM group and 8.68% in the 10 μM group. At the 4th day, the trends had intensified. The G2/M phase cells of the 5 and 10 μM groups were 28.11 and 29.41%, respectively, which were both more than 11.88% of the control group, while the S phase cells of the 5 and 10 μM group were 13.12 and 2.05%, respectively, which were obviously less than 30.1% of the control group. In other words, the data demonstrated that psoralen arrested HepG2 cells at the G2 phase.
Figure 5.
Effects of psoralen on the cell cycle in HepG2 cells. Cells were treated with psoralen at 5 or 10 μM for 1 or 4 days, respectively. Cell cycle stages (G0/G1, S, and G2/M) were measured using a Countstar Rigel image-based cytometer.
3. Discussion
In this study, the accumulating effect of long-term exposure of psoralen in female rats and its toxicity to HepG2 cells were explored. Pharmacokinetic parameters, such as AUC, MRT, t1/2, Tmax, VZ/F, CLZ/F, and Cmax, are significantly different between single and repeated administration of psoralen using the same dose. What is more, low doses of psoralen did not induce cell death of HepG2 in the 1st day, but cell viability was significantly decreased upon its accumulation by days of administration, which is probably due to the arrest of HepG2 cells at the G2 phase as shown in this study.
Compared to male rats, female rats are more sensitive to hepatotoxicity induced by Fructus Psoraleae.28 In a previous study, psoralen caused remarkable liver damage after long-term repetitive administration to female rats.29 Thus, female rats (5 weeks old) were chosen to study the hepatotoxicity of psoralen in this study in vivo. Plasma concentration of psoralen was determined by UPLC. Before psoralen administration, the effects of two solvents, acetonitrile and methanol, on the precipitation of drug-combined plasma proteins were compared. Acetonitrile required 1.5 times volume of the plasma to precipitate the protein, while methanol required three times the volume. Although a higher plasma volume is needed, methanol is easier to operate. So, methanol was finally chosen as the extraction solvent.
MRT(0-t) is dose-dependent and administration period-dependent as shown in this study, and MRT(0-t) in the 28 and 56 mg/kg groups were significantly higher than that of the 14 mg/kg group regardless of single administration or multiple administrations. It has been revealed that high doses of psoralen prolong its retention time in the body, which could cause drug accumulation. In addition, MRT(0-t) of the multiple doses groups significantly increased, compared with those of the single dose groups. t1/2 also increased, but it is not statistically significant because homogeneity of the variances was significant, compared with those of the single dose counterpart groups of 28 and 56 mg/kg. These results indicate that psoralen is incompletely eliminated when it was used at a high dosage (more than 28 mg/kg). Moreover, the Tmax of the multiple doses group significantly increased as the dose was increased, which indicates that the time required to reach the peak concentration is prolonged. The plasma psoralen level in multiple administrations groups was still over 6000 ng/mL 24 h after the last administration. In summary, there is accumulation of psoralen in the body after 3 months administration (i.g., once every day) with a dosage of more than 28 mg/kg.
Psoralen was deemed to be innoxious at low doses in previous publications.30,31 Also, a recent clinical study reported that low-dose and low-frequency oral psoralen–UV-A treatment are effective to treat early-stage mycosis fungoides.32 However, other reports suggested that the effect of drug accumulation in the body should not be ignored.33,34We found that the t1/2 of psoralen of repetitive administration was significantly longer than that of the single dose. The plasma concentrations of psoralen remained over 6000 ng/mL after 24 h of the last dose of repetitive administration at 56 mg/kg. It means that the plasma concentrations of psoralen were kept at over 6000 ng/mL during the whole administration process. Upon given multiple doses of psoralen at 28 mg/kg, the plasma concentrations were kept at over 2440 ng/mL. Meanwhile, the plasma concentrations of the single dose groups were less than the LLOQ. The results indicated that psoralen accumulated in the body because repetitive administration suppressed the ability of its metabolism and excretion, thus causing toxicity.
It had been reported that psoralen had cytotoxicity in some cell lines,23 but there is no study about the effect of its long-term exposure in low doses. According to calculations, 6000 and 2440 ng/mL psoralen in plasma was about 32.23 and 13.12 μM in cells, respectively. We tested the cytotoxicity effects of 5–40 μM psoralen in the human hepatocytes cell line HepG2 cells, while we estimated the drug concentration in the liver using the plasma concentration. In our study, 5 μM psoralen did not induce cell death in the 1st day, but cell viability was significantly decreased after 3 days with 5 μM psoralen exposure. This long-term toxicity in vitro would be overlooked if the observation is between 24 and 48 h as commonly used in cell experiments. Our results however showed that psoralen is of significant cytotoxicity under long-term but low-dose exposure and under short-term but high-dose exposure. Psoralen not only induces apoptosis, endoplasmic reticulum stress but also arrests the cell cycle at the G2 phase. Previous studies have reported that psoralen arrests MCF-7 cells in the G0/G1 phase, arrests MDA-MB-231 cells in the G2/M phase, and arrests L02 cells in the S phase.24,35 Our results added new evidence to the ability of psoralen to be able to block the cell cycle and inhibit cell proliferation.
4. Conclusions
This study developed a methodology to rapidly study plasma pharmacokinetics following oral administration of psoralen with high sensitivity. Our results in this study indicated that multiple administrations led to accumulation of psoralen in the body, which account for its hepatotoxicity. The plasma concentration of psoralen remained at over 6000 ng/mL for 24 h of the last dose of repetitive administration, which was equivalent to 32.23 μM in the cells. Our results showed that psoralen had significant cytotoxicity under long-term and low-dose exposure, with cells arrested at the G2 phase. The results provided useful information to better understand the toxicity of psoralen due to its accumulation.
5. Materials and Methods
5.1. Chemicals and Reagents
Psoralen (purity, >98%) was purchased from Chengdu Pufei De Biotech Co., Ltd. (Chengdu, Sichuan, China). Chloramphenicol was purchased from China National Institute for the Control of Pharmaceutical and Biological Products Biotechnology Co (Beijing, China). Acetonitrile and methanol were purchased from Fisher Scientific Ltd. (Shanghai, China). Fetal bovine serum (FBS), DMEM high-glucose medium, and 0.25% trypsin were obtained from GIBCO (Gainthersburg, MD, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (Shanghai, China). The image-based cell cycle kit was purchased from Ruiyu Biotech Co., Ltd. (Shanghai, China).
5.2. Instruments and Conditions
The chromatographic analysis of psoralen was performed on a Shimadzu LC-30 UPLC with a photodiode array detector (Shimadzu, Japan). The liquid chromatograph column was a Shim-pack GIST-HP-C18 (2.1 mm × 100 mm, 3 μm). The mobile phase is water (A) and acetonitrile (B). The gradient elution is as follows: 0–4.0 min, 18% B; 4.0–7.0 min, 18–26% B; 7.0–10.0 min, 26% B; and 10.0–14.0 min, 26–18% B. The column temperature was set to 40 °C, the flow rate was set to 0.5 mL/min, and the injection volume was set to 10 μL.
5.3. Preparation of Standard Solutions
Psoralen was prepared in MeOH at a concentration of 1 mg/mL. Chloramphenicol (IS) solution was prepared in MeOH at a concentration of 1 mg/mL. Appropriate aliquots of individual stock solutions were mixed together to prepare a mixed stock solution. All the stock solutions were stored at 4 °C.
5.4. Sample Preparation
The plasma sample (100 μL) was mixed with 10 μL of internal standard solution (25 μg/mL) and then vortexed for 30 s. Proteins were then precipitated using 1 mL of methanol and collected by centrifugation at 8000 rpm for 10 min. The supernatant (900 μL) was transferred to a new tube and dried in a vacuum concentrator (Shanghai Zander Medical Devices Co Ltd.). The residues were then dissolved in 200 μL of mobile phase and then vortexed for 3 min and centrifuged at 128,000 rpm for another 10 min. Aliquots of a 10 μL resuspended solution were injected into the UPLC system for analysis.
5.5. Method Validation
5.5.1. Specificity
The specificity was evaluated by analyzing the blank plasma samples (n = 6), which were compared to those plasma samples added with psoralen and chloramphenicol (IS) and those plasma samples after oral administration of psoralen.
5.5.2. Linearity and Sensitivity
Calibration standards were prepared in the same way as in section 5.4. For the calibration curve, nine concentrations of calibration standards (0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10, and 20 μg/mL) were processed and determined using UPLC. The calibration curves for psoralen are constructed by plotting peak area ratios of the analyte to IS against plasma concentrations. The lower limit of detection (LLOD) was defined as the lowest concentration level resulting in a signal-to-noise ratio (S/N) of 3:1.
5.5.3. Precision and Accuracy
Intra-day accuracy and precision were evaluated from replicate analysis (n = 5) of quality control (QC) samples at different concentrations (0.2, 2.0, and 8.0 ng/mL) on the same day. Inter-day accuracy and precision were also assessed from the analysis of the same QC samples on three consecutive days in replicate (n = 5). QC samples are analyzed against calibration curves. Mean, standard deviation (SD), and relative standards deviation (RSD) were calculated and used to estimate the intra- and inter-day precision. Accuracy was assessed by comparing the calculated mean concentrations against the known concentrations.
5.5.4. Extraction Recovery
The extraction recoveries was determined by comparing the peak area of analytes in QC samples with those of the pure standard solutions in MeOH containing equivalent amounts of analytes at three different levels (0.2, 2.0, and 8.0 ng/mL). Recoveries for plasma were examined at three QC concentrations (n = 5).
5.5.5. Stability
The extracted samples were run immediately after preparation and after 24 h of storage at room temperature to test the room temperature stability at three concentrations (0.2, 2.0, and 8.0 ng/mL). The stability was tested by subjecting plasma samples to three freeze–thaw cycles. Long-term cycle stability was tested by subjecting plasma samples to the freezer for 1 month at −20 °C.
5.6. Pharmacokinetic Study
5.6.1. Animals Experiments and Sample Collection
A total of 36 female Sprague Dawley (SD) rats weighing between 130 and 150 g were purchased from Beijing HFK Bioscience Technology Co. Ltd. (Beijing, China). The rats were housed at room temperature and 50–60% humidity. The animals had access to standard chow and water ad libitum. All of the experimental animals were housed for a week of acclimation and then fasted overnight before the experiments. The animal experiment protocols were approved by the Laboratory Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine (permit number: TCM-LAEC 2016002).
The single dose groups include three groups, which were administrated with 14, 28, and 56 mg/kg psoralen intragastrically, respectively, with six rats in each group. Then, blood was collected 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 12, and 24 h after administration.
The multiple doses groups were also further divided into three groups, which were administrated with 14, 28, and 56 mg/kg psoralen intragastrically once every day for 3 months, respectively, with six rats in each group. Then, blood was collected 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 12, and 24 h after administration.
Blood samples were taken from the choroidal bulb vein into a heparinized tube, and then blood samples were centrifuged at 3500 rpm for 10 min. Plasma in the supernatant was transferred to another centrifuge tubes and stored at −20 °C until analysis.
5.6.2. Pharmacokinetic Analysis
All the pharmacokinetic parameters were calculated using DAS 3.0 software (Drug and Statistics 3.0, Mathematical Pharmacology Professional Committee of China, Shanghai, China) by non-compartment model analysis. The plasma concentration of psoralen was expressed as mean ± SEM, and the curves of mean concentration–time were plotted.
5.7. In Vitro Experiments
5.7.1. HepG2 Cell Lines and Culture
Human HepG2 cells were obtained from Shanghai Cell Bank of Chinese Academy of Science (Shanghai, China). HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium high-glucose medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Waltham, MA, United States), 100 U/mL penicillin, and 100 U/mL streptomycin. Cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
5.7.2. MTT Assay
HepG2 cells were seeded at a density of 5 × 104 cells/mL in 96-well plates and incubated for 24 h. Cells were treated with 0.1% dimethyl sulfoxide (DMSO, control group) or 5, 10, 20, or 40 μM psoralen dissolved in DMEM for 1, 2, 3, or 4 days, respectively. Cell DMEM medium was replaced every 24 h. After treatment, cells were incubated with 0.5 mg/mL MTT for 4 h. The supernatant was removed, after which the formazan was dissolved in 100 μL of DMSO. The absorbance of each well at 570 nm was recorded using a multilayer reader (VictorX5, Perkin Elmer, USA).
5.7.3. Cell Cycle Analysis
Cell cycle stages were analyzed using a Countstar Rigel cytometer (Ruiyu Biotech Co., Shanghai, China) according to the manufacturer’s protocol. Briefly, HepG2 cells were seeded in 6-well plates at a density of 1 × 105 cells/mL and then treated with DMSO or 5 or 10 μM psoralen in DMSO for 1 or 4 days, respectively. After harvesting, cells were resuspended and incubated with 70% ice-cold ethanol overnight. Next, cells were washed with PBS and treated with propidium iodide (PI) for 30 min in the dark. The morphological changes, PI-positive cells, and cell cycle stages were monitored and analyzed using the Countstar Rigel-based cytometer.
5.8. Statistical Analysis
The pharmacokinetic parameters of six groups were analyzed by two-way ANOVA to explore the significant effect of the administration period (single dose or multiple doses) and dose and were analyzed by one-way ANOVA to confirm the differences between two groups. The MTT and cell cycle results were from at least three independent experiments, and data were analyzed by one-way ANOVA to test the significant differences between control and drug-treated groups. p < 0.05 was considered as statistically significant. The statistics analysis was performed using SPSS23 software.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (no. 81673826) and National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2014ZX09304307-001-005). The authors are grateful to Xiao-mei Yuan, Ya-nan Bi, Yue-fei Wang, and Bin Yu for their help in this work.
Glossary
Abbreviations
- FBS
fetal bovine serum
- DMEM
high glucose medium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyl-tetrazolium bromide
- UPLC
ultra-high-Performance liquid chromatography
Author Contributions
# L.Y. and Y.-l.Y. contributed equally to this work.
The authors declare no competing financial interest.
References
- Xin D.; Wang H.; Yang J.; Su Y. F.; Fan G. W.; Wang Y. F.; Zhu Y.; Gao X. M. Phytoestrogens from Psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine. 2010, 17, 126–131. 10.1016/j.phymed.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Vrtačnik P.; Ostanek B.; Mencej-Bedrač S.; Marc J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. 10.11613/BM.2014.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. D.; Yan C. P.; Wu Y.; Weng Z. B.; Yin F. Z.; Yang G. M.; Cai B. C.; Chen Z. P. Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine. 2014, 21, 400–405. 10.1016/j.phymed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Khatune N. A.; Islam M. E.; Haque M. E.; Khondkar P.; Rahman M. M. Antibacterial compounds from the seeds of Psoralea corylifolia. Fitoterapia 2004, 75, 228–230. 10.1016/j.fitote.2003.12.018. [DOI] [PubMed] [Google Scholar]
- He N.; Zhou J.; Hu M.; Ma C.; Kang W. The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L. Open Chem. 2018, 16, 882–889. 10.1515/chem-2018-0091. [DOI] [Google Scholar]
- Li A.; Gao M.; Zhao N.; Li P.; Zhu J.; Li W. Acute liver failure associated with Fructus Psoraleae: a case report and literature review. BMC Complement Altern Med. 2019, 19, 84. 10.1186/s12906-019-2493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa J. P.; Gerken G.; Canbay A. Acute Liver Failure - It’s Just a Matter of Cell Death. Dig Dis. 2016, 34, 423–428. 10.1159/000444557. [DOI] [PubMed] [Google Scholar]
- Liu R.; Li A.; Sun A.; Kong L. Preparative isolation and purification of psoralen and isopsoralen from Psoralea corylifolia by high-speed counter-current chromatography. J Chromatogr a. 2004, 1057, 225–228. 10.1016/j.chroma.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Li H. N.; Wang C. Y.; Wang C. L.; Chou C. H.; Leu Y. L.; Chen B. Y. Antimicrobial Effects and Mechanisms of Ethanol Extracts of Psoralea corylifolia Seeds Against Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus. Foodborne Pathog Dis. 2019, 573. 10.1089/fpd.2018.2595. [DOI] [PubMed] [Google Scholar]
- Seo E.; Lee E. K.; Lee C. S.; Chun K. H.; Lee M. Y.; Jun H. S. Psoralea corylifolia L. seed extract ameliorates streptozotocin-induced diabetes in mice by inhibition of oxidative stress. Oxid Med Cell Longev. 2014, 2014, 897296. 10.1155/2014/897296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Kong L.; Su X.; Pan C.; Ye M.; Zou H. Integration of ion-exchange chromatography fractionation with reversed-phase liquid chromatography-atmospheric pressure chemical ionization mass spectrometer and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for isolation and identification of compounds in Psoralea corylifolia. J Chromatogr a. 2005, 1089, 87–100. 10.1016/j.chroma.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Ruan B.; Kong L. Y.; Takaya Y.; Niwa M. Studies on the chemical constituents of Psoralea corylifolia L. J. Asian Nat. Prod. Res. 2007, 9, 41–44. 10.1080/10286020500289618. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Huang C.; Shan Z.; Xiang B.; Mei L. Fingerprint analysis of Psoralea corylifolia L. by HPLC and LC-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2005, 821, 67–74. 10.1016/j.jchromb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Wu C.; Sun Z.; Ye Y.; Han X.; Song X.; Liu S. Psoralen inhibits bone metastasis of breast cancer in mice. Fitoterapia 2013, 91, 205–210. 10.1016/j.fitote.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Kassahun G. A.; Wijerathne T. D.; Kim J. H.; Kim M. J.; Seo C.-S.; Shin H.-K.; Lee K. P. Psoralea corylifolia extract induces vasodilation in rat arteries through both endothelium-dependent and -independent mechanisms involving inhibition of TRPC3 channel activity and elaboration of prostaglandin. Pharm Biol. 2016, 55, 2136–2144. 10.1080/13880209.2017.1383484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdem L. K.; Liu Y.; Stearns V.; Kadlubar S. A.; Ramirez J.; Jeter S.; Shahverdi K.; Ward B. A.; Ogburn E.; Ratain M. J.; Flockhart D. A.; Desta Z. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br. J. Clin. Pharmacol. 2010, 70, 854–869. 10.1111/j.1365-2125.2010.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M. J.; Chen M. K.; Yu Y. Y.; Sheu G. T.; Chiou H.-L. Psoralen reverses docetaxel-induced multidrug resistance in A549/D16 human lung cancer cells lines. Phytomedicine 2014, 21, 970–977. 10.1016/j.phymed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Tang D.-Z.; Yang F.; Yang Z.; Huang J.; Shi Q.; Chen D.; Wang Y.-J. Psoralen stimulates osteoblast differentiation through activation of BMP signaling. Biochem. Biophys. Res. Commun 2011, 405, 256–261. 10.1016/j.bbrc.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M.; Cui Y.; Liu C.; Li C.; Liu Z.; Kang W. CYPs-mediated drug-drug interactions on psoralidin, isobavachalcone, neobavaisoflavone and daidzein in rats liver microsomes. Food chem. toxicol. 2020, 136, 111027. 10.1016/j.fct.2019.111027. [DOI] [PubMed] [Google Scholar]
- Hao B.; Chen Z. W.; Zhou X. J.; Zimin P. I.; Miljanich G. P.; Wulff H.; Wang Y. X. Identification of phase-I metabolites and chronic toxicity study of the Kv1.3 blocker PAP-1 (5-(4-phenoxybutoxy)psoralen) in the rat. Xenobiotica 2011, 41, 198–211. 10.3109/00498254.2010.532886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.; Wang X.; Cheng K.; Zhao W.; Hua Y.; Xu C.; Yang Z. Psoralen reverses the P-glycoprotein-mediated multidrug resistance in human breast cancer MCF-7/ADR cells. Mol. Med. Rep. 2016, 13, 4745–4750. 10.3892/mmr.2016.5098. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zhao W.; Wang Y.; Lu J.; Chen X. The Chemical Constituents and Bioactivities of Psoralea corylifolia Linn.: A Review. Am J Chin Med. 2016, 44, 35–60. 10.1142/S0192415X16500038. [DOI] [PubMed] [Google Scholar]
- Xia Q.; Wei L.; Zhang Y.; Kong H.; Shi Y.; Wang X.; Chen X.; Han L.; Liu K. Psoralen Induces Developmental Toxicity in Zebrafish Embryos/Larvae Through Oxidative Stress, Apoptosis, and Energy Metabolism Disorder. Front Pharmacol. 2018, 9, 1457. 10.3389/fphar.2018.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.; Chen X.; Zhao G.; Xu D.; Jiang Z.; Zhang L.; Wang T. Psoralen Induced Liver Injury by Attenuating Liver Regenerative Capability. Front Pharmacol. 2018, 9, 1179. 10.3389/fphar.2018.01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wang Q.; Wang Z. X.; Bi Y. N.; Yuan X. M.; Song L.; Jiang M. M.; Sun L. K.; Zhou K. A Study of NMR-Based Hepatic and Serum Metabolomics in a Liver Injury Sprague-Dawley Rat Model Induced by Psoralen. Chem. Res. Toxicol. 2018, 31, 852–860. 10.1021/acs.chemrestox.8b00082. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Yu R.; Men W.; Zhang P.; Zhang Y.; Song L.; Zhou K. Psoralen induces hepatic toxicity through PERK and ATF6 related ER stress pathways in HepG2 cells. Toxicol. Mech. Methods 2019, 39–47. 10.1080/15376516.2019.1650150. [DOI] [PubMed] [Google Scholar]
- Pisani A.; Sabbatini M.; Imbriaco M.; Riccio E.; Rubis N.; Prinster A.; Perna A.; Liuzzi R.; Spinelli L.; Santangelo M.; Remuzzi G.; Ruggenenti P.; Pisani A.; Visciano B.; Amicone M.; Dipietro R.; Mozillo G.; Riccio E.; Rossana R.; Spinelli L.; Santangelo M.; Rubis N.; Diadei O.; Calini W.; Calini W.; Villa A.; Sabatella M.; Ene-Iordache B.; Carminati S.; Martinetti D.; Giuliano G. A.; Perna A.; Liuzzi R.; Remuzzi A.; Imbriaco M.; Prinster A.; Altiero M.; Boccardo P.; Peracchi S. Long-term Effects of Octreotide on Liver Volume in Patients With Polycystic Kidney and Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 1022–1030.e4. 10.1016/j.cgh.2015.12.049. [DOI] [PubMed] [Google Scholar]
- Wang J.; Jiang Z.; Ji J.; Li Y.; Chen M.; Wang Y.; Zhang Y.; Tai T.; Wang T.; Zhang L. Evaluation of hepatotoxicity and cholestasis in rats treated with EtOH extract of Fructus Psoraleae. J. Ethnopharmacol. 2012, 144, 73–81. 10.1016/j.jep.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Wang P.; Yu R.; Lu J.; Jiang M.; Zhou K. Long-Term Exposure of Psoralen and Isopsoralen Induced Hepatotoxicity and Serum Metabolites Profiles Changes in Female Rats. Metabolites 2019, 9, 263. 10.3390/metabo9110263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L.; Yu B.; Yang L.; Wang Z. X.; Zhang Y.; Yu Y. L.; Zhou K. The mechanism of Psoralen and Isopsoralen hepatotoxicity as revealed by hepatic gene expression profiling in SD rats. Basic Clin. Pharmacol. Toxicol. 2019, 527. 10.1111/bcpt.13287. [DOI] [PubMed] [Google Scholar]
- Wu C. R.; Chang C. L.; Hsieh P. Y.; Lin L. W.; Ching H. Psoralen and isopsoralen, two coumarins of Psoraleae Fructus, can alleviate scopolamine-induced amnesia in rats. Planta Med. 2007, 73, 275–278. 10.1055/s-2007-967127. [DOI] [PubMed] [Google Scholar]
- Vieyra-Garcia P.; Fink-Puches R.; Porkert S.; Lang R.; Pöchlauer S.; Ratzinger G.; Tanew A.; Selhofer S.; Paul-Gunther S.; Hofer A.; Gruber-Wackernagel A.; Legat F.; Patra V.; Quehenberger F.; Cerroni L.; Clark R.; Wolf P. Evaluation of Low-Dose, Low-Frequency Oral Psoralen-UV-A Treatment With or Without Maintenance on Early-Stage Mycosis Fungoides: A Randomized Clinical Trial. Jama Dermatol. 2019, 155, 538–547. 10.1001/jamadermatol.2018.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.; Zhang Y.; Deng Y.; Jiang W.; Zhao Y.; Geng J.; Ding L.; Ren H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang H.; Jiang J. M.; Zheng D.; Chen Y. Y.; Wan S. J.; Tan H. S.; Tang L. M.; Xu H. X. Hepatotoxicity induced by psoralen and isopsoralen from Fructus Psoraleae: Wistar rats are more vulnerable than ICR mice. Food Chem. Toxicol. 2019, 125, 133–140. 10.1016/j.fct.2018.12.047. [DOI] [PubMed] [Google Scholar]
- Wang X.; Xu C.; Hua Y.; Cheng K.; Zhang Y.; Liu J.; Han Y.; Liu S.; Zhang G.; Xu S.; Yang Z. Psoralen induced cell cycle arrest by modulating Wnt/β -catenin pathway in breast cancer cells. Sci. Rep. 2018, 8, 14001. 10.1038/s41598-018-32438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]