Abstract
Background
Human leukocyte antigen‐identical sibling donor (ISD)‐hematopoietic stem cell transplantation (SCT) is a potentially curative treatment for high‐risk pediatric acute myeloid leukemia (AML). A haploidentical donor (HID) is readily available to almost all children. Previous studies have demonstrated that patients with HID‐SCT had similar outcomes compared to ISD‐SCT for pediatric and adult AML. However, the role of HID‐SCT in high‐risk pediatric AML is unclear.
Methods
To compare the overall survival of high‐risk AML children who underwent either HID‐SCT or ISD‐SCT, we analyzed 179 cases of high‐risk AML patients under 18 years of age treated with either ISD‐SCT (n = 23) or HID‐SCT (n = 156). Granulocyte colony‐stimulating factor plus anti‐thymocyte globulin‐based regimens were used for HID‐SCT. We also analyzed the subgroup data of AML patients at first complete remission (CR1) before SCT with known cytogenetic risk.
Results
The numbers of adverse cytogenetic risk recipients were 8 (34.8%) and 13 (18.8%) in the ISD‐SCT group and the HID‐SCT group, and the number of patients with disease status beyond CR1 were 6 (26.1%) and 14 (20.3%) in the two groups. The cumulative rates of grades II‐IV acute graft‐versus‐host disease (GVHD) were 13.0% in the ISD‐SCT group and 34.8% in the HID‐SCT group (P = 0.062), with a three‐year cumulative rates of chronic GVHD at 14.1% and 34.9%, respectively (P = 0.091). The relapse rate in the ISD‐SCT group was significantly higher than that in the HID‐SCT group (39.1% vs. 16.4%, P = 0.027); with non‐relapse mortality at 0.0% and 10.6% (P = 0.113), respectively. The three‐year overall survival rates were 73.0% for the ISD‐SCT group and 74.6% for the HID‐SCT group (P = 0.689). In subgroup analysis, the three‐year relapse rate in the ISD‐SCT group was higher than that in the HID‐SCT group (50.0% vs. 9.2%, P = 0.001) and the three‐year DFS in the ISD‐SCT group (50.0%) was lower than that in the HID‐SCT group (81.2%) (P = 0.021).
Conclusions
Unmanipulated HID‐SCT achieved DFS and OS outcomes comparable to those of ISD‐SCT for high‐risk pediatric AML patients with potentially higher rate but manageable GVHD.
Keywords: Acute myeloid leukemia, high-risk, pediatric, transplantation, haploidentical, identical sibling, propensity score matching, graft-versus-host disease, overall survival, disease-free survival
Abbreviations
- AML
acute myeloid leukemia
- Ara‐C
cytarabine
- ATG
anti‐human thymocyte globulin
- BM
bone marrow
- Bu
busulfan
- CB
cord blood
- CBMTCG
Chinese Bone Marrow Transplant Cooperative Group
- CR
complete remission
- CSA
cyclosporine A
- CTX
cyclophosphamide
- DFS
disease‐free survival
- G‐CSF
granulocyte colony‐stimulating factor
- GVHD
graft‐versus‐host disease
- GVL
graft versus leukemia
- HID
haploidentical donor
- HLA
human leukocyte antigen
- Hu
hydroxycarbamide
- ISD
identical sibling donor
- M‐CCNU
simustine
- MMF
mycophenolate mofetil
- MNC
mononuclear cell
- MRD
minimal residual disease
- MTX
methotrexate
- OS
overall survival
- PBSC
peripheral blood stem cell
- PT/Cy
post‐transplantation cyclophosphamide
- SCT
hematopoietic stem cell transplantation
- URD
unrelated matched donor
- WBC
white blood cell
1. BACKGROUND
The survival of pediatric acute myeloid leukemia (AML) has been increased up to 60%–70% after using more intensive chemotherapy and better supportive care [1, 2, 3]. Allogeneic hematopoietic stem cell transplantation (SCT) is a potentially curative treatment for high‐risk or chemotherapy‐refractory AML [4]. Although a human leukocyte antigen (HLA)‐identical sibling donor (ISD) would be the first‐line of choice for SCT, for patients lacking a suitable ISD, the haploidentical donor (HID)‐SCT may represent a valid alternative option. HID‐SCT has advantages such as rapid and universal availability as well as the ability to provide additional donor cells when needed for subsequent adoptive immunotherapies. Over the past decade, great progress has been made in the use of HID‐SCT which has led to improvements in patients survival and increase in the number of HID‐SCT procedures. According to a report from the European Society for Blood and Marrow Transplantation [5], the number of HID‐SCT procedures grew by 291% from 2005 to 2015. The three most commonly used strategies are the granulocyte colony‐stimulating factor (G‐CSF) and anti‐human thymocyte globulin (ATG)‐based protocol [6], the post‐transplantation cyclophosphamide (PT/Cy)‐based protocol [7], and the T‐cell depletion protocol [8]. HID‐SCT could be employed in the absence of an ISD or for those in urgent need of a transplant.
Liu et al. [9] reported a five‐year overall survival (OS) rate of 73.3% with acceptable non‐relapse mortality of 12.6% in pediatric AML patients who underwent T cell replete HID‐SCT. Recently, investigators have compared HID‐SCT and HLA‐matched SCT in adult AML. Bashey et al. [10] reported comparable results between PT/Cy‐based HID‐SCT and HLA‐matched SCT, where the three‐year OS, disease‐free survival (DFS), non‐relapse mortality and cumulative rates of relapse in the HID‐SCT and the HLA‐matched SCT were 67% and 62%, 58% and 51%, 9% and 17%, and 33% and 32%, respectively. Another promising result reported by Wang et al. [11] showed that HID‐SCT with a G‐CSF and ATG‐based protocol achieved outcomes similar to those of ISD‐SCT for adult AML patients in their first complete remission (CR1), where the three‐year OS, DFS, non‐relapse mortality, and relapse rates in the HID‐SCT and ISD‐SCT groups were 79% and 82%, 74% and 78%, 13% and 8%, 15% and 15%, respectively. However, previous studies either included both AML and acute lymphoblastic leukemia or high‐risk and standard‐risk AML [9, 12], as such data comparing HID‐SCT with ISD‐SCT in children with high‐risk AML are lacking.
Herein, we performed a multi‐center retrospective matched‐pair study to compare the survival outcomes between high‐risk AML children who underwent either unmanipulated HID‐SCT or ISD‐SCT at three large Chinese SCT centers.
2. PATIENTS AND METHODS
2.1. Patients
According to our previous study which enrolled 410 patients between January 2000 and October 2012 [13], in comparison with HID‐SCT, cord blood (CB)‐SCT had a lower one‐year OS (56.8% vs. 73.0%) and higher one‐year non‐relapse mortality (35.1% vs. 18.0%). Furthermore, high‐risk patients usually had insufficient time to wait for an unrelated matched donor (URD); thus, the proportions of both URD‐SCT and CB‐SCT cases were small in our centers. Therefore, we ruled out URD‐SCT and CB‐SCT cases. Patients aged 1‐18 years who underwent transplant between January 1, 2013 and January 11, 2017 were enrolled in this study. Each SCT procedure took place in one of the following three transplantation centers: the Department of Bone Marrow Transplantation, Peking University People's Hospital (Beijing, China); Department of Hematology, Xinqiao Hospital, Army Military Medical University (Chongqing, China); or Department of Pediatrics, Nanfang Hospital, Southern Medical University (Guangzhou, China). HLA‐ISD was the first choice for SCT donors. If HLA‐ISD was unavailable, HIDs were deemed eligible for the patients. The last follow‐up date was December 1, 2018. The study protocol was approved by the ethics committee at each local institution. Informed consent was obtained from all the patients’ guardians.
2.2. Transplant regimen
The treatment protocol is shown in Figure 1.The conditioning regimens for HID‐SCT were as follows: cytarabine (Ara‐C, 4 g/m2/d, intravenous infusion, Pfizer Pharmaceuticals Ltd., New York, USA) on days −10 (defined as 10 days before transplantation day) to −9; busulfan (Bu, 3.2 mg/kg/d, intravenous infusion, Otsuka Pharmaceutical Co. Ltd., Naruto, Tokushima, Japan) on days −8 to −6; cyclophosphamide (CTX, 1.8 g/m2/d, intravenous infusion, Baxter Oncology GmbH, Frankfurt, Hesse‐Darmstadt, Germany) on days −5 to −4; simustine (M‐CCNU, 250 mg/m2, oral administration, ZheJiang Ruixin Pharmaceutical Co. Ltd., Lishui, Zhejiang, China) on day −3; and rabbit thymoglobulin (ATG, 2.5 mg/kg/d, intravenous infusion, Genzyme Polyclonals S.A.S., Lyon, Rhône, France) on days −5 to −2. Patients in the ISD‐SCT group received the same treatment as the HID‐SCT patients except for the following differences: the patients were additionally treated with hydroxycarbamide (Hu, 80 mg/kg, oral administration, Qilu Pharmaceutical Co. Ltd., Jinan, Shandong, China) on day −10 and Ara‐C (2 g/m2/d) on day −9, and the patients were not treated with ATG. All patients received cyclosporine A (CSA, intravenous infusion, Novartis Pharma Ltd., Basel, Switzerland), mycophenolate mofetil (MMF, oral administration, Roche Pharmaceutical Ltd., Basel, Switzerland), and short‐term methotrexate (MTX, intravenous infusion, Pfizer Pharmaceuticals Ltd., New York, USA) as a prophylaxis for graft‐versus‐host disease (GVHD). CSA was used from day −9, of which the concentration was adjusted to 150‐250 ng/mL. From day −9, 250‐500 mg of MMF was administered every 12 hours until day +30. After graft infusion, a dose of 15 mg/m2 MTX was administered on day +1 as well as a dose of 10 mg/m2 on days +3 and +6 for the ISD‐SCT recipients and on days +3, +5 and +11 for the HID‐SCT recipients [14]. All recipients received G‐CSF‐mobilized (Chugai Pharmaceutical Co. Ltd., Tokyo, Japan) (5 μg/kg/d, subcutaneously for donor on day −3 to transplantation day), fresh, and unmanipulated (without T cell depletion of graft in vitro) bone marrow cells plus peripheral blood stem cells (PBSCs) or PBSCs alone. G‐CSF (5 μg/kg/d) was provided to all HID‐SCT recipients from day +6 until their white blood cell (WBC) count exceeded 2 × 109/L for three consecutive days [6, 14].
FIGURE 1.
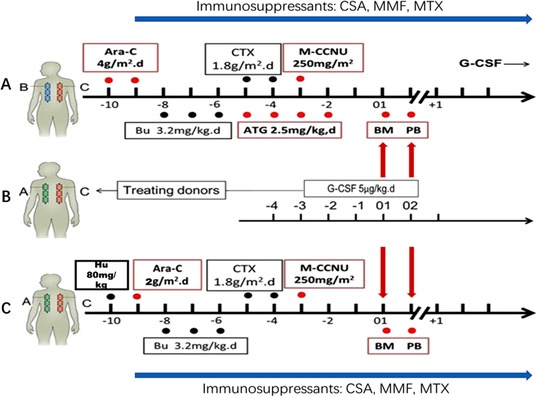
Protocol of conditioning regimen for pediatric AML patients who underwent ISD‐SCT or HID‐SCT (A). Transplantation protocol for HID‐SCT: Ara‐C (4 g/m2/d) on days −10 to −9; Bu (3.2 mg/kg/d) on days −8 to −6; CTX (1.8 g/m2/d) on days −5 to −4; M‐CCNU(250 mg/m2) on day −3; and ATG (2.5 mg/kg/d) on days −5 to −2. (B). Donor and stem cell harvesting: All donors received G‐CSF (5 μg/kg/d) on days −3 to transplantation day. (C). Transplantation protocol for ISD‐SCT: Bu(80 mg/kg) on day −10; Ara‐C (2 g/m2/d) on day −9; Bu (3.2 mg/kg/d) on days −8 to −6; CTX (1.8 g/m2/d) on days −5 to −4; M‐CCNU (250 mg/m2) on day −3. All recipients received G‐CSF‐mobilized, fresh, and unmanipulated bone marrow cells plus peripheral blood stem cells or peripheral blood stem cells alone. The GVHD prophylaxis plan included ATG, CSA, MMF and short‐term MTX [39]
Abbreviations: /d, per day; SCT, stem cell transplantation; ISD, identical sibling donor; HID, haploidentical donor; Ara‐C, cytarabine; Bu, busulfan; CTX, cyclophosphamide; M‐CCNU, simustine; Hu, hydroxycarbamide; ATG, anti‐human thymocyte globulin; G‐CSF, granulocyte colony‐stimulating factor; GVHD, graft‐versus‐host‐disease; CSA, cyclosporine A; MMF, mycophenolate mofetil; MTX, methotrexate; BM, bone marrow; PB, peripheral blood.
2.3. Definition and assessments
According to published criteria, “high‐risk” was defined as follows: 1) relapsed AML [15, 16]; 2) therapy‐related or myelodysplastic syndrome‐derived AML [15, 16]; 3) ≥ l5.0% blasts in bone marrow (BM) smear or ≥ 0.1% blasts in a BM sample tested by flow cytometry after two cycles of induction [17]; 4) AML with adverse cytogenetic features [17]; 5) ≥ 0.1% blasts in a BM sample tested by flow cytometry after three cycles of chemotherapy [15]; and 6) FLT3‐ITD without NPM1 mutation [17]. Adverse cytogenetic features were defined as abnormal 3q, monosomy 5, monosomy 7,monosomy 17, deletions of 5q, deletion of 7q, t(6;9)(q23;q34), t(9;22)(q34;q11), MLL re‐arrangement except t(9;11), and complex cytogenetic abnormalities which was defined as at least four unrelated cytogenetic abnormalities [17]. Patients with t(16;16)(p13;q22) and t(8;21)(q22;q22) were considered to have favorable cytogenetic features [17]. All other patients were considered to have intermediate risk for cytogenetic disease.
Neutrophil engraftment was defined as the first day of neutrophil count of 0.5 × 109/L or more for three consecutive days, and platelet engraftment was defined as the first day of a platelet count of 20 × 109/L or more for seven consecutive days without transfusion. Primary engraftment failure was defined as the absence of donor‐derived myeloid cells at day 60 in patients surviving beyond day 28 after transplantation or as the need for a second allogeneic transplant or reconstitution with autologous cells. ABO major mismatch was defined as: either group O patients who received grafts from group A, group B, or group AB donors; or group A and group B patients who received grafts from group AB donors. ABO minor mismatch was defined as: either group AB patients who received grafts from non‐group AB donors or group A and group B patients who received grafts from group O donors. Bidirectional mismatch was defined as: either group A patients who received grafts from group B donors or group B patients who received grafts from group A donors. The diagnosis of acute GVHD (aGVHD) was in accordance with the Glucksberg criteria [18]. Chronic GVHD (cGVHD) was classified as mild, moderate, or severe according to the National Institutes of Health consensus criteria [19].
OS was defined as the time from transplantation to death from any cause. DFS was defined as survival in continuous CR. CR was defined as BM blasts at ≤ 5.0%. CR1 was defined as the first complete remission. Relapse was defined as recurrence of BM blasts at > 5.0%, the reappearance of blasts in the blood or development of extramedullary disease infiltrates at any site. Non‐relapse mortality was defined as death after SCT without disease progression or relapse.
2.4. Statistical analysis
Data were censored at the time of death or last contact. Propensity score matching was performed to reduce or eliminate confounding effects. Each ISD‐SCT case was matched with three HID‐SCT cases using the nearest neighbor‐matching method. Age and sex of patients, disease status, cytogenetic risk, sex of donor, and graft type were included in the propensity score model. Continuous variables were compared using the Mann‐Whitney U‐test. Categorical variables were compared using the χ 2 and Fisher's exact tests. Survival functions were estimated using the Kaplan‐Meier method. Competing risk analysis was used to calculate the cumulative rates of GVHD, relapse, and non‐relapse mortality, and the Gray's test was used to test the differences between the HID‐SCT and ISD‐SCT groups. All reported P values were based on two‐sided hypothesis tests, and P < 0.05 was considered as having statistical significance. Data analyses were primarily conducted with the SPSS software package 22.0 (SPSS Inc., Chicago, IL, USA), and R software (version 3.3.1; http://www.r-project.org) was used for propensity score matching [20], competing risk analysis [21] and estimating survival.
3. RESULTS
3.1. Patient characteristics
As a result, 23 cases of ISD‐SCT and 156 cases of HID‐SCT were enrolled in the study. In total, we matched 23 ISD‐SCT patients with 69 HID‐SCT patients, and the characteristics are summarized in Table 1. The characteristics of CR1 patients in the two groups are shown in Table 2. There were 9 and 18 recipients who died in the ISD‐SCT group and the HID‐SCT group, respectively. The median follow‐up period after SCT in survivors was 39.6 months (range: 15.0‐65.1 months) for the ISD‐SCT group and 43.6 months (range: 10.8‐70.8 months) for the HID‐SCT group (P = 0.153).
TABLE 1.
Characteristics of pediatric AML patients who underwent ISD‐SCT or HID‐SCT
| ISD‐SCT | HID‐SCT | ||
|---|---|---|---|
| Characteristic | (n = 23) | (n = 69) | P value |
| Age of recipients [years, median (range)] | 11 (4‐18) | 12 (1‐16) | 0.805 |
| Sex of recipients [cases (%)] | 1.000 | ||
| Male | 14 (60.9%) | 42 (60.9%) | |
| Female | 9 (39.1%) | 27 (39.1%) | |
| WBC at diagnosis (range, × 109/L) | 19.9 (2.1‐227.9) | 19.9 (0.3‐404.0) | 0.334 |
| Time from diagnosis to SCT (range, months) | 5.5 (1.0‐30.0) | 6.0 (1.0‐84.0) | 1.000 |
| Cytogenetic risk | 0.126 | ||
| Favorable | 3 (13.0%) | 3 (4.3%) | |
| Intermediate | 8 (34.8%) | 40 (58.0%) | |
| Adverse | 8 (34.8%) | 13 (18.8%) | |
| Unknown | 4 (17.4%) | 13 (18.8%) | |
| Diseases status | 0.569 | ||
| CR1 | 17 (73.9%) | 55 (79.7%) | |
| Beyond CR1 | 6 (26.1%) | 14 (20.3%) | |
| ABO incompatibility | 0.007 | ||
| Match | 21 (91.3%) | 36 (52.2%) | |
| Minor mismatch | 0 | 21 (30.4%) | |
| Major mismatch | 1 (4.3%) | 8 (11.6%) | |
| Bidirectional mismatch | 1 (4.3%) | 4 (5.8%) | |
| Age of donors (range, years) | 16 (6‐31) | 37 (11‐52) | <0.001 |
| Sex of donors | 0.159 | ||
| Male | 9 (39.1%) | 39 (56.5%) | |
| Female | 14 (60.9%) | 30 (43.5%) | |
| Graft source | 0.307 | ||
| BM+PBSC | 14 (60.9%) | 50 (72.5%) | |
| PB | 9 (39.1%) | 19 (27.5%) | |
| Median MNC (range, × 108/kg) | 8.48 (5.43‐15.82) | 9.15 (3.19‐29.60) | 0.630 |
| Median CD34(range, × 106/kg) | 3.29 (1.10‐6.20) | 3.29 (0.52‐10.00) | 1.000 |
| Transplant center | 0.505 | ||
| Peking University People's Hospital | 14 (60.9%) | 39 (56.5%) | |
| Xinqiao Hospital, Army Military Medical University | 7 (30.4%) | 17 (24.6%) | |
| Nanfang Hospital, Southern Medical University | 2 (8.7%) | 13 (18.8%) |
AML, acute myeloid leukemia; SCT, stem cell transplantation; ISD, identical sibling donor; HID, haploidentical donor; WBC, white blood cell; CR1, first complete remission; BM, bone marrow; PBSC, peripheral blood stem cell; MNC, mononuclear cell.
TABLE 2.
Characteristics of pediatric AML patients who underwent ISD‐SCT or HID‐SCT in the subgroup for patients in CR1 with known cytogenetics
| Characteristic | ISD‐SCT (n = 14) | HID‐SCT (n = 44) | P value |
|---|---|---|---|
| Age of recipients (range, years) | 14 (4‐16) | 12 (2‐16) | 0.741 |
| Sex of recipients | 0.380 | ||
| Male | 8 (57.1%) | 21 (47.7%) | |
| Female | 6 (42.9%) | 23 (52.2%) | |
| WBC at diagnosis (range, × 109/L) | 19.9 (2.1‐55.7) | 25.1 (1.0‐404.0) | 0.357 |
| Time from diagnosis to SCT (range, months) | 4.0(1.0‐31.0) | 5.2 (1.0‐60.0) | 0.440 |
| Cytogenetic risk | 0.172 | ||
| Favorable | 2 (14.3%) | 3 (6.8%) | |
| Intermediate | 6 (42.9%) | 31 (70.5%) | |
| Adverse | 6 (42.9%) | 10 (22.7%) | |
| Resistant to first cycle of chemotherapy | 5 (35.7%) | 19 (43.2%) | 0.547 |
| Number of chemotherapy cycles (range) | 4.5 (1‐16) | 4 (2‐20) | 0.525 |
| ABO incompatibility | 0.026 | ||
| Match | 13 (92.8%) | 22 (50.0%) | |
| Minor mismatch | 0 | 5 (11.4%) | |
| Major mismatch | 0 | 14 (31.8%) | |
| Bidirectional mismatch | 1 (7.1%) | 3 (6.8%) | |
| Age of donors (range, years) | 16 (10‐31) | 38.5 (11‐52) | 0.002 |
| Sex of donors | 0.540 | ||
| Male | 6 (42.9%) | 25 (56.8%) | |
| Female | 8 (57.1%) | 19(43.2%) | |
| Graft source | 0.086 | ||
| BM+PBSC | 10 (71.4%) | 40 (90.9%) | |
| PB | 4 (28.6%) | 4 (9.1%) | |
| Median MNC (range, × 108/kg) | 9.01 (5.43‐15.82) | 8.95 (3.19‐16.66) | 0.759 |
| Median CD34 (range, × 106/kg) | 3.12 (1.10‐6.20) | 3.09 (0.52‐10.00) | 0.759 |
AML, acute myeloid leukemia; SCT, stem cell transplantation; CR, complete remission; ISD, identical sibling donor; HID, haploidentical donor; WBC, white blood cell; BM, bone marrow; PBSC, peripheral blood stem cell; MNC, mononuclear cell.
3.2. Engraftment
All patients achieved donor cell engraftment except one HID‐SCT patient who died of cerebral hemorrhage on day +8. All patients achieved platelet engraftment, except one HID‐SCT patient who died of thrombotic thrombocytopenic purpura on day +106. The median time to achieve neutrophil engraftment were 15 days (range: 11‐23 days) and 13 days (range: 10‐46 days) for the ISD‐SCT group and HID‐SCT group, respectively (P = 0.002). Platelet engraftment occurred at a median time of 12 days (range: 10‐26 days) for the ISD‐SCT group and 13 days (range: 7‐61 days) for the HID‐SCT group (P = 0.026).
3.3. GVHD
The cumulative rate of GVHD in HID‐SCT group may be higher than that in the ISD‐SCT group but there was no statistical difference observed between the two groups. At day +100, the cumulative rates of grades II‐IV aGVHD were 13.0% (95% CI, 3.1%–30.2%) in the ISD‐SCT group and 34.8% (95% CI, 23.7%–46.1%) in the HID‐SCT group (P = 0.062) (Figure 2A). Meanwhile, the cumulative rates of grades III‐IV aGVHD were 0.0% and 11.6% (95% CI, 5.4%–20.4%) in the ISD‐SCT group and the HID‐SCT group, respectively (P = 0.090).
FIGURE 2.
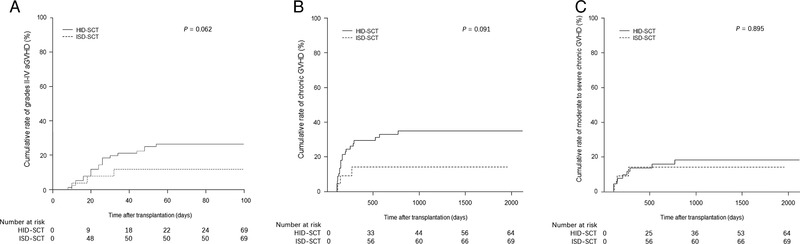
Illustration of the (A). Cumulative rate of grades II‐IV acute GVHD, (B). chronic GVHD, and (C). moderate to severe chronic GVHD in the entire study population cohort
Abbreviations: ISD, identical sibling donor; HID, haploidentical donor; GVHD, graft‐versus‐host disease.
The three‐year cumulative rates of cGVHD were 14.1% (95% CI, 3.4%–32.4%) in the ISD‐SCT group, and 34.9% (95% CI, 23.2%–46.8%) in the HID‐SCT group respectively (P = 0.091) (Figure 2B). The three‐year cumulative rates of moderate to severe cGVHD were 14.1% (95% CI, 3.4%–32.4%) in the ISD‐SCT group and 16.2% (95% CI, 8.2%–26.6%) in the HID‐SCT group (P = 0.895) (Figure 2C). The three‐year cumulative rates of severe cGVHD were 5.0% (95% CI, 0.3%–21.1%) and 10.0% (95% CI, 4.0%–19.3%) in the ISD‐SCT group and the HID‐SCT group, respectively (P = 0.494).
3.4. Relapse and non‐relapse mortality
The three‐year relapse rate in the ISD‐SCT group was significantly higher than that in the HID‐SCT group (39.1% [95% CI, 19.4%–58.4%] vs. 16.4% [95% CI, 8.7%–26.3%], P = 0.027) (Figure 3A). Causes of death for relapse and non‐relapse mortality are shown in Table 3. The three‐year cumulative rates of non‐relapse mortality were 0.0% in the ISD‐SCT group and 10.6 (95% CI, 4.7%–19.7%) in the HID‐SCT group (P = 0.113).
FIGURE 3.
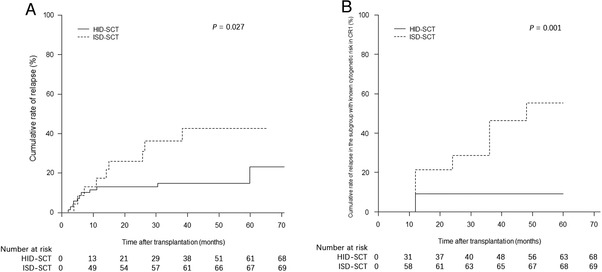
Illustration showing the (A). Cumulative relapse rate in the entire study population, and (B). rate of relapse in the subgroup with known cytogenetic risk in CR1
Abbreviations: ISD, identical sibling donor; HID, haploidentical donor; SCT, stem cell transplantation.
TABLE 3.
Causes of death in pediatric AML patients who underwent ISD‐SCT or HID‐SCT
| Causes of death | ISD‐SCT [cases (%)] | HID‐SCT [cases (%)] |
|---|---|---|
| Total | 9 | 18 |
| AML Relapse | 9 (100.0) | 7 (38.9) |
| Infections | 0 | 4 (22.2) |
| aGVHD | 0 | 0 |
| cGVHD | 0 | 1 (5.6) |
| Organ failure | 0 | 2 (11.1) |
| Others * | 0 | 4 (22.2) |
Included one case of diffuse alveolar hemorrhage, one case of cerebral hemorrhage and two cases with missing data.
AML, acute myeloid leukemia; ISD, identical sibling donor; SCT, stem cell transplantation; HID, haploidentical donor; aGVHD, acute graft‐versus‐host disease; cGVHD, chronic‐graft‐versus host disease.
Subgroup analysis of patients with known cytogenetic risk in CR1 showed that the three‐year relapse rate in the ISD‐SCT group was significantly higher than that in the HID‐SCT group (50.0% [95% CI, 21.5%–73.2%] vs. 9.2% [95% CI, 2.8%–20.1%]; P = 0.001) (Figure 3B), whereas the non‐relapse mortality rates were 0.0% and 9.5% (95% CI, 3.0%–20.9%) in the ISD‐SCT group and the HID‐SCT group, respectively (P = 0.256).
3.5. Survival
There was no significant difference in the three‐year DFS and OS rates between the ISD‐SCT and the HID‐SCT group. Their three‐year DFS rates were 60.9% (95% CI, 40.9%–80.9%) and 72.9% (95% CI, 62.1%–83.7%; P = 0.297) (Figure 4A) and their three‐year OS rates were 73.0% (95% CI, 54.4%–91.6%) and 74.6% (95% CI, 64.0%–85.2%; P = 0.689), respectively (Figure 4B).
FIGURE 4.
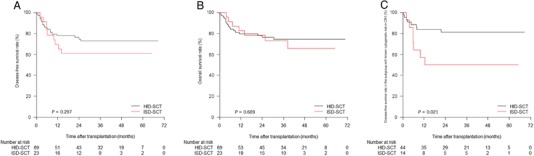
Illustration of the (A). disease‐free survival and (B). overall survival rate in the entire study population, and (C). disease‐free survival rate in the subgroup with known cytogenetic risk in CR1
Abbreviations: ISD, identical sibling donor; HID, haploidentical donor; CR1, first complete remission.
Subgroup analysis of patients with known cytogenetic risk in CR1 showed that the three‐year DFS rate in the HID‐SCT group was significantly higher than that in the ISD‐SCT group (81.2% [95% CI, 69.4%–93.0%] vs. 50.0% [95% CI, 23.7%–76.3%]; P = 0.021) (Figure 4C), whereas the OS rates were comparable between the two groups (81.5% [95% CI, 69.9%–93.1%] vs. 68.8% [95% CI, 42.7%–94.9%]; P = 0.196).
4. DISCUSSION
The present study compared the survival outcomes between undergoing HID‐SCT or ISD‐SCT in children with high‐risk AML. Matched‐pair analyses showed that, in high‐risk pediatric AML, relapse rate among ISD‐SCT recipients was significantly higher than that among HID‐SCT recipients despite having comparable OS and DFS rates and that the subgroup of patients with known cytogenetic risk and transplanted in CR1 had higher relapse rate and lower DFS rate in ISD‐SCT compared with HID‐SCT. Our study findings also demonstrated that HID‐SCT was comparable to ISD‐SCT in terms of OS rate, DFS rate, and non‐relapse mortality in pediatric high‐risk AML patients. Therefore, the results showed the possibility for exploring the currently undefined role of T‐cell‐replete HID‐SCT in pediatric high‐risk AML.
In the current study, the relapse rate was 16.4% in the HID‐SCT group, which is comparable to a recent study that enrolled 58 ISD‐SCT patients, 75 URD‐SCT patients, and 8 HID‐SCT patients in the allogenic SCT group [22]. Mo et al. [23] observed that the relapse rates were comparable (22.2% vs. 7.6%) between the two groups that were resistant and sensitive to the first course of induction chemotherapy in pediatric AML patients, suggesting that HID‐SCT could improve the outcomes of high‐risk AML. In the current study, the proportion of HID‐SCT recipients who were resistant to the first course of induction chemotherapy in the subgroup with known cytogenetic risk in CR1 was 43.2% and the relapse rate of the subgroup was 9.2%, which aligned with the results of a previous study [23]. However, in the current study, the strikingly low relapse rate in the HID‐SCT might be explained by the fact that, in HID‐SCT, the proportions of favorable and intermediate‐risk patients were 62.3% in the whole population and 77.3% in the subgroup; whereas in ISD‐SCT, the proportions of these two cytogenetic‐risk groups were 47.8% in the whole population and 57.2% in the subgroup. Furthermore, the current study demonstrated that in both the entire population and the subgroup of patients with known cytogenetic risk in CR1, patients in the ISD‐SCT group had higher relapse rate than those in the HID‐SCT group. Chang et al. [24] reported that patients receiving HID‐SCT experienced lower relapse rate than those who underwent ISD‐SCT (19% vs. 57% in a retrospective cohort and 13% vs. 36% in a prospective cohort). In that study [24], among the patients receiving ISD‐SCT, relapse rate was higher in the minimal residual disease (MRD)‐positive group than in the MRD‐negative group (36% vs. 7%), whereas relapse rates were comparable between the MRD‐positive group and MRD‐negative group among patients who received HID‐SCT (13% vs. 7%); suggesting that HID‐SCT had a stronger graft versus leukemia (GVL) effect than ISD‐SCT [24]. The results from other studies also confirmed the stronger GVL effect in HID‐SCT than in ISD‐SCT [25, 26]. In theory, in HID‐SCT, stronger alloimmune response would be achieved by leukemic cells which express HLA mismatched from donors and result in a decrease in relapse rate. However, since little is known about the mechanisms underlying the stronger GVL effect of HID‐SCT compared to that of ISD‐SCT [27, 28, 29], there may be some other possible reasons for the higher relapse rate in the ISD‐SCT group compared to the HID‐SCT group in the current study. First, in the present study, both groups of patients received CSA, MTX, and MMF as GVHD prophylaxis. However, whether a strong immunosuppression strategy is also applicable to high‐risk pediatric AML patients who underwent ISD‐SCT needs to be reevaluated. Second, there were almost twice as many cytogenetically adverse‐risk patients in the ISD‐SCT group than in the HID‐SCT group (34.8% vs. 18.8%). Third, in the subgroup of ISD‐SCT, only 10 patients had MRD data: six were MRD positive before ISD‐SCT, and four relapsed after SCT. The higher percentages of pre‐MRD‐positive patients in the ISD‐SCT subgroup may explain the higher rate of relapse rate in the CR1 subgroup than in the whole population of ISD‐SCT. To better understand the cause of the higher relapse rate of patients after ISD‐SCT than HID‐SCT warrants larger samples size of case‐control studies and further biological experiments.
Liu et al. [9] demonstrated that the non‐relapse mortality was 7.1% and 12.6% among ISD‐SCT and HID‐SCT groups. In our study, non‐relapse mortality was 10.6% in the HID‐SCT group, which was in agreement with a previous report [9]. All ISD‐SCT recipients died of relapse which might be due to the relatively small sample size of this group. Meanwhile, no patients died of infections in the ISD‐SCT group, whereas infection was the common cause of death in the HID‐SCT group. This may be due to the intensive immunosuppressive regimen in the HID‐SCT group which causes delayed immune reconstitution compared to the ISD‐SCT group [30]. However, as the data of the present study was derived from multicenter database, details on immune reconstitution were not available. Furthermore, in the HID‐SCT group, only 1% of HID‐SCT recipients died of GVHD. Although the GVHD rate may be potentially higher in the HID‐SCT group than in the ISD‐SCT group, severe GVHD was manageable in our protocol. In addition, up to 39% of HID‐SCT recipients died of relapse, which suggested that it might be necessary to improve the transplantation protocol to reduce the relapse of this highly malignant disease, for instance by optimizing the conditioning regimen and applying targeted drugs as maintenance therapy.
One of the major concerns regarding HID‐SCT is the increased possibility of GVHD. In the current study, the difference in the cumulative incidences of grades II‐IV and III‐IV aGVHD and cGVHD was not significant between the ISD‐SCT group and the HID‐SCT group but there were trends that HID‐SCT led to higher rates of GVHD than ISD‐SCT, which were aligned with the findings from a previous study [9]. However, our previous studies showed that HID‐SCT resulted in a comparable rate of GVHD as ISD‐SCT in pediatric and adult recipients [31, 32, 33]. This may be due to the small number of patients, especially in the ISD‐SCT group. Despite potentially higher rate of GVHD, as discussed above, the mortality of GVHD was low (1/18), suggesting that the prophylaxis strategies for GVHD that combined in vivo T cell depletion with ATG [34], CSA, MTX, MMF, and transfusing G‐CSF mobilized PBSC [35] were effective for overcoming the HLA barrier in HID‐SCT. Currently, PT/Cy prophylaxis is widely used due to its effectiveness in decreasing the incidence of GVHD. Our results showed that, despite the fact that the incidence of grades II‐IV aGVHD was consistent with those in previous studies that employed PT/Cy prophylaxis for HID‐SCT recipients (27%–33%) [36, 37], the rates of grades III‐IV aGVHD (11.6%) and cGVHD (34.9%) of present study were higher than that for the PT/Cy protocols (5.0% for grades III‐IV aGVHD and 13%‐23% for cGVHD) [36, 37]. This may be partly explained by the fact that all recipients received PBSC infusion in our study, whereas 7.5% of patients were infused PBSC in one of the previous studies [36], and all recipients in another study were only infused with BM as the stem cell source [37].
The major limitation of our study was its retrospective design. Some of the HID‐SCT recipients had advanced disease status, lacked an ISD and could not wait for a matched unrelated donor, thus it would be difficult to conduct a randomized study to compare the efficacy of HID‐SCT and ISD‐SCT. Second, not all the MRD statuses were available in our study. As discussed above, MRD status before SCT could impact the outcome of SCT [12, 24]. Third, the information of donor lymphocyte infusion was invalid. Yan et al. [38] found that DLI could reduce relapse and increase survival in patients with refractory/relapsed acute leukemia after SCT with the ATG and G‐CSF‐based protocol. Future studies should involve both MRD and information of donor lymphocyte infusion to analyze data in more details. Last, although we conducted matched pair analysis to reduce selection bias, the sample size of the ISD‐SCT group was small, which could partially explain the slightly different results with our previous studies [9, 31, 32, 33].
5. CONCLUSIONS
In conclusion, our findings underline that HID‐SCT had comparable OS and DFS to ISD‐SCT with acceptable complications in high‐risk pediatric AML patients, and that HID‐SCT can be a valid alternative option in this population. A prospective study to investigate the role of HID‐SCT in this setting is warranted for confirmation.
DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the ethics committee of the local institution. Informed consent was obtained from all patients’ guardians. All authors vouch for the accuracy and completeness of the reported data and analyses.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was partly supported by grants from the National Key Research and Development Program of China (Grant No.: 2017YFA0104500) from the Ministry of Science and Technology, National Natural Science Foundation of China (Grant No.: 81770189, 81621001, and 81530046), the Science and Technology Project of Guangdong Province of China (Grant No.: 2016B030230003), Peking University Clinical Scientist Program(Grant No.: BMU2019LCKXJ003), the Fundamental Research Funds for the Central Universities, and the project of health collaborative innovation of Guangzhou city (Grant No.: 201704020214).
AUTHORS' CONTRIBUTIONS
XJH and YW designed the research; FMZ, XZ, CFL, YFC, LG, and YLH collected the clinical data; FMZ, XZ and CFL performed the statistical analyses and wrote the report. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank all patients who shared their data for this study and the study coordinators from each institute.
Zheng F-M, Zhang X, Li C-F, et al. Haploidentical- versus identical-sibling transplant for high-risk pediatric AML: A multi-center study. 2020;40:93–104. 10.1002/cac2.12014
Contributor Information
Yu Wang, Email: ywyw3172@sina.com.
Xiao-Jun Huang, Email: huangxiaojun@bjmu.edu.cn.
REFERENCES
- 1. Creutzig U, van den Heuvel‐Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120(16):3187‐205. 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 2. Kaspers GJ, Creutzig U. Pediatric acute myeloid leukemia: international progress and future directions. Leukemia. 2005;19(12):2025‐9. 10.1038/sj.leu.2403958. [DOI] [PubMed] [Google Scholar]
- 3. Rasche M, Zimmermann M, Borschel L, Bourquin JP, Dworzak M, Klingebiel T et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML‐BFM trials from 1987 to 2012. Leukemia. 2018;32(10):2167‐77. 10.1038/s41375-018-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanakry CG, de Lima MJ, Luznik L. Alternative Donor Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia. Semin Hematol. 2015;52(3):232‐42. 10.1053/j.seminhematol.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52(6):811‐7. 10.1038/bmt.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Haploidentical hematopoietic stem cell transplantation without in vitro T‐cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38(4):291‐7. 10.1038/sj.bmt.1705445. [DOI] [PubMed] [Google Scholar]
- 7. O'Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M et al. Nonmyeloablative bone marrow transplantation from partially HLA‐mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377‐86. 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 8. Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S et al. Full haplotype‐mismatched hematopoietic stem‐cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23(15):3447‐54. 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 9. Liu DH, Xu LP, Liu KY, Wang Y, Chen H, Han W et al. Long‐term outcomes of unmanipulated haploidentical HSCT for paediatric patients with acute leukaemia. Bone Marrow Transplant. 2013;48(12):1519‐24. 10.1038/bmt.2013.99. [DOI] [PubMed] [Google Scholar]
- 10. Bashey ZA, Zhang X, Brown S, Jackson K, Morris LE, Holland HK et al. Comparison of outcomes following transplantation with T‐replete HLA‐haploidentical donors using post‐transplant cyclophosphamide to matched related and unrelated donors for patients with AML and MDS aged 60 years or older. Bone Marrow Transplant. 2018;53(6):756‐63. 10.1038/s41409-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X et al. Haploidentical vs identical‐sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956‐62. 10.1182/blood-2015-02-627786. [DOI] [PubMed] [Google Scholar]
- 12. Chang YJ, Zhao XS, Wang Y, Liu YR, Xu LP, Zhang XH et al. Effects of pre‐ and post‐transplantation minimal residual disease on outcomes in pediatric patients with acute myeloid leukemia receiving human leukocyte antigen‐matched or mismatched related donor allografts. Am J Hematol. 2017;92(12):E659‐E61. 10.1002/ajh.24910. [DOI] [PubMed] [Google Scholar]
- 13. Mo XD, Zhao XY, Liu DH, Chen YH, Xu LP, Zhang XH et al. Umbilical cord blood transplantation and unmanipulated haploidentical hematopoietic SCT for pediatric hematologic malignances. Bone Marrow Transplant. 2014;49(8):1070‐5. 10.1038/bmt.2014.109. [DOI] [PubMed] [Google Scholar]
- 14. Lai YR, Chen YH, Hu DM, Jiang M, Liu QF, Liu L et al. Multicenter phase II study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG). J Hematol Oncol. 2014;7:59 10.1186/s13045-014-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leung W, Campana D, Yang J, Pei D, Coustan‐Smith E, Gan K et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high‐risk leukemia. Blood. 2011;118(2):223‐30. 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J et al. Minimal residual disease‐directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543‐52. 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasle H. A critical review of which children with acute myeloid leukaemia need stem cell procedures. Br J Haematol. 2014;166(1):23‐33. 10.1111/bjh.12900. [DOI] [PubMed] [Google Scholar]
- 18. Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft‐versus‐host disease in human recipients of marrow from HL‐A‐matched sibling donors. Transplantation. 1974;18(4):295‐304. 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 19. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945‐56. 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20. Randolph J. J. FK, Manuel A. K., Balloun J. L. A step‐by‐step guide topropensity score matching in R. Practical Assessment Research and Evaluation 2014. [Google Scholar]
- 21. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381‐7. 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 22. Locatelli F, Masetti R, Rondelli R, Zecca M, Fagioli F, Rovelli A et al. Outcome of children with high‐risk acute myeloid leukemia given autologous or allogeneic hematopoietic cell transplantation in the aieop AML‐2002/01 study. Bone Marrow Transplant. 2015;50(2):181‐8. 10.1038/bmt.2014.246. [DOI] [PubMed] [Google Scholar]
- 23. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H et al. Unmanipulated Haploidentical Hematopoietic Stem Cell Transplantation in First Complete Remission Can Abrogate the Poor Outcomes of Children with Acute Myeloid Leukemia Resistant to the First Course of Induction Chemotherapy. Biol Blood Marrow Transplant. 2016;22(12):2235‐42. 10.1016/j.bbmt.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 24. Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre‐transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10(1):134 10.1186/s13045-017-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mariotti J, Devillier R, Bramanti S, Sarina B, Furst S, Granata A et al. T Cell‐Replete Haploidentical Transplantation with Post‐Transplantation Cyclophosphamide for Hodgkin Lymphoma Relapsed after Autologous Transplantation: Reduced Incidence of Relapse and of Chronic Graft‐versus‐Host Disease Compared with HLA‐Identical Related Donors. Biol Blood Marrow Transplant. 2018;24(3):627‐32. 10.1016/j.bbmt.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 26. Gauthier J, Poire X, Gac AC, Leclerc M, Guillaume T, Chalandon Y et al. Better outcome with haploidentical over HLA‐matched related donors in patients with Hodgkin's lymphoma undergoing allogeneic haematopoietic cell transplantation‐a study by the Francophone Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transplant. 2018;53(4):400‐9. 10.1038/s41409-017-0018-z. [DOI] [PubMed] [Google Scholar]
- 27. Casucci M, Perna SK, Falcone L, Camisa B, Magnani Z, Bernardi M et al. Graft‐versus‐leukemia effect of HLA‐haploidentical central‐memory T‐cells expanded with leukemic APCs and modified with a suicide gene. Mol Ther. 2013;21(2):466‐75. 10.1038/mt.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Locatelli F, Pende D, Falco M, Della Chiesa M, Moretta A, Moretta L. NK Cells Mediate a Crucial Graft‐versus‐Leukemia Effect in Haploidentical‐HSCT to Cure High‐Risk Acute Leukemia. Trends Immunol. 2018;39(7):577‐90. 10.1016/j.it.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 29. Whitehill GD, Amarnath S, Muranski P, Keyvanfar K, Battiwalla M, Barrett AJ et al. Adenosine Selectively Depletes Alloreactive T Cells to Prevent GVHD While Conserving Immunity to Viruses and Leukemia. Mol Ther. 2016;24(9):1655‐64. 10.1038/mt.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang YJ, Zhao XY, Huang XJ. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(4):440‐9. 10.1016/j.bbmt.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett. 2018;438:63‐75. 10.1016/j.canlet.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 32. Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA‐mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA‐identical sibling transplantation. Blood. 2006;107(8):3065‐73. 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M et al. Haploidentical transplant for myelodysplastic syndrome: registry‐based comparison with identical sibling transplant. Leukemia. 2016;30(10):2055‐63. 10.1038/leu.2016.110. [DOI] [PubMed] [Google Scholar]
- 34. Nachbaur D, Eibl B, Kropshofer G, Meister B, Mitterschiffthaler A, Schennach H et al. In vivo T cell depletion with low‐dose rabbit antithymocyte globulin results in low transplant‐related mortality and low relapse incidence following unrelated hematopoietic stem cell transplantation. J Hematother Stem Cell Res. 2002;11(4):731‐7. 10.1089/15258160260194884. [DOI] [PubMed] [Google Scholar]
- 35. Morton J, Hutchins C, Durrant S. Granulocyte‐colony‐stimulating factor (G‐CSF)‐primed allogeneic bone marrow: significantly less graft‐versus‐host disease and comparable engraftment to G‐CSF‐mobilized peripheral blood stem cells. Blood. 2001;98(12):3186‐91. 10.1182/blood.v98.12.3186. [DOI] [PubMed] [Google Scholar]
- 36. Klein OR, Buddenbaum J, Tucker N, Chen AR, Gamper CJ, Loeb D et al. Nonmyeloablative Haploidentical Bone Marrow Transplantation with Post‐Transplantation Cyclophosphamide for Pediatric and Young Adult Patients with High‐Risk Hematologic Malignancies. Biol Blood Marrow Transplant. 2017;23(2):325‐32. 10.1016/j.bbmt.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Munchel A, Kesserwan C, Symons HJ, Luznik L, Kasamon YL, Jones RJ et al. Nonmyeloablative, HLA‐haploidentical bone marrow transplantation with high dose, post‐transplantation cyclophosphamide. Pediatr Rep. 2011;3 (Suppl 2):e15 10.4081/pr.2011.s2.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan CH, Liu QF, Wu DP, Zhang X, Xu LP, Zhang XH et al. Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft‐versus‐Host Disease‐Guided Multiple DLIs Could Improve Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory/Relapsed Acute Leukemia. Biol Blood Marrow Transplant. 2017;23(8):1311‐9. 10.1016/j.bbmt.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 39. Chang YJ, Zhao XY, Huang XJ. Granulocyte Colony‐Stimulating Factor‐Primed Unmanipulated Haploidentical Blood and Marrow Transplantation. Front Immunol. 2019;10:2516 10.3389/fimmu.2019.02516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding authors on reasonable request.


