Abstract
The in situ hydrogel drug delivery system is a hot research topic in recent years. Combining both properties of hydrogel and solution, in situ hydrogels can provide many advantages for drug delivery application, including easy application, high local drug concentration, prolonged drug retention time, reduced drug dose in vivo, good biocompatibility and improved patient compliance, thus has potential in tumor treatment. In this paper, the related literature reports in recent years were reviewed to summarize and discuss the research progress and development prospects in the application of in situ hydrogels in tumor treatment.
Keywords: In situ hydrogel, tumor treatment, drug delivery system, progress
1. Introduction
Tumors are one type of the malignant diseases that threaten human health most. With the aging of the world’s population and changing in living environment and daily habits, the number of people died from tumors has been increasing dramatically every year, and the world has fallen into conflict with the terrible war of tumors (Shaker et al., 2016; Siegel et al., 2019; Feng et al., 2019). The treatment of tumors has become an important task for medical researchers. At present, the main treatments for tumors include surgical resection, chemotherapy, and radiotherapy (Mao et al., 2016; Watanabe et al., 2017). Among them, chemotherapy is a conventional method and plays an important irreplaceable role in tumor treatment. However, many candidate compounds or drugs currently developed or used clinically in tumor treatment have many problems, including strong hydrophobicity, low bioavailability, instability, greater toxicity and side effects, and lack of targeting, etc., thus fails to fully meet the clinical needs of tumor treatment (Kakinoki et al., 2007; Almeida et al., 2014; Fu & Wu, 2014; Lu et al., 2015; Khaliq et al., 2017; Cullen et al., 2019). Therefore, it is an urgent task to design and construct new delivery system of antitumor drugs for tumor treatment with high efficiency and low toxicity. Sustained and/or targeted release formulations can make the tumor tissues exposed to high-concentration antitumor drugs for a long time, thus avoiding the inconvenience caused by continuous administration, and minimizing the toxic and side effects of antitumor drugs because of relatively low drug uptake by systemic normal cells thanks to tumor local administration (Jung et al., 2018; Chen et al., 2019; Fan et al., 2019; Gibbens-Bandala et al., 2019).
Based on the problems mentioned above, the in situ hydrogel drug delivery system has attracted attention from more and more researchers in recent years. Being made of polymer materials with solution, suspension or semi-solid state, the in-situ hydrogel system can undergo phase change at the site of administration immediately after administration, making the solution or suspension transformed into a semi-solid or solid state (Chu et al., 2013; Morsi et al., 2016). Due to its liquid nature, it is easy to apply to drug absorption sites and convenient to deliver drugs to wanted sites of patients after the formulation is formed (Paulsamy et al., 2018). Hydrogel formation depends on several factors including pH changes, temperature adjustment and the presence of ions or light. Because of the advantages of this system including local and specific site effects, prolonged drug delivery, reduced drug dosage, increased bioavailability, reduced side effects, and improved patient comfort and compliance (Shen et al., 2012; Lu et al., 2015; Ellah et al., 2018), in-situ hydrogels have become a research hotspot for scientific researchers in the treatment of tumors. The researchers in the related fields have tried to develop in situ hydrogel system achieving two main goals of drug administration: the first one is to specifically and safely deliver and target the drug to its site of action through local drug delivery (intratumor/paratumor) to maximize its therapeutic efficacy while minimizing its toxicity, based on enhanced antitumor activity with increasing concentration of anti-tumor drugs at the target site (tumor); the second one is to extend the duration of the tumor’s exposure to the drug and maintain its release from the formulated drug carrier to help maintain its therapeutic effect after administration. The in situ hydrogel forms a drug depot at the injection site, which may reduce the total number of injections throughout the drug treatment process (Bendas et al., 2013; Moghassemi & Hadjizadeh, 2014; Shaker et al., 2015; Xing et al., 2015, 2019; Omidi et al., 2020). In order to summarize and improve study the application of in situ hydrogel system in tumor treatment, this paper focuses on the recent research progress in related areas.
2. Types and characteristics of in situ hydrogels
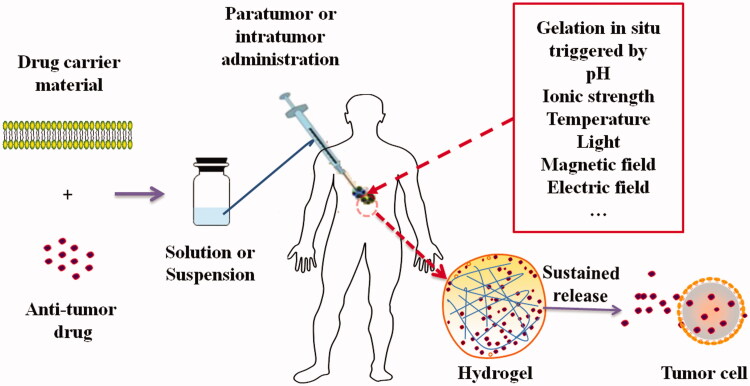
Because of the benefits from both advantages of solutions and hydrogels, in situ hydrogel system has the characteristics of local administration at the disease site, prolonged release cycle, reduced administration dose, and avoiding surgical implantation pain as a novel drug delivery system (Chen et al., 2019; Wei et al., 2019). The in situ hydrogel delivery system for anti-tumor drugs before administration is usually in the form of solution or suspension, which was formulated by dissolving or suspending anti-tumor drugs in water solution of drug carrier materials. When the solution or suspension was intratumorally or paratumorly administered, phase transition triggered by external conditions such as pH, temperature, ionic strength, light, magnetic field, and electric field can occur immediately and the hydrogel can form in situ. The three-dimensional network structure of the hydrogel can make the anti-tumor drugs incorporated in the in situ hydrogel constrained in or near the tumor tissue and sustained released from the hydrogels into tumor cells, thus may improve the anti-tumor effects and limit side-effects of the drugs. The process of formation of in situ hydrogel and sustained release of drugs from the in situ hydrogel into tumor cells was schematically presented in Figure 1. The advantages of an ideal in situ hydrogel as a pharmaceutical preparation can include but not limit to (Cho et al., 2015; Ding et al., 2016; Meng et al., 2019; Zhang et al., 2019): (1) easy to functionalize and clear functional units; (2) biodegradable; (3) good biocompatibility; (4) suitable gelling speed and gel strength; (5) low immunogenicity and toxicity; (6) responsiveness to rapid stimuli from the external environment. With the deepening of researches in related areas, current in situ hydrogels can be divided into pH-, temperature-, ionic strength-, temperature-pH-, and light-sensitive and other types. We have consulted the literature on in situ hydrogels and their application in anti-tumor drugs for in vitro and in vivo tumor treatment studies, and summarized their gelling mechanism, drug carrier materials, therapeutic agents, in vitro and in vivo model into Table 1.
Figure 1.
Schematic process of formation of in situ hydrogel and sustained release of drugs from the hydrogel into tumor cells.
Table 1.
The in situ hydrogel systems in tumor treatment studied in recent years.
| Gelling mechanism | Materials | Therapeutic agents | Cancer cell (in vitro) | Tumor model (in vivo) | References |
|---|---|---|---|---|---|
| pH | Hyaluronic acid and fluorescein isothiocyanate conjugated mesoporous silica nanocomposites | Doxorubicin | SKBR3 | – | Chen and Liu (2016) |
| pH | N-carboxyethyl chitosan and dibenzaldehyde-terminated polyethylene glycol | Doxorubicin | HepG2 | – | Qu et al. (2017) |
| pH | Chitosan-grafted dihydrocaffeic acid and oxidized amylopectin | Doxorubicin | HCT116 | – | Liang et al. (2019) |
| pH | Dextran phosphate | Prospidine | HeLa and HepG2 | Liver cancer | Solomevich et al. (2019) |
| Temperature | Polyethylene glycol and monomethoxy poly(ethylene glycol)-poly(caprolactone) | Cisplatin and Paclitaxel | A549 | Lung cancer | Wu et al. (2014) |
| Temperature | Tamoxifen gallate liposomes | Tamoxifen | – | Breast cancer | Shaker et al. (2016) |
| Temperature | Graphene oxide, folic acid and hyaluronic acid-chitosan-g-poly (N-isopropylacrylamide) | Doxorubicin | MCF-7 | Breast cancer | Fong et al. (2017) |
| Temperature | Negatively charged survivin antisense oligonucleotide and positively charged | Survivin antisense oligonucleotide | MCF-7 | Breast cancer | Zhao et al. (2019) |
| Temperature | Chitosan, β-glyceryl phosphate and polyethylene imine modified superparamagnetic graphene oxide | Doxorubicin | MCF-7 | Breast cancer | Zhu et al. (2015) |
| Temperature | Oxaliplatin and tannic acid polymer nanoparticles | Oxaliplatin and Tannic Acid | HCT26 | Colorectal cancer | Ren et al. (2019) |
| Magnetic field | PEGylated iron oxide nanoparticles | Paclitaxel | – | Breast cancer | Wu et al. (2018) |
| Magnetic field | Alginate and xanthan gum | Levodopa | SH-SY5Y | – | Kondaveeti et al. (2018) |
| Magnetic field | α-amino acid residues, CoFe2O4 and Fe3O4 | Doxorubicin | HeLa | – | Casolaro et al. (2014) |
| Light | Hemoglobin and polyethylene glycol | Near infrared | A549 | Lung cancer | Lee et al. (2018) |
| Light | Hyaluronic acid, catechol compounds, gallic acid and iron ions | Near infrared | KB, 4T1 and A375 | breast cancer, and melanoma | Ko et al. (2019) |
| Light | Azobenzene and DNA | Doxorubicin | CEM | – | Kang et al. (2011) |
| Temperature-pH | Poly-N-isopropylacrylamide polymer | Anastrozole | MCF-7 | – | Singh et al. (2019) |
| Temperature-pH | PEG monomethacrylate, and acrylic acid | 5-fluorouracil | HepG2 | – | Yue et al. (2019) |
| Temperature-pH | Plunik and polyacrylic acid | Epirubicin | – | Colon adenocarcinoma | Lo et al. (2012) |
| Temperature-magnetic field | Magnetic iron oxide nanoparticles | Magnetic heat | U87-MG | Glioblastoma | Zhang and Song (2016) |
| Ion strength/pH | Self-assembling peptide RADA16-I | Emodin | A549 and HepG2 | – | Wei et al. (2019) |
| Ion strength/pH | Self-assembling peptide RADA16-I | Mangiferin | DLD-1 and KYSE 30 | – | Meng et al. (2019) |
| pH-light | Black phosphorus nanosheets, dibenzaldehyde functionalized polymers and polyaspartic hydrazide polymers | Doxorubicin | MDA-MB-231 | Breast cancer | Wu et al. (2019) |
2.1. pH-sensitive in situ hydrogel
The molecular skeleton of the materials made of pH-sensitive hydrogel contains a large number of dissociable groups. With the change of the environmental pH and the repulsion between the charges, the molecular chains are stretched, expanded and tangled with each other to form a hydrogel (Yan & Jin, 2012; Sahoo et al., 2013; Shi et al., 2016; Demirdirek & Uhrich, 2017; Rizwan et al., 2017). Such hydrogels usually contain –COOH or –NH3, which can form ions with different surrounding pH levels and promote effective shrinkage or expansion of the hydrogel. In the acidic environment, new hydrogen bonds may be formed between the –COOH groups, and the composite hydrogen bonds promote the contraction of the hydrogel and prevent more water from being absorbed, making it difficult to release the loaded drug; while –COOH groups are ionized into carboxyl ions (–COO−) under alkaline conditions, and the electrostatic repulsion between the –COO− groups is strengthened, which causes the polymer chain to stretch and then the hydrogel expands and thus water can be absorbed in their 3 D grids, allowing drugs incorporated in the hydrogel to be released quickly. In contrast, the –NH3 group shrinks in an alkaline environment, but expands under acidic conditions.
2.2. Temperature-sensitive in situ hydrogel
Temperature-sensitive in situ hydrogels are liquid or semi-solid at room temperature. After administration, as the temperature rises from room temperature to body temperature, a phase transition occurs immediately at the site of application and the liquid or semi-solid form solidifies into hydrogel, resulting in good adhesion and slow release effects (Chu et al., 2013; Lin et al., 2014; Rarokar et al., 2016; Cao et al., 2019).
2.3. Ionic strength sensitive in situ hydrogel
Ionic strength-sensitive in situ hydrogel refers to a kind of hydrogel that can be formed through conformational changes in response to cations such as K+, Na+, and Ca2+ at the administration site. Typical materials used in this kind of hydrogel include alginate, deacetylated gellan gum, etc. When a dilute solution of alginate is introduced to solutions of monovalent or divalent metal ions (K+, Na+, Ca2+), a translucent hydrogel can immediately form in situ. Deacetylated gellan gum can immediately form a three-dimensional gel network structure when it encounters monovalent or divalent cations (K+, Na+, Ca2+), thus can also be used in situ hydrogel anti-tumor drug delivery system (Rupenthal et al., 2011; Janga et al., 2018; Yang et al., 2018). Due to the good biocompatibility, hydrophilicity and low toxicity related to commonly biogenic nature of drug carrier materials, this type of hydrogel deserves indepth studies and exploitment (Pareek et al., 2017; Shang et al., 2018).
2.4. Light-sensitive in situ hydrogel
Light-sensitive in situ hydrogels are prepared by incorporating photosensitive functional groups into a hydrogel network. In response to light signals, this type of hydrogel can change physical or chemical properties, including viscosity, conductivity, pH, solubility, wettability, mechanical properties, polymer morphology, etc. Under the light stimulus (ultraviolet, infrared, etc.) in the environment, the chromophore undergoes isomerization, cracking or dimerization through the induction of the chromophore in the photoreceptor. Then, the light signals can be transformed into a chemical signal, which in turn affects or changes the structure and performance of this type of hydrogel (Rastogi et al., 2018).
2.5. Other types of in situ hydrogels
Electric field sensitive in situ hydrogel means that the hydrogel can respond and deform quickly when the hydrogel is in an electric field, and the electric field responsive in situ hydrogels usually have good water-swelling performance (Kushwaha et al., 2012; Jiang et al., 2019); Magnetic field sensitive in situ hydrogel usually consists of a three-dimensional polymeric network and magnetic nanoparticles, and its swelling and shrinking can change with the change of the magnetic field (Liu et al., 2017; Liu et al., 2019).
2.6. Dual sensitive in situ hydrogel
The double-sensitive in situ hydrogel is formed by grafting polymerization of the two sensitive polymers or connecting them with an interpenetrating network, where each polymer chain also independently has different stimulus response (Norouzi et al., 2016; Wang et al., 2017; Fathi et al., 2019). The dual sensitive in-situ hydrogels, such as temperature-pH, temperature-ion or pH-magnetic field sensitive in-situ hydrogel, not only retain the responsiveness of single sensitive hydrogel, but also have complementary effects on different combinations of them, thus have great potential for increasing applications in various areas.
3. Application of in situ hydrogel delivery system in tumor treatment
3.1. pH-sensitive in situ hydrogel
Solid tumors usually show a slightly acidic environment with pH between 6.5 and 7.2. pH-sensitive nano-drug carriers are thus designed to release the drug under the conditions of the tumor’s slightly acidic environment, achieving passively targeting to the tumors (Bai et al., 2018; Wang et al., 2018; Ata et al., 2019; Xie et al., 2018). Injectable hydrogels with tissue adhesion and pH sensitivity are also highly needed for local drug delivery (Wu et al., 2018; Rakhshaei et al., 2019; Solomevich et al., 2019). Chen and Liu (2016) synthesized a mesoporous silica nanocomposite (MSN) of hyaluronic acid (HA) and fluorescein isothiocyanate (FITC), which can self-assemble into hydrogels in situ around tumor tissue by pH-responsive interactions (hydrogen bonds) between HA. The hydrogel can indicate the location of the entire tumor and stay in the microenvironment for a long time, providing a wealth of anti-cancer drugs in and around tumor tissue to avoid recurrence. Cytotoxicity experiments showed that FITC-HA-MSN nanohydrogels loaded with doxorubicin (Dox) had increased cytotoxicity to tumor cells with less toxicity to normal cells. Qu et al. (2017) prepared a series of pH-sensitive self-healing (means materials can restore their structures and functionalities after damage) injectable hydrogel by N-carboxyethyl chitosan (CEC) and dibenzaldehyde-terminated polyethylene glycol (PEGDA) synthesized by Michael reaction in aqueous solution. The dynamic covalent Schiff base bond between the amine group of CEC and the benzaldehyde group of PEGDA makes the hydrogel exhibit rapid self-repairing performance. The results of the cytotoxicity test showed that Dox released from the hydrogel matrix significantly inhibited the proliferation of HepG2 cells: at concentrations below 0.1 μg/mL, the hydrogel groups showed a stronger inhibitory effect than the free Dox group; at relatively high levels (0.1 μg/mL and 0.25 μg/mL), the inhibitory effect in the hydrogel group was the same as that in the free Dox group. This indicated that pH-responsive self-healing injectable hydrogels can be excellent candidates for carriers for liver cancer drugs. Liang et al. (2019) developed a series of multifunctional injectable pH-responsive hydrogel based on chitosan-grafted dihydrocaffeic acid (CS-DA) and oxidized amylopectin (OP) via Schiff base reaction. These hydrogels exhibited good injectability, suitable gelation time, in vitro pH-dependent equilibrated swelling ratios, morphologies, rheological characteristics, and desirable in vitro pH-sensitive drug release behavior. Cell studies showed that the DOX-loaded hydrogel groups had significant inhibitory effect on HCT116 cells better than that in the free DOX groups at same concentration of DOX. This shows that CS-DA/OP hydrogel has great potential as an anti-cancer drug delivery system for colon cancer treatment.
3.2. Temperature-sensitive in situ hydrogel
Wu et al. (2014) prepared a cisplatin (DDP)- containing thermosensitive poly(ethylene glycol)-poly(ε-caprolactone)-poly(ethylene glycol) (PEG-PCL-PEG/DDP, PECE/DDP) hydrogel and a paclitaxel (PTX)-loaded monomethoxy poly(ethylene glycol)-poly(caprolactone) (MPEG-PCL) micelle to establish in-situ hydrogel dual drug delivery system (PEG-PCL-PEG/DDP + MPEG-PCL/PTX, PDMP). PDMP shows a solution state at room temperature and forms a stationary hydrogel at body temperature, making it a promising drug reservoir for orthotopic treatment of lung cancer. In vivo lung cancer xenograft model tests showed that PDMP could inhibit tumor growth and prolong survival in tumor-bearing BALB/c nude mice with longer survival time (53 days versus 40 days in the free DDP + PTX group, 26 days in the blank control group and 25 days in the normal saline group). Shaker et al. (2016) prepared tamoxifen citrate liposomes by membrane hydration technology, and incorporated them into Planck’s thermosensitive hydrogel by cold method. Planck’s thermosensitive hydrogel was composed mainly of poloxamer, a kind of polymer nonionic surfactant, polyoxyethylene ether block copolymer, and the gelation temperature of the hydrogel system can be modified to optimal gelation at physiological temperature (34–37 °C) by changing the type and composition of the poloxamer (such as poloxamer 188 or 407). The higher viscosity and elasticity of the hydrogel at physiological temperature than that at room temperature or lower are the keys to controlling the release of the incorporated drug. In vitro release data showed the controlled release of tamoxifen (TMC) by the hydrogel for several consecutive days. The final tumor inhibition rates of free tamoxifen, tamoxifen liposomes, and tamoxifen thermosensitive gels were 68.54%, 84.04%, and 97.4%, respectively. The concentrations of tamoxifen in the tumors of mice in the liposome and hydrogel groups were 6 times and 14 times of that in the free drug group, respectively, and the drug concentrations in blood and liver of mice in the liposome and hydrogel groups were both significantly lower than those in the free drug group. The thermosensitive hydrogel immediately formed at body temperature can make the drug concentrated within the tumor site and less transferred to other organs and tissues, thus lessening damage to normal organs and tissues. Fong et al. (2017) prepared the folic acid (FA) conjugated graphene oxide (GO) (GOFA) for targeted delivery of the chemotherapy drug DOX by using the pH-sensitive drug release characteristics of GO after intracellular uptake. GOFA-DOX is further encapsulated in injectable in situ formed thermosensitive hyaluronic acid-chitosan-g-poly (N-isopropylacrylamide) (HACPN) hydrogel. Temperature-sensitive HACPN can provide rapid sol-gel phase transition kinetics near the normal temperature of human body to form the hydrogel in situ. The gelled HACPN can release drugs continuously to deliver DOX within the tumor. MTT experiments showed that the cytotoxicity of GOFA-DOX/HACPN on MCF-7 cells was dose and time dependents. In vivo anti-tumor experiments showed that the intratumor delivery of GOFA DOX/HACPN can be used as a safe and effective drug delivery system for breast cancer chemotherapy.
3.3. Magnetic field sensitive in situ hydrogel
The local recurrence of tumors after surgical resection is still a major challenge for medical field. Wu et al. (2018) prepared a shear-thinning injectable magnetic hydrogel using an inclusion complex between PEGylated iron oxide (Fe3O4) nanoparticles and α-cyclodextrin (α-CD). Self-assembling magnetic supramolecular hydrogel (MSH) was designed by partially inclusion complexes with α-CD penetrating the copolymer part on the surface of PEGylated Fe3O4 nanoparticles, so as to achieve shear thinning injection and controlled thermoreversible gel-sol transition. Experiment results showed that when MSH was evenly injected into the postoperative wound, MSH could flow freely and match the irregular cavity after tumor resection due to the magnetothermal gel-sol transition when exposed to an alternating current magnetic field (ACMF). In addition, biocompatible supramolecular hydrogels could co-load hydrophobic molecules such as PTX into the lipid layer of magnetic nanoparticles (MNPs). The dual structure of MSH can obtain different release processes of multiple drug molecules from a single sample, providing certain concentration of anticancer drugs for a long time. During the gel-sol transition, MNPs-mediated induction heat can exert heat-induced cell damage to tumor cells and trigger the release of anti-tumor drugs. In vivo experiments on tumor-bearing animal models showed that MSH has the trustful potential to synergistically eliminate tumors and completely prevent local recurrence of breast cancer after surgical resection. Kondaveeti et al. (2018) prepared a magnetically responsive hydrogel consisting of alginate (Alg) and xanthan gum (XG), which was cross-linked with Ca2+ ions and modified by the formation of in-situ magnetic nanoparticles (MNP). Compared with magnetic Alg hydrogel, due to the high charge density and molecular weight of XG, magnetic Alg-XG hydrogel had excellent mechanical and swelling properties, which made it significantly more efficient to load levodopa (LD). Under the static electromotive force (EMF) stimulation of 0.4 T, the magnetic stimulus release time of LD from Alg-XG/MNP hydrogel exceeds 30 h, which indicated the potential of sustained release.
3.4. Temperature-pH dual-sensitive in situ hydrogel
Being sensitive to temperature and pH, poly-N-isopropylacrylamide (PNIPAM) polymer can undergo phase change process at body temperature (37 °C) and/or in slightly acidic environment. Singh et al. (2019) prepared temperature-/pH-triggered PNIPAM smart nanogel systems (NPs) loaded with anastrozole (ANST) by solvent evaporation method for pH and thermally responsive drug delivery. The releases of ANST from this formulation at pH 5.0 were much faster than at pH 7.4 which may indicate ideal release in tumor microenvironment. In vitro cytotoxicity showed that the NPs have higher cell uptake, better anti-cancer effects on MCF-7 cells than that of free ANST. Yue et al. (2019) prepared a heat and pH sensitive hydrogel (PAA) through a free-radical copolymerization reaction in water by using PEG monomethacrylate (PEGMA) and acrylic acid (AAc) as monomers, potassium persulfate (K2S2O8), and sodium thiosulfate (Na2S2O3) as initiators. The ideal intestinal adhesion and amphiphilic properties of PEGMA thanks to its microstructure of a hydrophobic polymethacrylate backbone with a hydrophilic PEG side chain can make the hydrogels sensitive to the temperature of the gastrointestinal tract; while the combination of AAc functional groups can make the hydrogels producing stronger physical cross-links through intra-chain and inter-chain associations with non-covalent forces, thus improving the pH sensitivity and mechanical properties of the hydrogels. The dual sensitivity of the PAA hydrogels was confirmed by swelling properties of the hydrogel in different temperatures and buffer solutions being measured by gravimetric analysis. The release profiles of hydrogel-encapsulated 5-fluorouracil (5-Fu) at different pH (stomach pH 1.2 and intestinal pH 7.4) and temperature (25 °C and 37 °C) showed that the release of 5-Fu from the hydrogels was faster at pH 7.4/25 °C than at pH 1.2/37 °C. Cytotoxicity experiments on normal cell lines LO2 and HepG2 cancer cell lines showed that the hydrogel had good cell compatibility and the 5-Fu-loaded hydrogels had a slightly weaker but sustained cell-inhibiting activity than free 5- Fu, which might be due to the slow and long-term release of 5-Fu from drug-loaded hydrogels. Lo et al. developed an oral in situ temperature and pH sensitive hydrogel formulation consisting of polyacrylic acid, for the delivery of the anticancer drug epirubicin (Epi). In vivo anti-CT-26 mouse colon adenocarcinoma experiments showed better effectiveness of oral administration of Epi in situ temperature and pH-sensitive hydrogel preparations in inhibiting tumor growth than that of intravenous or other oral preparations. Generally, in situ hydrogels require injection or topical application. This oral in situ temperature and pH sensitive hydrogel formulation may provide a promising basis for future use of oral in situ hydrogels in cancer treatment.
3.5. Light-sensitive in situ hydrogel
Lee et al. (2019) prepared a hemoglobin (Hb) hydrogel that exhibited excellent photothermal therapy (PTT) effects in vitro and in vivo without obvious toxicity observed in major organs. It showed that the therapeutic effect of near infrared (NIR) irradiation on Hb-80 hydrogel was obviously stronger than that without NIR irradiation. Hb hydrogel combined with NIR irradiation is expected to be used in PTT system to improve the anticancer activity of Hb hydrogel loaded drugs. According to relevant reports (Raymond et al., 2015), microorganisms such as bacteria, yeast and fungi can produce iron carriers, which are used as iron chelators for intracellular transport, while carboxyl groups and catechol iron carriers have been proven to have natural iron chelating ability. This indicates that hyaluronic acid (HA) and catechol compound gallic acid (GA) may have iron coordination activity. Therefore, Ko et al. (2019) developed an injectable and photosensitive hydrogel based on the ability of HA and GA to form coordination bonds with iron ions (Fe3+). The conjugate of HA and GA (HA-GA) immediately formed a hydrogel in the presence of iron ions, and showed photothermal characteristics in the NIR response. Studies showed that after HA-GA was injected subcutaneously into mice, HA-GA and Fe3+ form a hydrogel and stay at the injection site for at least 8 days; HA-GA/Fe hydrogel was injected intratumorally into the mouse, and radiation with NIR showed that NIR irradiation caused complete ablation of tumors in KB cancer cell xenograft mouse and inhibited lung metastasis of 4T1-Luc orthotopic breast tumors. The hydrogel was applied to the skin of an A375 melanoma transplanted tumor, and then subjected to NIR irradiation, which also completely ablated the tumor. The results showed that HA-GA/Fe hydrogel has photothermal anticancer effects on solid tumors and skin cancer. Kang et al. (2011) developed a photoresponsive DNA-cross-linked hydrogel by incorporating photosensitive azobenzene moieties into DNA strands as crosslinkers to make their hybridization to complementary DNAs (cDNAs) responding differently to different wavelengths of light. The hydrogel was utilized for controllable encapsulation and release of fluorescein, horseradish peroxidase, gold nanoparticles and chemotherapy drug doxorubicin by taking advantage of the photoinduced reversible sol-gel conversion. Doxorubicin was encapsulated inside the hydrogel at 450 nm and then released by photons at 350 nm. The in vitro release results showed a net drug release rate of 65% within 10 min of doxorubicin with maintained therapeutic effect. This indicated the potential of the hydrogel system to be a promising platform for anti-tumor drug delivery in targeted therapy.
3.6. Other types of in situ hydrogels
At present, magnetothermal therapy (MHT) has been studied as an effective and noninvasive treatment for cancer. However, the short retention time of magnetic nanoparticles in tumor targets hinders their potential for repeatable treatment. Zhang and Song (2016) developed a biodegradable, heat-sensitive and superparamagnetic iron oxide nanoparticle-supported nanocapsule hydrogel (SPION-NHs) system. SPION-loaded nanocapsule solutions could be converted into hydrogel at body temperature through hydrophobic interactions. MTT results showed that compared to normal MHT, SPION-NHs made U87-MG cells nearly half died after heating at 50 °C for 25 min; confocal microscopy images further verified that cells underwent significant cell death induced by MHT and magnetic thermal ablation (MTA) under an alternating magnetic field (AMF). In vivo results from xenograft model showed that after a single injection of SPION-NHs, SPIONs remain in the tumor for more than three weeks, so MHT can be repeated multiple times. The magnatic resonance imaging (MRI) results showed that SPION-NHs was injected into the tumor once and the anti-tumor effect was particularly obvious after four MHT cycles without significant influence on the surrounding normal tissues. Studies showed that classic ion-complementary self-assembling peptide RADA16-I can encapsulate hydrophobic antitumor drugs such as PTX (Liu et al., 2011) and 5-Fu (Ashwanikumar et al., 2016). Recent studies further demonstrated that the peptide RADA16-I and RVDV16-I can stabilize hydrophobic anti-tumor drugs mangiferin and emodin and maintain or enhance their antitumor effects with the suspension-in situ hydrogel drug delivery system (Meng et al., 2019; Wei et al., 2019).
3.7. FDA approved in situ hydrogels
Compared with conventional parenteral and intravenous route of drug administration, the in situ hydrogel can be locally injected or targeted to the lesion site, allowing the diseased tissue to be in contact with the drug at a high concentration for a long time, avoiding the inconvenience caused by continuous administration. The local application of in situ hydrogel can also limit the toxicity and side effects of the drug (Huang et al., 2016; Demirdirek & Uhrich, 2017; Ailincai et al., 2018). Polyethylene glycol (PEG) (Zhang et al., 2015; Chin et al., 2018), hydroxyethyl methacrylate (HEMA) (Bonifacio et al., 2017), gellan gum (GG) (Boazak et al., 2019), poly-(DL-lactic-co-glycolic acid) (PLGA) (Kempe & Mäder, 2012; Park et al., 2019), lactide/glycolide copolymers and lactide/caprolactone copolymers (Singh et al., 2012) and block copolymers of polyethylene oxide (PEO) and polypropylene oxide (PPO) (Laddha & Mahajan, 2017) have been approved by the US Food and Drug Administration (FDA) as drug carrier materials for in situ hydrogel drug delivery system because of their biodegradable properties and nontoxic degradation products. Table 2 contains some of the FDA approved commercially available in situ hydrogels. Though only two of them are indicated for cancer, the desirable formulation characteristics and drug delivery performance of these in situ hydrogels also indicate that in-situ hydrogel can be widely applied to clinical treatment of tumor and other diseases.
Table 2.
List of some FDA approved in situ hydrogels.
| Product name | Gelling mechanism | Materials | Active ingredient | Indication | Approved |
|---|---|---|---|---|---|
| Atridox | Temperature | PLGA | Doxycycline hyclate | Adult parodontitis | 1998 |
| Sandostatin | Temperature | PLGA | Octreotide acetate | Acromegaly | 1988 |
| Atrisorb D | Temperature | PLGA | Doxycycline hyclate | Periodontal tissue regeneration | 2000 |
| Sublocad | Temperature | PLGA | Buprenorphine | Analgesic | 2017 |
| Perseris | Temperature | PLGA | Risperidone | Acute and chronic schizophrenia | 2018 |
| Azasite | Temperature | Poloxamer 407 | Lidocaine hydrochloric acid | Bacterial conjunctivitis | 2007 |
| Lupron depot | pH | PLGA | Leuprolide acetate | Advanced prostate cancer | 1995 |
| Eligard | pH | PLGA | Leuprolide acetate | Advanced prostate cancer | 2002 |
| Pilopine HS | pH | Carbopol 940 | Pilocarpine hydrochloric acid | Glaucoma | 1984 |
| Timoptic XE | Ion strength | Gellan gum | Timolol Maleate | Glaucoma | 1993 |
4. Conclusions
In-situ hydrogels are made of polymer materials in a solution, suspension or semi-solid state, and drugs involving anti-tumor drugs with various properties can be freely loaded into them. After being administered in solution or suspension form, they can immediately undergo phase transformation through different gelling mechanisms at the site of application to form semi-solid or solid hydrogels, which can prolong drug delivery, reduce drug dose, improve bioavailability, and limit side effects of anti-tumor drugs. In-situ hydrogel drug delivery systems have been continuously affirmed in tumor treatment research areas and can be successfully applied to various anti-tumor drugs, but most of them are still in the preliminary research stage as a new type of controlled drug delivery system, and the existing problems still need to be further intensively addressed. For example, clinically applied materials for drug carriers require good biocompatibility, biodegradability and relatively cheap material costs. Therefore, research and development on relatively cheap materials with good biocompatibility, biodegradability and appropriate controlled-release performance may be a major task for future research on in situ hydrogels, making it a new clinically applicable drug delivery system to contribute to improved clinical treatment of malignant tumors and serve tumor patients better.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (NO: 31460246), the Guizhou Provincial Science and Technology Foundation (NO: Qiankehe LH [2014] 7564), the Project of Special Funds for Science and Technology Cooperation in Guizhou Provinces and Zunyi City (Shengshikehe [2015] 53), the Education Department of Guizhou Province (GNYL (2017) 006 and YLXKJS-YS-05), Collaborative Innovation Center of Guizhou Traditional Chinese Medicine and Ethnic medicine (NO: Qianjiaokeyanfa [2012] 311).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ailincai D, Tartau Mititelu L, Marin L. (2018). Drug delivery systems based on biocompatible imino-chitosan hydrogels for local anticancer therapy. Drug Deliv 25:1080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida H, Amaral MH, Lobao P, et al. (2014). In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov Today 19:400–12. [DOI] [PubMed] [Google Scholar]
- Ashwanikumar N, Kumar NA, Saneesh Babu PS, et al. (2016). Self-assembling peptide nanofibers containing phenylalanine for the controlled release of 5-fluorouracil. IJN 11:5583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ata S, Rasool A, Islam A, et al. (2019). Loading of Cefixime to pH sensitive chitosan based hydrogel and investigation of controlled release kinetics. Int J Biol Macromol. [DOI] [PubMed] [Google Scholar]
- Bai MY, Tang SL, Chuang MH, et al. (2018). Evaluation of chitosan derivative microparticles encapsulating superparamagnetic iron oxide and doxorubicin as a pH-sensitive delivery carrier in hepatic carcinoma treatment: an in vitro Comparison Study. Front Pharmacol 9:1025–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendas ER, Abdullah H, El-Komy MHM, et al. (2013). Hydroxychloroquine niosomes: a new trend in topical management of oral lichen planus. Int J Pharm 458:287–95. [DOI] [PubMed] [Google Scholar]
- Boazak EM, Greene VK, Auguste DT. (2019). The effect of heterobifunctional crosslinkers on HEMA hydrogel modulus and toughness. PLoS One 14:e0215895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacio MA, Gentile P, Ferreira AM, et al. (2017). Insight into halloysite nanotubes-loaded gellan gum hydrogels for soft tissue engineering applications. Carbohydr Polym 163:280–91. [DOI] [PubMed] [Google Scholar]
- Cao M, Wang Y, Hu X, et al. (2019). Reversible thermoresponsive peptide-PNIPAM hydrogels for controlled drug delivery. Biomacromolecules 20:3601–10. [DOI] [PubMed] [Google Scholar]
- Casolaro M, Casolaro I, Bottari S, et al. (2014). Long-term doxorubicin release from multiple stimuli-responsive hydrogels based on α-amino-acid residues. Eur J Pharm Biopharm 88:424–33. [DOI] [PubMed] [Google Scholar]
- Chen Q, Wang C, Zhang X, et al. (2019). In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol 14:89–97. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu Z. (2016). A pH-responsive hydrogel based on a tumor-targeting mesoporous silica nanocomposite for sustained cancer labeling and therapy. Macromol Rapid Commun 37:1533–9. [DOI] [PubMed] [Google Scholar]
- Chin SY, Poh YC, Kohler AC, et al. (2018). An additive manufacturing technique for the facile and rapid fabrication of hydrogel-based micromachines with magnetically responsive components. J Vis Exp 18:56727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho EJ, Sun B, Doh KO, et al. (2015). Intraperitoneal delivery of platinum with in-situ crosslinkable hyaluronic acid gel for local therapy of ovarian cancer. Biomaterials 37:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Chen L, Xu W, et al. (2013). Preparation of a paeonol-containing temperature-sensitive in situ gel and its preliminary efficacy on allergic rhinitis. IJMS 14:6499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JK, Simmons JL, Parsons PG, et al. (2019). Topical treatments for skin cancer. Adv Drug Deliv Rev. [DOI] [PubMed] [Google Scholar]
- Demirdirek B, Uhrich KE. (2017). Novel salicylic acid-based chmically crosslinked pH-sensitive hydrogel as potential drug delivery systems. Int J Pharm 528:406–15. [DOI] [PubMed] [Google Scholar]
- Ding W, Li Y, Hou X, et al. (2016). Bleomycin A6-loaded anionic liposomes with in situ gel as a new antitumoral drug delivery system. Drug Deliv 23:88–94. [DOI] [PubMed] [Google Scholar]
- Ellah NHA, Abouelmagd SA, Abbas AM, et al. (2018). Dual-responsive lidocaine in situ gel reduces pain of intrauterine device insertion. Int J Pharm 538:279–86. [DOI] [PubMed] [Google Scholar]
- Fan DY, Tian Y, Liu ZJ. (2019). Injectable hydrogels for localized cancer therapy. Front Chem 7:675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi M, Barar J, Erfan-Niya H, et al. (2019). Methotrexate-conjugated chitosan-grafted pH- and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int J Biol Macromol 48:111–20. [DOI] [PubMed] [Google Scholar]
- Feng RM, Zong YN, Cao SM, et al. (2019). Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 39:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong YT, Chen CH, Chen JP. (2017). Intratumoral delivery of doxorubicin on folate-conjugated graphene oxide by in-situ forming thermo-sensitive hydrogel for breast cancer therapy. Nanomaterials 7:388–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SZ, Wu JB. (2014). P204 A paclitaxel-cisplatin loaded thermosensitive hydrogel for in situ treatment of lung cancer. Eur J Cancer 50:e66. [Google Scholar]
- Gibbens-Bandala B, Morales-Avila E, Ferro-Flores G, et al. (2019). Lu-Bombesin-PLGA (paclitaxel): a targeted controlled-release nanomedicine for bimodal therapy of breast cancer. Mater Sci Eng C Mater Biol Appl 105:e110043. [DOI] [PubMed] [Google Scholar]
- Huang P, Song H, Zhang Y, et al. (2016). Bridging the gap between macroscale drug delivery systems and nanomedicines: a nanoparticle-assembled thermosensitive hydrogel for peritumoral chemotherapy. ACS Appl Mater Interfaces 8:29323–33. [DOI] [PubMed] [Google Scholar]
- Janga KY, Tatke A, Balguri SP, et al. (2018). Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: in vitro permeability, cytotoxicity and in vivo evaluation. Artif Cells Nanomed Biotechnol 46:1039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang Y, Li Q, et al. (2019). Natural polymer-based stimuli-responsive hydrogels. Curr Med Chem 27:1–26. [DOI] [PubMed] [Google Scholar]
- Jung YS, Koo DH, Yang JY, et al. (2018). Peri-tumor administration of 5-fluorouracil sol-gel using a hollow microneedle for treatment of gastric cancer. Drug Deliv 25:872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinoki S, Taguchi T, Saito H, et al. (2007). Injectable in situ forming drug delivery system for cancer chemotherapy using a novel tissue adhesive: characterization and in vitro evaluation. Eur J Pharm Biopharm 66:383–90. [DOI] [PubMed] [Google Scholar]
- Kang H, Liu H, Zhang X, et al. (2011). Photoresponsive DNA-cross-linked hydrogels for controllable release and cancer therapy. Langmuir 27:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe S, Mäder K. (2012). In situ forming implants - an attractive formulation principle for parenteral depot formulations. J Control Release 161:668–79. [DOI] [PubMed] [Google Scholar]
- Khaliq NU, Oh KS, Sandra FC, et al. (2017). Assembly of polymer micelles through the sol-gel transition for effective cancer therapy. J Control Release 255:258–69. [DOI] [PubMed] [Google Scholar]
- Ko S, Park JY, Oh YK. (2019). A microbial siderophore-inspired self-gelling hydrogel for noninvasive anticancer phototherapy. Cancer Res 79:6178–89. [DOI] [PubMed] [Google Scholar]
- Kondaveeti S, Semeano ATS, Cornejo DR, et al. (2018). Magnetic hydrogels for levodopa release and cell stimulation triggered by external magnetic field. Colloids Surf B Biointerfaces 167:415–24. [DOI] [PubMed] [Google Scholar]
- Kushwaha SK, Saxena P, Rai A. (2012). Stimuli sensitive hydrogels for ophthalmic drug delivery: a review. Int J Pharm Investig 2:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddha UD, Mahajan HS. (2017). An insight to ocular in situ gelling systems. Int J Adv in Pharm 06:31–40. [Google Scholar]
- Lee C, Lim K, Kim SS, et al. (2019). Near infrared light-responsive heat-emitting hemoglobin hydrogels for photothermal cancer therapy. Colloids Surf B Biointerfaces 176:156–66. [DOI] [PubMed] [Google Scholar]
- Liang Y, Zhao X, Ma PX, et al. (2019). pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J Colloid Interface Sci 536:224–34. [DOI] [PubMed] [Google Scholar]
- Lin Z, Gao W, Hu H, et al. (2014). Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: high drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J Control Release 174:161–70. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang L, Yang Z, et al. (2011). Controlled release of paclitaxel from a self-assembling peptide hydrogel formed in situ and antitumor study in vitro. Int J Nanomed 6:2143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li H, Lam KY. (2017). Development of a multiphysics model to characterize the responsive behavior of magnetic-sensitive hydrogels with finite deformation. J Phys Chem B 121:5633–46. [DOI] [PubMed] [Google Scholar]
- Liu Q, Li H, Lam KY. (2019). Modeling of a fast-response magnetic-sensitive hydrogel for dynamic control of microfluidic flow. Phys Chem Chem Phys 21:1852–62. [DOI] [PubMed] [Google Scholar]
- Lo YL, Hsu CY, Lin HR. (2012). pH-and thermo-sensitive pluronic/poly (acrylic acid) in situ hydrogels for sustained release of an anticancer drug. J Drug Target 21:54–66. [DOI] [PubMed] [Google Scholar]
- Lu C, Liu M, Fu H, et al. (2015). Novel thermosensitive in situ gel based on poloxamer for uterus delivery. Eur J Pharm Sci 77:24–8. [DOI] [PubMed] [Google Scholar]
- Mao Y, Li X, Chen G, et al. (2016). Thermosensitive hydrogel system with paclitaxel liposomes used in localized drug delivery system for in situ treatment of tumor: better antitumor efficacy and lower toxicity. J Pharm Sci 105:194–204. [DOI] [PubMed] [Google Scholar]
- Meng C, Wei W, Wang Y, et al. (2019). Study on the interaction between self-assembling peptide and mangiferin and in vitro release of mangiferin from in-situ hydrogel. Int J Nanomedicine 14:7447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghassemi S, Hadjizadeh A. (2014). Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release 185:22–36. [DOI] [PubMed] [Google Scholar]
- Morsi N, Ghorab D, Refai H, et al. (2016). Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int J Pharm 506:57–67. [DOI] [PubMed] [Google Scholar]
- Norouzi M, Nazari B, Miller DW. (2016). Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today 21:1835–49. [DOI] [PubMed] [Google Scholar]
- Omidi S, Pirhayati M, Kakanejadifard A. (2020). Co-delivery of doxorubicin and curcumin by a pH-sensitive, injectable, and in situ hydrogel composed of chitosan, graphene, and cellulose nanowhisker. Carbohydr Polym 231:115745. [DOI] [PubMed] [Google Scholar]
- Pareek A, Maheshwari S, Cherlo S, et al. (2017). Modeling drug release through stimuli responsive polymer hydrogels. Int J Pharm 532:502–10. [DOI] [PubMed] [Google Scholar]
- Park K, Skidmore S, Hadar J, et al. (2019). Injectable, long-acting PLGA formulations: analyzing PLGA and understanding microparticle formation. J Control Release 304:125–34. [DOI] [PubMed] [Google Scholar]
- Paulsamy M, Ponnusamy C, Palanisami M, et al. (2018). Nepafenac loaded silica nanoparticles dispersed in-situ gel systems: development and characterization. Int J Biol Macromol 110:336–45. [DOI] [PubMed] [Google Scholar]
- Qu J, Zhao X, Ma PX, et al. (2017). pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater 58:168–80. [DOI] [PubMed] [Google Scholar]
- Rakhshaei R, Namazi H, Hamishehkar H, et al. (2019). Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties. Int J Biol Macromol. [DOI] [PubMed] [Google Scholar]
- Rarokar NR, Saoji SD, Raut NA, et al. (2016). Nanostructured cubosomes in a thermoresponsive depot system: an alternative approach for the controlled delivery of docetaxel. AAPS Pharm Sci Tech 17:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi SK, Anderson HE, Lamas J, et al. (2018). Enhanced release of molecules upon UV light irradiation from photoresponsive hydrogels prepared from bifunctional azobenzene and four-arm poly (ethylene glycol). ACS Appl Mater Interfaces 10:30071–80. [DOI] [PubMed] [Google Scholar]
- Raymond KN, Allred BE, Sia AK. (2015). Coordination chemistry of microbial iron transport. Acc Chem Res 48:2496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Li X, Han B, et al. (2019). Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur J Pharm Sci 128:279–89. [DOI] [PubMed] [Google Scholar]
- Rizwan M, Yahya R, Hassan A, et al. (2017). pH Sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupenthal ID, Green CR, Alany RG. (2011). Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: physicochemical characterisation and in vitro release. Int J Pharm 411:69–77. [DOI] [PubMed] [Google Scholar]
- Sahoo B, Devi KS, Banerjee R, et al. (2013). Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl Mater Interfaces 5:3884–93. [DOI] [PubMed] [Google Scholar]
- Shaker DS, Shaker MA, Hanafy MS. (2015). Cellular uptake, cytotoxicity and in-vivo evaluation of Tamoxifen citrate loaded niosomes. Int J Pharm 493:285–94. [DOI] [PubMed] [Google Scholar]
- Shaker DS, Shaker MA, Klingner A, et al. (2016). In situ thermosensitive tamoxifen citrate loaded hydrogels: an effective tool in breast cancer loco-regional therapy. J Drug Deliv Sci Technol 35:155–64. [Google Scholar]
- Shang J, Theato P. (2018). Smart composite hydrogel with pH-, ionic strength- and temperature-induced actuation. Soft Matter 14:8401–7. [DOI] [PubMed] [Google Scholar]
- Shen N, Hu J, Zhang L, et al. (2012). Doxorubicin-loaded zein in situ gel for interstitial chemotherapy of colorectal cancer. Acta Pharm Sin B 2:610–4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang X, Deng X, et al. (2016). Release profile of insulin from pH-sensitive hydrogel and its hypoglycemic effect by oral administration. J Biomater Sci Polym Ed 27:86–96. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. (2019). Cancer statistics, 2019. CA A Cancer J Clin 69:7–34. [DOI] [PubMed] [Google Scholar]
- Singh A, Vaishagya K, Verma RK, et al. (2019). Temperature/pH-triggered PNIPAM-based smart nanogel system loaded with anastrozole delivery for application in cancer chemotherapy. AAPS Pharm Sci Tech 20:213–26. [DOI] [PubMed] [Google Scholar]
- Singh K, HariKumar SL. (2012). Injectable in-situ gelling controlled release drug delivery system. Int J Drug Dev & Res 4:56–69. [Google Scholar]
- Solomevich SO, Bychkovsky PM, Yurkshtovich TL, et al. (2019). Biodegradable pH-sensitive prospidine-loaded dextran phosphate based hydrogels for local tumor therapy. Carbohydr Polym 226:115308. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang G, Liu G, et al. (2017). Photo- and thermo-responsive multicompartment hydrogels for synergistic delivery of gemcitabine and doxorubicin. J Control Release 259:149–59. [DOI] [PubMed] [Google Scholar]
- Wang Z, Deng X, Ding J, et al. (2018). Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: a review. Int J Pharm 535:253–60. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Furuse J, Okano N, et al. (2017). A pathological complete response after combined chemotherapy of gemcitabine and S-1 in advanced biliary tract cancer with para-aortic lymph nodes metastasis: a case report. Surg Case Rep 3:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Meng C, Wang Y, et al. (2019). The interaction between self - assembling peptides and emodin and the controlled release of emodin from in-situ hydrogel. Artif Cells Nanomed Biotechnol 47:3961–75. [DOI] [PubMed] [Google Scholar]
- Wu H, Song L, Chen L, et al. (2018). Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents the post-operative recurrence in a breast cancer model. Acta Biomater 74:302–11. [DOI] [PubMed] [Google Scholar]
- Wu RS, Lin J, Xing YM, et al. (2019). pH-sensitive black phosphorous incorporated hydrogel as novel implant for cancer treatment. J Pharm Sci 108:2542–51. [DOI] [PubMed] [Google Scholar]
- Wu Z, Zou X, Yang L, et al. (2014). Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer. Colloids Surf B Biointerfaces 122:90–8. [DOI] [PubMed] [Google Scholar]
- Xie AJ, Yin HS, Liu HM, et al. (2018). Chinese quince seed gum and poly (N,N-diethylacryl amide-co-methacrylic acid) based pH-sensitive hydrogel for use in drug delivery. Carbohydr Polym 185:96–104. [DOI] [PubMed] [Google Scholar]
- Xie MH, Ge M, Peng JB, et al. (2019). In-vivo anti-tumor activity of a novel poloxamer-based thermosensitive in situ gel for sustained delivery of norcantharidin. Pharm Dev Technol 24:1–22. [DOI] [PubMed] [Google Scholar]
- Xing J, Qi X, Jiang Y, et al. (2015). Topotecan hydrochloride liposomes incorporated into thermosensitive hydrogel for sustained and efficient in situ therapy of H22 tumor in Kunming mice. Pharm Dev Technol 20:812–9. [DOI] [PubMed] [Google Scholar]
- Yan H, Jin B. (2012). Influence of environmental solution pH and microstructural parameters on mechanical behavior of amphoteric pH-sensitive hydrogels. Eur Phys J E Soft Matter 35:36–46. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang X, Yang F, et al. (2018). Highly elastic and ultratough hybrid Ionic-covalent hydrogels with tunable structures and mechanics. Adv Mater 30:e1707071. [DOI] [PubMed] [Google Scholar]
- Yue Z, Che YJ, Jin Z, et al. (2019). A facile method to fabricate thermo- and pH-sensitive hydrogels with good mechanical performance based on poly (ethylene glycol) methyl ether methacrylate and acrylic acid as a potential drug carriers. J Biomater Sci Polym Ed 30:1375–98. [DOI] [PubMed] [Google Scholar]
- Zhang K, Shi X, Lin X, et al. (2015). Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv 22:375–82. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhou L, Chen F, et al. (2019). Injectable gel self-assembled by paclitaxel itself for in situ inhibition of tumor growth. J Control Release 315:197–205. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Song SC. (2016). Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 106:13–23. [DOI] [PubMed] [Google Scholar]
- Zhao D, Song H, Zhou X, et al. (2019). Novel facile thermosensitive hydrogel as sustained and controllable gene release vehicle for breast cancer treatment. Eur J Pharm Sci 134:145–52. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang H, Huang H, et al. (2015). Functionalized graphene oxide-based thermosensitive hydrogel for magnetic hyperthermia therapy on tumors. Nanotechnology 26:365103. [DOI] [PubMed] [Google Scholar]