ABSTRACT
Acinetobacter baumannii, a Gram-negative opportunistic pathogen, is a leading cause of hospital- and community-acquired infections. Acinetobacter baumannii can rapidly acquire diverse resistance mechanisms and undergo genetic modifications that confer resistance and persistence to all currently used clinical antibiotics. In this study, we found exogenous L-lysine sensitizes Acinetobacter baumannii, other Gram-negative bacteria (Escherichia coli and Klebsiella pneumoniae) and a Gram-positive bacterium (Mycobacterium smegmatis) to aminoglycosides. Importantly, the combination of L-lysine with aminoglycosides killed clinically isolated multidrug-resistant Acinetobacter baumannii and persister cells. The exogenous L-lysine can increase proton motive force via transmembrane chemical gradient, resulting in aminoglycoside acumination that further accounts for reactive oxygen species production. The combination of L-lysine and antibiotics highlights a promising strategy against bacterial infection.
KEYWORDS: L-Lysine, aminoglycosides, Acinetobacter baumannii, proton motive force, reactive oxygen species
Introduction
Acinetobacter baumannii (A. baumannii) is a notorious nosocomial infection pathogen and a major source of hospital-acquired pathogen in intensive care units (ICUs) [1–3]. It provokes a 26% and 43% mortality rate in hospital settings and ICUs, respectively [1]. In addition, most clinical A. baumannii isolates are naturally competent [4], and they can rapidly acquire genetic elements for drug resistance and undergo genetic modifications that make bacteria resistant to all currently used clinical antibiotics [5–7]. Therefore, it is particularly problematic and extremely difficult to treat A. baumannii infection. From the recently published Tigecycline Evaluation and Surveillance Trial (TEST) data, 44% of A. baumannii shows multidrug-resistant (MDR) characteristics, which is the highest rate among Gram-negative pathogens [8]. Because of this, multidrug-resistant A. baumannii especially carbapenem-resistant A. baumannii is classified at a threat level of “urgent” pathogen by the United States Centers for Disease Control and Prevention. It requires more and urgent attention for discovering new antibiotics or adjuvant for antibiotics against A. baumannii infections [9–12].
Aminoglycoside antibiotics (AGs) are widely used to treat Gram-negative infections including Escherichia coli (E. coli), Enterobacteriaceae spp, Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae (K. pneumoniae) and Acinetobacter baumannii (A. baumannii), and some Gram-positive infections [13]. During AG treatment, diverse mechanisms, including inactivation of drug target, toxin–antitoxin modules and drug efflux pumps-induced multidrug resistant bacteria (MDR) and persister formation, can cause chronic bacterial infection [14–17]. One of the typical examples of resistance is that any influence on the bacterial proton motive force (PMF), including Δψ (the electrical potential across the membrane) and ΔpH (the transmembrane difference in the H+ concentration) can significantly change the bactericidal activity of AGs [18]. Novel approaches that target this phenotypic resistance mechanism to restore the cell’s initial susceptibility to antibiotics may be an alternative approach to solve drug resistance [19–23]. Specific metabolites, such as glucose, fructose, mannitol, or pyruvate, promote NADH production through glycolysis, resulting in an increased PMF production through the electron transport chain [24–26]. Jean-Marc Ghigo et al reported that the effect of basic amino acid L-arginine on gentamicin against bacterial infection probably relies on the ΔpH-dependent transmembrane difference in the H+ ion concentration rather than membrane electrical potential [27], suggesting pH-mediated potentiation of AGs is effective against nosocomial pathogens.
An interesting study has demonstrated that L-lysine could enhance the antifungal effect of Amphotericin B (AmB) against fungal infections, such as Candida albicans, Candida parapsilosis and Cryptococcus neoformans, compared with AmB alone [28]. Schweizer and coworkers described a novel class of tobramycin-lysine conjugates containing an optimized amphiphilic tobramycin-C12 tether that sensitizes Gram-negative bacteria to legacy antibiotics [29]. These studies provide a promising strategy for the therapy of fungal and bacterial infection by using antibiotics together with L-lysine. Whether L-lysine could potentiate AGs against bacteria, especially drug-resistant bacteria, needs to be elucidated. In this study, we found that exogenous L-lysine stimulates the bactericidal ability of AGs against A. baumannii (persisters and clinical MDR strains). The mechanism underlying this process is that L-lysine promotes transmembrane pH difference (ΔpH), which, in turn, increases PMF and stimulates uptake of AGs. During antibiotic stress, endogenous reactive oxygen species (ROS) is also induced simultaneously to kill the bacteria. Importantly, L-lysine increases the efficiency of AGs against both a Gram-negative bacteria (E. coli and K. pneumoniae) and a Gram-positive bacterium Mycobacterium smegmatis (M. smegmatis). In summary, this work establishes a strategy for eradicating multidrug-resistant bacteria and bacterial persisters, highlighting the synergistic effects of L-lysine on antibiotics against bacterial infection.
Materials and methods
Strains and culturing conditions
A. baumannii ATCC19606 and clinically isolated multidrug-resistant A. baumannii 18030945 and 16010214, E. coli ATCC 25922, K. pneumoniae ATCC700603 and M. smegmatis MC2-155 were used in this study. M. smegmatis MC2-155 was grown in Middlebrook 7H9 broth medium supplemented with 0.05% Tween 80 and 0.5% glycerol. The experimental and stationary phase bacteria were grown in the following way: bacteria from frozen stock were grown at 37°C, 220 rpm in Luria–Bertani (LB) broth overnight. Cells were then diluted 1:1000 in 50 ml LB to an optical density (OD600) of 0.6 or grown for 16 h at 37°C, 250 rpm in 250 mL flasks, respectively. The cultures were washed with phosphate buffer solution (PBS), suspended in M9 minimal medium supplemented with 10 mM acetate, 1 mM MgSO4 and 100 mM CaCl2 (referred to M9 medium in the text) and treated with different antibiotics with or without L-lysine using the indicated concentrations.
Antibiotics and chemicals
Kanamycin (Kan), gentamicin (Gen) and amikacin (Ami) were purchased from Sangon Biotech Co. (Shanghai, China), and their stock solutions were freshly prepared, filter-sterilized and used at the indicated concentrations. L-lysine was obtained from Sigma-Aldrich (USA). A stock solution of amino acids (2 mM) was prepared in ddH2O, stored at −20°C and used at the indicated concentrations. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)), 2′,7′-dichlorofluorescin diacetate (DCFH-DA) and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (USA). A stock solution of CCCP was dissolved in DMSO 500 mM and stored at 4°C, and fresh ammonium sulphate (AS) was dissolved in water. Antibiotics were filtered a hydrophilic PVDF membrane with a 0.22 μm pore size.
MIC determination
Bacteria were grown to log-phase. The MIC was performed by 2-fold dilutions of the antibiotics in 96-well polystyrene microtiter plates (Corning), the bacteria were incubated with indicated antibiotics at 37°C, overnight. The MIC was determined as the concentration of antibiotics that inhibit bacterial growth. All MICs were tested in duplicate at least three times. The MIC of each antibiotic for different bacteria is shown in Table 1.
Table 1. MIC (μg/ml) for strains used in this study.
| Antibiotics MIC (μg/ml) | |||
|---|---|---|---|
| Strain | Kanamycin | Gentamicin | Amikacin |
| A. baumannii ATCC19606 | 8 | 4 | 4 |
| E. coli ATCC 25922 | 8 | 2 | 2 |
| K. pneumoniae ATCC700603 | 20 | 4 | 2 |
| M. smegmatis MC2-155 | 4 | 2 | 2 |
Persister cells isolation
Persisters can be induced in response to antibiotics, pre-treatment of E. coli with low levels of ciprofloxacin induced the formation of persisters to higher doses of ciprofloxacin [30]. Previous work has demonstrated that treatment the E. coli with 5 μg/ml ofloxacin for 3 h eliminates all susceptible non-persister cells [31]. For A. baumannii persister assays, bacteria were grown to stationary phase and diluted 50-fold with LB medium. Cultures were then treated with 5 μg/ml ciprofloxacin at 37°C for 4 h. Surviving cells were pelleted and suspended in M9 minimal medium. We verified that remaining cells were persisters by increasing the concentration of ciprofloxacin up to 125-fold MIC and noted no further decrease in viability (Figure S1).
Antibiotic survival assay
To obtain exponential- and mid-stationary-phase cultures, overnight cultures (16 h) were diluted 1:1000 in fresh medium and grown into OD600 = 0.8 and 1.6, respectively. Cultures were centrifuged at 5000 rpm for 5 min, and the pellets were washed twice with PBS and re-suspended in M9 medium at OD600 = 0.1. For antibiotic treatment, bacteria were kept at the volumes of 1 ml in 12-well plate without shaking or 1 ml in sterilized glass tube with shaking. The cells were treated with kanamycin, gentamicin or amikacin with or without L-lysine in 37°C for 4 h. After treatment, 100 μl samples were serially diluted, and 10 μl aliquots were plated on LB agar plates. The results are averages from four biological replicates, and error bars represent standard deviations.
Proton motive force quantification
Determination of PMF was performed using DiBAC4(3), as previously described [24,26]. Briefly, DiBAC4(3) was re-suspended in DMSO to form a 1 mM stock and then diluted to a final concentration of 1 mM. Bacteria were grown to stationary phase and were re-suspended in M9 minimal medium with OD600 = 0.3 for 6 h with various concentrations of L-lysine and/or Gen, Kan and Ami, as described above. DiBAC4(3) was added for an additional 10 min, and the cells were washed and analysed using BD FACSCANTO II Flow Cytometer for fluorescein isothiocyanate (FITC)-A fluorescence.
ROS determination
ROS was measured by flow cytometry using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Exponential-phase cultures were washed twice with 1×PBS and re-suspended in M9 minimal medium, they were treated with Gen, Kan and Ami in the presence or absence of 20 mM L-lysine. Then, the cells were washed twice with PBS and incubated with 10 mM DCFH-DA at 37°C for 20 min. The cells were then washed twice with PBS, re-suspended in 200 μl PBS and analysed using a flow cytometer for FITC-A fluorescence.
Ethidium bromide accumulation assay
The ethidium bromide (EB) accumulation assay was used to measure the florescence intensity with minor modifications [32]. Briefly, mid-log-phase cultures were washed with PBS containing 0.05% Tween 80 (PBST) and then re-suspended in M9 minimal media supplemented with or without L-lysine for 6 h. EB (1 μg/ml) was used for accumulation assays. In all assays, the cells were incubated in 96-well plates, and the analysis was performed at the indicated time points by excitation at 544 nm and emission at 590 nm on a FLUOstar OPTIMA Microplate Reader (BMG Labtech). All data were normalized to the time zero time point reading of each well.
NAD+ and NADH measurements
A. baumannii culture was collected and diluted with M9 minimal media cultures to an OD600 = 0.3 and incubated with L-lysine at 37°C for 6 h. The NAD+/NADH assay was performed following the manufacturer’s protocol (EnzyChrom NAD/NADH Assay Kit, BioAssay Systems).
Metabolon-based energy metabolism detection
Logarithmic phase cells were washed with PBS and re-suspended in M9 media with or without L-lysine. After 6 h incubation, the cells were pelleted (5 min at 140,00g, 4°C), washed with cold PBS, and snap frozen in liquid nitrogen. Sextuplicate samples were collected and sent for analysis by Metabolon-associated energy metabolism (Applied Protein Technology, Shanghai, China). A homogenate of 100 mg of sample mixed with 1 ml of cold methanol/acetonitrile/H2O (2:2:1, v/v/v) was sonicated at a low temperature (30 min/once, twice) and then centrifuged for 20 min (140,00g, 4°C). The supernatant was dried in a vacuum centrifuge. For LC-MS analysis, the dried samples were dissolved in 100 μl acetonitrile/water (1:1, v/v), adequately vortexed and then centrifuged (140,00 rpm, 4°C, 15 min). The supernatants were collected for the LC-MS/MS analysis. Analyses were performed using an UHPLC (1290 Infinity LC, Agilent Technologies) coupled to a QTRAP (AB Sciex 5500).
Data Analysis and Statistics
FlowJo V10 was used for processing flow cytometric data, GraphPad prism was used to plot survival assays. All the data are analysed by GraphPad prism and shown as the mean ± SD of triplicate wells. Similar results were obtained in three independent experiments. *P < 0.05; **P < 0.01, ***P < 0.001. All figures were formatted with Adobe Illustrator.
Results
Exogenous L-lysine increases the susceptibility of A. baumannii to AGs
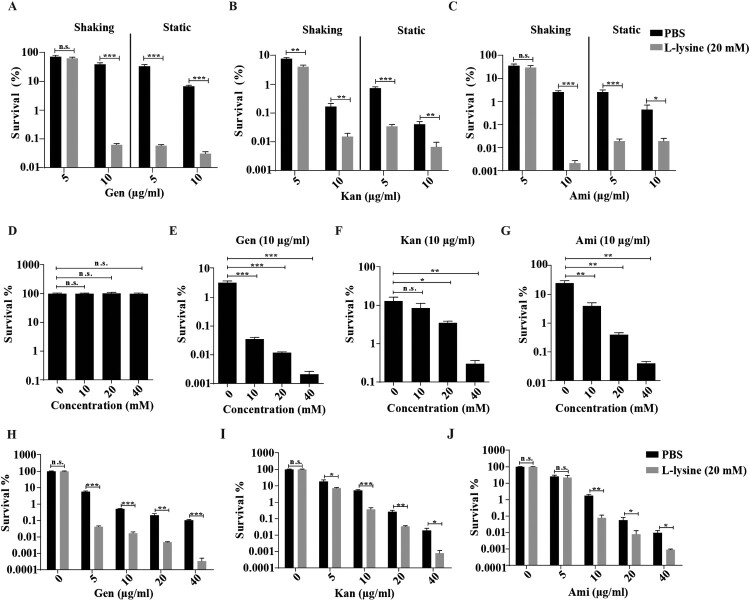
It has been previously shown that the effect of L-arginine on AGs was more potent in anaerobic conditions, the effect of L-lysine on AGs against A. baumannii in different culture conditions was tested. We found that antibiotics cause less cell death on shaking-grown A. baumannii than bacteria cultured in static cultivation in the presence of L-lysine (Figure 1(A–C)). Although L-lysine promotes Kan against the bacteria, there is no potentiation of L-lysine on Gen and Ami at the finial concentration of 5 μg/ml when the bacteria cultured with shaking. However, this disappeared potentiation of L-lysine on antibiotics recovers when we raised the finial concentration of antibiotics into 10 μg/ml. In particular, the combination of L-lysine with AGs at the finial concentration of 5 μg/ml or 10 μg/ml induces comparable cell death of bacteria cultured in static cultivation. Thus, we treated the bacteria with AGs in the presence of L-lysine in static cultivation through all the experiments. Log-phase growing A. baumannii cultures were treated with AGs in the presence of or absence of L-lysine as indicated concentration that did not affect bacterial growth (Figure 1(D)). The survival of the bacteria is decreased in an L-lysine dose-dependent manner for AGs, including Gen (Figure 1(E)), Kan (Figure 1(F)) or Ami (Figure 1(G)). Next, we treated exponential-phase bacteria with antibiotics in the presence of 20 mM L-lysine, the survival of the bacteria is significantly reduced by treatment with Gen (Figure 1(H)), Kan (Figure 1(I)) and Ami (Figure 1(J)) in the presence of L-lysine. Moreover, the mortality of stationary-phase bacteria exposed to antibiotics in the presence of L-lysine is similar to the results observed using exponential-phase cultures (Figure S2). In these cases, antibiotic-mediated cell killing is more severe in the presence of L-lysine. These results prompt us to explore why the supplementary of L-lysine is useful for AGs against bacteria.
Figure 1.
L-lysine increases the susceptibility of exponential-phase A. baumannii 19606 to aminoglycoside antibiotics, including gentamicin (Gen), kanamycin (Kan), and amikacin (Ami). (A–C) The survival of A. baumannii 19606 in the presence of or absence of 20 mM L-lysine and 5 μg/ml or 10 μg/ml Gen (A), Kan (B) and Ami (C) with shaking or static cultivation for 6 h. (D) Percentage survival of A. baumannii 19606 after treatment with different concentrations of L-lysine for 6 h. (E–G) A. baumannii 19606 culture was incubated with increasing concentrations of L-lysine in the presence of 10 µg/ml Gen (E), 10 µg/ml Kan (F) and 10 µg/ml Ami (G) for 6 h. (H–J). An exponentially growing A. baumannii culture was treated with increasing concentrations of Gen (H), Kan (I) or Ami (J) for 6 h in the presence of L-lysine (20 mM). The cultures were platted onto LB agar to determine the survival of bacteria.
L-lysine-induced PMF production is independent of TCA cycle
Previous studies have reported that glucose or glucose plus alanine promotes the uptake of AGs by increasing the PMF [25,26]. We found that L-lysine promotes PMF production of A. baumannii in the presence or in the absence of antibiotics (Figure 2(A)). The electrical potential (ΔΨ) driven by tricarboxylic acid cycle (TCA) control is responsible for generation of PMF, which is known to drive the uptake of AGs [18]. To disrupt PMF on the L-lysine-enhanced antibiotics killing, we used CCCP, a proton ionophore that can abolish Δψ [33]. Consistent with previous studies, the addition of CCCP partially reduces antibacterial activity of AGs in the presence of L-lysine against E. coli (Figures S3A-D) and M. smegmatis (Figures S3E-H). Strikingly, A. baumannii displays high mortality by antibiotic killing when exposed to L-lysine in the presence of CCCP, suggesting that L-lysine-induced PMF is independent of ΔΨ (Figure 2(B–E)). To further determine whether L-lysine increased the PMF is associated with TCA control, the metabolomics was analysed using LC/MS/MS. Unsupervised hierarchical clustering and Z scores were used to rank metabolites whose abundance differed significantly in the bacteria in the presence of or absence of L-lysine (Figure S4A). However, the accumulation of metabolites associated with TCA is decreased in L-lysine-treated bacteria in comparison to bacteria alone (Figures S4B and D). In addition, the intracellular NADH and NAD+ levels were measured by the EnzyChrom NAD+/NADH Assay Kit after the bacteria were incubated with 20 mM L-lysine for 6 h. The concentration of NADH and NAD+ is lower in the L-lysine-treated bacteria than that in control group concentration. The NAD+/NADH ratio is increased in the L-lysine-treated bacteria due to a greater decrease in the NADH concentration (Figure S4C). These data suggest that L-lysine-induced PMF production is independent of TCA cycle.
Figure 2.
L-lysine increases PMF of A. baumannii which is independent on TCA cycle. (A) PMF in A. baumannii 19606 in the presence of 20 mM L-lysine and 10 μg/ml Gen, 10 μg/ml Kan and 10 μg/ml Ami, respectively. (B–E) Survival of exponential-phase A. baumannii 19606 in the presence or absence of 20 mM L-lysine and 20 µM CCCP (B), and in the presence of 10 μg/ml Gen (C), 10 μg/ml Kan (D), or 10 μg/ml Ami (E). Cultures were platted onto LB agar to determine the survival of bacteria.
L-lysine induces transmembrane pH difference of PMF and promotes AGs uptake
PMF includes both the Δψm and transmembrane pH difference (ΔpH) [33]. The uptake of tetracyclines, such as minocycline, is known to be driven by ΔpH of PMF [34]. We found that L-lysine promotes PMF production in the presence or in the absence of antibiotics, while it is reduced in the presence of ΔpH inhibitor AS (Figure 3A–D). Moreover, the addition of AS also partially recovers the survival of the bacteria from L-lysine and AGs treatment (Figure 3E–H). These results suggest that the importance of the ΔpH of PMF induced by L-lysine in increasing the uptake of AGs. The effectiveness of antibiotics mainly depends on their intracellular concentration. Mechanisms that lead to a higher drug permeability could be an alternative pathway to enhance susceptibility to antibiotics [35–38]. Then, we determined the effects of L-lysine on the cell permeability. Interestingly, the antibiotic killing enhanced by L-lysine can be detected after 45 min when A. baumannii was pretreated with L-lysine for 4 h (Figure 4(A–C)), while L-lysine has no effect on antibiotic killing within 3 h in the bacteria without L-lysine pretreating (Figure S5). L-lysine can increase the cell permeability of bacteria, including A. baumannii 19606 (Figure 4(D)), E. coli ATCC 25922 (Figure 4(E)) and K. pneumoniae ATCC700603 (Figure 4(F)). These results suggest that L-lysine increases cell permeability and antibiotic uptake.
Figure 3.
L-lysine-stimulated PMF production depends on transmembrane pH difference (ΔpH). (A–D) PMF in A. baumannii 19606 in the presence of or absence of 20 mM L-lysine and/or 10 mM AS (A), and in the presence of 10 µg/ml Gen (B), 10 µg/ml Kan (C), or 10 µg/ml Ami (D). (E–H) Percent survival of A. baumannii 19606 in the presence or absence of AS and when treated with L-lysine (E), and in the presence of 10 µg/ml Gen (F), 10 µg/ml Kan (G), or 10 µg/ml Ami (H). The cultures were platted onto LB agar to determine the survival of bacteria.
Figure 4.
L-lysine treatment affects the cell permeability of bacteria. (A–C). Pretreatment of A. baumannii 19606 with 20 mM L-lysine for 3 h accelerated antibiotic killing of 25 μg/ml Gen (A), 30 μg/ml Kan (B), or 20 μg/ml Ami (C). (D–F) L-lysine increased the cell permeability of A. baumannii 19606 (D), E. coli ATCC 25922 (E) and K. pneumoniae ATCC700603 (F).
L-lysine promotes endogenous ROS to alter the susceptibility of A. baumannii to AGs
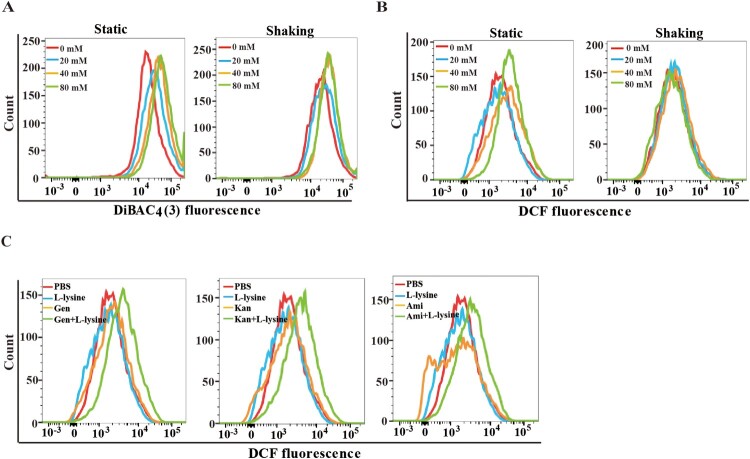
To examine this difference in bacterial killing, we compared the PMF in L-lysine-treated bacteria with shaking or static cultivation. L-lysine induces PMF in both shaking and static cultured bacteria, although L-lysine causes slightly higher PMF in static cultured bacteria than shaking cultured bacteria (Figure 5(A)). These data suggest there could be other underlying mechanism in which L-lysine enabled antibiotic killing. As antibiotics kill bacteria, in part, by inducing ROS [39–41], we reasoned that L-lysine might also target the microbial ROS production to potentiate antibiotic activity. We next compared the production of ROS in A. baumannii cultured in static cultivation and shaking in the presence of different concentration of L-lysine. ROS production is only found in static cultured A. baumannii treated by L-lysine at the concentration of 80 mM (Figure 5(B)). Interestingly, higher ROS accumulation in A. baumannii was induced by 20 mM L-Lysine plus antibiotic treatment in static cultivation (Figure 5(C)). These results suggested that L-lysine not only accelerated PMF production but also altered ROS accumulation under antibiotic stress.
Figure 5.
L-lysine regulates cooperatively PMF and intracellular ROS production. (A) PMF in A. baumannii 19606 in the presence of different concentration of L-lysine for 4 h with shaking or static cultivation. (B) ROS in A. baumannii 19606 in the presence of different concentration of L-lysine for 4 h with shaking or static cultivation. (C) ROS in A. baumannii 19606 in the presence of 20 mM L-lysine and 10 μg/ml Gen, 10 μg/ml Kan and 10 μg/ml Ami, respectively. The cultures were platted onto LB agar to determine the survival of bacteria.
L-lysine potentiates AGs against clinically relevant multidrug-resistant bacteria and other bacteria
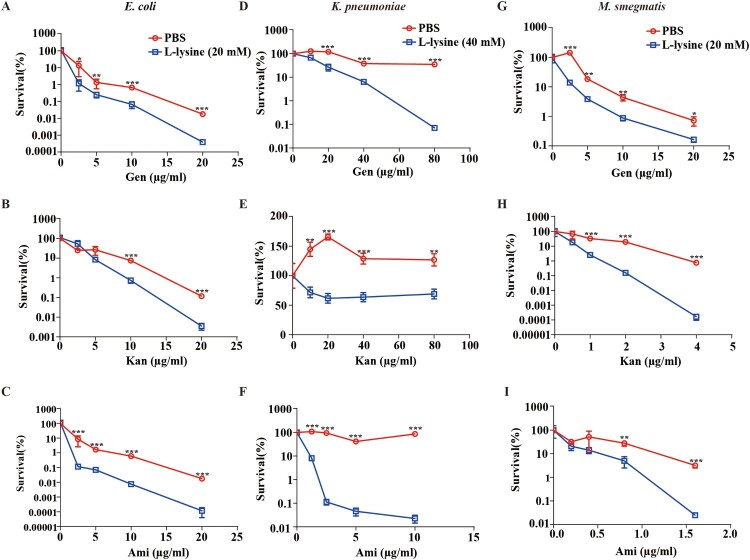
Multidrug-resistant bacteria and persister cell-related infections are a great concern in clinical facilities [42–44], and it would be clinically useful if multidrug-resistant bacteria and persisters were more susceptible to killing by AGs in the presence of L-lysine. A. baumannii CRAb18030945 and CRAb16010214 isolated from patients are two multidrug-resistant strain that confers resistance to amikacin, ampicillin\sulbactam cefepime, ceftazidime, ceftriaxone, ciprofloxacin, gentamicin, imipenem, levofloxacin and piperacillin/tazobactam (Table S1). We identified L-lysine act in a synergistic manner with AGs against A. baumannii (Figure 1(H–J)), so two MDR A. baumannii strains were co-incubated with L-lysine and challenged with different AGs, because L-lysine increased the efficiency of AGs against CRAb18030945 (Figure 6(A–C)) and CRAb16010214 (Figure 6(D–F)). Persisters are pre-existing and formed randomly in microbial populations that are extremely tolerant to antibiotics [45,46]. Joseph Bigger originally referred a small subpopulation of Staphylococcus aureus survived from a lethal dose of penicillin as persisters [47]. Bacterial persisters have been shown to be highly tolerant to antimicrobials and have been reported to be the cause of persistent and difficult-to-treat infections [42]. When combined with AGs and L-lysine, the frequency of A. baumannii persisters was decreased 25-fold in the presence of antibiotic plus L-lysine (Figure 6(G–I)), suggesting that L-lysine promotes the antibiotic susceptibility of both multidrug-resistant clinical isolates and bacterial persisters. In addition to A. baumannii, other relevant Gram-negative bacteria, including E. coli (Figure 7(A–C)) and K. pneumoniae (Figure 7(D–F)), and a Gram-positive bacterium M. smegmatis (Figure 7(G–I)), are susceptible to killing by AGs with L-lysine. Together, L-lysine by itself is incapable of preventing pathogenic infections, while it will enhance the action of AGs to combat pathogenic infections.
Figure 6.
L-lysine elevates the susceptibility of clinically isolated multidrug resistance A. baumannii and persisters to aminoglycoside antibiotics. (A–C) Clinically isolated multidrug resistance A. baumannii CRAb16010214 was treated with 40 mM L-lysine with an increasing concentration of Gen (A), Kan (B) and Ami (C), respectively. (D–F) The survival of another clinically isolated multidrug resistance A. baumannii CRAb18030945 in the presence of 40 mM L-lysine and Gen (D), Kan (E) and Ami (F), respectively. (G–I) Persisters were treated with different doses of Gen (G), Kan (H) and Ami (I) in the presence of 40 mM L-lysine. Clinically isolates and persisters were mixed L-lysine and antibiotics as indicated overnight. The cultures were platted onto LB agar to determine the survival of bacteria.
Figure 7.
L-lysine potentiates aminoglycosides against other Gram-negative and Gram-positive bacteria. (A–C) E. coli ATCC 25922 was treated with 20 mM L-lysine with an increasing concentration of Gen (A), Kan (B) and Ami (C). (D–F) The survival of Gram-negative baterium K. pneumoniae ATCC700603 in the presence of 40 mM L-lysine and Gen (D), Kan (E) and Ami (F). (G–I) Percent survival of Gram-positive bacterium M. smegmatis MC2-155 in the presence of 20 mM L-lysine and Gen (G), Kan (H) and Ami (I). Logarithmic growth phase bacteria cells were re-suspended in M9 medium with an OD600 of 0.2. The re-suspended cells were mixed with L-lysine plus antibiotics as indicated at 37°C for 4 h. For the E. coli ATCC 25922 and K. pneumoniae ATCC700603, cultures were platted onto LB agar to determine the survival of bacteria. For M. smegmatis MC2-155, cells were plotted onto 7H9 agar to determine the survival of bacteria.
Discussion
The ever-increasing incidence of antibiotic-resistant infections combined with a weak pipeline of new antibiotics has created a global public health crisis [48]. Accordingly, novel strategies for enhancing antibiotic arsenal are needed. AGs have shown their efficacy in the treatment of Gram-positive and -negative infections, while the emergence of AGs-resistant bacteria requires us to develop additional ways to combat antibiotic resistance [13]. A combination of compounds that enhance drug susceptibility and antibiotics represent a promising, effective and feasible way to improve the efficacy of existing antibiotics [49–51]. Here, we identified that exogenous L-lysine serves as a non-toxic adjuvant of AGs to kill A. baumannii (Figure 1), especially multidrug-resistant A. baumannii (Figure 6(A–F)) and persisters (Figure 6(G–I)). Similar results were observed in other Gram-negative bacteria (K. pneumoniae and E. coli) and a Gram-positive bacterium (M. smegmatis) (Figure 7).
The antimicrobial activity of AGs is enhanced by PMF via Δψ or ΔpH [18,52]. Fructose or mannitol [25] and alanine and/or glucose [26] stimulates PMF production relies on an increase in Δψ. Consistent with previous study [26], CCCP reduces L-lysine-mediated AGs against E. coli (Figures S3A-D) and M. smegmatis (Figures S3E-H). Unfortunately, the mortality of A. baumannii exposed to AGs in the presence of CCCP and L-lysine is higher than the addition of AGs and L-lysine, which is not due to the toxicity of CCCP itself (Figure 2(B-F)). Studies have shown that an excess of multidrug efflux pumps in A. baumannii is responsible for acquired multidrug [53,54]. CCCP has been reported to serve as efflux pumps inhibitor to reduce the susceptibility of A. baumannii to antimicrobial agents [55]. Therefore, we supposed efflux pumps inhibitor CCCP cooperates L-lysine to promote AGs against A. baumannii. Our results also demonstrated that L-lysine mediates PMF production via transmembrane ΔpH rather than Δψ (Figure 3). AS, an inhibitor of transmembrane pH, is observed to decrease L-lysine-induced PMF production significantly. The mortality of A. baumannii towards AGs plus L-lysine is decreased in the presence of AS (Figure 3). Together, our results implied that L-lysine increases ΔpH of PMF, resulting in antibiotics uptake and accumulation of antibiotics (Figure 4). Although a similar result demonstrated that the pH-mediated L-arginine effect probably relies on transmembrane ΔpH difference rather than on Δψ, the mechanistic aspects of this effect are still currently under investigation.
Moreover, L-lysine-mediated mortality of A. baumannii in the presence of AGs is more potent in static cultivation than shaking, while PMF production is comparable in both conditions (Figure 5(A)). We speculated that other contributing factors may present in L-lysine-induced AGs potentiation against A. baumannii in static cultivation. ROS accumulation within bacteria can trigger cell death or arrest cell growth via damaging proteins, DNA, RNA, and membrane lipids [56,57]. An increase in endogenous microbial ROS can increase the efficiency of antibiotic killing and is a well-known strategy against both Gram-positive and -negative bacteria [58]. Most of the bactericidal antibiotics that target E. coli induce lethality by a common mechanism despite having different primary targets [40,59–62]. Intracellular ROS production is induced in A. baumannii in the presence of higher concentration of L-lysine (Figure 5(B)), while ROS is quickly accumulated in A. baumannii treated with AGs in the presence of lower concentration of L-lysine in static cultivation (Figure 5(C)). Nevertheless, it is possible assumed that L-lysine-induced PMF production promotes intracellular AG accumulation, contributing to cellular ROS production.
L-lysine, one of the essential amino acids, which is necessary for many vital processes and needs to be obtained from dietary sources. It should be mentioned that most people can take L-lysine up to 3 g (about 20 mM) per day without any side effects (https://www.medicalnewstoday.com/articles/324019.php). We provided evidence that L-lysine (20 mM) is enough to enhance the in vitro antimicrobial activity AGs. However, the efficacy and the safety for the dose L-lysine supplement demands further investigation in vivo. In summary, this work established a strategy for eradicating bacterial infections and highlighted L-lysine serves as a promising adjuvant candidate of AGs or antibiotics against bacterial infection, especially clinical multidrug resistant bacteria or persister.
Supplementary Material
Acknowledgements
These experiments were conceived and designed by DWY and LQX. DWY, JX, FTW, ZZ, SH, ZPF performed the experiments. DWY and LQX carried out data analysis and wrote the paper. XJP, RH, HP and LQ modified the paper. All authors have read and approved the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant numbers 81701979, 81660331); the Chongqing Science and Technology Commission (grant numbers cstc2017jcyjA0560, cstc2018jcyjAX0027, Cstc2018jscx-msybX0376 and cstc2018jcyjAX0667); Venture and Innovation Support Program for Chongqing Overseas Returnees (grant numbers Cx2017106, Cx2019070). Wanyan Deng is sponsored by the China Postdoctoral Science Foundation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Greene C, Vadlamudi G, Newton D, et al. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am J Infect Control. 2016;44(5):e65–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demirdal T, Sari US, Nemli SA.. Is inhaled colistin beneficial in ventilator associated pneumonia or nosocomial pneumonia caused by Acinetobacter baumannii? Ann Clin Microbiol Antimicrob. 2016;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayobami O, Willrich N, Harder T, et al. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: a systematic review and meta-analysis. Emerg Microbes Infect. 2019;8(1):1747–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domingues S, Rosário N, Cândido Â, et al. Competence for natural transformation is common among clinical strains of resistant Acinetobacter spp. Microorganisms. 2019;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peleg AY, Seifert H, Paterson DL.. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijkshoorn L, Nemec A, Seifert H.. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5(12):939–951. [DOI] [PubMed] [Google Scholar]
- 7.Perez F, Hujer AM, Hujer KM, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51(10):3471–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giammanco A, Calà C, Fasciana T, et al. Global assessment of the activity of tigecycline against multidrug-resistant gram-negative pathogens between 2004 and 2014 as part of the tigecycline evaluation and surveillance trial. mSphere. 2017;2(1):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon SL, Oliver KB.. Antibiotic resistance threats in the United States: stepping back from the brink. Am Fam Physician. 2014;89(12):938–941. [PubMed] [Google Scholar]
- 10.Boucher HW, Talbot G, Bradley J, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. [DOI] [PubMed] [Google Scholar]
- 11.Harding CM, Hennon SW, Feldman MF.. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol. 2018;16(2):91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC . Antibiotic resistance threats in the United States, 2019; 2019.
- 13.Garneau-Tsodikova S, Labby KJ.. Mechanisms of resistance to aminoglycoside antibiotics: overview and perspectives. Medchemcomm. 2016;7(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harms A, Maisonneuve E, Gerdes K.. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354(6318):1390–1390. [DOI] [PubMed] [Google Scholar]
- 15.Shan Y, Brown Gandt A, Rowe SE, et al. ATP-dependent persister formation in Escherichia coli. mBio. 2017;8(1):e02267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKay SL, Portnoy DA.. Ribosome hibernation facilitates tolerance of stationary-phase bacteria to aminoglycosides. Antimicrob Agents Chemother. 2015;59(11):6992–6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pu Y, Zhao Z, Li Y, et al. Enhanced efflux activity facilitates drug tolerance in dormant bacterial cells. Mol Cell. 2016;62(2):284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taber HW, Mueller JP, Miller PF, et al. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987;51(4):439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock RE, Nijnik A, Philpott DJ.. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012;10(4):243–254. [DOI] [PubMed] [Google Scholar]
- 20.Lee HH, Collins JJ.. Microbial environments confound antibiotic efficacy. Nat Chem Biol. 2011;8(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roemer T, Boone C.. Systems-level antimicrobial drug and drug synergy discovery. Nat Chem Biol. 2013;9(4):222–231. [DOI] [PubMed] [Google Scholar]
- 22.Meylan S, Andrews IW, Collins JJ.. Targeting antibiotic tolerance, pathogen by pathogen. Cell. 2018;172(6):1228–1238. [DOI] [PubMed] [Google Scholar]
- 23.Dewachter L, Fauvart M, Michiels J.. Bacterial heterogeneity and antibiotic survival: understanding and combatting persistence and heteroresistance. Mol Cell. 2019;76(2):255–267. [DOI] [PubMed] [Google Scholar]
- 24.Meylan S, Porter CBM, Yang JH, et al. Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chem Biol. 2017;24(2):195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison KR, Brynildsen MP, Collins JJ.. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng B, Su Y-b, Li H, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015;21(2):249–262. [DOI] [PubMed] [Google Scholar]
- 27.Lebeaux D, Chauhan A, Létoffé S, et al. pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. J Infect Dis. 2014;210(9):1357–1366. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L, Jiang J, Zhu Z, et al. Lysine enhances the effect of amphotericin B against Candida albicans in vitro. Acta Biochim Biophys Sin (Shanghai). 2016;48(2):182–193. [DOI] [PubMed] [Google Scholar]
- 29.Lyu Y, Yang X, Goswami S, et al. Amphiphilic tobramycin-lysine conjugates sensitize multidrug resistant gram-negative bacteria to rifampicin and minocycline. J Med Chem. 2017;60(9):3684–3702. [DOI] [PubMed] [Google Scholar]
- 30.Dorr T, Lewis K, Vulic M.. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 2009;5(12):e1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keren I, Kaldalu N, Spoering A, et al. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230(1):13–18. [DOI] [PubMed] [Google Scholar]
- 32.Chuang YM, Bandyopadhyay N, Rifat D, et al. Deficiency of the novel exopolyphosphatase Rv1026/PPX2 leads to metabolic downshift and altered cell wall permeability in Mycobacterium tuberculosis. MBio. 2015;6(2):e02428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun M, Wartel M, Cascales E, et al. Motor-driven intracellular transport powers bacterial gliding motility. Proc Natl Acad Sci USA. 2011;108(18):7559–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi A, Ohmori H, Kaneko-Ohdera M, et al. Delta pH-dependent accumulation of tetracycline in Escherichia coli. Antimicrob Agents Chemother. 1991;35(1):53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez JL. The antibiotic resistome: challenge and opportunity for therapeutic intervention. Future Med Chem. 2012;4(3):347–359. [DOI] [PubMed] [Google Scholar]
- 36.Lomovskaya O, Bostian KA.. Practical applications and feasibility of efflux pump inhibitors in the clinic–a vision for applied use. Biochem Pharmacol. 2006;71(7):910–918. [DOI] [PubMed] [Google Scholar]
- 37.Drawz SM, Bonomo RA.. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23(1):160–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez JL, Rojo F, Vila J.. Are nonlethal targets useful for developing novel antimicrobials? Future Microbiol. 2011;6(6):605–607. [DOI] [PubMed] [Google Scholar]
- 39.Foti JJ, Devadoss B, Winkler JA, et al. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336(6079):315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohanski MA, Dwyer DJ, Hayete B, et al. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. [DOI] [PubMed] [Google Scholar]
- 41.Kohanski MA, Dwyer DJ, Wierzbowski J, et al. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135(4):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen NR, Lobritz MA, Collins JJ.. Microbial persistence and the road to drug resistance. Cell Host Microbe. 2013;13(6):632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corona F, Martinez JL.. Phenotypic resistance to antibiotics. Antibiotics (Basel). 2013;2(2):237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drenkard E, Ausubel FM.. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416(6882):740–743. [DOI] [PubMed] [Google Scholar]
- 45.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. [DOI] [PubMed] [Google Scholar]
- 46.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(1):48–56. [DOI] [PubMed] [Google Scholar]
- 47.Defraine V, Fauvart M, Michiels J.. Fighting bacterial persistence: current and emerging anti-persister strategies and therapeutics. Drug Resist Updat. 2018;38:12–26. [DOI] [PubMed] [Google Scholar]
- 48.Arias CA, Murray BE.. Antibiotic-resistant bugs in the 21st century – a clinical super-challenge. N Engl J Med. 2009;360(5):439–443. [DOI] [PubMed] [Google Scholar]
- 49.Cottarel G, Wierzbowski J.. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol. 2007;25(12):547–555. [DOI] [PubMed] [Google Scholar]
- 50.Pieren M, Tigges M.. Adjuvant strategies for potentiation of antibiotics to overcome antimicrobial resistance. Curr Opin Pharmacol. 2012;12(5):551–555. [DOI] [PubMed] [Google Scholar]
- 51.Worthington RJ, Melander C.. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013;31(3):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilman S, Saunders VA.. Accumulation of gentamicin by Staphylococcus aureus: the role of the transmembrane electrical potential. J Antimicrob Chemother. 1986;17(1):37–44. [DOI] [PubMed] [Google Scholar]
- 53.Vila J, Marti S, Sanchez-Cespedes J.. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007;59(6):1210–1215. [DOI] [PubMed] [Google Scholar]
- 54.Rumbo C, Gato E, López M, et al. Contribution of efflux pumps, porins, and beta-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57(11):5247–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ardehali SH, Azimi T, Fallah F, et al. Role of efflux pumps in reduced susceptibility to tigecycline in Acinetobacter baumannii. New Microbes New Infect. 2019;30:100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Zhao X, Malik M, et al. Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death. J Antimicrob Chemother. 2010;65(3):520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. [DOI] [PubMed] [Google Scholar]
- 58.Brynildsen MP, Winkler JA, Spina CS, et al. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat Biotechnol. 2013;31(2):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imlay JA, Chin SM, Linn S.. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240(4852):640–642. [DOI] [PubMed] [Google Scholar]
- 60.Dwyer DJ, Belenky PA, Yang JH, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci USA. 2014;111(20):E2100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belenky P, Ye JD, Porter CBM, et al. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 2015;13(5):968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasouly A, Nudler E.. Antibiotic killing through oxidized nucleotides. Proc Natl Acad Sci USA. 2018;115(9):1967–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.