Abstract
Ginseng roots, Panax ginseng C.A. Meyer, obtained from cultivated ginseng grown in the Kaesong province (North Korea) and Primorye (Russia) were extracted using the supercritical CO2 extraction method. The extracts were subsequently analyzed by high-performance liquid chromatography with tandem mass spectrometry identification. The results showed the spectral peaks of typical ginsenosides with some other minor groups, and major differences were observed between the spectra of the two ginseng samples. The use of a pressure of 400 bar and higher allowed an increase in the yield of ginsenosides in comparison with similar previous studies
Keywords: ginseng, supercritical extraction, HPLC-MS/MS, ginsenosides, bioactive substances
1. Introduction
Supercritical fluid solvents represent interesting alternatives to conventional solvents for producing high-quality natural food products without toxic residues [1,2]. The introduction of supercritical fluid extraction (SFE) has led to a novel technology that is being continually developed [3,4]. High-pressure SFE can be used to produce natural thermolabile compounds, leaving no organic solvent residues in food products, which are commonly observed with conventional extraction methods using methanol and hexane. Easy solvent removal from the final product, high selectivity, and moderate temperatures during the extraction process are the major advantages of SFE, leading to a significant increase in research focused on its use in the food, cosmetic, and pharmacological industries.
SFE has been used for extracting many natural products, including the fruits of Schisandra chinensis [5], microalgae rich in polyunsaturated fatty acids [6,7], lutein from the microalgae Scenedesmus almeriensis [8], lipid extraction [9], nimbin from Neem tree seeds [10], antioxidants from coriander seeds [11], ginger oleoresin (turpentine) from ginger [12], essential oils from the leaves of Juniperus rigida [13], triterpenic acids from Eucalyptus globulus [14], and many other compounds from plant matrices.
Far Eastern ginseng Panax ginseng C.A. Meyer (P. ginseng) is a perennial plant that has been used for millennia in traditional oriental medicine. The most studied biologically active components of ginseng, ginsenosides, are a homologous series of triterpene saponins with different glycosylation profiles [15]. Ginsenosides have been reported to exhibit diverse positive effects, including antitumor, chemopreventive, immunomodulating, and antidiabetic effects [16,17,18].
However, because of the temperature instability of ginsenosides, the production and quality of P. ginseng extracts depend on the extraction method [19]. Conventional extraction methods require long extraction times and large solvent volumes, which can lead to the thermal destruction of biologically active compounds. In addition, additional filtration and/or concentration procedures are often required to remove solid residues [20]. Supercritical extraction is an ideal solution to preserve the extractable target in a non-toxic and efficient manner.
The generation of metabolic profiles is a difficult task during the analysis of biologically active substances contained in plant matrices. The identification of detected compounds is commonly achieved by high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). Technological advances have allowed for an expansion of the range of analytes and, most importantly, made it possible to identify these new compounds by accurate mass analysis (sixth decimal weight accuracy) [21,22].
Liquid chromatography combined with tandem mass spectrometry using an electrospray ionization source (LC-ESI-MS/MS) is a powerful tool for analyzing ginsenosides. Ji et al. used this method to study the composition of P. ginseng roots, combining HPLC studies with MS analysis [23,24,25].
Kite et al. used HPLC-MS to investigate melon ginsenosides and verify their authenticity [26], and Morinaga et al. identified ginsenosides in the pulp of American ginseng berries.
For compound identification, the elemental composition of the metabolite must be determined from high-accuracy mass data within 5 ppm of the theoretical mass [27]. It should be noted that a single mass value may correspond to more than one ginsenoside. Previously, more than 136 different ginsenosides with 62 unique elemental compositions have been identified in a single study [28].
2. Results and Discussion
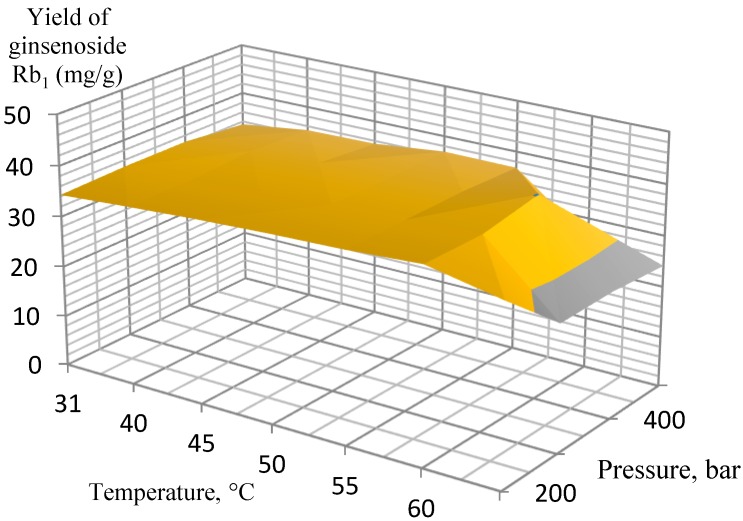
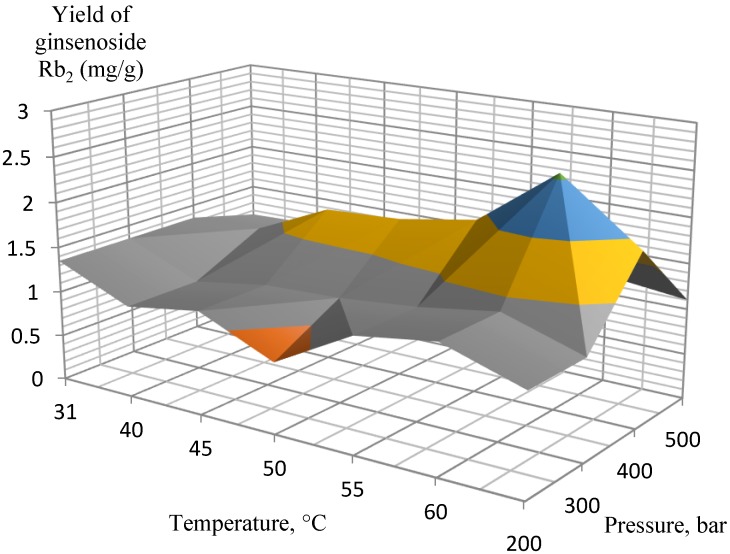
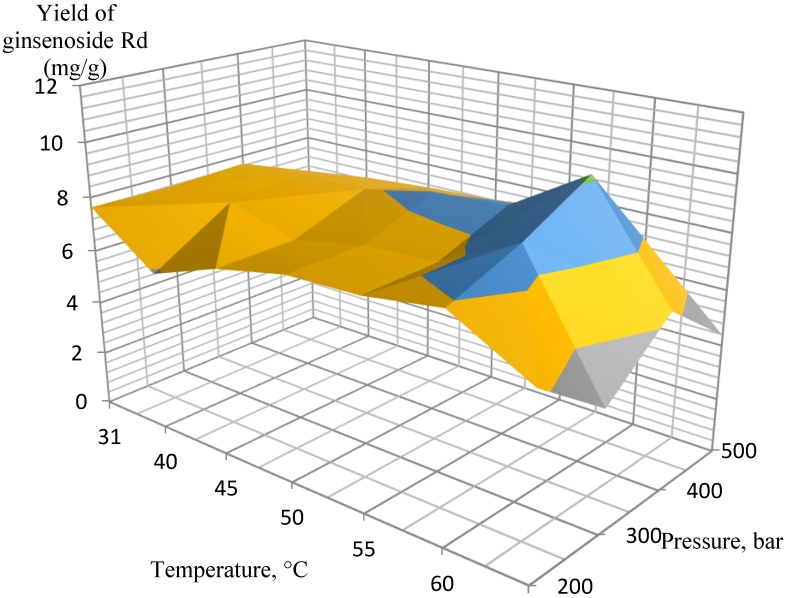
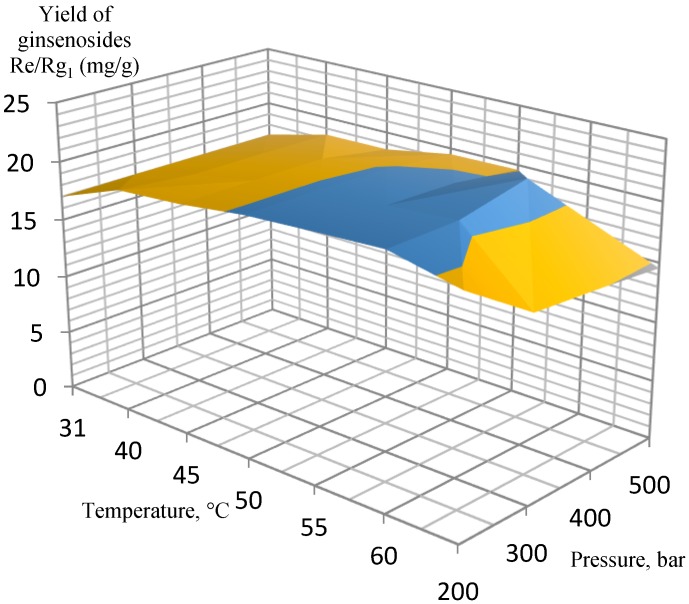
Several experimental conditions were investigated in the pressure range of 200–500 bar, 3.4% of co-solvent (ethanol, EtOH) in the liquid phase at 31–70 °C. After testing a wide range of pressures and temperatures, the most efficient extraction conditions were determined for extracting the target analytes from the ginseng roots. According to the experimental data, the orthogonal projections of the graphs were constructed separately for ginsenosides Rb1, Rb2, Rd, and Rg1/Re (Figure 1, Figure 2, Figure 3 and Figure 4, respectively).
Figure 1.
Orthogonal projection representing the results of extraction of ginsenoside Rb1 at a 200 to 500 bar and 3.4% EtOH co-solvent.
Figure 2.
Orthogonal projection representing the results of extraction of ginsenoside Rb2 at 200 to 500 bar and 3.4% EtOH co-solvent.
Figure 3.
Orthogonal projection representing the results of extraction of ginsenoside Rd at 200 to 500 bar and 3.4% EtOH co-solvent.
Figure 4.
Orthogonal projection representing the results of extraction of ginsenosides Re and Rg1 at 200 to 500 bar and 3.4% EtOH co-solvent.
These ginsenosides were chosen because their quantity and ratio were previously shown to be an effective marker for determining quality from different species, geographic environments, and cultivation cultures [29].
It is well known that the disadvantage of using pure CO2 for extraction and fractionation is that there is no pure dipole moment, and CO2 is an ineffective solvent for highly polar materials. To overcome this drawback, modifiers (ethanol, methanol, or n-hexane) can be used to increase the overall polarity of the liquid phase during extraction. In addition, modifiers allow for a more efficient extraction of solid materials by disrupting the interactions between the solutes and solid matrix. Many researchers have reported this synergistic effect in previous studies using supercritical CO2 extraction [30,31]. In spite of the fact that the co-solvent methanol is most often used for qualitative analysis, we chose ethanol as the co-solvent since extraction in a closed-cycle plant was carried out in this work, and the main idea was not just a qualitative analysis, but an analysis of the applicability of technology for the food industry [19,32,33]. Moreover, we decided to use the minimum amount of co-solvent, which gave a significant increase in the yield of the product. On the one hand, the further addition of the amount of a co-solvent shifted the system too much from the supercritical state since for ethanol the supercritical state occurs above 240 °C, and on the other hand, the further addition of ethanol did not give a significant increase in the extraction yield.
The extraction results for the isolated ginsenosides separately supported the initial conclusion that the optimal extraction conditions were 400 bar at 60 °C. When these parameters were achieved, a substantial increase in extract yields occurred, while a further increase in pressure and temperature did not affect the yields so significantly, and, therefore, it was not economical. In particular, this conclusion is the most pronounced in Figure 2 (ginsenoside Rb2 extraction) and Figure 3 (ginsenoside Rd extraction).
Obtaining chemical profiles is extremely important for the analysis of biological systems. The most commonly used methods in this regard are nuclear magnetic resonance (NMR) and HPLC-MS. Herein, HPLC-ESI-MS/MS with additional ionization and analysis of fragmented ions was used to obtain chemical profiles. High-accuracy mass spectrometric data were recorded using an ion trap amaZon SL equipped with an ESI source in negative ion mode with two-stage ion separation (MS/MS mode).
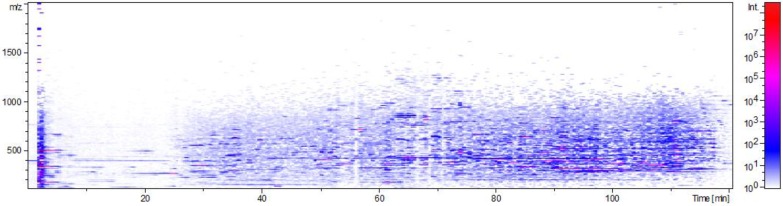
Figure 5 shows the distribution density of the analyzed chemical profiles in the ion chromatogram of the wild ginseng supercritical CO2 extract from Russia (HPLC ESI MS/MS). Visually, a rather high-density distribution of the target analytes in the analyzed extract was observed.
Figure 5.
The distribution density of the analyzed chemical profiles in the ion chromatogram of the wild ginseng supercritical CO2 extract (Russia).
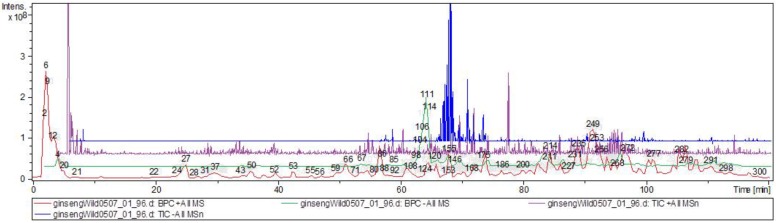
The chemical profiles of all the samples were obtained by HPLC-ESI-MS/MS. A total of 300 peaks were detected in the chromatogram (Figure 6), and 28 components were authenticated as ginsenosides by comparing the retention times, m/z values, and fragment ions with the literature data [34,35,36,37,38,39,40,41,42].
Figure 6.
Representative chemical profiles of the wild ginseng’s (Russia) total ion chromatogram from the supercritical CO2 extract.
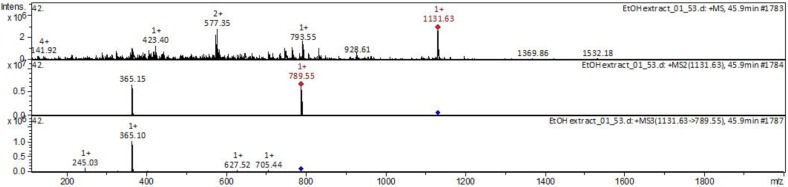
The collision-induced dissociation (CID) spectrum obtained in positive ion mode for triterpene glycoside ginsenoside Rb1 from Russian P. ginseng is shown in Figure 7.
Figure 7.
CID (collision-induced dissociation) spectra of ginsenoside Rb1 from wild ginseng (Russia), m/z 1131.63.
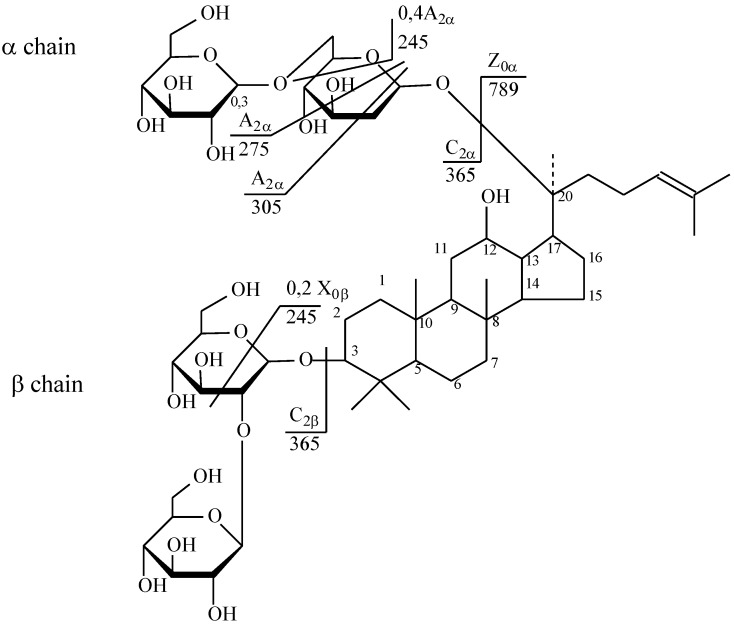
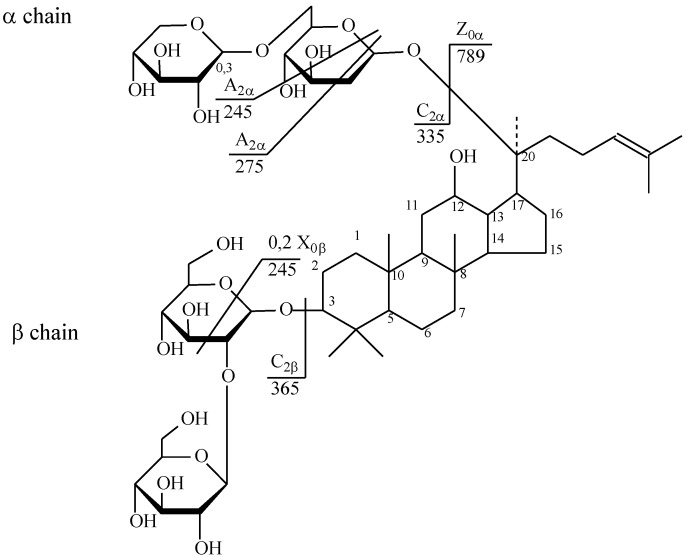
The [M + Na]+ ion produced two fragments—Z_0α at m/z 789.55 via loss of two hexose residues and dihexose C_2α at m/z 365.10 because of the higher reactivity of C20 composed to C3 (Figure 8). Z_0α also yielded a daughter ion at m/z 365.10 (C_2β), and the mass difference of 424.45 Da between the m/z 789.55 and 365.10 ions corresponded to the mass of panaxadiol with the loss of two water molecules. The C_2β ion mainly produced X_0β (m/z 245.03) by cross-ring cleavage, indicating that the β-chain consisted of two hexoses connected to each other at the 1,2 position. Thus, it was clarified that the hydrolysis of the oligosaccharide residue first occurs at the C-20 aglycone [40].
Figure 8.
Structure of ginsenoside Rb1 from wild ginseng (Russia).
The fragmentation of ginsenoside Rb2 proceeded similarly, and its structure is shown in Figure 9.
Figure 9.
Structure of ginsenoside Rb2 from wild ginseng (Russia).
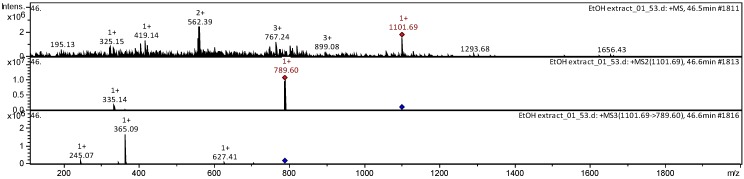
The positive ion mode CID spectra of triterpene glycoside ginsenoside Rb2 from Russian P. ginseng is shown in Figure 10.
Figure 10.
CID spectrum of the ginsenoside Rb2 from wild ginseng (Russia), m/z 1101.69.
The molecular masses of the target analytes presented in the supercritical extract of wild ginseng P. ginseng (Russia) and analyzed by HPLC with tandem mass spectrometry are listed in Table 1 for easy identification.
Table 1.
Components identified from the supercritical extract of P. ginseng (Russia).
| № | Identity | Molecular Formula | Adducts | MS (m/z) | MS2 (m/z) | MS3 (m/z) |
|---|---|---|---|---|---|---|
| Triterpene Glycosides (Dammarane Type) | ||||||
| 1 | Ginsenoside Rk3 | C36H60O8 | [M − H]− | 619.21 | 421.22 | 229.06; 347.07; 403.19 |
| 2 | Malonyl ginsenoside Rb1 | C57H94O26 | [M − H]− | 1149.81 | 1107.65 | 459.31; 621.44; 783.46; 945.52 |
| 3 | Malonyl ginsenoside Rb1 isomer | C57H94O26 | [M − H]− | 1193.7 | 1151.72 | 604.33; 826.59; 946.58; 1109.59 |
| 4 | Ginsenoside Rg1 | C42H72O14 | [M − H + HCOOH]− | 845.79 | 799.65 | 475.45; 637.61 |
| 5 | Ginsenoside Rd isomer | C48H82O18 | [M − H + HCOOH]− | 991.83 | 945.73 | 391.43; 475.5; 637.62; 783.68 |
| 6 | Ginsenoside Rg6 | C42H70O12 | [M + Na]+ | 765.41 | 405.39 | 171.07; 281.12 |
| 7 | Acetyl ginsenoside Rg1 isomer | C44H74O15 | [M + Na]+ | 841.55 | 661.5 | 481.53; 573.28; 643.32 |
| 8 | Ginsenoside Rf | C42H72O14 | [M − H]− | 846.81 | 799.65 | 391.34; 475.46; 545.54; 637.55 |
| 9 | (Yesanchinoside d isomer | C44H74O15 | [M + Na]+ | 841.62 | 661.47 | 481.48; 541.46; 571.59; 601.27; 643.42 |
| 10 | Ginsenoside Rb1 | C54H92O23 | [M − H]− | 1107.88 | 783.7 | 621.57; 460.52 |
| Ginsenoside Rb1 | C54H92O23 | [M + Na]+ | 1131.63 | 789.55 | 245.03; 365.10; 627.52; 705.44 | |
| 11 | Ginsenoside Rd | C48H82O18 | [M − H]− | 945.93 | 783.65 | 621.63; 459.39 |
| 12 | Ginsenoside 20-glc-Rf | C48H82O19 | [M − H]− | 961.84 | 915.76 | 292.31; 375.99; 459.51; 621.51; 783.75 |
| 13 | Ginsenoside 25-OH-Rh4 | C36H62O9 | [M − H]− | 637.6 | 239.14 | |
| 14 | Ginsenoside 20(R)-Rh1 | C36H62O9 | [M − H]− | 683.65 | 475.4 | 375.38 |
| 15 | Ginsenoside 20(S)-Rh1 | C36H62O9 | [M − H]− | 683.64 | 637.59 | 375.42; 475.48; 549.31 |
| 16 | Ginsenoside Rc | C53H90O22 | [M − H]− | 1077.86 | 783.59 | 621.66 |
| 17 | Ginsenoside Rb2 | C53H90O22 | [M + Na]+ | 1101.69 | 789.60 | 245.07; 365.09 |
| 18 | Ginsenoside 20(S)-Rf | C42H72O14 | [M + Na]+ | 800.94 | 782.93 | 474.96; 307.83 |
| 19 | Ginsenoside Rk2 | C42H72O15 | [M + Na]+ | 663.20 | 543.26 | 287.04; 367.26; 499.21 |
| 20 | 3,12-dihydroxydammar-20(22)E,24-diene-6-o--d-xylopyranosyl-(12)-O--d-glucopyranoside (DHDXG) | C42H70O12 | [M + Na]+ | 751.19 | 631.31 | 243.08; 367.12; 455.2; 587.21 |
| 21 | Ginsenoside Rg9 | C42H72O13 | [M + Na]+ | 781.79 | 707.44 | 377.14; 671.18 |
| Oleanolic Acid Pentaterpene Glycosides | ||||||
| 22 | Ro | C48H76O19 | [M − H]− | 955.57 | 793.41 | 455.29; 613.38; 731.42 |
| 23 | Methyl ester Ro | C49H78O19 | [M + Na]+ | 969.48 | 364.96 | 304.95 |
| 24 | Chikusetsusaponin IVA | C42H66O14 | [M − H]− | 793.36 | 334.97 | 274.94 |
| 25 | Methyl ester chikusetsusaponin IVA | C42H66O14 | [M + Na]+ | 807.38 | 627.34 | 203.05; 285.14; 361.77; 488.93 |
| 26 | Silphioside G | C42H66O14 | [M − H]− | 793.7 | 613.49 | 483.3 |
| 27 | Zingibroside R1 | C42H66O14 | [M − H]− | 793.56 | 481.43 | 275.07 |
A total of 27 ginsenosides were isolated from wild ginseng (Russia) via column chromatography and mass spectrometry. The structures were elucidated via stepwise ion fragmentation MS/MS and compared with the typical structural features from the literature data. In the wild ginseng, pairs of 20 R/S isomers, i.e., 20 R/S–acetyl Rg1, 20 R/S–Rh1, 20 R/S–Rf, 20 R/S–Rg3, and 20 R/S–Rh2, were isolated. This series of 20R ginsenosides, which are not often found in wild ginseng, are most likely obtained by the attack on the hydroxyl group at C-20 after selective deglycosylation (Kang et al., 2007). Ginsenosides Rg9, Rk2, and Rk3 and hydroxylases at C-25, i.e., 25–OH–20 R/S–Rh4, were also obtained. These compounds could be prepared by combining dehydration and hydration during heating and acid or enzymatic hydrolysis [43]. Thus, exhaustive supercritical extraction using ethanol could be used to obtain ginsenoside extracts from the ginseng crown. As well as using methanol as a co-solvent, it allows one to achieve good qualitative and quantitative indicators, while the use of ethanol in the food industry is more preferable compared to methanol [19,32]. We isolated 21 ginsenosides, which is a quarter more than previously isolated under supercritical extraction conditions; we attribute this to two factors. The first is that the conditions for the growth of ginseng in the northern regions are more contrasting in temperature, and the second, that we used pressures of up to 500 bar, while previously the researchers were limited to 220 bars [32,33].
The molecular masses of the target analytes isolated from the supercritical extract of ginseng P. ginseng (North Korea, Kaesong) are listed in Table 2 for easy identification.
Table 2.
Components identified from the supercritical extract of P. ginseng (North Korea, Kaesong).
| № | Identity | Molecular Formula | Adducts | MS (m/z) | MS2 (m/z) | MS3 (m/z) |
|---|---|---|---|---|---|---|
| Triterpene Glycosides (Dammarane Type) | ||||||
| 1 | Ginsenoside 20(R)-Rh1 | C36H62O9 | [M − H]− | 637.38 | 597.32 | 375.42; 475.48 |
| 2 | Ginsenoside 20(S)-Rh1 | C36H62O9 | [M − H]− | 637.39 | 597.33 | 375.42; 475.49 |
| 3 | Ginsenoside 20(R)-Rh2 | C36H62O8 | [M − H]− | 621.32 | 580.2 | 390.33 |
| 4 | Ginsenoside 20(S)-Rh2 | C36H63O10 | [M − H]− | 621.32 | 580.24 | 390.34 |
| 5 | Ginsenoside 25-OH-(S)-Rh1 | C36H63O10 | [M − H]− | 654.41 | 375.15 | 332.26 |
| 6 | Ginsenoside Rg1 | C42H72O14 | [M + HCOO]− | 845.26 | 501.18 | 485.17 |
| 7 | Ginsenoside F2 | C42H72O13 | [M + H]+ | 785.55 | 783.6 | 375.27; 459.33; 537.37; 621.36 |
| 8 | Ginsenoside 20(S)-Rf2 | C42H74O14 | [M − H]− | 801.80 | 767.68 | 378.21; 671.55 |
| 9 | Ginsenoside 20-glu-Rf | C48H82O19 | [M − H]− | 961.59 | 681.45 | 637.44; 357.14; 401.12 595.46 |
| 10 | Ginsenoside 20(R/S)-Rg2 | C42H72O13 | [M − H]− | 783.54 | 529.38 | 429.21 |
| 11 | Ginsenoside 20(R/S)-Rg3 | C42H72O13 | [M − H]− | 783.68 | 737.87 | 694.71 |
| 12 | Ginsenoside 20(R/S)-Rf | C42H72O14 | [M − H]− | 799.80 | 544.49 | 227.21; 280.18; 379.14 |
| 13 | Notoginsenoside Rw2 | C41H70O14 | [M + Na]− | 809.81 | 544.50 | 227.21; 280.18; 379.15 |
| Oleanolic Acid Pentaterpene Glycosides | ||||||
| 14 | Silphioside G | C42H66O14 | [M − H]− | 793.49 | 613.3 | 407.21; 509.33 |
| 15 | Chikusetsusaponin IVA | C42H66O14 | [M + H]+ | 795.67 | 631.39 | 511.18 |
A total of 15 ginsenosides were isolated from Korean ginseng (North Korea, Kaesong) via column chromatography and MS. In the Korean ginseng, pairs of 20 R/S isomers, i.e., 20 R/S–Rh1, 20 R/S–Rh2, 20 R/S–Rf, 20 R/S–Rg2, 20 R/S–Rg3, and 20 R/S–Rf2, were isolated. Ginsenosides that were hydrolyzed at C-25, i.e., 25–OH–20 R/S–Rh1, were also found. These compounds could be prepared by combining dehydration and hydration during heating and acid or enzymatic hydrolysis [43]. Oleanolic acid pentaterpene glycosides chikusetsusaponin IV A and silphioside G were also isolated (Table 2).
3. Materials and Methods
3.1. Materials
Samples of P. ginseng were purchased from the Lazovsky district of Primorye, Russia, and cultivated ginseng (P. ginseng) was obtained from the province of Kaesong, North Korea. All samples were morphologically authenticated according to the current Russian Pharmacopeia standards [44].
3.2. Chemicals and Reagents
HPLC-grade acetonitrile was purchased from Fisher Scientific (Southborough, UK), and MS-grade formic acid was obtained from Sigma-Aldrich (Steinheim, Germany). Ultra-pure water was prepared using a SIEMENS ULTRA clear instrument (SIEMENS Water Technologies, Berlin, Germany), and all other chemicals were of analytical grade.
3.3. Liquid Chromatography
HPLC was performed using a Shimadzu LC-20 Prominence system (Shimadzu, Kyoto Japan) equipped with ZORBAX Eclipse XDB C18 (150 × 4.6 mm, particle size: 5 μm) reverse-phase column for the separation of the multicomponent mixtures. The gradient elution program was as follows: 0.01–4 min, 100% A; 4–60 min, 100–25% A; 60–75 min, 25–0% A; control washing 75–120 min, 0% A. Solvent A - deionized water, solvent B - acetonitrile. The entire HPLC analysis was performed using a UV-VIS detector SPD-20A (Shimadzu, Kyoto Japan) at wavelengths of 230 and 330 nm at 17 °C provided with column oven CTO-20A (Shimadzu, Kyoto Japan) with an injection volume of 20 μL.
3.4. Supercritical Fluid Extraction
Supercritical CO2 extraction was performed using the TharSCF SFE-500 system (Waters, Pittsburgh, PA, USA) supercritical pressure extraction apparatus. System options include a co-solvent pump (Thar Waters P-50 High-Pressure Pump) for extracting polar samples; CO2 flow meter (Siemens, Berlin, Germany) to measure the amount of CO2 being supplied to the system; multiple extraction vessels to extract different sample sizes or to increase the throughput of the system. The flow rate was 50 mL/min for liquid CO2 and 1.76 mL/min for EtOH. For extraction, samples of 10 g of ginseng root were used. The extraction time was counted after reaching the set pressure and equilibrium flow, and it was 6 h for each sample.
3.5. Mass Spectrometry
MS analysis was performed using an ion trap amaZon SL (BRUKER DALTONIKS, Berlin, Germany) equipped with an ESI source in negative ion mode. The optimized parameters were as follows: ionization source temperature, 70 °C; gas flow, 4 L/min; nebulizer gas (atomizer), 7.3 psi; capillary voltage, 4500 V; endplate bend voltage, 1500 V; fragmentation voltage, 280 V; collision energy, 60 eV. An ion trap was used in the m/z 100–1700 scan range for MS and MS/MS analyses. The capture rate was one spectrum/s for MS and two spectra/s for MS/MS. Data collection was controlled by Windows software for BRUKER DALTONIKS. All experiments were performed in triplicate, and a two-stage ion separation mode (MS/MS mode) was implemented.
4. Conclusions
Supercritical CO2 extraction is a soft extractive method that can be executed at fairly low temperatures with a very sparing effect on the plant matrix. In addition, it features easy removal of the solvent from the resulting extract and is environmentally friendly, yielding a richer extractive material.
Over the past decade, more than 400 novel ginsenoside compounds have been reported. The use of newly developed methods for studying the chemical structure of ginsenosides, including HPLC-MS/MS, can significantly improve the accuracy of identification methods, facilitating further discoveries of biologically active compounds from plant matrices.
Ginseng roots, Panax ginseng C.A. Meyer, obtained from cultivated ginseng grown in the Kaesong province (North Korea) and Primorye (Russia) were extracted using the supercritical CO2 extraction method. We chose the conditions for extraction that are most suitable for subsequent scaling for food industrial extraction. The optimal co-solvent ethanol at a concentration of 3.4% was selected. The optimum temperature was determined to be 60 °C. The results showed the spectral peaks of typical ginsenosides with some other minor groups, and major differences were observed between the spectra of the two ginseng samples.
Author Contributions
Conceptualization, G.C. and K.G.; methodology, A.Z.; software, M.R.; validation, M.R. and T.-S.S.; formal analysis, M.R.; investigation, M.R. and A.Z.; resources, K.G. and A.Z.; data curation, T.-S.S.; writing—original draft preparation, M.R.; writing—review and editing, T.-S.S. and G.C.; visualization, M.R.; supervision, K.G.; project administration, A.Z.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Council on Grants of the President of the Russian Federation (CΠ-3156.2019.4).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Subra P., Castellani S., Jestin P., Aoufi A. Extraction of β-carotene with supercritical fluids. J. Supercrit. Fluids. 1998;12:261–269. doi: 10.1016/S0896-8446(98)00085-0. [DOI] [Google Scholar]
- 2.Casas L., Mantell C., Rodríguez M., Torres A., Macías F.A., De La Ossa E.M., Cardoso L.C. Extraction of natural compounds with biological activity from sunflower leaves using supercritical carbon dioxide. Chem. Eng. J. 2009;152:301–306. doi: 10.1016/j.cej.2007.06.027. [DOI] [Google Scholar]
- 3.Herrero M., Mendiola J.A., Cifuentes A., Ibáñez E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A. 2010;1217:2495–2511. doi: 10.1016/j.chroma.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Plaza M., Herrero M., Cifuentes A., Ibáñez E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009;57:7159–7170. doi: 10.1021/jf901070g. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y.H., Kim J., Jeon S.H., Yoo K.-P., Lee H.-K. Optimum SFE condition for lignans of Schisandra chinensis fruits. Chromatographia. 1998;48:695–699. doi: 10.1007/BF02467601. [DOI] [Google Scholar]
- 6.Macías-Sánchez M.D., Serrano C.M., Rodríguez-Rodríguez M., De La Ossa E.M., Lubián L.M., Montero O. Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J. Sep. Sci. 2008;31:1352–1362. doi: 10.1002/jssc.200700503. [DOI] [PubMed] [Google Scholar]
- 7.Gouveia L., Nobre B., Marcelo F., Mrejen S., Cardoso M., Palavra A., Mendes R. Functional food oil coloured by pigments extracted from microalgae with supercritical CO2. Food Chem. 2007;101:717–723. doi: 10.1016/j.foodchem.2006.02.027. [DOI] [Google Scholar]
- 8.Mehariya S., Iovine A., Di Sanzo G., LaRocca V., Martino M., Leone G.P., Casella P., Karatza D., Marino T., Musmarra D., et al. Supercritical fluid extraction of lutein from Scenedesmus almeriensis. Molecules. 2019;24:1324. doi: 10.3390/molecules24071324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahena F., Zaidul I.S.M., Jinap S., Karim A.A., Abbas K.A., Norulaini N.A.N., Omar A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009;95:240–253. doi: 10.1016/j.jfoodeng.2009.06.026. [DOI] [Google Scholar]
- 10.Tonthubthimthong P., Douglas P.L., Douglas S., Luewisutthichat W., Teppaitoon W., Pengsopa L.-E. Extraction of nimbin from neem seeds using supercritical CO2 and a supercritical CO2–methanol mixture. J. Supercrit. Fluids. 2004;30:287–301. doi: 10.1016/j.supflu.2003.07.007. [DOI] [Google Scholar]
- 11.Yepez B., Espinosa M., López S., Bolaños G. Producing antioxidant fractions from herbaceous matrices by supercritical fluid extraction. Fluid Phase Equilibria. 2002;194:879–884. doi: 10.1016/S0378-3812(01)00707-5. [DOI] [Google Scholar]
- 12.Zancan K.C., Marques M.O.M., Petenate A.J., Meireles M.A. Extraction of ginger (Zingiber officinale Roscoe) oleoresin with CO2 and co-solvents: A study of the antioxidant action of the extracts. J. Supercrit. Fluids. 2002;24:57–76. doi: 10.1016/S0896-8446(02)00013-X. [DOI] [Google Scholar]
- 13.Meng X., Li D.-W., Zhou D., Wang N., Liu Q., Fan S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016;194:698–705. doi: 10.1016/j.jep.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 14.De Melo M., Oliveira E., Silvestre A., Silva C.M. Supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark. J. Supercrit. Fluids. 2012;70:137–145. doi: 10.1016/j.supflu.2012.06.017. [DOI] [Google Scholar]
- 15.Elyakov G.B., Strigina L.I., Khorlin A.Y., Kochetkov H.K. Glycosides of ginseng (Panax ginseng C. A. Mey) Russ. Chem. Bull. 1962;11:1055. doi: 10.1007/BF00905239. [DOI] [Google Scholar]
- 16.Brekhman I., Dardymov I.V., Dobriakov I. On the pharmacology of individual glycosides from the roots of Panax ginseng C.A. Mey. Farmakol. Toksikol. 1966;29:167–171. [PubMed] [Google Scholar]
- 17.Park E.-K., Shin Y.-W., Lee H.-U., Kim S.-S., Lee Y.-C., Lee B.-Y., Kim N.-H. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Boil. Pharm. Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 18.Bao C., Wang Y., Min H., Zhang M., Du X., Han R., Liu X. Combination of ginsenoside Rg1 and bone marrow mesenchymal stem cell transplantation in the treatment of cerebral ischemia reperfusion injury in rats. Cell. Physiol. Biochem. 2015;37:901–910. doi: 10.1159/000430217. [DOI] [PubMed] [Google Scholar]
- 19.Wood J., Bernards M.A., Wan W.-K., Charpentier P.A. Extraction of ginsenosides from North American ginseng using modified supercritical carbon dioxide. J. Supercrit. Fluids. 2006;39:40–47. doi: 10.1016/j.supflu.2006.01.016. [DOI] [Google Scholar]
- 20.Wang L., Weller C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- 21.Kochkin D.V., Kachala V.V., Shashkov A.S., Chizhov A.O., Chirva V.Y., Nosov A.M. Malonyl-ginsenoside content of a cell-suspension culture of Panax japonicus var. repens. Phytochemistry. 2013;93:18–26. doi: 10.1016/j.phytochem.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Stavrianidi A., Baygildiev T.M., Stekolshchikova E.A., Shpigun O.A., Rodin I.A. New approaches to the determination and group identification of physiologically active compounds in plant materials and commercial products by high-performance liquid chromatography–mass spectrometry. J. Anal. Chem. 2019;74:58–70. doi: 10.1134/S1061934819010106. [DOI] [Google Scholar]
- 23.Ji Q.C., Harkey M.R., Henderson G.L., Gershwin M.E., Stern J.S., Hackman R.M. Quantitative determination of ginsenosides by high-performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. 2001;12:320–326. doi: 10.1002/pca.593. [DOI] [PubMed] [Google Scholar]
- 24.Ligor T., Ludwiczuk A., Wolski T., Buszewski B. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal. Bioanal. Chem. 2005;383:1098–1105. doi: 10.1007/s00216-005-0120-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Sakuma T., Asafu-Adjaye E., Shiu G.K. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal. Chem. 1999;71:1579–1584. doi: 10.1021/ac980890p. [DOI] [PubMed] [Google Scholar]
- 26.Kite G.C., Howes M.-J.R., Leon C.J., Simmonds M.S. Liquid chromatography/mass spectrometry of malonyl-ginsenosides in the authentication of ginseng. Rapid Commun. Mass Spectrom. 2003;17:238–244. doi: 10.1002/rcm.899. [DOI] [PubMed] [Google Scholar]
- 27.Brown P.N. Determination of ginsenoside content in Asian and North American ginseng raw materials and finished products by high-performance liquid chromatography: Single-laboratory validation. J. AOAC Int. 2011;94:1391–1399. doi: 10.1093/jaoac/94.5.1391. [DOI] [PubMed] [Google Scholar]
- 28.Buckingham J. Dictionary of Natural Products. CRC Press/Taylor and Francis Group; London, UK: 1993. [Google Scholar]
- 29.Yang L., Li C.-L., Cheng Y.-Y., Tsai T.-H., Tsai T.-H. Development of a validated UPLC-MS/MS method for analyzing major ginseng saponins from various ginseng species. Molecules. 2019;24:4065. doi: 10.3390/molecules24224065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo S.-D., Park S.-J., Kim J.-W., Kim J.-C. Critical properties of carbon dioxide + methanol, + ethanol, + 1-propanol, and + 1-butanol. J. Chem. Eng. Data. 2000;45:932–935. doi: 10.1021/je000104p. [DOI] [Google Scholar]
- 31.Brunner G., Machado N. Process design methodology for fractionation of fatty acids from palm fatty acid distillates in countercurrent packed columns with supercritical CO2. J. Supercrit. Fluids. 2012;66:96–110. doi: 10.1016/j.supflu.2012.02.012. [DOI] [Google Scholar]
- 32.Huang Y., Zhang T., Zhao Y., Zhou H., Tang G., Fillet M., Crommen J., Jiang Z. Simultaneous analysis of nucleobases, nucleosides and ginsenosides in ginseng extracts using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2017;144:213–219. doi: 10.1016/j.jpba.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 33.Samimi R., Xu W.Z., AlSharari Q., Charpentier P.A. Supercritical fluid chromatography of North American ginseng extract. J. Supercrit. Fluids. 2014;86:115–123. doi: 10.1016/j.supflu.2013.12.004. [DOI] [Google Scholar]
- 34.Xie Y.-Y., Luo D., Cheng Y.-J., Ma J.-F., Wang Y.-M., Liang Q., Luo G.-A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MSn-based multicomponent quantification fingerprint. J. Agric. Food Chem. 2012;60:8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- 35.Yang H., Lee N.Y., Kang K.B., Kim J.Y., Kim S.O., Yoo Y.H., Sung S.H. Identification of ginsenoside markers from dry purified extract of Panax ginseng by a dereplication approach and UPLC–QTOF/MS analysis. J. Pharm. Biomed. Anal. 2015;109:91–104. doi: 10.1016/j.jpba.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 36.Lee J., Choi B.-R., Kim Y.-C., Choi D.J., Lee Y.-S., Kim G.-S., Baek N.-I., Kim S.-Y., Lee D. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. 2017;22:2147. doi: 10.3390/molecules22122147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y., Zhao Z., Chen H., Yi T., Qin M., Liang Z. Chemical differentiation and quality evaluation of commercial Asian and American ginsengs based on a UHPLC-QTOF/MS/MS metabolomics approach. Phytochem. Anal. 2014;26:145–160. doi: 10.1002/pca.2546. [DOI] [PubMed] [Google Scholar]
- 38.Wang H.-P., Zhang Y.-B., Yang X., Zhao D., Wang Y.-P. Rapid characterization of ginsenosides in the roots and rhizomes of Panax ginseng by UPLC-DAD-QTOF-MS/MS and simultaneous determination of 19 ginsenosides by HPLC-ESI-MS. J. Ginseng Res. 2015;40:382–394. doi: 10.1016/j.jgr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li F., Lv C., Li Q., Wang J., Song D., Liu P., Zhang D., Lu J. Chemical and bioactive comparison of flowers of Panax ginseng Meyer, Panax quinquefolius L., and Panax notoginseng Burk. J. Ginseng Res. 2016;41:487–495. doi: 10.1016/j.jgr.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S., Cui M., Liu Z., Song F., Mo W. Structural analysis of saponins from medicinal herbs using electrospray ionization tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2004;15:133–141. doi: 10.1016/j.jasms.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Q.-L., Zhu D.-N., Yang X., Xu W., Wang Y.-P. Development and validation of a UFLC–MS/MS method for simultaneous quantification of sixty-six saponins and their six aglycones: Application to comparative analysis of red ginseng and white ginseng. J. Pharm. Biomed. Anal. 2018;159:153–165. doi: 10.1016/j.jpba.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 42.Karpova R.V., Shevchenko V.E., Bocharov E.V., Sheychenko O.P., Bocharova O.A., Kucheryanu V.G., Bykov V.A. Ginsenosides definition in plant extracts by means of high through liquid chromatography with tandem mass-spectrometry. Russ. J. Biother. 2016;15:36–46. doi: 10.17650/1726-9784-2016-15-2-36-46. [DOI] [Google Scholar]
- 43.Li S.-L., Lai S.-F., Song J.-Z., Qiao C.-F., Liu X., Zhou Y., Cai H., Cai B., Xu H.-X. Decocting-induced chemical transformations and global quality of Du–Shen–Tang, the decoction of ginseng evaluated by UPLC–Q-TOF-MS/MS based chemical profiling approach. J. Pharm. Biomed. Anal. 2010;53:946–957. doi: 10.1016/j.jpba.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Russia State Pharmacopoeia XII. Scientific Center of Expertise of Medical Products; Moscow, Russia: 2008. pp. 26–121. [Google Scholar]