ABSTRACT
Helicobacter pylori (H. pylori) recurrence remains a significant public health concern. The study aimed to assess H. pylori reinfection rate and identify its risk factors in China. This prospective open cohort, observational study was performed at 18 hospitals across 15 provinces in China. Consecutive patients who received the successful initial eradication during 1 January 2012 and 31 December 2018 were eligible for enrolment. H. pylori recurrence was defined as reinfection that occurred at more than the 12-month interval after successful initial eradication. Surveyed risk factors that might be associated with reinfection were preliminarily estimated by log-rank test and further determined by Cox regression model to calculate the hazard ratio (HR) and 95% confidence interval (CI). A total of 5193 subjects enrolled in the study. The follow-up intervals varied from 6 to 84 months with a general follow-up rate of 67.9%. Annual reinfection rate was 1.5% (95% CI: 1.2–1.8) per person-year. H. pylori reinfection was independently associated with the following five risk factors: minority groups (HR = 4.7, 95% CI: 1.6–13.9), the education at lower levels (HR = 1.7, 95% CI: 1.1–2.6), a family history of gastric cancer (HR = 9.9, 95% CI: 6.6–14.7), and the residence located in Western China (HR = 5.5, 95% CI: 2.6–11.5) following by in Central China (HR = 4.9, 95% CI: 3–8.1) (all P < 0.05). Reinfection rate of H. pylori in China is relatively low. Patients with specific properties of ethnic groups, education level, family history, or residence location appear to be at higher risk for reinfection.
KEYWORDS: Helicobacter pylori, recurrence, reinfection, risk factor, epidemiology
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative bacterial pathogen that infects more than half of the human population worldwide [1]. The Kyoto global consensus report has defined H. pylori as a common source of infection [2], whose modes of transmission include oral-oral, faecal-oral and gastro-oral [3]. H. pylori is usually acquired during childhood and able to establish lifelong chronic infection [4]. Infected patients are asymptomatic in most cases but infection has been directly linked to chronic gastritis, peptic ulcer, non-ulcer dyspepsia, mucosa-associated lymphoid tissue lymphoma, and gastric cancer [5]. On the basis of compelling evidence, the World Health Organization has classified H. pylori as a group I carcinogen leading to gastric adenocarcinoma and recently highlighted the ranking of H. pylori in the priority list of research [6]. In addition, the extra-gastric manifestations also represent indeed one of the most fascinating and appealing issues of the whole history of H. pylori [7]. Therefore, Eradication of H. pylori is an effective strategy to prevent related gastrointestinal diseases and reduces the risk of relapse, such as peptic ulcer and gastric cancer [8].
At present, recommended treatment strategies usually comprise two antibiotics, a proton-pump inhibitor, and, in some regimens, bismuth salts [9]. These treatment protocols achieve eradication rates ∼90% [10]. To prevent progression of premalignant histological changes (such as atrophic gastritis and intestinal metaplasia) and recurrent peptic ulcer diseases, it is important to maintain H. pylori eradication status after successful treatment [11]. However, a negative result of a follow-up test after H. pylori treatment does not guarantee subsequent persistent eradication status in the future, even with the most effective treatment regimen currently available [12]. Studies suggest that the number of infected people has persisted or even increased over the past three decades because of H. pylori may be detected again after successful eradication [13]. Such a case is usually considered a recurrence. H. pylori recurrence also remains a significant public health concern in the management of H. pylori infection besides increasing antibiotic resistance [14].
The recurrence of H. pylori infection involves two distinct mechanisms: recrudescence and reinfection [15]. Recrudescence refers to the recurrence of the original strain of H. pylori that remains temporarily suppressed and undetectable posttreatment, whereas reinfection refers to infection by a new strain of H. pylori after successful eradication [16]. Obviously, the former is closely related to the failure of eradication treatment, while the latter is related to the transmission of infection again after successful eradication. Stewardship of H. pylori reinfection is more difficult than that of H. pylori recurrence, because the latter can be improved partially by adjusting the regimens. It is worth mentioning that the published rate and related factors of reinfection varied greatly among different countries depending on the survey region, population groups, sample size, socioeconomic status, research period, investigation methods, etc [13,17,18]. China has a vast territory and a large H. pylor-infected population that may exhibit complicated features of H. pylori reinfection. There has been no nationwide reinfection report of H. pylori in China till now, except for a few scattered regional studies [19–22]. The aim of the present nationwide survey was to assess the reinfection rate of H. pylori after successful initial eradication in China. In addition, we investigated the correlation between a series of potential risk factors and reinfection of H. pylori.
Patients and methods
Study design and protocol
This prospective open cohort, observational study was performed at 18 hospitals across 15 provinces in China. The potential risk factors that might be associated with the reinfection of H. pylori were determined by literature review and national conditions. To facilitate the collection and filling of concerned data, a structured case report form (CRF) was jointly designed by an expert panel, all of them are the members of the Chinese Study Group on Helicobacter pylori and Peptic Ulcer. The normative CRF mainly consisted of the following four sections: (1) Demographic information; (2) Socioeconomic status; (3) Individual behaviour; (4) Medical records. Each study centre used this uniform CRF, in which all formatted questions was required and standardized response options were provided. Because of the descriptive, exploratory nature of this observational study without statistical parameters, no pilot sample size and power calculations were performed.
Study subjects
Consecutive patients with initial H. pylori infection were successfully eradicated in each study centre between 1 January 2012 and 31 December 2018 are eligible for enrolment. Moreover, all participants must also be in accordance with the following two criteria for enrolment and during the course of follow-up.
- Inclusion criteria:
- Volunteering for participation;
- Age between 18 and 65 years;
- Absence of malignant disease, neuropsychiatric disorders or other serious chronic diseases;
- Eradication regimens were limited to triple therapy, quadruple therapy (bismuth containing, sequential, concomitant, hybrid) or others recommended in the Maastricht III-V/Florence Consensus Report or Third-Fifth Chinese National Consensus Report on the management of H. pylori infection.
- Cooperation to finish the detailed questions about the potential risk factors of H. pylori reinfection as required in designed CRF;
- Agree to re-check the negative status of H. pylori again at 4 weeks after the initial confirmation of successful eradication;
- Willing to comply with follow-up plan to reconfirm the status of H. pylori at specified interval which also stated by telephone notification in advance.
- Exclusion criteria:
- Prepare for pregnancy or lactation duration;
- A history of gastric surgery or received endoscopic therapy (e.g. endoscopic mucosal resection or endoscopic submucosal dissection);
- Therapies containing other compounds such as probiotics, Chinese patent drugs or herbs, etc.;
- Failure to understand correctly or express clearly during interviews;
- Out of the study area or touch;
- Oral or informal reconfirm report of H. pylori infection status.
Follow-up schedule
All participants for each centre were interviewed by a well-trained interviewer to complete the designed questions and options regarding demographic information, socioeconomic status, individual behaviour, and medical records as required in CRF. The following specific variables were explored from each participant during the enrolment process:
Demographic information: gender, ethnicity, age, educational level, marital status, the geographical location of permanent residence.
Socioeconomic status: occupation class, the average monthly earnings, living environment, sanitation conditions, family size, per capita residential floor space (number of people sleeping in a house divided by the number of bedrooms).
Individual behaviour: personal hygiene awareness, sharing the same glass, washing before eating, washing after defecation, tobacco use, drinking/alcohol intake;
Medical records: a family history of gastric cancer, other family members infected with H. pylori, relevant diagnosis, eradication regimens, duration of regimens, types of proton pump inhibitors, combinations of antibiotics.
The follow-up interval to reconfirm the status of H. pylori was set as 6 months for the first time point after successful eradication of H. pylori infection and every 12 months thereafter. The criterion for terminating the follow-up was the recurrence of H. pylori infection at any point during the course of follow-up.
Definitions
Successful eradication: the initial eradication of H. pylori infection before follow-up schedule is defined as the achievement of negative H. pylori status at least 4 weeks after treatment in a previously H. pylori-infected patient. And the re-checking results at another 4 weeks after that will be deemed as a real negative-status of H. pylori after initial eradication treatment.
Diagnostic criterion: the status of H. pylori infection during the follow-up period was directly determined by one or more of standard detection methods (limited to urea breath tests, histological staining, bacterial culture or faecal antigen testing). Diagnosis of H. pylori infection should meet the corresponding criteria in consensus. The performance of reconfirming within 4 weeks of receiving proton-pump inhibitors, H2 receptor antagonists, antibiotics, bismuth salts or endoscopy should be avoid. If this happens, it should be executed again at an appropriate time (at least 4 weeks later).
H. pylori recurrence: H. pylori recurrence referred to the situation of H. pylori status became positive again in a patient with previously confirmed successful eradication. The recurrence of H. pylori was defined as a recrudescence that occurred during the 6–12 months period immediately after successful eradication. The recurrence of H. pylori was defined as reinfection that occurred more than 12 months after successful eradication.
Review statement and informed consent
This observational study was free of charge, including the medical cost of re-examination of H. pylori during the follow-up period. The study protocol was reviewed and approved by the Chinese Medical Association & Chinese Society of Gastroenterology and the institutional review board of each participating centre. The objective of the study was explained to all patients before their participation, and written informed consent was obtained from all participants.
Data processing and statistical analysis
The data of CRF were entered in a structure form with Epi InfoTM software for Windows (version 7.2, CDC, Atlanta, GA). The primary endpoint was recurrence cases. As the follow-up time markedly varies among different published studies, the risk of reinfection is better expressed as “yearly” infection. Therefore, the annual reinfection rate of H. pylori was calculated as percentage per person-year: [number of participants who became positive status of H. pylori during the defined period/sum of all participants during the observation years] × 100%.
Data of potential risk factors were presented as the mean ± standard deviation for continuous variables followed a normal distribution and as number (%) for categorical variables. Surveyed risk factors that might be associated with reinfection were preliminarily estimated by the log-rank test. Multivariable analyses with the Cox regression model was used to verify the independent predictors that influenced the reinfection events, in which the results were reported as hazard ratio (HR) and 95% confidence interval (CI). The Kaplan-Meier survival curve was used to depict events of the reinfection and remaining negative of H. pylori over time. The SPSS statistical software package for Windows (version 25.0, Inc., Chicago, IL, USA) was performed for all calculations. Differences were considered to be statistically significant when the P-value was 0.05 or less. All statistical tests were two-sided.
Results
Characteristics of participants in follow-up
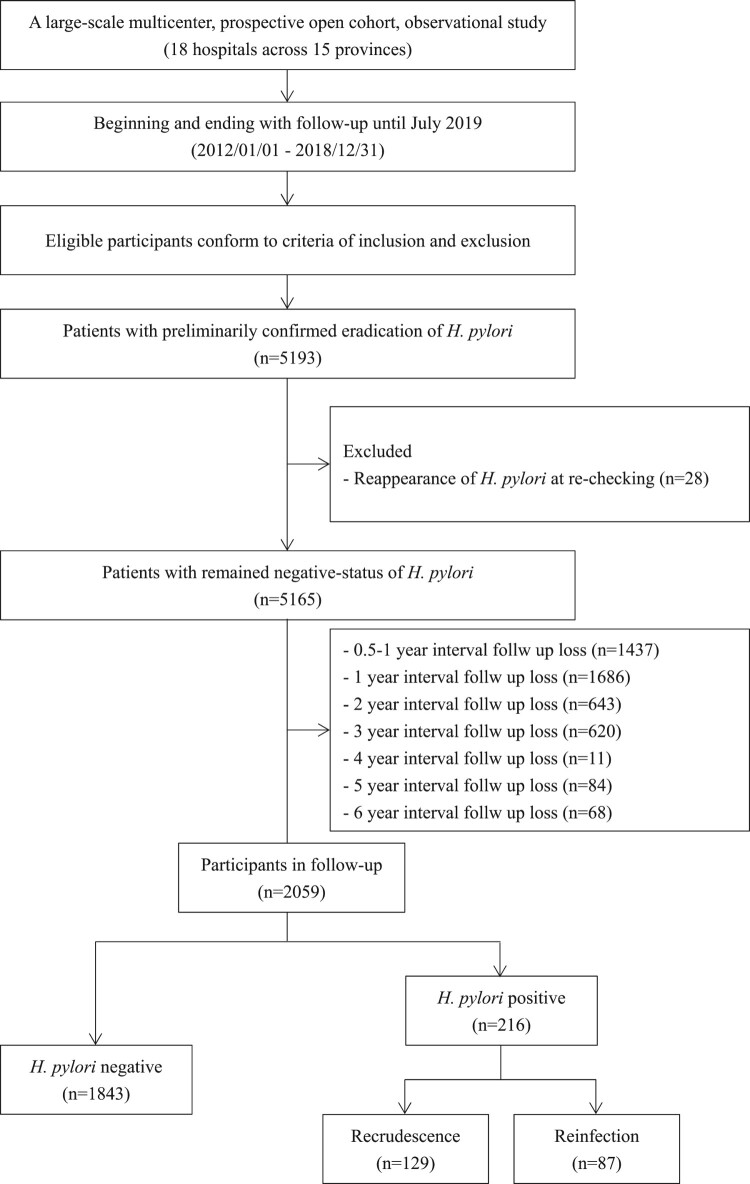
A total of 5193 subjects enrolled in a prospective open cohort during the enrolment process. However, 28 of these subjects were excluded from the further visit cohort for the reasons of H. pylori reappearance during re-checking at 8 weeks after successful eradication, and 1437 were excluded for the follow-up loss at first visit point. Therefore, a total of 3728 participants that remained negative status in a prospective open cohort were eligible for the follow-up. During the follow-up period, the number of participants who moved from the study area or were lost to follow-up at each visit interval was 1686, 643, 620, 11, 84, 11, and 68, respectively. Therefore, the overall follow-up rate in a prospective open cohort was 67.9% (range, 36.4–95.6%). Follow-up interval after successful eradication varied from 6 to 84 months and median follow-up duration was 58.2 ± 13.6 months. Ultimately, a total of 2059 participants in the prospective open cohort, with a median age of 47.3 ± 14.8 years, including 946 (46%) females and 1113 (54%) males, completed the follow-up at least once. A flow diagram of the subject’s progress through the phases of the study is shown in Figure 1.
Figure 1.
Study flow chart with criteria of inclusion and exclusion.
H. pylori reinfection
During the follow-up, a total of 216 participants who experienced successful eradication of H. pylori in the prospective open cohort showed H. pylori-positive again (as recurrence cases). Among which, 129 participants showed as recrudescence cases of H. pylori infection at the 6-month interval after successful eradication, while 87 participants showed as reinfection cases of H. pylori infection at every 12-month interval thereafter. Because of a long follow-up period, the number of participants had decreased in each visit interval to certain extents. Based on the above number of participants we followed, a 1.5% per person-year (87/5707.5 person-year) (95%CI: 1.2–1.8) was calculated as the annual reinfection rate of H. pylori. The follow-up interval and the time when recurrences were found are summarized in Table 1.
Table 1. Annual reinfection rate of Helicobacter pylori.
| Follow-up | Reinfection (n) | Person-years | Annual reinfection rate (%) | |||
|---|---|---|---|---|---|---|
| Time interval | Ideality (n) | Reality (n) | Rate (%) | |||
| 1≤year<2 | 1930 | 1287 | 66.7 | 21 | 1930.5 | 1.1 |
| 2≤year<3 | 1257 | 637 | 50.7 | 24 | 1592.5 | 1.5 |
| 3≤year<4 | 252 | 241 | 95.6 | 17 | 843.5 | 2 |
| 4≤year<5 | 224 | 140 | 62.5 | 9 | 630 | 1.4 |
| 5≤year<6 | 131 | 120 | 91.6 | 13 | 660 | 2 |
| 6≤year<7 | 107 | 39 | 36.4 | 3 | 253.5 | 1.2 |
| Total | 3728 | 2059 | 55.2 | 87 | 5910 | 1.5a |
aThere was no statistically significant difference in reinfection rate between each follow-up period (P = 0.842).
Risk factors for the reinfection of H. pylori
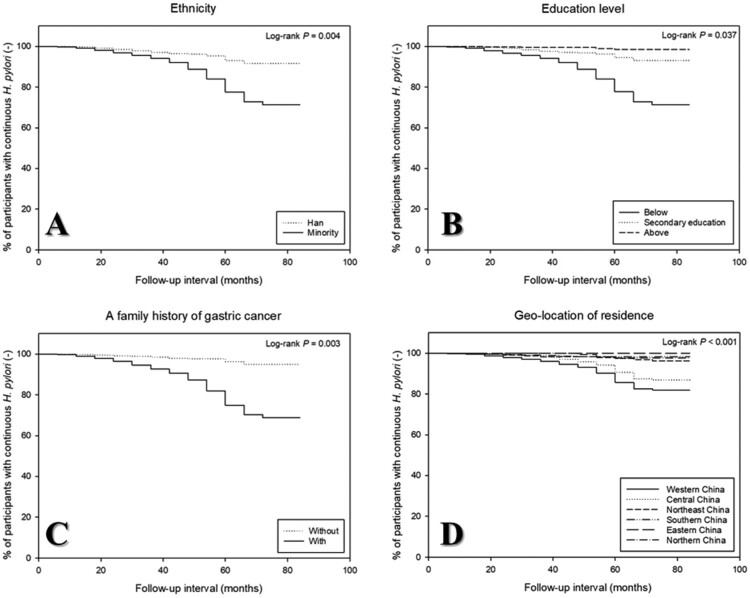
The potential risk factors affecting the recurrence of H. pylori infection were explored from each participant during the enrolment process, in which the survey had an overall response rate of ≥80%. Univariate analysis revealed that potential risk factors, including ethnicity, educational level, geo-location of residence, a family history of gastric cancer (all P < 0.1), suggesting that these factors possibly affected H. pylori infection recurrence after successful eradication therapy (Table 2). Further multivariate analysis showed that H. pylori reinfection was independently associated with the following five risk factors: minority groups (HR = 4.7, 95% CI: 1.6–13.9), the education at lower levels (HR = 1.7, 95% CI: 1.1–2.6), a family history of gastric cancer (HR = 9.9, 95% CI: 6.6–14.7), and the residence located in Western China (HR = 5.5, 95% CI: 2.6–11.5) following by in Central China (HR = 4.9, 95% CI: 3–8.1) (all P < 0.05), as shown in Table 3. Differences between the reinfection and non-reinfection groups were also observed when Kaplan-Meier curves were compared depending on these independent risk factors, and the curve of reinfection occurrence was depicted in Figure 2(A–D).
Table 2. Univariate analysis of risk factors associated with Helicobacter pylori reinfection.
| Factors | Category | Total N (%) |
Reinfection | P-valuea | |
|---|---|---|---|---|---|
| Yes n (%) |
No n (%) |
||||
| Total | 2059 (100) | 87 (100) | 1972 (100) | ||
| Gender | 2059 (100) | 87 (100) | 1972 (100) | ||
| Male | 1113 (54.1) | 42 (3.8) | 1071 (96.2) | 0.269 | |
| Female | 946 (45.9) | 45 (4.8) | 901 (95.2) | ||
| Ethnicity | 2059 (100) | 87 (100) | 1972 (100) | ||
| Minority | 21 (1) | 4 (19.1) | 17 (80.9) | 0.004 | |
| Han | 2038 (99) | 83 (4.1) | 1955 (95.9) | ||
| Age (years) | 2059 (100) | 87 (100) | 1972 (100) | ||
| 18–25 | 62 (3) | 4 (6.5) | 58 (93.5) | 0.877 | |
| 26–35 | 414 (20.1) | 16 (3.9) | 398 (96.1) | ||
| 36–45 | 550 (26.7) | 24 (4.4) | 526 (95.6) | ||
| 46–55 | 682 (33.1) | 30 (4.4) | 652 (95.6) | ||
| 56–65 | 298 (14.5) | 10 (3.4) | 288 (96.6) | ||
| 66–70 | 53 (2.6) | 3 (5.7) | 50 (94.3) | ||
| Educational level | 2023 (98.3) | 86 (98.9) | 1937 (98.2) | ||
| Below | 726 (35.9) | 42 (5.8) | 684 (94.2) | 0.037 | |
| Secondary education | 906 (44.8) | 30 (3.3) | 876 (96.7) | ||
| Above | 391 (19.3) | 14 (3.6) | 377 (96.4) | ||
| Marital status | 2010 (97.6) | 85 (97.7) | 1925 (97.6) | ||
| Yes | 1902 (94.6) | 78 (4.1) | 1824 (95.9) | 0.342 | |
| No | 108 (5.4) | 7 (6.5) | 101 (93.5) | ||
| Occupational class | 2031 (98.6) | 82 (94.3) | 1949 (98.8) | ||
| Unemployed | 362 (17.8) | 10 (2.8) | 352 (97.2) | 0.609 | |
| Farmer | 699 (34.4) | 32 (4.6) | 667 (95.4) | ||
| Worker | 319 (15.7) | 17 (5.3) | 302 (94.7) | ||
| Merchant | 267 (13.1) | 10 (3.7) | 257 (96.3) | ||
| Officer | 204 (10) | 8 (3.9) | 196 (96.1) | ||
| Professional | 180 (8.9) | 5 (2.8) | 175 (97.2) | ||
| The average monthly earnings (RMB, ¥) | 2059 (100) | 87 (100) | 1972 (100) | ||
| <1000 | 486 (23.6) | 31 (6.4) | 455 (93.6) | 0.076 | |
| 1000–3000 | 959 (46.6) | 33 (3.4) | 926 (96.6) | ||
| >3000 | 614 (29.8) | 23 (3.7) | 591 (96.3) | ||
| Geo-location of residence | 2059 (100) | 87 (100) | 1972 (100) | ||
| Eastern China | 1003 (48.7) | 24 (2.4) | 979 (97.6) | <0.001 | |
| Western China | 34 (1.7) | 4 (11.8) | 30 (88.2) | ||
| Southern China | 173 (8.4) | 9 (5.2) | 164 (94.8) | ||
| Northern China | 209 (10.2) | 9 (4.3) | 200 (95.7) | ||
| Northeast China | 398 (19.3) | 23 (5.8) | 375 (94.2) | ||
| Central China | 242 (11.8) | 18 (7.4) | 224 (92.6) | ||
| Living environment | 2037 (98.9) | 87 (100) | 1950 (98.9) | ||
| Urban | 755 (37.1) | 42 (5.6) | 713 (94.4) | 0.027 | |
| Rural | 1282 (62.9) | 45 (3.5) | 1237 (96.5) | ||
| Sanitary level around residence | 2011 (97.7) | 85 (97.7) | 1926 (97.7) | ||
| High | 629 (31.3) | 17 (2.7) | 612 (97.3) | 0.063 | |
| Medium | 814 (40.5) | 42 (5.2) | 772 (94.8) | ||
| Low | 568 (28.2) | 26 (4.6) | 542 (95.4) | ||
| Family size | 2050 (99.6) | 87 (100) | 1963 (99.5) | ||
| 1 | 21 (1.0) | 1 (4.8) | 20 (95.2) | 0.267 | |
| 2 | 254 (12.4) | 6 (2.4) | 248 (97.6) | ||
| 3–4 | 955 (46.6) | 38 (4.0) | 917 (96.0) | ||
| ≥5 | 820 (40.0) | 42 (5.1) | 778 (94.9) | ||
| Per capita living space (m2) | 2031 (98.6) | 86 (98.9) | 1945 (98.6) | ||
| <10 | 138 (6.8) | 5 (3.6) | 133 (96.4) | 0.159 | |
| 10–30 | 1130 (55.6) | 58 (5.1) | 1072 (94.9) | ||
| 31–50 | 702 (34.6) | 21 (3) | 681 (97) | ||
| >50 | 61 (3.0) | 2 (3.3) | 59 (96.7) | ||
| Personal hygiene awareness | 2025 (98.3) | 85 (97.7) | 1940 (98.4) | ||
| High | 316 (15.6) | 9 (2.8) | 307 (97.2) | 0.196 | |
| Medium | 918 (45.3) | 46 (5) | 872 (95) | ||
| Low | 791 (39.1) | 30 (3.8) | 761 (96.2) | ||
| Sharing the same glass | 2039 (99) | 87 (100) | 1952 (99) | ||
| Always | 1209 (59.3) | 45 (3.7) | 1164 (96.3) | 0.142 | |
| Seldom | 830 (40.7) | 42 (5.1) | 788 (94.9) | ||
| Washing before eating | 2050 (99.6) | 86 (98.9) | 1964 (99.6) | ||
| Always | 1889 (92.6) | 75 (4) | 1814 (96) | 0.125 | |
| Seldom | 161 (7.9) | 11 (6.8) | 150 (93.2) | ||
| Washing after defecation | 2050 (99.6) | 84 (96.6) | 1966 (99.7) | ||
| Always | 1879 (91.7) | 73 (3.9) | 1806 (96.1) | 0.159 | |
| Seldom | 171 (8.3) | 11 (6.4) | 160 (93.6) | ||
| Tobacco use | 2056 (99.9) | 85 (97.7) | 1971 (99.9) | ||
| Yes | 1965 (95.6) | 79 (4) | 1886 (96) | 0.349 | |
| No | 91 (4.4) | 6 (6.6) | 85 (93.4) | ||
| Drinking/alcohol intake | 2056 (99.9) | 85 (97.7) | 1971 (99.9) | ||
| Yes | 1729 (84.1) | 66 (3.8) | 1663 (96.2) | 0.131 | |
| No | 327 (15.9) | 19 (5.8) | 308 (94.2) | ||
| A family history of gastric cancer | 2018 (98) | 86 (98.9) | 1932 (98) | ||
| With | 79 (3.9) | 9 (11.4) | 70 (88.6) | 0.003 | |
| Without | 1939 (96.1) | 77 (4) | 1862 (96) | ||
| A family member with H. pylori | 2053 (99.7) | 86 (98.9) | 1967 (99.7) | ||
| Yes | 327 (15.9) | 18 (5.5) | 309 (94.5) | 0.252 | |
| No | 1726 (84.1) | 68 (3.9) | 1658 (96.1) | ||
| Relevant diagnosis | 2059 (100) | 87 (100) | 1972 (100) | ||
| Chronic gastritis | 859 (41.7) | 30 (3.5) | 829 (96.5) | 0.183 | |
| Peptic ulcer | 1034 (50.2) | 52 (5) | 982 (95) | ||
| Others | 166 (8.1) | 5 (3) | 161 (97) | ||
| Eradication regimens | 2059 (100) | 87 (100) | 1972 (100) | ||
| Triple therapy | 724 (35.2) | 23 (3.2) | 701 (96.8) | 0.104 | |
| Quadruple therapy | 1335 (64.8) | 64 (4.8) | 1271 (95.2) | ||
| Duration of regimens | 2059 (100) | 87 (100) | 1972 (100) | ||
| 7 d | 691 (33.6) | 28 (4.1) | 663 (95.9) | 0.165 | |
| 10 d | 323 (15.7) | 8 (2.5) | 315 (97.5) | ||
| 14 d | 1045 (50.8) | 51 (4.9) | 994 (95.1) | ||
| Types of proton pump inhibitors | 2059 (100) | 87 (100) | 1972 (100) | ||
| Ilaprazole | 25 (1.2) | 0 (0) | 25 (100) | 0.119 | |
| Rabeprazole | 494 (24.0) | 18 (3.6) | 476 (96.4) | ||
| Lansoprazole | 134 (6.5) | 5 (3.7) | 129 (96.3) | ||
| Pantoprazole | 606 (29.4) | 32 (5.3) | 574 (94.7) | ||
| Omeprazole | 164 (8.0) | 2 (1.2) | 162 (98.8) | ||
| Esomeprazole | 536 (26.0) | 30 (5.6) | 506 (94.4) | ||
| Combinations of antibiotics | 2059 (100) | 87 (100) | 1972 (100) | ||
| Amoxicillin + Clarithromycin | 650 (31.6) | 23 (3.5) | 627 (96.5) | 0.296 | |
| Amoxicillin + Furazolidone | 1094 (53.1) | 55 (5) | 1039 (95) | ||
| Amoxicillin + Tetracycline | 84 (4.1) | 4 (4.8) | 80 (95.2) | ||
| Amoxicillin + Metronidazole | 68 (3.3) | 0 (0) | 68 (100) | ||
| Amoxicillin + Levofloxacin | 102 (5) | 3 (2.9) | 99 (97.1) | ||
| Others | 61 (3) | 2 (3.3) | 59 (96.7) | ||
aLog-rank test.
Table 3. Multivariate analysis of risk factors associated with Helicobacter pylori reinfection.
| Risk factors | Pa | HR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Geo-location of residence | ||||
| Northern China | Reference | |||
| Central China | <0.001 | 4.9 | 3.0 | 8.1 |
| Northeastern China | 0.052 | 1.9 | 1.0 | 3.8 |
| Southern China | 0.64 | 0.6 | 0.1 | 4.6 |
| Western China | <0.001 | 5.5 | 2.6 | 11.5 |
| Eastern China | 0.578 | 1.9 | 0.2 | 19.0 |
| Ethnicity | ||||
| Han | 0.005 | 5 | 1.6 | 10 |
| Minority | ||||
| Education level | ||||
| Above | Reference | |||
| Secondary education | 0.444 | 0.7 | 0.3 | 1.7 |
| Below | 0.027 | 1.7 | 1.1 | 2.6 |
| A family history of gastric cancer | ||||
| With | <0.001 | 9.9 | 6.6 | 14.7 |
| Without | ||||
aCox regression model.
Figure 2.
Kaplan–Meier curves for Helicobacter pylori reinfection according to ethnicity (A), educational level (B), a family history of gastric cancer (C), and geo-location of residence (D).
Discussion
Effective eradication of H. pylori is essential for the risk reduction of developing gastric cancer [23]. However, certain patients will infection again after successful eradication [13,24,25]. It remains a serious problem worldwide, especially in less-developed areas with a high prevalence of H. pylori infection or gastric cancer [26]. Because of the rate of H. pylori reinfection is low, perhaps diseases associated with the infection will also decline accordingly [27]. The major results of this prospective open cohort study performed at 18 hospitals across 15 provinces with a long-term follow-up (6–84 months) in China were that the annual reinfection rate was 1.5% (95% CI: 1.2–1.8) per person-year.
As outlined in a recent comprehensive meta-analysis covering 132 studies (53,934 person-year) from 45 countries or regions by Hu et al., the global annual reinfection rate of H. pylori was 3.1% (95% CI: 2–5), which remained relatively stable over the past few decades but varied across different regions [25]. In this study, it can be seen that the current annual reinfection rate in China is lower than the global level (1.5% vs. 3.1%). Similarly, we also found that the reinfection case could occur at each interval during follow-up and these differences were not statistically significant (P < 0.05).
There were reports that the incidence of H. pylori reinfection varies with regional development [28]. Gisbert et al [13] reviewed the annual recurrence rate after eradication was about 3.4% in developed countries and 8.7% in developing countries, respectively. The main reason for this difference is that recurrence of H. pylori usually has been considered to be due to recrudescence in developed countries. Whereas studies from developing countries suggest the main cause for recurrence of H. pylori is reinfection [16]. Distinguishing between reinfection and recrudescence can be a challenge in H. pylori infection without DNA fingerprints [29]. However, it is not easy to perform in clinical practice. Reports have shown that the recurrence rates of H. pylori decrease with time and decline sharply after the first year and come close to the rate of natural acquisition of H. pylori infection in adulthood [13,16,30]. Reinfection contributes to 62.5% of cases of H. pylori recurrence in the first 6 months after eradication, as well as most cases in the first year [31]. Furthermore, this definition is supported by data obtained using DNA analysis that the cause of H. pylori recurrence after the first year is reinfection [16].
In our study with large sample size, the re-checking results at 8 weeks after eradication treatment will be used as the real status of H. pylori infection to eliminate the false-negative cases (0.54%, 28/5193). Like the reasons mentioned above, we distinguished between recrudescence and reinfection in recurrence cases with a one-year interval after successful eradication. Therefore, our criteria to judge recurrence are consistent with their definitions that identical strains were detected in a significant proportion of H. pylori which became re-positive at early follow up (at 6–12 months), while H. pylori which became re-positive at later follow up (>1-year interval) were all different strains [32]. Therefore, the results of this study are comparable to published data from similar studies.
Recrudescence is most common when low-efficiency therapies are used, while reinfection requires re-exposure and is, therefore, more likely in countries with high H. pylori prevalence and poor sanitation [33]. As for the lower annual reinfection rate in China, it might be caused by the significant improvements in the socio-economic situation as well as health and living conditions over the past decades. Analogously, it may be not surprising that such an inconsistent result of the annual reinfection rate has emerged in Asia-Pacific countries. A long-term prospective study with 1609 patients followed for up to 12.5 years (mean 4.7 years) in Japan showed the reinfection rate of H. pylori was very low (0.22%) [27], while similar long-term follow-up study (18–95 months) in Korea showed the annual reinfection rate was 3.51% per year [34]. Moreover, the recurrence rate also varies (0–2.3%) among developed western countries or community (e.g. the United States, European Union and Australia) [25,35,36].
H. pylori recurrence rate is inversely correlated with its regional Human Development Index (HDI) and sanitation conditions [25,28]. Currently, following factors have been proposed as risk factor for reinfection, including age, dental plaque, close contacts, contaminated endoscopic equipment, eating habits, drinking water, low income, etc [37–44]. In our study, there are five risk factors: minority groups (HR = 4.7, 95% CI: 1.6–13.9), the education at lower levels (HR = 1.7, 95% CI: 1.1–2.6), a family history of gastric cancer (HR = 9.9, 95% CI: 6.6–14.7), and the residence located in Western China (HR = 5.5, 95% CI: 2.6–11.5) following by in Central China (HR = 4.9, 95% CI: 3–8.1), while other reported factors were not found to be the independent affecting factors in our study. Nevertheless, the findings of this study are generally consistent with the HDI. However, the underlying mechanism of its correlation needs further modelling analysis and in-depth clinical verification.
The present study still has some limitations. Firstly, DNA fingerprint technology is the golden standard for identifying the difference between recrudescence and reinfection. Secondly, despite efforts made by each centre to recruit all participants during the enrolment process, some participants did not complete a follow-up plan ideally. Therefore, we have a certain degree of computational bias in evaluating the rate of H. pylori reinfection. Thirdly, the re-treatment information was not available in the current medical records, and we could not evaluate the effectiveness of subsequent regimens in reinfection patients. That topic is what we will focus on in the future. Finally, because reinfection cases are much less than non-recurrence cases in practice, type I statistical errors are prone to be made in judging the relevant risk factors.
Conclusion
In conclusion, long-term follow-up of the present open prospective cohort study shows that the annual reinfection rate (1.5% per person-year) of H. pylori after initial eradication in China is relatively low when compared with other developing countries. However, we still need to be wary of qualitative changes arising from quantitative translation. Patients with specific properties of residential location, ethnic groups, education level, or family history appear to be at higher risk for reinfection. Therefore, different strategic surveillance and appropriate reassessment should be taken to prevent the reinfection of H. pylori in high-risk populations.
Supplementary Material
Funding Statement
Funding was provided by National Key Research and Development Program of China [grant number 2016YFC1302201]; National Natural Science Foundation of China [grant number 81970502, grant number 81860107, and grant number 81260076]; Natural Science Foundation of Fujian Province of China [grant number 2017J01347]; and Leading Talent Training Plan of the Gan-Po Outstanding Talents 555 Project of Jiangxi Province [grant number 2010-3-61].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Acknowledgements
Yong Xie and Conghua Song equally contributed to this work for analyzing the data and writing the manuscript. Nonghua Lu designed the study and reviewed the manuscript as the corresponding author. Yong Xie, Conghua Song, Hong Cheng, Canxia Xu, Zhenyu Zhang, Jiangbin Wang, Lijuan Huo, Qin Du, Jianming Xu, Ye Chen, Xiaomei Zhang, Guoxin Zhang, Guibin Yang, Xiuli Zuo, Tao Guo, Yapi Lu, Fen Wang, Xuehong Wang, Kun Zhuang, and Shiyao Chen performed the protocol including data collection and manuscript preparation. The authors had full access to the data and take full responsibility for the integrity of the data. All the authors gave their approval for the submission of the final manuscript.
References
- 1.Ailloud F, Didelot X, Woltemate S, et al. Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps. Nat Commun. 2019;10(1):2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leja M, Axon A, Brenner H.. Epidemiology of Helicobacter pylori infection. Helicobacter. 2016;21(Suppl. 1):3–7. [DOI] [PubMed] [Google Scholar]
- 4.Malaty HM, El-Kasabany A, Graham DY, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359(9310):931–935. [DOI] [PubMed] [Google Scholar]
- 5.Crowe SE. Helicobacter pylori infection. N Engl J Med. 2019;380(12):1158–1165. [DOI] [PubMed] [Google Scholar]
- 6.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. [DOI] [PubMed] [Google Scholar]
- 7.Harmati M, Gyukity-Sebestyen E, Dobra G, et al. Binary mixture of Satureja hortensis and Origanum vulgare subsp. hirtum essential oils: in vivo therapeutic efficiency against Helicobacter pylori infection. Helicobacter. 2017;22(2):e12350. [DOI] [PubMed] [Google Scholar]
- 8.Wu JY, Lee YC, Graham DY.. The eradication of Helicobacter pylori to prevent gastric cancer: a critical appraisal. Expert Rev Gastroenterol Hepatol. 2019;13(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor A, Lamarque D, Gisbert JP, et al. Treatment of Helicobacter pylori infection 2017. Helicobacter. 2017;22(Suppl. 1):e12410. [DOI] [PubMed] [Google Scholar]
- 11.Corral JE, Mera R, Dye CW, et al. Helicobacter pylori recurrence after eradication in Latin America: Implications for gastric cancer prevention. World J Gastrointest Oncol. 2017;9(4):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohr UR, Malfertheiner P.. Eradication of H. pylori infection: the challenge is on if standard therapy fails. Therap Adv Gastroenterol. 2009;2(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gisbert JP. The recurrence of Helicobacter pylori infection: incidence and variables influencing it. A critical review. Am J Gastroenterol. 2005;100(9):2083–2099. [DOI] [PubMed] [Google Scholar]
- 14.Shah E, Chey WD.. Editorial: recurrence of Helicobacter pylori infection-still the same after all these years. Aliment Pharmacol Ther. 2018;47(1):131–132. [DOI] [PubMed] [Google Scholar]
- 15.Moya DA, Crissinger KD.. Helicobacter pylori persistence in children: distinguishing inadequate treatment, resistant organisms, and reinfection. Curr Gastroenterol Rep. 2012;14(3):236–242. [DOI] [PubMed] [Google Scholar]
- 16.Zhang YY, Xia HH, Zhuang ZH, et al. Review article: ‘true’ re-infection of Helicobacter pylori after successful eradication–worldwide annual rates, risk factors and clinical implications. Aliment Pharmacol Ther. 2009;29(2):145–160. [DOI] [PubMed] [Google Scholar]
- 17.Xia HX, Talley NJ, Keane CT, et al. Recurrence of Helicobacter pylori infection after successful eradication: nature and possible causes. Dig Dis Sci. 1997;42(9):1821–1834. [DOI] [PubMed] [Google Scholar]
- 18.Bruce MG, Bruden DL, Morris JM, et al. Reinfection after successful eradication of Helicobacter pylori in three different populations in Alaska. Epidemiol Infect. 2015;143(6):1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell HM, Hu P, Chi Y, et al. A low rate of reinfection following effective therapy against Helicobacter pylori in a developing nation (China). Gastroenterology. 1998;114(2):256–261. [DOI] [PubMed] [Google Scholar]
- 20.Zhou LY, Song ZQ, Xue Y, et al. Recurrence of Helicobacter pylori infection and the affecting factors: a follow-up study. J Dig Dis. 2017;18(1):47–55. [DOI] [PubMed] [Google Scholar]
- 21.Zhou LY, Lin SR, Shen ZY, et al. Five-year follow-up study after Helicobacter pylori eradication: reinfection and peptic ulcer status. Chin J Dig Dis. 2003;4(1):45–48. [Google Scholar]
- 22.Xue Y, Zhou LY, Lu HP, et al. Recurrence of Helicobacter pylori infection: incidence and influential factors. Chin Med J. 2019;132(7):765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, Mori H.. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53(3):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayali S, Manfredi M, Gaiani F, et al. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Biomed. 2018;89:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Wan JH, Li XY, et al. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Therap. 2017;46(9):773–779. [DOI] [PubMed] [Google Scholar]
- 26.Megraud F, Lamouliatte H.. Helicobacter pylori infection relapse after eradication is not a problem in developed countries. Nat Clin Pract Gastroenterol Hepatol. 2006;3(9):484–485. [DOI] [PubMed] [Google Scholar]
- 27.Take S, Mizuno M, Ishiki K, et al. Reinfection rate of Helicobacter pylori after eradication treatment: a long-term prospective study in Japan. J Gastroenterol. 2012;47(6):641–646. [DOI] [PubMed] [Google Scholar]
- 28.Yan TL, Hu QD, Zhang Q, et al. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Therap. 2013;37(10):963–968. [DOI] [PubMed] [Google Scholar]
- 29.Bamford KB, Bickley J, Collins JS, et al. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Gut. 1993;34(10):1348–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peitz U, Hackelsberger A, Malfertheiner P.. A practical approach to patients with refractory Helicobacter pylori infection, or who are re-infected after standard therapy. Drugs. 1999;57(6):905–920. [DOI] [PubMed] [Google Scholar]
- 31.Okimoto T, Murakami K, Sato R, et al. Is the recurrence of Helicobacter pylori infection after eradication therapy resultant from recrudescence or reinfection, in Japan. Helicobacter. 2003;8(3):186–191. [DOI] [PubMed] [Google Scholar]
- 32.Kim SY, Hyun JJ, Jung SW, et al. Helicobacter pylori recurrence after first- and second-line eradication therapy in Korea: the problem of recrudescence or reinfection. Helicobacter. 2014;19(3):202–206. [DOI] [PubMed] [Google Scholar]
- 33.Raymond J, Thiberge JM, Dauga C.. Diagnosis of Helicobacter pylori recurrence: relapse or reinfection? Usefulness of molecular tools. Scand J Gastroenterol. 2016;51(6):672–678. [DOI] [PubMed] [Google Scholar]
- 34.Kim MS, Kim N, Kim SE, et al. Long-term follow-up Helicobacter pylori reinfection rate and its associated factors in Korea. Helicobacter. 2013;18(2):135–142. [DOI] [PubMed] [Google Scholar]
- 35.Abu-Mahfouz MZ, Prasad VM, Santogade P, et al. Helicobacter pylori recurrence after successful eradication: 5-year follow-up in the United States. Am J Gastroenterol. 1997;92(11):2025–2028. [PubMed] [Google Scholar]
- 36.Borody TJ, Andrews P, Mancuso N, et al. Helicobacter pylori reinfection rate, in patients with cured duodenal ulcer. Am J Gastroenterol. 1994;89(4):529–532. [PubMed] [Google Scholar]
- 37.Gomez Rodriguez BJ, Rojas Feria M, Garcia Montes MJ, et al. Incidence and factors influencing on Helicobacter pylori infection recurrence. Rev Esp Enferm Dig. 2004;96(9):620–623; 424–627. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu T, Yarita Y, Kaneko K, et al. Case of intrafamilial Helicobacter pylori reinfection after successful eradication therapy. Pediatr Infect Dis J. 2000;19(9):901–903. [DOI] [PubMed] [Google Scholar]
- 39.Nam JH, Ryu KH, Park BJ, et al. Rate and predictive factors of Helicobacter pylori recurrence: analysis of a screening cohort. Saudi J Gastroenterol. 2019;25(4):251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gisbert JP, Arata IG, Boixeda D, et al. Role of partner’s infection in reinfection after Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2002;14(8):865–871. [DOI] [PubMed] [Google Scholar]
- 41.Tongtawee T, Wattanawongdon W, Simawaranon T.. Effects of periodontal therapy on eradication and recurrence of Helicobacter pylori infection after successful treatment. J Int Med Res. 2019;47(2):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karczewska E, Konturek JE, Konturek PC, et al. Oral cavity as a potential source of gastric reinfection by Helicobacter pylori. Dig Dis Sci. 2002;47(5):978–986. [DOI] [PubMed] [Google Scholar]
- 43.Kilmartin CM. Dental implications of Helicobacter pylori. J Can Dent Assoc. 2002;68(8):489–493. [PubMed] [Google Scholar]
- 44.Sugiyama T, Naka H, Yachi A, et al. Direct evidence by DNA fingerprinting that endoscopic cross-infection of Helicobacter pylori is a cause of postendoscopic acute gastritis. J Clin Microbiol. 2000;38(6):2381–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.