Abstract
A practical approach for the regioselective synthesis of 3-arylthioindoles has been accomplished using a combination of 1-aryltriazene/CS2 as a new sulfenylation source. The methodology employs molecular iodine as a catalyst and is compatible with a variety of structurally diverse reactants.
Introduction
Sulfenylated organic molecules manifest significant biological activity and are prospective candidates for drug discovery programs (Figure 1).1 Particularly, the C(sp2)–S bond has a definite role in the functional expression of various natural products and biologically active moieties.2 Indoles constitute a section of most important heterocycles and are amenable to many substitutions so as to comprise the core structure of various bioactive molecules and natural products.3 The difficulty in synthesizing selected functionalized indoles4 using pre-eminent traditional methods has, thus, led to the concept of selective substitution.5
Figure 1.
Some biologically active sulfenylated molecules.
3-Sulfenylindole derivatives have attained considerable pharmacological significance in the treatment of various diseases, including HIV, heart diseases, cancer, bacterial infection, etc.6 They have also been explored as potent inhibitors of tubulin polymerization.7 As a result, the synthesis of 3-sulfenylindoles has garnered a great deal of current interest concerning various protocols.8 The most eminent strategies for desired functionalization, however, comprise direct sufenylation of the indole core, employing sulfenylating agents, such as thiols,9 arylsulfonyl hydrazides,10 sulfinates,11 disulfides,12N-thioimides,13 sulfonium salts,14 quinone mono-O, S-acetals,15 sulfenyl halides,16 arylsulfonyl chloride,17 etc. From this perspective, the use of arylsulfonyl hydrazides by the Tian group10d and, more recently, the use of arylsulfonyl chlorides by the Radosevich group17a as sulfur electrophiles for the sulfenylation of indoles have drawn special attention (Scheme 1). Nevertheless, a practical and mild synthetic strategy employing a readily available and inexpensive sulfenylating source is still in demand.
Scheme 1. Synthesis of 3-Arylthioindoles.
1-Aryltriazenes have recently evolved as excellent synthons and are used as masked arenediazonium salts.18,19 Besides their use as an aryl and diazenyl source, 1-aryltriazenes are also employed as a versatile directing group for selective C–H activation.20 Easy preparation from commercially available arylamines and the stability of 1-aryltriazenes under air/moisture lend them additional advantages and application expediency over the arenediazonium salts.
In the pursuit of an external sulfur source, highly available carbon disulfide has been newly established as an efficient sulfur source for many organic transformations.21 Inspired by the recent reports and considering the propensity of 1-aryltriazenes to generate aryl radicals,22 it was envisioned to design a reaction module for in situ generation of thiyl radicals via the combination of aryl radicals with CS2 followed by dethiocarbonylation,21a which may eventually lead to a new means of sulfenylation of organic molecules.
In view of the above and as a part of our ongoing research on C–S bond formation,23 we report herein a molecular-iodine-catalyzed facile synthesis of 3-arylthioindoles, employing a blend of 1-aryltriazenes and carbon disulfide as a new sulfenylation source in ethanol at 70 °C (Scheme 1).
Results and Discussion
To comprehend our idea and to optimize the reaction conditions, a model reaction involving 1H-indole (1a) and 1-(phenyldiazenyl)pyrrolidine (2a) was thoroughly investigated by varying different parameters, such as the catalyst, sulfur source, solvent, and temperature (Table 1).
Table 1. Optimization of Reaction Conditionsa.
| entry | catalyst | sulfur source | solvent | temp. (°C) | yield (%)b |
|---|---|---|---|---|---|
| 1 | I2 | CS2 | DMSO | rt | 46 |
| 2 | I2 | CS2 | DMSO | 50 | 63 |
| 3 | I2 | CS2 | DMSO | 70 | 73 |
| 4 | I2 | CS2 | DMSO | 80 | 71 |
| 5 | I2 | CS2 | DMSO | 70 | 47c |
| 6 | CS2 | DMSO | 70 | 0 | |
| 7 | KI | CS2 | DMSO | 70 | 43 |
| 8 | NH4I | CS2 | DMSO | 70 | 41 |
| 9 | NIS | CS2 | DMSO | 70 | 28 |
| 10 | TBAI | CS2 | DMSO | 70 | trace |
| 11 | DIB | CS2 | DMSO | 70 | trace |
| 12 | I2 | CS2 | DMF | 70 | 25 |
| 13 | I2 | CS2 | CH3CN | 70 | 46 |
| 14d | I2 | CS2 | EtOH | 70 | 86 |
| 15 | I2 | CS2 | dioxane | 70 | 42 |
| 16 | I2 | CS2 | toluene | 70 | 37 |
| 17 | I2 | CS2 | DCE | 70 | 49 |
| 18 | I2 | CS2 | THF | 70 | 32 |
| 19 | I2 | CS2 | H2O | 70 | 0 |
| 20 | I2 | CS2 | EtOH/H2O (2:1) | 70 | 46 |
| 21 | I2 | Na2S | EtOH | 70 | trace |
| 22 | I2 | S8 | EtOH | 70 | 0 |
Reaction conditions: 1a (1.0 mmol), 2a (1.2 mmol), catalyst (20 mol %), sulfur source (3.0 mmol), solvent (2 mL), 70 °C (in a sealed tube), 8 h.
Isolated yield after column chromatography.
Using 10 mol % I2.
The bold entries (entry 14) represents the optimized reaction conditions.
The studies commenced with a preliminary reaction setup by taking a mixture of 1a (1.0 mmol), 2a (1.2 mmol), CS2 (3.0 mmol), molecular iodine (20 mol %), and DMSO with stirring in a sealed glass vessel at room temperature for 8 h, which led to the formation of the desired product 3-(phenylthio)-1H-indole (3a) in 46% yield (Table 1, entry 1). An appreciable surge in the product yield was witnessed with an increase in the temperature up to 70 °C, beyond which there was no further increase in the product yield (entries 2–4). A much lower product yield (47%) was obtained on lowering the catalyst loading (10 mol %, entry 5). Notably, no product formation was observed in the absence of the I2 catalyst (entry 6). Further, a number of other iodine catalysts, viz., KI, NH4I, NIS, TBAI, and DIB were screened (entries 7–11), out of which only KI, NH4I, and NIS could cause apparent desired conversion, while TBAI and DIB failed. Among the various solvents tried (entries 12–20), ethanol happened to be the best to afford 86% product yield (entry 14). The attempt to utilize other sulfur sources, namely, Na2S and S8, also remained futile (entries 21 and 22).
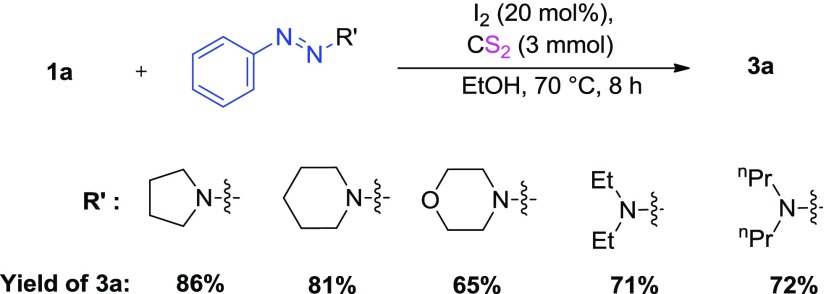
Under the established reaction conditions (Table 1, entry 14), various secondary amines, viz., piperidine, morpholine, diethylamine, and dipropylamine, employed as the N-substituted moiety of 1-phenyltriazenes, were tested for their efficacy on the product yield (Scheme 2). Although all secondary amines afforded good product yields, pyrrolidine remained to be the best option.
Scheme 2. Reaction of 1a with Different 1-Phenyltriazenes.
Finally, the scope and generality of the reaction was systematically examined using a variety of indoles (1) and 1-aryltriazenes (2) with different substitution patterns, and the results are given in Table 2.
Table 2. Scope of the Reactiona,b.
Reaction conditions: 1a (1.0 mmol), 2a (1.2 mmol), CS2 (3.0 mmol), I2 (20 mol %), EtOH (2 mL), 70 °C (in a sealed tube), 8 h.
Isolated yield after column chromatography.
Gratifyingly, 1-aryltriazenes containing electron-donating (Me, OMe) as well as electron-withdrawing (F, Cl, Br, I, CF3, NO2) groups at diverse positions on the aryl ring endured smoothly when reacted with indole (1a) under the stipulated conditions to afford the sulfenylation products (3a–3n) in excellent yields. It was observed that the presence of electron-donating groups on the aromatic ring of 1-aryltriazenes afforded somewhat higher yield in comparison to those bearing electron-withdrawing groups (3b–3f vs 3g–3n). A heteroaromatic 1-aryltriazene, namely, 3-(pyrrolidin-1-yldiazenyl)pyridine, however, failed to afford the desired sulfenylated product (3o). Conversely, indole derivatives containing dissimilar electron-donating as well as electron-withdrawing substituents also participated well in the reaction and offered the desired sulfenylated products (3p–3u) in very high yields. Interestingly, the C2-substituted indoles, such as 2-methylindole and 2-phenylindole, also performed well in the reaction to give the corresponding products 3q and 3r in very good yields. However, when a C3-substituted indole, viz., 3-methylindole, was screened under the stipulated conditions, no conversion was observed.
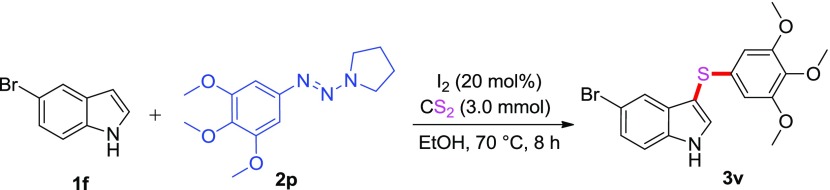
To demonstrate the viability and usefulness of the realized sulfenylation reaction, a well-known antitumor compound, viz., RS 2518 (5-bromo-3-((3,4,5-trimethoxyphenyl)thio)-1H-indole) (3v),24 was also effectively synthesized in 65% yield from 1-((3,4,5-trimethoxyphenyl)pyrrolidine and 5-bromo-1H-indole under the established conditions (Scheme 3).
Scheme 3. Synthesis of the Antitumor Compound 3v.
Encouraged by the results obtained with indoles, the sulfenylation strategy was further applied to other electron-rich arenes, such as 1,3,5-trimethoxybenzene (4a) and 2-naphthol (5a), which also efficiently afforded the likely sulfenylated products (6 and 7) in high yields under the standard conditions (Scheme 4). However, the desired sulfenylation of 1-naphthol under the stipulated conditions remained futile and instead gave rise to an azo dye as the major product.
Scheme 4. Sulfenylation of 4a and 5a.
All products were fully characterized on the basis of their physical and spectral data. The structure of a representative product 3c was conclusively confirmed by single-crystal X-ray diffraction (see the Supporting Information for details).
To gain insight into the reaction mechanism, a number of control experiments were carried out (Scheme 5). The typical reaction involving 1H-indole (1a) and 1-(phenyldiazenyl)pyrrolidine (2a) under the standard conditions was fully suppressed in the presence of radical inhibitors, such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), 2,6-di-tert-butyl-4-methylphenol (BHT), and ascorbic acid, thereby suggesting a radical pathway. Further, the reaction of a representative diazonium salt, viz., p-methoxybenzenediazonium tetrafluoroborate (8a), with indole (1a) under the standard conditions also gave rise to the corresponding product 3e (90%), suggestive of the intermediacy of the arenediazonium cation during the course of the reaction. Further, the reaction of 1-(phenyldiazenyl)pyrrolidine (2a) under the standard conditions gave rise to 1,2-diphenyldisulfane (2aa, 92%), which when made to react with indole (1a) under the standard conditions also afforded the desired product (3a, 83%), implying the involvement of disulfide in the reaction.
Scheme 5. Control Experiments.
Based on control experiments, isolation of products, and existing literature,21a,22,25 a plausible mechanism is outlined in Scheme 6.
Scheme 6. Plausible Reaction Mechanism.
The reaction is assumed to begin with the I2-assisted formation of arenediazonium ion A with the removal of N-iodopyrrolidine. The diazonium ion A is then reduced by the iodide ion to the aryl radical B, which adds on to carbon disulfide to form the radical intermediate C, followed by the loss of carbon monosulfide to provide the thiyl radical D. An electrophilic species F is subsequently formed either via the direct combination of thiyl radical D with the iodide radical or via the formation of dimer E followed by its reaction with molecular iodine. Finally, the substrate indole 1 undergoes electrophilic substitution involving the species F (ArSI) to afford the sulfenylated product 3.
Conclusions
In conclusion, a new and efficient sulfenylation strategy has been developed for the regioselective thiolation of indoles to provide 3-arylthiolindoles, employing an inexpensive and readily available 1-aryltriazene/CS2 combination. The protocol employs mild and easy-to-handle reaction conditions and offers a wide range of substrate scope and functional group tolerance.
Experimental Section
General Information
1H, 13C, and 19F spectra were recorded on a JEOL ECZ 500R FTNMR spectrometer (1H NMR at 500 MHz, 13C NMR at 125 MHz, and 19F NMR at 470 MHz). Chemical shifts are reported in parts per million (ppm) using tetramethylsilane (TMS) as an internal reference. 1H NMR chemical shifts are given in ppm with respect to the residual CHCl3 peak (δ 7.26 ppm), and 13C NMR chemical shifts are given in ppm with respect to CDCl3 (δ 77.16 ppm). NMR data are represented as follows: chemical shift, multiplicity (s = singlet, brs = broad singlet, d = doublet, dd = double doublet, t = triplet, q = quartet, and m = multiplet), coupling constant (J) (Hz), and integration. Mass spectra were recorded on a SCIEX X500R QTOF mass spectrometer. Analytical thin-layer chromatography (TLC) was performed on Merck Kieselgel 60 GF254 plates (thickness 0.25 mm). Visualization was accomplished using a 254 nm UV lamp and by staining in an I2 chamber. Organic solutions were concentrated under reduced pressure using a Büchi rotary evaporator. Purification of the crude products was done by column chromatography using a silica gel 100–200 mesh. All reactions were carried out in an oven-dried glass sealed tube. Yield refers to the isolated analytically pure material.
Materials
1-Aryltriazene derivatives were prepared adopting an earlier method.20a The rest of the reagents were purchased from Sigma-Aldrich and Merck chemical co. and were used without further purification. Solvents were purified by standard methods.
General Experimental Procedure for the Synthesis of Products
A mixture of indole (1, 1.0 mmol), 1-aryltriazene (2, 1.2 mmol), CS2 (3.0 mmol), I2, and EtOH (2 mL), contained in a sealed tube, was stirred at 70 °C for 8 h. After completion of the reaction (monitored through TLC), ethanol was removed under reduced pressure, and the resulting mixture was successively worked-up using aqueous solution of sodium thiosulfate pentahydrate-ethyl acetate. The organic phase was then dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The resulting crude product was finally purified by silica gel column chromatography using n-hexane and ethyl acetate as the eluent to afford the pure product 3.
3-(Phenylthio)-1H-indole (3a)17a
White solid; mp 151–152 °C, yield = 194 mg (86%); 1H NMR (500 MHz, CDCl3): δ 8.35 (brs, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 2.5 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.27 (t, J = 7.5 Hz, 1H), 7.17–7.09 (m, 5H), 7.05–7.02 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 139.4, 136.6, 130.8, 129.2, 128.8, 126.0, 124.9, 123.2, 121.0, 119.8, 111.7, 103.0.
3-(p-Tolylthio)-1H-indole (3b)17a
White solid; mp 124–125 °C, yield = 213 mg (89%); 1H NMR (500 MHz, CDCl3): δ 8.26 (brs, 1H), 7.62 (d, J = 8.0 Hz, 1H), 7.41 (d, J = 2.5, 1H), 7.39 (d, J = 8.5 Hz, 1H), 7.25 (t, J = 7.5 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 7.03 (d, J = 8.0 Hz, 2H), 6.97 (d, J = 8.0 Hz, 2H), 2.23 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 136.6, 135.6, 134.8, 130.6, 129.6, 129.2, 126.4, 123.1, 121.0, 119.8, 111.7, 103.6, 21.0.
3-(m-Tolylthio)-1H-indole (3c)9b
White solid; mp 126–127 °C, yield = 208 mg (87%); 1H NMR (500 MHz, CDCl3): δ 8.30 (brs, 1H), 7.62 (d, J = 7.5 Hz, 1H), 7.42 (d, J = 2.0, 1H), 7.40 (d, J = 8.5 Hz, 1H), 7.26 (t, J = 7.5 Hz, 1H), 7.17 (t, J = 7.5 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 6.97 (s, 1H), 6.88 (t, J = 6.0 Hz, 2H), 2.21 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 139.1, 138.6, 136.6, 130.8, 129.3, 128.7, 126.6, 125.9, 123.1, 121.0, 119.8, 111.7, 103.0, 21.5.
3-(o-Tolylthio)-1H-indole (3d)9b
White solid; mp 112–113 °C, yield = 203 mg (85%); 1H NMR (500 MHz, CDCl3): δ 8.38 (brs, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.44 (d, J = 9.0 Hz, 2H), 7.28 (t, J = 7.5 Hz, 1H), 7.17–7.12 (m, 2H), 6.98 (t, J = 7.5 Hz, 1H), 6.90 (t, J = 7.5 Hz, 1H), 6.72 (d, J = 8.0 Hz, 1H), 2.49 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 138.4, 136.7, 134.5, 130.9, 130.0, 129.4, 126.4, 125.4, 124.6, 123.2, 121.0, 119.8, 111.7, 102.5, 20.0.
3-((4-Methoxyphenyl)thio)-1H-indole (3e)10d
Orange solid; mp 114–115 °C, yield = 235 mg (92%); 1H NMR (500 MHz, CDCl3): δ 8.29 (brs, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.41 (d, J = 2.5 Hz, 1H), 7.39 (d, J = 8.0 Hz, 1H), 7.27 (t, J = 7.5 Hz, 1H), 7.19–7.14 (m, 3H), 6.77–6.74 (m, 2H), 3.73 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 157.9, 136.5, 130.2, 129.6, 129.1, 128.7, 123.0, 120.9, 119.7, 114.6, 111.7, 104.6, 55.4.
3-((3,4-Dimethoxyphenyl)thio)-1H-indole (3f)
White solid; mp 137–138 °C, yield = 271 mg (95%); 1H NMR (500 MHz, CDCl3): δ 8.44 (brs, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.45 (d, J = 2.5 Hz, 1H), 7.40 (d, J = 8.5 Hz, 1H), 7.25 (t, J = 7.5 Hz, 1H), 7.17 (t, J = 7.5 Hz, 1H), 6.80 (s, 1H), 6.68 (s, 2H), 3.78 (s, 3H), 3.72 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 149.3, 147.3, 136.6, 130.2, 129.1, 123.1, 120.9, 119.7, 119.5, 112.0, 111.7, 110.9, 104.3, 56.1, 55.9. High-resolution mass spectrometry (HRMS) (electrospray ionization time-of-flight (ESI-TOF)) calcd for C16H14NO2S– (M – H)− 284.0750; found: 284.0742.
3-((4-Fluorophenyl)thio)-1H-indole (3g)17a
White solid; mp 134–135 °C, yield = 182 mg (75%); 1H NMR (500 MHz, CDCl3): δ 8.37 (brs, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 3.0 Hz, 1H), 7.43 (d, J = 8.0 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 7.11–7.07 (m, 2H), 6.88–6.84 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 162.0 (d, J = 242.5 Hz), 136.6, 134.2 (d, J = 2.5 Hz), 130.6, 129.0, 128.1 (d, J = 7.9 Hz), 123.3, 121.1, 119.7, 116.0 (d, J = 21.1 Hz), 111.8, 103.6. 19F NMR (470 MHz, CDCl3): δ −118.1.
3-((4-Chlorophenyl)thio)-1H-indole (3h)10d
White solid; mp 130–131 °C, yield = 199 mg (77%); 1H NMR (500 MHz, CDCl3): δ 8.43 (brs, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.50 (d, J = 2.5 Hz, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.29 (t, J = 7.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.13–7.10 (m, 2H), 7.03–7.00 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 138.0, 136.7, 130.8, 130.7, 129.0, 128.9, 127.3, 123.4, 121.2, 119.7, 111.8, 102.7.
3-((3-Chlorophenyl)thio)-1H-indole (3i)9e
White solid; mp 77–78 °C, yield = 197 mg (76%); 1H NMR (500 MHz, CDCl3): δ 8.44 (brs, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.49 (d, J = 2.5 Hz, 1H), 7.45 (d, J = 8.0 Hz, 1H), 7.29 (t, J = 7.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.07–7.04 (m, 2H), 7.01 (d, J = 8.0 Hz, 1H), 6.96 (d, J = 7.5 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 141.7, 136.6, 134.8, 131.1, 129.8, 129.0, 125.6, 125.1, 124.0, 123.4, 121.3, 119.6, 111.8, 102.0.
3-((4-Bromophenyl)thio)-1H-indole (3j)10d
White solid; mp 141–142 °C, yield = 239 mg (79%); 1H NMR (500 MHz, CDCl3): δ 8.42 (brs, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.49 (d, J = 2.5 Hz, 1H), 7.46 (d, J = 8.5 Hz, 1H), 7.29 (t, J = 7.5 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.12–7.10 (m, 2H), 7.03–7.00 (m, 2H). 13C NMR (125 MHz, CDCl3): δ 138.0, 136.7, 130.8, 130.7, 128.9, 128.9, 127.3, 123.4, 121.2, 119.7, 111.8, 102.6.
3-((2-Bromophenyl)thio)-1H-indole (3k)9b
White solid; mp 148–149 °C, yield = 236 mg (78%); 1H NMR (500 MHz, CDCl3): δ 8.43 (brs, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.45–7.39 (m, 3H), 7.24 (t, J = 7.5 Hz, 1H), 7.13 (t, J = 7.5 Hz, 1H), 6.92 (t, J = 7.5 Hz, 1H), 6.85 (t, J = 7.5 Hz, 1H), 6.57 (d, J = 7.5 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 140.6, 136.7, 132.7, 131.4, 129.1, 127.6, 126.5, 125.8, 123.4, 121.3, 119.8, 111.8, 102.1.
3-((2-Iodophenyl)thio)-1H-indole (3l)
Reddish brown solid; mp 151–152 °C, yield = 277 mg (79%); 1H NMR (500 MHz, CDCl3): δ 8.39 (brs, 1H), 7.69 (d, J = 7.5 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.42–7.37 (m, 2H), 7.23 (t, J = 7.5 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 6.94 (t, J = 7.5 Hz, 1H), 6.68 (t, J = 7.5 Hz, 1H), 6.54 (d, J = 8.0 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ 143.9, 139.3, 136.7, 131.3, 128.9, 128.4, 126.0, 126.0, 123.4, 121.3, 119.8, 111.8, 103.5, 94.4. HRMS (ESI-TOF) calcd for C14H11INS+ (M + H)+ 351.9652; found: 351.9638.
3-((3-(Trifluoromethyl)phenyl)thio)-1H-indole (3m)12a
Brown viscous liquid; yield = 214 mg (73%); 1H NMR (500 MHz, CDCl3): δ 8.48 (brs, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.49 (d, J = 2.5 Hz, 1H), 7.46 (t, J = 8.0 Hz, 2H), 7.31 (t, J = 7.0 Hz, 2H), 7.25–7.19 (m, 3H). 13C NMR (125 MHz, CDCl3): δ 141.1, 136.6, 131.2 (q, J = 32.3 Hz), 129.2, 128.9, 128.8, 125.1, 123.4 (q, J = 270.5 Hz), 122.4 (d, J = 3.4 Hz), 121.6 (d, J = 3.6 Hz), 121.3, 119.5, 111.9, 101.5. 19F NMR (470 MHz, CDCl3): δ −62.60.
3-((4-Nitrophenyl)thio)-1H-indole (3n)10d
Yellow solid; mp 174–175 °C, yield = 192 mg (71%); 1H NMR (500 MHz, CDCl3): δ 8.75 (brs, 1H), 7.96 (d, J = 9.0 Hz, 2H), 7.51 (t, J = 4.0 Hz, 2H), 7.48 (d, J = 8.0 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.19 (t, J = 7.5 Hz, 1H), 7.10 (d, J = 9.0 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 150.2, 144.9, 136.7, 131.5, 128.5, 125.2, 123.9, 123.6, 121.4, 119.2, 112.1, 99.9.
1-Methyl-3-(phenylthio)-1H-indole (3p)10d
White solid; mp 83–84 °C, yield = 198 mg (83%); 1H NMR (500 MHz, CDCl3): δ 7.63 (d, J = 8.0 Hz, 1H), 7.41 (d, J = 8.5 Hz, 1H), 7.35 (s, 1H), 7.32 (t, J = 8.0 Hz, 1H), 7.19–7.14 (m, 3H), 7.11–7.10 (m, 2H), 7.07–7.03 (m, 1H), 3.86 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 139.8, 137.7, 135.2, 130.0, 128.8, 125.8, 124.8, 122.7, 120.6, 119.9, 109.8, 100.6, 33.3.
2-Methyl-3-(phenylthio)-1H-indole (3q)10d
White solid; mp 112–113 °C, yield = 175 mg (73%); 1H NMR (500 MHz, CDCl3): δ 8.25 (brs, 1H), 7.55 (d, J = 7.5 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.21–7.11 (m, 4H), 7.05 (t, J = 7.0 Hz, 3H), 2.52 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 141.2, 139.5, 135.6, 130.4, 128.8, 125.6, 124.6, 122.3, 120.8, 119.2, 110.7, 99.6, 12.3.
2-Phenyl-3-(phenylthio)-1H-indole (3r)12f
Light yellow oil; yield = 205 mg (68%); 1H NMR (500 MHz, CDCl3): δ 8.48 (brs, 1H), 7.69 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 7.5 Hz, 1H), 7.39–7.31 (m, 4H), 7.22 (t, J = 7.0 Hz, 1H), 7.11–7.07 (m, 3H), 7.03 (d, J = 7.0 Hz, 2H), 6.99–6.96 (m, 1H). 13C NMR (125 MHz, CDCl3): δ 142.2, 139.4, 136.0, 131.6, 131.3, 128.9, 128.8, 128.3, 125.7, 124.8, 123.5, 121.3, 120.1, 111.3, 99.6.
5-Methoxy-3-(phenylthio)-1H-indole (3s)10d
Light yellow liquid; yield = 244 mg (88%); 1H NMR (500 MHz, CDCl3): δ 8.26 (brs, 1H), 7.37 (d, J = 3.0 Hz, 1H), 7.25 (d, J = 8.5 Hz, 1H), 7.10 (t, J = 8.0 Hz, 2H), 7.03 (d, J = 7.5 Hz, 2H), 6.99–6.96 (m, 2H), 6.85 (dd, J = 2.0, 2.0 Hz, 1H), 3.71 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 155.3, 139.5, 131.5, 131.4, 130.1, 128.8, 125.9, 124.9, 113.8, 112.5, 102.5, 101.0, 56.0.
5-Bromo-3-(phenylthio)-1H-indole (3t)10d
White solid; mp 121–122 °C, yield = 254 mg (84%); 1H NMR (500 MHz, CDCl3): δ 8.47 (brs, 1H), 7.75 (s, 1H), 7.48 (d, J = 2.5 Hz, 1H), 7.36–7.30 (m, 2H), 7.19 (t, J = 8.0 Hz, 2H), 7.09 (d, J = 7.5 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ 138.8, 135.2, 131.9, 131.1, 128.9, 126.2, 126.0, 125.1, 122.3, 114.6, 113.1, 103.0.
3-(Phenylthio)-1H-indole-5-carbonitrile (3u)9a
White solid; mp 129–130 °C, yield = 180 mg (72%); 1H NMR (500 MHz, CDCl3): δ 8.77 (brs, 1H), 7.88 (s, 1H), 7.55 (d, J = 2.5 Hz, 1H), 7.45 (q, J = 8.5 Hz, 2H), 7.13 (t, J = 8.0 Hz, 2H), 7.04 (t, J = 7.5 Hz, 3H). 13C NMR (125 MHz, CDCl3): δ 138.4, 138.0, 132.8, 129.2, 129.1, 126.5, 126.2, 125.6, 125.5, 120.4, 112.8, 105.0, 104.3.
5-Bromo-3-((3,4,5-trimethoxyphenyl)thio)-1H-indole (3v)6a
White solid; mp 153–154 °C, yield = 255 mg (65%); 1H NMR (500 MHz, CDCl3): δ 8.74 (brs, 1H), 7.78 (s, 1H), 7.49 (d, J = 2.0 Hz, 1H), 7.35–7.26 (m, 2H), 6.36 (s, 2H), 3.79 (s, 3H), 3.68 (s, 6H). 13C NMR (125 MHz, CDCl3): 153.6, 136.0, 135.2, 133.8, 132.0, 131.0, 126.2, 122.3, 114.5, 113.3, 103.7, 103.1, 61.0, 56.2.
Phenyl(2,4,6-trimethoxyphenyl)sulfane (6a)26
White solid; mp 93–94 °C, yield = 196 mg (71%); 1H NMR (500 MHz, CDCl3): δ 7.15 (t, J = 8.0 Hz, 2H), 7.02 (d, J = 7.5 Hz, 3H), 6.20 (s, 2H), 3.85 (s, 3H), 3.79 (s, 6H). 13C NMR (125 MHz, CDCl3): δ 163.0, 162.6, 138.8, 128.6, 125.7, 124.5, 98.8, 91.3, 56.4, 55.5.
p-Tolyl(2,4,6-trimethoxyphenyl)sulfane (6b)26
White solid; mp 112–113 °C, yield = 223 mg (77%); 1H NMR (500 MHz, CDCl3): δ 6.97–6.93 (m, 4H), 6.20 (s, 2H), 3.85 (s, 3H), 3.80 (s, 6H), 2.24 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 162.9, 162.6, 135.1, 134.2, 129.4, 126.1, 99.5, 91.3, 56.4, 55.5, 21.0.
1-(Phenylthio)naphthalen-2-ol (7a)26
White solid; mp 66–67 °C, yield = 169 mg (67%); 1H NMR (500 MHz, CDCl3): δ 8.25 (d, J = 8.5 Hz, 1H), 7.92 (d, J = 8.0 Hz, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.52 (t, J = 7.5 Hz, 1H), 7.40–7.35 (m, 2H), 7.20–7.16 (m, 3H), 7.12 (d, J = 6.5 Hz, 1H), 7.05 (d, J = 7.5 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ 157.1, 135.6, 135.5, 133.0, 129.6, 129.3, 128.7, 128.1, 126.5, 126.0, 124.8, 124.0, 117.0, 108.2.
1-(p-Tolylthio)naphthalen-2-ol (7b)26
White solid; mp 78–79 °C, yield = 189 mg (71%); 1H NMR (500 MHz, CDCl3): δ 8.23 (d, J = 8.5 Hz, 1H), 7.89 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.5 Hz, 1H), 7.37–7.31 (m, 2H), 7.20 (s, 1H), 6.98–6.93 (m, 4H), 2.23 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 157.0, 136.0, 135.6, 132.8, 131.9, 130.1, 129.6, 128.7, 128.0, 126.8, 124.8, 123.9, 117.0, 108.8, 21.0.
1,2-Diphenyldisulfane (2aa)23a
White solid; mp 58–59 °C, yield = 201 mg (92%); 1H NMR (500 MHz, CDCl3): δ 7.55 (d, J = 8.0 Hz, 4H), 7.35–7.26 (m, 6H). 13C NMR (125 MHz, CDCl3): δ 137.2, 129.2, 127.6, 127.3.
Acknowledgments
We are grateful to the CSIR, New Delhi (Scheme No. 02(0285)/16/ EMR-II), for financial assistance. A.K.P. is grateful to the DST, New Delhi, for an INSPIRE fellowship and S.C. is grateful to the CSIR, New Delhi, for a JRF.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00472.
The authors declare no competing financial interest.
Supplementary Material
References
- a Ghosh P.; Nandi A. K.; Chhetri G.; Das S. Generation of ArS- and ArSe-Substituted 4-Quinolone Derivatives Using Sodium Iodide As an Inducer. J. Org. Chem. 2018, 83, 12411–12419. 10.1021/acs.joc.8b01426. [DOI] [PubMed] [Google Scholar]; b Patil R.; Ghosh A.; Cao P. S.; Sommer R. D.; Grice K. A.; Waris G.; Patil S. Novel 5-arylthio-5H-chromenopyridines as a new class of anti-fibrotic agents. Bioorg. Med. Chem. Lett. 2017, 27, 1129–1135. 10.1016/j.bmcl.2017.01.089. [DOI] [PubMed] [Google Scholar]; c Bang-Andersen B.; Ruhland T.; Jørgensen M.; Smith G.; Frederiksen K.; Jensen K. G.; Zhong H.; Nielsen S. M.; Hogg S.; Mørk A.; Stensbøl T. B. Discovery of 1-[2-(2,4-Dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): A Novel Multimodal Compound for the Treatment of Major Depressive Disorder. J. Med. Chem. 2011, 54, 3206–3221. 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]; d La Regina G.; Gatti V.; Famiglini V.; Piscitelli F.; Silvestri R. Venting-while-Heating Microwave-Assisted Synthesis of 3-Arylthioindoles. ACS Comb. Sci. 2012, 14, 258–262. 10.1021/co200165j. [DOI] [PubMed] [Google Scholar]; e Campbell J. A.; Bordunov V.; Broka C. A.; Browner M. F.; Kress J. M.; Mirzadegan T.; Ramesha C.; Sanpablo B. F.; Stabler R.; Takahara P.; Villasenor A.; Walker K. A. M.; Wang J.-H.; Welch M.; Weller P. Rational design of 6-methylsulfonylindoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 4741–4745. 10.1016/j.bmcl.2004.06.075. [DOI] [PubMed] [Google Scholar]; f Silvestri R.; Martino G. D.; Regina G. L.; Artico M.; Massa S.; Vargiu L.; Mura M.; Loi A. G.; Marceddu T.; Colla P. L. Novel Indolyl Aryl Sulfones Active against HIV-1 Carrying NNRTI Resistance Mutations: Synthesis and SAR Studies. J. Med. Chem. 2003, 46, 2482–2493. 10.1021/jm0211063. [DOI] [PubMed] [Google Scholar]
- a Guo Y.; Zhong S.; Wei L.; Wan J.-P. Transition-metal-free synthesis of 3-sulfenylated chromones via KIO3-catalyzed radical C(sp2)-H sulfenylation. Beilstein J. Org. Chem. 2017, 13, 2017–2022. 10.3762/bjoc.13.199. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu Y.; Xiang J.; Wei L. Recent Advances in the C(sp2)-S Bond Formation Reactions by Transition Metal-Free C(sp2)-H Functionalization. Chin. J. Org. Chem. 2017, 37, 1667–1680. 10.6023/cjoc201702009. [DOI] [Google Scholar]; c Halimehjani A. Z.; Shokrgozar S.; Beier P. Transition-Metal-Free Coupling Reaction of Dithiocarbamates with Indoles: C–S Bond Formation. J. Org. Chem. 2018, 83, 5778–5783. 10.1021/acs.joc.8b00206. [DOI] [PubMed] [Google Scholar]
- a Katritzky A. R.; Pozharskii A. F.. Handbook of Heterocyclic Chemistry, 3rd ed., Elsevier: Oxford, 2010. [Google Scholar]; b Ban F.; Leblanc E.; Li H.; Munuganti R. S.; Frewin K.; Rennie P. S.; Cherkasov A. Discovery of 1H-Indole-2-carboxamides as Novel Inhibitors of the Androgen Receptor Binding Function 3 (BF3). J. Med. Chem. 2014, 57, 6867–6872. 10.1021/jm500684r. [DOI] [PubMed] [Google Scholar]; c Kaila N.; Follows B.; Leung L.; Thomason J.; Huang A.; Moretto A.; Janz K.; Lowe M.; Mansour T. S.; Hubeau C.; Page K.; Morgan P.; Fish S.; Xu X.; Williams C.; Saiah E. Discovery of Isoquinolinone Indole Acetic Acids as Antagonists of Chemoattractant Receptor Homologous Molecule Expressed on Th2 Cells (CRTH2) for the Treatment of Allergic Inflammatory Diseases. J. Med. Chem. 2014, 57, 1299–1322. 10.1021/jm401509e. [DOI] [PubMed] [Google Scholar]; d Tzvetkov N. T.; Hinz S.; Kuppers P.; Gastreich M.; Muller C. E. Indazole- and Indole-5-carboxamides: Selective and Reversible Monoamine Oxidase B Inhibitors with Subnanomolar Potency. J. Med. Chem. 2014, 57, 6679–6703. 10.1021/jm500729a. [DOI] [PubMed] [Google Scholar]; e Zandt M. C. V.; Jones M. L.; Gunn D. E.; Geraci L. S.; Jones J. H.; Sawicki D. R.; Sredy J.; Jacot J. L.; Dicioccio A. T.; Petrova T.; Mischler A.; Podjarny A. D. Discovery of 3-[(4,5,7-Trifluorobenzothiazol-2-yl)methyl]indole-N-acetic Acid (Lidorestat) and Congeners as Highly Potent and Selective Inhibitors of Aldose Reductase for Treatment of Chronic Diabetic Complications. J. Med. Chem. 2005, 48, 3141–3152. 10.1021/jm0492094. [DOI] [PubMed] [Google Scholar]; f Casapullo A.; Bifulco G.; Bruno I.; Riccio R. New Bisindole Alkaloids of the Topsentin and Hamacanthin Classes from the Mediterranean Marine Sponge Rhaphisia lacazei. J. Nat. Prod. 2000, 63, 447–451. 10.1021/np9903292. [DOI] [PubMed] [Google Scholar]
- Taber D. F.; Tirunahari P. K. Indole synthesis: a review and proposed classification. Tetrahedron 2011, 67, 7195–7210. 10.1016/j.tet.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini M.; Eichholzer A. Catalytic Functionalization of Indoles in a New Dimension. Angew. Chem., Int. Ed. 2009, 48, 9608–9644. 10.1002/anie.200901843. [DOI] [PubMed] [Google Scholar]
- a Regina G. L.; Edler M. C.; Brancale A.; Kandil S.; Coluccia A.; Piscitelli F.; Hamel E.; Martino G. D.; Matesanz R.; Díaz J. F.; Scovassi A. I.; Prosperi E.; Lavecchia A.; Novellino E.; Artico M.; Silvestri R. Arylthioindole Inhibitors of Tubulin Polymerization. 3. Biological Evaluation, Structure-Activity Relationships and Molecular Modeling Studies. J. Med. Chem. 2007, 50, 2865–2874. 10.1021/jm061479u. [DOI] [PubMed] [Google Scholar]; b Ragno R.; Coluccia A.; Regina G. L.; Martino G. D.; Piscitelli F.; Lavecchia A.; Novellino E.; Bergamini A.; Ciaprini C.; Sinistro A.; Maga G.; Crespan E.; Artico M.; Silvestri R. Design, Molecular Modeling, Synthesis, and Anti-HIV-1 Activity of New Indolyl Aryl Sulfones. Novel Derivatives of the Indole-2-carboxamide. J. Med. Chem. 2006, 49, 3172–3184. 10.1021/jm0512490. [DOI] [PubMed] [Google Scholar]; c Funk C. D. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat. Rev. Drug Discovery 2005, 4, 664–672. 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]; d de Martino G.; Regina G. L.; Coluccia A.; Edler M. C.; Barbera M. C.; Brancale A.; Wilcox E.; Hamel E.; Artico M.; Silvestri R. Arylthioindoles, Potent Inhibitors of Tubulin Polymerization. J. Med. Chem. 2004, 47, 6120–6123. 10.1021/jm049360d. [DOI] [PubMed] [Google Scholar]
- La Regina G.; Bai R.; Rensen W.; Coluccia A.; Piscitelli F.; Gatti V.; Bolognesi A.; Lavecchia A.; Granata I.; Porta A.; Maresca B.; Soriani A.; Iannitto M. L.; Mariani M.; Santoni A.; Brancale A.; Ferlini C.; Dondio G.; Varasi M.; Mercurio C.; Hamel E.; Lavia P.; Novellino E.; Silvestri R. Design and Synthesis of 2-Heterocyclyl-3-arylthio-1H-indoles as Potent Tubulin Polymerization and Cell Growth Inhibitors with Improved Metabolic Stability. J. Med. Chem. 2011, 54, 8394–8406. 10.1021/jm2012886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nalbandian C. J.; Miller E. M.; Toenjes S. T.; Gustafson J. L. A conjugate Lewis base-Brønsted acid catalyst for the sulfenylation of nitrogen containing heterocycles under mild conditions. Chem. Commun 2017, 53, 1494–1497. 10.1039/C6CC09998J. [DOI] [PubMed] [Google Scholar]; b Wang F.-X.; Zhou S.-D.; Wang C.; Tian S.-K. N-Hydroxy sulfonamides as new sulfenylating agents for the functionalization of aromatic compounds. Org. Biomol. Chem. 2017, 15, 5284–5288. 10.1039/C7OB01390F. [DOI] [PubMed] [Google Scholar]; c Li J.; Li C.; Yang S.; An Y.; Wu W.; Jiang H. Palladium-Catalyzed Oxidative Sulfenylation of Indoles and Related Electron-Rich Heteroarenes with Aryl Boronic Acids and Elemental Sulfur. J. Org. Chem. 2016, 81, 7771–7783. 10.1021/acs.joc.6b01428. [DOI] [PubMed] [Google Scholar]; d Liu J.; Li P.; Chen W.; Wang L. An efficient synthesis of 2-bromo(chloro)-3-selenyl(sulfenyl) indoles via tandem reactions of 2-(gem-dibromovinyl)anilines with diselenides(disulfides). Chem. Commun. 2012, 48, 10052–10054. 10.1039/c2cc34800d. [DOI] [PubMed] [Google Scholar]; e Du H.-A.; Tang R.-Y.; Deng C.-L.; Liu Y.; Li J.-H.; Zhang X.-G. Iron-Facilitated Iodine-Mediated Electrophilic Annulation of N,N-Dimethyl-2-alkynylanilines with Disulfides or Diselenides. Adv. Synth. Catal. 2011, 353, 2739–2748. 10.1002/adsc.201100349. [DOI] [Google Scholar]; f Chen Y.; Cho C.-H.; Larock R. C. A Novel Synthetic Route to 3-Sulfenyland 3-Selenylindoles by n-Bu4NI-Induced Electrophilic Cyclization. Org. Lett. 2009, 11, 173–176. 10.1021/ol8021287. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Chen Y.; Cho C.-H.; Shi F.; Larock R. C. Synthesis of 3-Sulfenyl- and 3-Selenylindoles by the Pd/Cu-Catalyzed Coupling of N,N-Dialkyl-2-iodoanilines and Terminal Alkynes, Followed by n-Bu4NI-Induced Electrophilic Cyclization. J. Org. Chem. 2009, 74, 6802–6811. 10.1021/jo9014003. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Guo Y.-J.; Tang R.-Y.; Li J.-H.; Zhong P.; Zhang X.-G. Palladium-Catalyzed Annulation of 2-(1- Alkynyl)benzenamines with Disulfides: Synthesis of 3-Sulfenylindoles. Adv. Synth. Catal. 2009, 351, 2615–2618. 10.1002/adsc.200900055. [DOI] [Google Scholar]
- a Chen M.; Luo Y.; Zhang C.; Guo L.; Wang Q.; Wu Y. Graphene Oxide Mediated Thiolation of Indoles in Water: A Green and Sustainable Approach to 3-Sulfenylindoles. Org. Chem. Front. 2019, 6, 116–120. 10.1039/C8QO00726H. [DOI] [Google Scholar]; b Bering L.; D’ Ottavio L.; Sirvinskaite G.; Antonchick A. P. Nitrosonium Ion Catalysis: Aerobic, Metal-Free Cross Dehydrogenative Carbon-Heteroatom Bond Formation. Chem. Commun. 2018, 54, 13022–13025. 10.1039/C8CC08328B. [DOI] [PubMed] [Google Scholar]; c Ohkado R.; Ishikawa T.; Iida H. Flavin–Iodine Coupled Organocatalysis for Aerobic Oxidative Direct Sulfenylation of Indoles with Thiols under Mild Conditions. Green Chem. 2018, 20, 984–988. 10.1039/C8GC00117K. [DOI] [Google Scholar]; d Bai F.; Zhang S.; Wei L.; Liu Y. Transition-Metal-Free Indole C3 Sulfenylation by KIO3 Catalysis. Asian J. Org. Chem. 2018, 7, 371–373. 10.1002/ajoc.201700677. [DOI] [Google Scholar]; e Song S.; Zhang Y.; Yeerlan A.; Zhu B.; Liu J.; Jiao N. Cs2CO3-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling of Thiols with Phosphonates and Arenes. Angew. Chem., Int. Ed. 2017, 56, 2487. 10.1002/anie.201612190. [DOI] [PubMed] [Google Scholar]; f Saima; Equbal D.; Lavekar A. G.; Sinha A. K. Cooperative Catalysis by Bovine Serum Albumin-Iodine Towards Cascade Oxidative Coupling-C(sp2)-H Sulfenylation of Indoles/Hydroxyaryls with Thiophenols on water. Org. Biomol. Chem. 2016, 14, 6111–6118. 10.1039/C6OB00930A. [DOI] [PubMed] [Google Scholar]; g Liu X.; Cui H.; Yang D.; Dai S.; Zhang G.; Wei W.; Wang H. Iodine-catalyzed Direct Thiolation of Indoles with Thiols Leading to 3-Thioindoles Using Air as the Oxidant. Catal. Lett. 2016, 146, 1743–1748. 10.1007/s10562-016-1798-2. [DOI] [Google Scholar]; h Zhang H.; Bao X.; Song Y.; Qu J.; Wang B. Iodine-catalysed versatile sulfenylation of indoles with thiophenols: controllable synthesis of mono- and bis-arylthioindoles. Tetrahedron 2015, 71, 8885–8891. 10.1016/j.tet.2015.09.070. [DOI] [Google Scholar]; i Liu Y.; Zhang Y.; Hu C.; Wan J.-P.; Wen C. Synthesis of 3-sulfenylated indoles by a simple NaOH promoted sulfenylation reaction. RSC Adv. 2014, 4, 35528–35530. 10.1039/C4RA05206D. [DOI] [Google Scholar]
- a Raghuvanshi D. S.; Verma N. Regioselective thiolation of electron rich arenes and heterocycle under recyclable catalytic media. RSC Adv. 2017, 7, 22860–22868. 10.1039/C7RA02350B. [DOI] [Google Scholar]; b Yang Y.; Zhang S.; Tang L.; Hu Y.; Zha Z.; Wang Z. Catalyst-free thiolation of indoles with sulfonyl hydrazides for the synthesis of 3-sulfenylindoles in water. Green Chem. 2016, 18, 2609–2613. 10.1039/C6GC00313C. [DOI] [Google Scholar]; c Kumaraswamy G.; Raju R. Copper(I) induced sulfenylation of H-phosphonates, -phosphonites and phosphine Oxides with aryl/alkylsulfonylhydrazides as a thiol surrogate. Adv. Synth. Catal. 2014, 356, 2591–2598. 10.1002/adsc.201400116. [DOI] [Google Scholar]; d Yang F.-L.; Tian S.-K. Iodine-Catalyzed Regioselective Sulfenylation of Indoles with Sulfonyl Hydrazides. Angew. Chem., Int. Ed. 2013, 52, 4929–4932. 10.1002/anie.201301437. [DOI] [PubMed] [Google Scholar]
- a Ji Y.-Z.; Li H.-J.; Zhang J.-Y.; Wu Y.-C. Switchable regioselection of C-H thiolation of indoles using different TMS counterions. Chem. Commun. 2019, 55, 11864–11867. 10.1039/C9CC05652A. [DOI] [PubMed] [Google Scholar]; b Ge X.; Sun F.; Liu X.; Chen X.; Qian C.; Zhou S. Combined Experimental/theoretical Study on D-Glucosamine Promoted Regioselective Sulfenylation of Indoles Catalyzed by Copper. New J. Chem. 2017, 41, 13175–13180. 10.1039/C7NJ02784B. [DOI] [Google Scholar]; c Xiao F.; Xie H.; Liu S.; Deng G.-J. Iodine-Catalyzed Regioselective Sulfenylation of Indoles with Sodium Sulfinates. Adv. Synth. Catal. 2014, 356, 364–368. 10.1002/adsc.201300773. [DOI] [Google Scholar]
- a Luz E. Q.; Seckler D.; Araújo J. S.; Angst L.; Lima D. B.; Rios E. A. M.; Ribeiro R. R.; Rampon D. S. Fe(III)-Catalyzed direct C3 chalcogenylation of indole: The effect of iodide ions. Tetrahedron 2019, 75, 1258–1266. 10.1016/j.tet.2019.01.037. [DOI] [Google Scholar]; b Yu Y.; Zhou Y.; Song Z.; Liang G. An Efficient t-BuOK Promoted C3-Chalcogenylation of Indoles with Dichalcogenides. Org. Biomol. Chem. 2018, 16, 4958–4962. 10.1039/C8OB00948A. [DOI] [PubMed] [Google Scholar]; c Rafique J.; Saba S.; Franco M. S.; Bettanin L.; Schneider A. R.; Silva L. T.; Braga A. L. Direct, Metal-free C(sp2)-H Chalcogenation of Indoles and Imidazopyridines with Dichalcogenides Catalysed by KIO3. Chem. - Eur. J. 2018, 24, 4173–4180. 10.1002/chem.201705404. [DOI] [PubMed] [Google Scholar]; d Thurow S.; Penteado F.; Perin G.; Alves D.; Santi C.; Monti B.; Schiesser C. H.; Barcellosd T.; Lenardão E. J. Selenium Dioxide-promoted Selective Synthesis of Mono and Bis-sulfenylindoles. Org. Chem. Front. 2018, 5, 1983–1991. 10.1039/C8QO00360B. [DOI] [Google Scholar]; e Azeredo J. B.; Godoi M.; Martins G. M.; Silveira C. C.; Braga A. L. A Solvent- and Metal-Free Synthesis of 3-Chacogenyl-indoles Employing DMSO/I2 as an Eco-friendly Catalytic Oxidation System.. J. Org. Chem. 2014, 79, 4125–4130. 10.1021/jo5000779. [DOI] [PubMed] [Google Scholar]; f Sang P.; Chen Z.; Zou J.; Zhang Y. K2CO3 Promoted Direct Sulfenylation of Indoles: A Facile Approach of 3-Sulfenylindoles. Green Chem. 2013, 15, 2096–2100. 10.1039/c3gc40724a. [DOI] [Google Scholar]; g Ge W.; Wei Y. Iodine-Catalyzed Oxidative System for 3-Sulfenylation of Indoles with Disulfides using DMSO as oxidant under Ambient Conditions. Green Chem. 2012, 14, 2066–2070. 10.1039/c2gc35337g. [DOI] [Google Scholar]
- a Marcantoni E.; Cipolletti R.; Marsili L.; Menichetti S.; Properzi R.; Viglianisi C. An Efficient Catalytic Method for Regioselective Sulfenylation of Electron-Rich Aza-Aromatics at Room Temperature. Eur. J. Org. Chem. 2013, 2013, 132–140. 10.1002/ejoc.201201100. [DOI] [Google Scholar]; b Silveira C. C.; Mendes S. R.; Wolf L.; Martins G. M. The use of anhydrous CeCl3 as a catalyst for the synthesis of 3-sulfenyl indoles. Tetrahedron Lett. 2010, 51, 2014–2016. 10.1016/j.tetlet.2010.02.038. [DOI] [Google Scholar]; c Tudge M.; Tamiya M.; Savarin C.; Humphrey G. R. Development of a Novel, Highly Efficient Halide-Catalyzed Sulfenylation of Indoles. Org. Lett. 2006, 8, 565–568. 10.1021/ol052615c. [DOI] [PubMed] [Google Scholar]
- Jain S.; Shukla K.; Mukhopadhyay A.; Suryawanshi S. N.; Bhakuni D. S. The Reaction of Acyloxysulfonium Salt with Cyclic Enol Ethers: Novel Synthesis of Vinyl Sulfides. Synth. Commun. 1990, 20, 1315–1320. 10.1080/00397919008052843. [DOI] [Google Scholar]
- a Matsugi M.; Murata K.; Nambu H.; Kita Y. An efficient sulfenylation of aromatics using highly activequinone mono O,S-acetal bearing a pentafluorophenylthio group. Tetrahedron Lett. 2001, 42, 1077–1080. 10.1016/S0040-4039(00)02186-9. [DOI] [Google Scholar]; b Matsugi M.; Murata K.; Gotanda K.; Nambu H.; Anilkumar G.; Matsumoto K.; Kita Y. Facile and Efficient Sulfenylation Method Using Quinone Mono-O,S-Acetals under Mild Conditions. J. Org. Chem. 2001, 66, 2434–2441. 10.1021/jo001710q. [DOI] [PubMed] [Google Scholar]; c Matsugi M.; Gotanda K.; Murata K.; Kita Y. A novel efficient sulfenylation method using quinone mono-O,S-acetals under mild conditions. Chem. Commun. 1997, 1387–1388. 10.1039/a702912h. [DOI] [PubMed] [Google Scholar]
- a Hamel P. Mechanism of the Second Sulfenylation of Indole. J. Org. Chem. 2002, 67, 2854–2858. 10.1021/jo0109220. [DOI] [PubMed] [Google Scholar]; b Gilow H. M.; Brown C. S.; Copeland J. N.; Kelly K. E. Sulfenylation of some pyrroles and indoles. J. Heterocycl. Chem. 1991, 28, 1025–1034. 10.1002/jhet.5570280432. [DOI] [Google Scholar]; c Raban M.; Chern L.-J. Reactions of Arenesulfenyl Chlorides with Indole. 13C and 1H Nuclear Magnetic Resonance Spectra of 3-(Arylthio)indoles. J. Org. Chem. 1980, 45, 1688–1691. 10.1021/jo01297a033. [DOI] [Google Scholar]
- a Ghosh A.; Lecomte M.; Kim-Lee S.-H.; Radosevich A. T. Organophosphorus-Catalyzed Deoxygenation of Sulfonyl Chlorides: Electrophilic (Fluoroalkyl)sulfenylation by PIII/PV=O Redox Cycling. Angew. Chem., Int. Ed. 2019, 58, 2864–2869. 10.1002/anie.201813919. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Equbal D.; Singh R.; Malik S.; Lavekar A. G.; Sinha A. K. Synergistic Dual Role of [hmim]Br-ArSO2Cl in Cascade Sulfenylation–Halogenation of Indole: Mechanistic Insight into Regioselective C–S and C–S/C–X (X = Cl and Br) Bond Formation in One Pot. J. Org. Chem. 2019, 84, 2660–2675. 10.1021/acs.joc.8b03097. [DOI] [PubMed] [Google Scholar]; c He Y.; Jiang J.; Bao W.; Deng W.; Xiang J. TBAI-mediated regioselective sulfenylation of indoles with sulfonylchlorides in one pot. Tetrahedron Lett. 2017, 58, 4583–4586. 10.1016/j.tetlet.2017.10.040. [DOI] [Google Scholar]; d Wu Z.; Li Y.-C.; Ding W.-Z.; Zhu T.; Liu S.-Z.; Ren X.; Zou L.-H. Copper-Catalyzed Regioselective Sulfenylation of Indoles with Arylsulfonyl Chlorides. Asian J. Org. Chem. 2016, 5, 625–628. 10.1002/ajoc.201600096. [DOI] [Google Scholar]; e Chen M.; Huang Z. T.; Zheng Q. Y. Visible light-induced 3-sulfenylation of N-methylindoles with arylsulfonyl chlorides. Chem. Commun. 2012, 48, 11686–11688. 10.1039/c2cc36866h. [DOI] [PubMed] [Google Scholar]
- a Suleymanov A. A.; Scopelliti R.; Tirani F. F.; Severin K. One-Pot Synthesis of Trisubstituted Triazenes from Grignard Reagents and Organic Azides. Org. Lett. 2018, 20, 3323–3326. 10.1021/acs.orglett.8b01214. [DOI] [PubMed] [Google Scholar]; b Daia W.-C.; Wang Z.-X. Palladium-catalyzed coupling of azoles with 1-aryltriazenes via C-H/C-N cleavage. Org. Chem. Front. 2017, 4, 1281–1288. 10.1039/C7QO00174F. [DOI] [Google Scholar]; c Li W.; Wu X. F. Palladium-Catalyzed Carbonylative Sonogashira Coupling between Aryl Triazenes and Alkynes. Org. Biomol. Chem. 2015, 13, 5090–5093. 10.1039/C5OB00502G. [DOI] [PubMed] [Google Scholar]; d Zhang Y.; Li Y.; Zhang X.; Jiang X. Sulfide Synthesis through Copper-Catalyzed C-S Bonds Formation under Biomolecule-Compatible Conditions. Chem. Commun. 2015, 51, 941–944. 10.1039/C4CC08367A. [DOI] [PubMed] [Google Scholar]
- a Zhang Y.; Hu H.; Liu C. J.; Cao D.; Wang B.; Sun Y.; Abdukader A. Highly Efficient Brønsted Acidic Ionic Liquid Promoted Direct Diazenylation of Pyrazolones with Aryltriazenes under Mild Conditions. Asian J. Org. Chem. 2017, 6, 102–107. 10.1002/ajoc.201600475. [DOI] [Google Scholar]; b Cao D.; Zhang Y.; Liu C.; Wang B.; Sun Y.; Abdukader A.; Hu H.; Liu Q. Ionic Liquid Promoted Diazenylation of N-Heterocyclic Compounds with Aryltriazenes under Mild Conditions. Org. Lett. 2016, 18, 2000–2003. 10.1021/acs.orglett.6b00605. [DOI] [PubMed] [Google Scholar]; c Liu C.; Lv J.; Luo S.; Cheng J. P. Sc(OTf)3-Catalyzed Transfer Diazenylation of 1,3-Dicarbonyls with Triazenes via N–N Bond Cleavage. Org. Lett. 2014, 16, 5458–5461. 10.1021/ol5027014. [DOI] [PubMed] [Google Scholar]
- a Wang C.; Sun H.; Fang Y.; Huang Y. General and Efficient Synthesis of Indoles through Triazene-Directed C–H Annulation. Angew. Chem., Int. Ed. 2013, 52, 5795–5798. 10.1002/anie.201301742. [DOI] [PubMed] [Google Scholar]; b Wang C.; Chen H.; Wang Z.; Chen J.; Huang Y. Rhodium(III)-Catalyzed CH Activation of Arenes Using a Versatile and Removable Triazene Directing Group. Angew. Chem., Int. Ed. 2012, 51, 7242–7245. 10.1002/anie.201203230. [DOI] [PubMed] [Google Scholar]; c Hafner A.; Bräse S. Ortho-Trifluoromethylation of Functionalized Aromatic Triazenes. Angew. Chem., Int. Ed. 2012, 51, 3713–3715. 10.1002/anie.201107414. [DOI] [PubMed] [Google Scholar]
- a Leng J.; Wang S.-M.; Qin H.-L. Chemoselective synthesis of diaryl disulfides via a visible light-mediated coupling of arenediazonium tetrafluoroborates and CS2. Beilstein J. Org. Chem. 2017, 13, 903–909. 10.3762/bjoc.13.91. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Firouzabadi H.; Iranpoor N.; Samadi A. One-pot synthesis of aryl alkyl thioethers and diaryl disulfidesusing carbon disulfide as a sulfur surrogate in the presence of diethylamine catalyzed by copper(I) iodide in polyethyleneglycol (PEG200). Tetrahedron Lett. 2014, 55, 1212–1217. 10.1016/j.tetlet.2014.01.001. [DOI] [Google Scholar]; c Zhao P.; Yin H.; Gao H.; Xi C. Cu-Catalyzed Synthesis of Diaryl Thioethers and S-Cycles by Reaction of Aryl Iodides with Carbon Disulfide in the Presence of DBU. J. Org. Chem. 2013, 78, 5001–5006. 10.1021/jo400709s. [DOI] [PubMed] [Google Scholar]; d Wang F.; Cai S.; Wang Z.; Xi C. Synthesis of 2-Mercaptobenzothiazoles via DBU-Promoted Tandem Reaction of o-Haloanilines and Carbon Disulfide. Org. Lett. 2011, 13, 3202–3205. 10.1021/ol2011105. [DOI] [PubMed] [Google Scholar]; e Chatterjee T.; Bhadra S.; Ranu B. C. Transition metal free procedure for the synthesis of S-aryl dithiocarbamates using aryl diazonium fluoroborate in water at room temperature. Green Chem. 2011, 13, 1837–1842. 10.1039/c1gc00001b. [DOI] [Google Scholar]; f Barba F.; Ranz F.; Batanero B. Electrochemical transformation of diazonium salts into diaryl disulfides. Tetrahedron Lett. 2009, 50, 6798–6799. 10.1016/j.tetlet.2009.09.102. [DOI] [Google Scholar]
- Harmata M.; Hong X.; Zheng P. ortho Substituent effect on a 1,5-H shift reaction during thermal decomposition of aryltriazenes. Tetrahedron Lett. 2006, 47, 7343–7347. 10.1016/j.tetlet.2006.08.017. [DOI] [Google Scholar]
- a Singh R.; Raghuvanshi D. S.; Singh K. N. Regioselective Hydrothiolation of Alkynes by Sulfonyl Hydrazides Using Organic Ionic BaseBrønsted Acid. Org. Lett. 2013, 15, 4202–4205. 10.1021/ol401925u. [DOI] [PubMed] [Google Scholar]; b Singh N.; Singh R.; Raghuvanshi D. S.; Singh K. N. Convenient MW-Assisted Synthesis of Unsymmetrical Sulfides Using Sulfonyl Hydrazides as Aryl Thiol Surrogate. Org. Lett. 2013, 15, 5874–5877. 10.1021/ol402948k. [DOI] [PubMed] [Google Scholar]; c Singh R.; Allam B. K.; Singh N.; Kumari K.; Singh S. K.; Singh K. N. A Direct Metal-Free Decarboxylative Sulfono Functionalization (DSF) of Cinnamic Acids to α,β-Unsaturated Phenyl Sulfones. Org. Lett. 2015, 17, 2656–2659. 10.1021/acs.orglett.5b01037. [DOI] [PubMed] [Google Scholar]; d Singh R.; Allam B. K.; Singh N.; Kumari K.; Singh S. K.; Singh K. N. Nickel-Catalyzed C–S Bond Formation: Synthesis of Aryl Sulfides from Arylsulfonyl Hydrazides and Boronic Acids. Adv. Synth. Catal. 2015, 357, 1181–1186. 10.1002/adsc.201400983. [DOI] [Google Scholar]; e Pandey A. K.; Kumar S.; Singh R.; Singh K. N. A practical synthesis of aryl sulfones via cross-coupling of sulfonylhydrazides with aryltriazenes using copper/ionic liquid combination. Tetrahedron 2018, 74, 6704–6709. 10.1016/j.tet.2018.09.059. [DOI] [Google Scholar]
- Giansanti V.; Piscitelli F.; Camboni T.; Prosper E.; Regina G. L.; Parks M.; Silvestri R.; Scovassi A. I. Arylthioindoles: Promising compounds against cancer cell proliferation. Oncol. Lett. 2010, 1, 109–112. 10.3892/ol_00000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H.; Zhang T.; Wan K.; Luo M. Catalytic Synthesis of 3-Thioindoles Using Bunte Salts as Sulfur Sources under Metal-Free Conditions. J. Org. Chem. 2016, 81, 4262–4268. 10.1021/acs.joc.6b00636. [DOI] [PubMed] [Google Scholar]
- Parumala S. K. R.; Peddinti R. K. Iodine catalyzed cross-dehydrogenative C–S coupling by C(sp2)-H bond activation: Direct access to aryl sulfides from aryl thiols. Green Chem. 2015, 17, 4068–4072. 10.1039/C5GC00403A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.