Dear Editor,
Alveolar macrophages (AM) express a unique repertoire of matrix metalloproteinases (MMPs) that have downstream effects on inflammatory mediators involved in acute respiratory distress syndrome (ARDS) pathogenesis. MMP28 is the newest member of the MMP family and has been shown to be upregulated in inflammatory conditions such as idiopathic pulmonary fibrosis [1]. In animal models of lung infection, MMP28 plays a key role in macrophage chemotaxis [2] and in modulating macrophage polarity [3]. AM polarization plays a central role in orchestrating alveolar inflammation and repair in ARDS, and we have previously shown that AM transcriptional programs are associated with ARDS clinical outcomes such as ventilator-free days (VFDs) [4]. Therefore, our primary hypothesis was that AM MMP28 gene expression is associated with VFDs in subjects with ARDS. Secondarily, we hypothesized that AM MMP28 gene expression and alveolar MMP28 concentrations are associated with PaO2/FiO2 ratio (P/F ratio), percentage alveolar neutrophils (% PMNs), and total protein levels.
We analyzed bronchoalveolar lavage fluid (BALF) from subjects previously enrolled in a phase-II trial [5] of omega-3 fatty acids for the treatment of ARDS (n = 76) (Table 1). In a subset of these patients (n = 25), AMs were purified from BALF by negative selection as previously described [4]. Samples were obtained from subjects within 48 h of ARDS onset and prior to them receiving study drug. We extracted RNA from isolated AMs, assessed it for purity, and then reverse-transcribed it into cDNA. RT-PCR was performed per the manufacturer’s instructions using HPRT and MMP28 (Hs01020031_m1) primer probe sets from Applied Biosystems. BALF MMP28 was measured using an ELISA (Cat #: LS-F12061) specific for human MMP28 per the manufacturer’s instructions (LifeSpan Biosciences). Specimens with an MMP28 concentration below the lower limit of detection (LLOD) were assigned an MMP28 concentration of 50% of the LLOD for analytical purposes. These data were analyzed with non-parametric tests. In primary analysis, we tested for associations between AM-specific MMP28 gene expression (relative quantification) and VFDs. In secondary analysis, we tested for associations between AM-specific MMP28 gene expression or BALF MMP28 protein levels and P/F ratio, % PMNs, and alveolar total protein levels.
Table 1.
Subject characteristics
| Characteristic | ARDS cohort (n = 76) |
|---|---|
| Demographic | |
| Age (mean ± SD) | 50 ± 16 |
| Sex (M/F) | 45/31 |
| Comorbidities | |
| Diabetes | 16 (22%) |
| Cirrhosis | 5 (7%) |
| Chronic renal insufficiency | 2 (3%) |
| ARDS risk factor*, n (%) | |
| Sepsis | 48 (64%) |
| Pneumonia | 31 (41%) |
| Trauma | 27 (37%) |
| Other | 8 (11%) |
| Physiologic | |
| P/F Ratio (median, IQR) | 156, (121–205) |
| APACHE II (mean ± SD) | 22 ± 7 |
| Outcome | |
| VFDs (median, IQR) | 14, (0–21) |
| Mortality (28-day) (n, %) | 11, 20% |
*ARDS risk factors are not mutually exclusive; APACHE Acute Physiology, Age, Chronic Health Evaluation, ARDS acute respiratory distress syndrome, IQR interquartile range, P/F ratio PaO2/FiO2 ratio, SD standard deviation, VFDs ventilator-free days—defined as the number of days a subject is alive and free from mechanical ventilation between day 1 and day 28 after enrollment. If a subject died before day 28, they were considered to have VFDs = 0
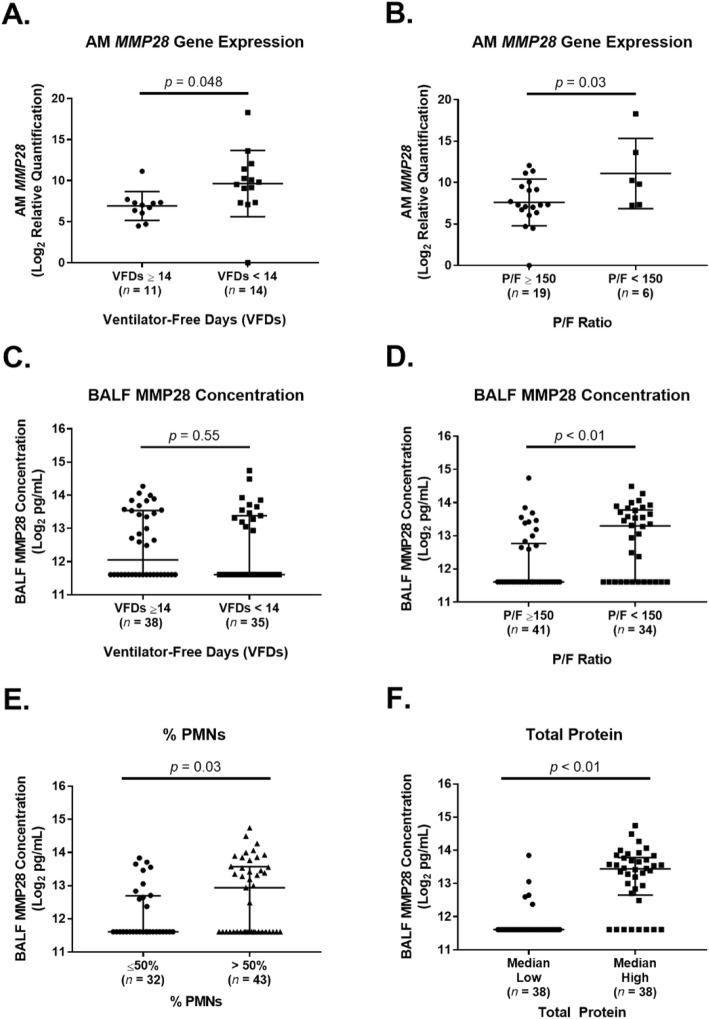
Higher AM MMP28 gene expression at the time of ARDS onset was associated with worse VFDs (Fig. 1a, groups were dichotomized by the median VFDs). We next tested whether AM MMP28 gene expression on day 1 was associated with P/F ratio to determine whether there was a link between AM MMP28 gene expression and a lung-specific endpoint. Higher AM MMP28 gene expression was associated with worse P/F ratio (Fig. 1b groups were divided into mild-moderate (P/F > 150) vs. moderate-severe (P/F < 150) based on a recent classification of ARDS severity [6]). In secondary analysis, we found that higher BALF MMP28 concentrations were associated with worse P/F ratio, but not VFDs (Fig. 1c, d). Increased BALF MMP28 concentrations were associated with increased % PMNs and total protein concentrations (Fig. 1e, f).
Fig. 1.
Alveolar MMP28 is associated with clinical outcomes in subjects with acute respiratory distress syndrome (ARDS). a AM-specific relative gene expression of MMP28 was higher in subjects with worse ventilator-free days (VFDs) (VFDs < 14) vs. better VFDs (VFDs ≥ 14) (p = 0.048, unpaired t test). Subjects were divided by the median VFDs (VFD = 14). Shown are the individual values, mean, and standard deviation. b AM-specific relative gene expression of MMP28 was higher in subjects with a P/F ratio < 150 vs. P/F ratio ≥ 150 (p = 0.03, unpaired t test). Shown are the individual values, mean, and standard deviation. C) BALF MMP28 concentrations were not different in subjects with worse VFDs (VFDs < 14) vs. better VFDs (VFDs ≥14) (p = 0.55, Wilcoxon rank test). Shown are the individual values, median, and interquartile range. d BALF MMP28 concentrations were higher in subjects with a P/F ratio < 150 vs. P/F ratio ≥ 150 (p < 0.01, Wilcoxon rank test). Shown are the individual values, median, and interquartile range. e BALF MMP28 concentrations were higher in subjects with % PMNs > 50% vs. subjects with % PMNs ≤50% (p = 0.03, Wilcoxon rank test). Shown are the individual values, median, and interquartile range. f BALF MMP28 concentrations were higher in subjects with higher alveolar total protein vs. subjects with lower alveolar total protein (p < 0.01, Wilcoxon rank test). Subjects were divided by the median alveolar total protein concentration (306.5 μg/mL). Shown are the individual values, median, and interquartile range
Our study is the first in humans to demonstrate that increased AM MMP28 gene expression within the first 48 h after ARDS onset is associated with worse VFDs. Future studies that employ alveolar sampling are needed to validate the findings from this single-cohort association study.
Abbreviations
- AM
Alveolar macrophage
- ARDS
Acute respiratory distress syndrome
- BALF
Bronchoalveolar lavage fluid
- ELISA
Enzyme-linked immunosorbent assay
- MMP
Matrix metalloproteinases
- P/F ratio
PaO2/FiO2 ratio
- RQ
Relative quantification
- VFD
Ventilator-free days
Authors’ contributions
A.M.M. contributed to the conception of the work. E.D.M., M.M.W., C.M., and A.M.M. contributed to the design of the work. E.D.M., K.G., S.K., M.M.W., C.M., and A.M.M. contributed to the acquisition, analysis, and interpretation of the data for the work. E.D.M., C.M., M.M.W., and A.M.M drafted and revised the manuscript for important intellectual content. E.D.M., K.G., S.K., M.M.W., C.M., and A.M.M. significantly contributed to and approved the final version of the manuscript for publication. E.D.M., K.G., S.K., M.M.W., C.M., and A.M.M. agree to be accountable for all aspects of the work. All author(s) read and approved the final manuscript.
Funding
NIH NHLBI P50 HL073996 (Dr. Wurfel), NIH K23 HL144916 and Francis Family Foundation/Parker B. Francis Fellowship (Dr. Morrell), NIH K23 HL120896 (Dr. Mikacenic), NIH R01 HL116514 (Dr. Manicone).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All studies were approved by the Human Subjects Division at the University of Washington.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maldonado M, Buendía-Roldán I, Vicens-Zygmunt V, Planas L, Molina-Molina M, Selman M, et al. Identification of MMP28 as a biomarker for the differential diagnosis of idiopathic pulmonary fibrosis. PLoS One. 2018;13:e0203779. doi: 10.1371/journal.pone.0203779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manicone AM, Birkland TP, Lin M, Betsuyaku T, van Rooijen N, Lohi J, et al. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182:3866–3876. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharib SA, Johnston LK, Huizar I, Birkland TP, Hanson J, Wang Y, et al. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J Leukoc Biol. 2014;95:9–18. doi: 10.1189/jlb.1112587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrell ED, Bhatraju PK, Mikacenic CR, Radella F, Manicone AM, Stapleton RD, et al. Alveolar macrophage transcriptional programs are associated with outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;200:732–741. doi: 10.1164/rccm.201807-1381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stapleton RD, Martin TR, Weiss NS, Crowley JJ, Gundel SJ, Nathens AB, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–1662. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiolo G, Collino F, Vasques F, Rapetti F, Tonetti T, Romitti F, et al. Reclassifying acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:1586–1595. doi: 10.1164/rccm.201709-1804OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.