Abstract
BACKGROUND
Little is published regarding surgery for transposition of the great arteries (TGA) in the developing world.
OBJECTIVES
We sought to identify patient characteristics, surgical interventions, institutional characteristics, risk factors for mortality, and outcomes among patients undergoing surgery for TGA in this setting.
METHODS
Developing world congenital heart surgical programs submitted de-identified data to a novel international collaborative database as part of a quality improvement (QI) project. We conducted a retrospective cohort study including all cases of TGA with intact ventricular septum (TGA/IVS) and ventricular septal defect (TGA/VSD) that were performed from 2010 to 2013. Demographic, surgical, and institutional characteristics and their associations with in-hospital mortality were identified.
RESULTS
There were 778 TGA operations at 26 centers, 480 (62%) for TGA/IVS and 298 (38%) for TGA/VSD. Most (80%) were single-stage arterial switch operations (ASO), but 20% were atrial baffling procedures (atrial switch [ATS]) or 2-stage repairs (pulmonary artery band followed by ASO). Age at operation was >30 days in half of the cases and did not vary significantly with operation type. Survival was 85% and did not significantly vary with age at operation or operation type. Preceding septostomy was infrequently reported (16%) and not associated with surgical mortality. Mortality was associated with lower World Health Organization weight/body mass index-for-age percentile and lower institutional volume of TGA repair.
CONCLUSIONS
Surgical repair of TGA performed in the developing world is associated with an early survival of 85%. Type of surgical repair and age at operation varied considerably, but no associations with mortality were identified. In contrast, poor nutrition and small surgical volume were most strongly associated with mortality. Multicenter collaborative QI efforts may benefit patients with TGA in the developing world.
Keywords: arterial switch operation, congenital heart surgery, international, mortality, resource limited
Surgical care for congenital heart disease (CHD) in the developing world is evolving rapidly, enhancing survival and improving quality of life for children with previously lethal cardiac malformations (1). This is important progress, but mortality associated with CHD and its surgical care remains challenging (2,3).
D-loop transposition of the great arteries (TGA) is the second most common form of cyanotic congenital disease (4). Left untreated, TGA is associated with mortality approaching 85% to 90% (5,6). The medical and surgical management of TGA is well-established in many North American and European countries; the usual practice being single-stage anatomic repair with arterial switch operation (ASO) in the first week of life (2,4,7,8). Thirty-day mortality is <3% and 20-year survival approaches 90% (7,9–11). Less is known, however, about surgery for TGA in the developing world.
The purpose of this study was to identify patient characteristics, surgical interventions, institutional characteristics, risk factors for mortality, and outcomes among patients with TGA in the developing world, using data from the International Quality Improvement Collaborative for Congenital Heart Surgery in Developing World Countries (IQIC), a novel, multicenter effort aimed at reducing mortality in developing world settings (12).
METHODS
Twenty-six IQIC sites in 15 countries contributed data to this study (Table 1). All sites self-identified as practicing in a resource-limited environment and participated voluntarily in IQIC. Sites submitted de-identified data pertaining to patients <18 years of age undergoing operative repair for CHD that were collected for quality improvement (QI) purposes. Data were verified using a random 10% sample of each site’s cases. Key variables audited included type of procedure, RACHS-1 (Risk Adjusted classification for Congenital Heart Surgery) category, age, prematurity, postoperative outcomes, and 30-day follow-up outcomes. Approval to conduct research using the IQIC database was obtained from the Boston Children’s Hospital Institutional Review Board. The IQIC database was searched for all instances of ASO, atrial switch (ATS), and TGA performed between January 2010 and December 2013. Associated cardiac lesions were noted.
TABLE 1.
IQIC Sites Contributing Data
| Institution | City, Country |
|---|---|
| Aga Khan University Hospital | Karachi, Pakistan |
| Amrita Institute of Medical Sciences | Kochi, India |
| Armed Forces Institute of Cardiology, National Institute of Heart Disease | Rawalpindi, Pakistan |
| Care Hospital | Hyderabad, India |
| Federal State Budgetary Institution “Research Institute for Complex Problems of Cardiovascular Diseases,” Siberian Branch of the Russian Academy of Medical Sciences | Kemerovo, Russia |
| First Hospital of Lanzhou University | Lanzhou, Gansu Province, China |
| Frontier Lifeline Hospital | Chennai, India |
| Fundación Cardioinfantil de Bogotá | Bogotá, Colombia |
| Fundación Cardiovascular Adulto-Pediátrica Clínica San Rafael | Bogotá, Colombia |
| Hospital da Criança e Maternidade de São José do Rio Preto | São José do Rio Preto, Brazil |
| Hospital de Niños | Córdoba, Argentina |
| Hospital Garrahan | Buenos Aires, Argentina |
| Innova Children’s Heart Hospital | Hyderabad, India |
| Institute of General & Urgent Surgery, Academy of Medical Sciences, | Kharkiv Ukraine |
| Instituto do Coracao do Hospital das Clinicas de Universidade de Sao Paulo | São Paulo, Brazil |
| Instituto Nacional de Pediatría | México City, México |
| Instituto Nacional del Corazón | Lima, Perú |
| Kokilaben Dhirubhai Ambani Hospital & Medical Research Center | Mumbai, India |
| Mother and Child Health Institute | Belgrade, Serbia |
| National Children’s Cardiac Medical Center | Minsk, Belarus |
| Nhi Dong 1 (Children’s Hospital #1) | Ho Chi Minh City, Vietnam |
| Shanghai Children’s Medical Center | Shanghai, China |
| Star Hospital | Hyderabad, India |
| Unidad de Cirugía Cardiovascular de Guatemala | Guatemala City, Guatemala |
| United Hospital | Dhaka, Bangladesh |
| West China Hospital, Sichuan University | Chengdu, China |
IQIC = International Quality Improvement Collaborative.
INCLUSION/EXCLUSION CRITERIA.
Operations for TGA with intact ventricular septum (TGA/IVS) and TGA with ventricular septal defect (TGA/VSD) were included. Patients entered into the database as having a double-outlet right ventricle who underwent ASO with VSD closure also were included as TGA/VSD.
Operations where the underlying anatomy included right- or left-sided obstructive lesions, including coarctation of the aorta, were excluded, as were operations involving complex intracardiac lesions, such as straddling atrioventricular valves. We also excluded operations without data pertaining to mortality.
STATISTICAL ANALYSIS.
Demographic, surgical, and institutional characteristics were summarized with frequencies and percentages. In order to account for the correlation among patients within the same institution, generalized estimating equation models were used to evaluate associations with in-hospital mortality. Risk factors significant at the 0.10 level in univariate analysis were considered for inclusion in a multivariable model; p < 0.05 was required for retention in the final model. Odds ratios and 95% confidence intervals (CIs) were estimated.
RESULTS
There were 778 operations for TGA from 2010 through 2013 (Table 2). Most surgeries (62%) were for TGA/IVS; the remainder (38%) were for TGA/VSD. The majority of surgeries (70%) were performed in males. Patients in a large proportion of cases (48%) were below the 5th percentile for World Health Organization (WHO) weight/body mass index (BMI)-for-age percentile. Surgeries in patients with identified chromosomal abnormalities, major noncardiac structural abnormalities, and prematurity were uncommon. Preceding septostomy was reported in 16% of cases.
TABLE 2.
Patient Characteristics, Operations, and Mortality
| Number | Mortality | |
|---|---|---|
| Total | 778 (100) | 119 (15) |
| TGA/IVS | 480 (62) | 67 (14) |
| TGA/VSD | 298 (38) | 52 (17) |
| Sex | ||
| Male | 542 (70) | 89 (16) |
| Female | 236 (30) | 30 (13) |
| Age at surgery | ||
| ≤7 days* | 83 (11) | 13 (16) |
| ≤30 days* | 384 (49) | 59 (15) |
| 31 days - <1 yr | 350 (45) | 56 (16) |
| 1–12 yrs | 44 (6) | 4 (9) |
| Nutrition status | ||
| Overweight | 3 (<1) | 0 (0) |
| Normal | 504 (65) | 71 (14) |
| Malnourished | 166 (21) | 38 (23) |
| Emaciated | 105 (13) | 10 (10) |
| Weight <3 kg | 225 (29) | 46 (20) |
| WHO weight/BMI-for-age percentile | ||
| <5th | 371 (48) | 72 (19) |
| 5–14th | 109 (14) | 17 (16) |
| ≥15th | 295 (38) | 29 (10) |
| Prematurity | 36 (5) | 3 (19) |
| Major noncardiac structural anomaly | 16 (2) | 2 (33) |
| Major chromosomal abnormality | 6 (1) | 14 (21) |
| Major medical illness | 68 (9) | 4 (13) |
| Visiting surgical group present | 30 (4) | 58 (20) |
| Open chest post-surgery | 284 (37) | 25 (14) |
| Major infection | 175 (22) | 25 (14) |
| Operation | ||
| Arterial switch | 382 (49) | 53 (14) |
| Atrial switch | 66 (8) | 7 (11) |
| 2-stage arterial switch | 32 (4) | 7 (22) |
| Arterial switch/VSD | 240 (31) | 44 (18) |
| Atrial switch/VSD | 35 (5) | 4 (11) |
| 2-stage arterial switch/VSD | 23 (3) | 4 (17) |
| Annual volume TGA repair | ||
| <10 | 16 (122)† | 36 (30) |
| 10 to 19 | 5 (243)† | 49 (20) |
| ≥20 | 5 (413)† | 34 (8) |
Values are n (%).
Age categories are not mutually exclusive.
Number of institutions (number of patients).
BMI = body mass index; IVS = intact ventricular septum; TGA = transposition of the great arteries; VSD = ventricular septal defect; WHO = World Health Organization.
Most surgeries (80%) were single-stage ASO, but atrial baffling procedures—Mustard or Senning ATS (13%) or 2-stage pulmonary artery banding followed by ASO (7%)—occurred frequently. Only 10% of operations were performed during the first week of life. The remaining 90% of operations were performed later, with a large proportion (51%) performed after 1 month of age. Among those in this latter group, 350 (45%) surgeries were performed between 1 month and 1 year of age, with 44 (6%) undertaken between 1 and 12 years of age. There was no significant association between patient age and choice of surgical repair.
The average annual institutional volume of TGA operations varied. Most centers (62%) performed fewer than 10 TGA repairs annually. There was no association between surgical volume and choice of surgical repair.
Univariate associations with mortality are shown in Table 3. The overall mortality rate was 15%. VSD closure as part of the operation, male sex, lower WHO weight/BMI-for-age percentile, weight <3 kg, and prematurity were all factors associated with higher in-hospital mortality. Average annual volume of TGA repair <10 cases or 10 to 19 cases were also associated with higher mortality relative to average annual volume ≥20 cases.
TABLE 3.
Univariate Associations with Mortality*
| OR | 95% CI | p Value | |
|---|---|---|---|
| Diagnosis | |||
| TGA/IVS | 1 | -- | -- |
| TGA/VSD | 1.30 | 1.00–1.69 | 0.05 |
| Male | 1.35 | 1.04–1.74 | 0.02 |
| WHO weight/BMI-for-age percentile | |||
| <5th | 2.20 | 1.48–3.29 | <0.001 |
| 5–15th | 1.69 | 0.92–3.10 | 0.09 |
| ≥15th | 1 | -- | -- |
| Weight <3 kg | 1.71 | 1.09–2.68 | 0.02 |
| Prematurity | 2.26 | 1.13–4.53 | 0.02 |
| Open chest post-surgery | 1.82 | 0.90–3.69 | 0.1 |
| Average annual volume of TGA repair | |||
| <10 | 4.66 | 2.08–10.4 | <0.001 |
| 10–19 | 2.82 | 1.12–7.09 | 0.03 |
| ≥20 | 1 | -- | -- |
No statistically significant associations with age at surgery, major noncardiac structural anomaly, major chromosomal abnormality, major medical illness, surgical procedure, whether a visiting surgical group was present, or presence of a major infection.
CI = confidence interval; OR = odds ratio; other abbreviations as in Table 2.
Age at operation and type of operation were not significantly associated with mortality, nor were chromosomal abnormalities or major noncardiac abnormalities. Many patients (22%) had a major infection and 9% were characterized as having a major medical illness, but neither condition was significantly associated with mortality. Preceding use of septostomy was not associated with mortality among the entire cohort, and analysis of septostomy among the TGA/IVS subgroup was also not associated with mortality (p = 0.11). Septostomy use varied widely among centers, with some reporting zero and others reporting 100% utilization; but, septostomy rate was not associated with mortality.
In multivariable analyses, lower WHO weight/BMI-for-age percentile and male sex were risk factors for death. Other patient risk factors identified in univariate analysis were no longer statistically significant after adjusting for these 2 factors. However, lower institutional volume did remain significant after adjusting for these factors (Table 4).
TABLE 4.
Multivariable Associations with Mortality
| OR | 95% CI | p Value | |
|---|---|---|---|
| WHO weight/BMI-for-age percentile | |||
| <5th | 2.23 | 1.48–3.33 | <0.001 |
| 5–15th | 1.66 | 0.91–3.04 | 0.1 |
| ≥15th | 1 | -- | -- |
| Male | 1.36 | 1.07–1.75 | 0.01 |
| Adding Annual TGA Volume: | |||
| WHO Weight/BMI-for-Age Percentile | |||
| <5th | 1.98 | 1.30–3.02 | 0.002 |
| 5–15th | 1.60 | 0.91–2.82 | 0.1 |
| ≥15th | 1 | -- | -- |
| Male | 1.45 | 1.10–1.90 | 0.008 |
| Average annual volume of TGA repair | |||
| <10 | 4.71 | 2.10–10.5 | <0.001 |
| 10–19 | 2.41 | 0.91–6.41 | 0.08 |
| ≥ 20 | 1 | -- | -- |
DISCUSSION
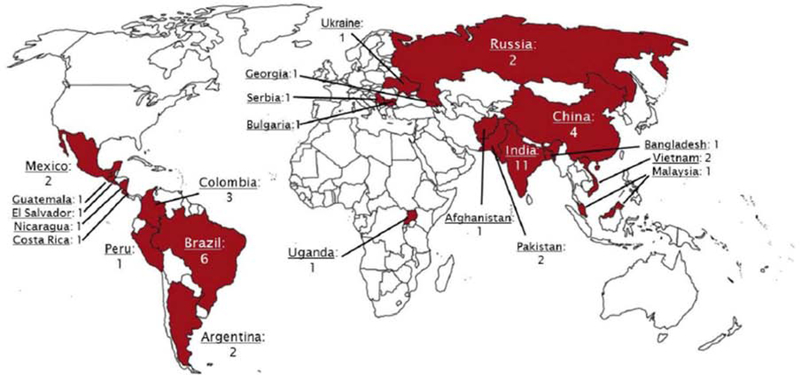
We reported operations for TGA over a 4-year period among 26 centers in the developing world. The phrase “developing world” is challenging and defies precise definition. Importantly, IQIC centers self-identify as working in a resource-constrained developing world environment, with the understanding that such limitations carry inherent risk (Figure 1). Indeed, these operations are performed across a broad spectrum of resources. Many centers have contemporary medication, equipment, and surgical technique, but geographic, socioeconomic, and even political limitations might affect outcomes. Despite this variation, we noted important common themes with implications for enhancing care.
Figure 1. Current International Quality Improvement Collaborative participant sites.
Includes sites added after data collection for this study. Abbreviations: Central Illustration
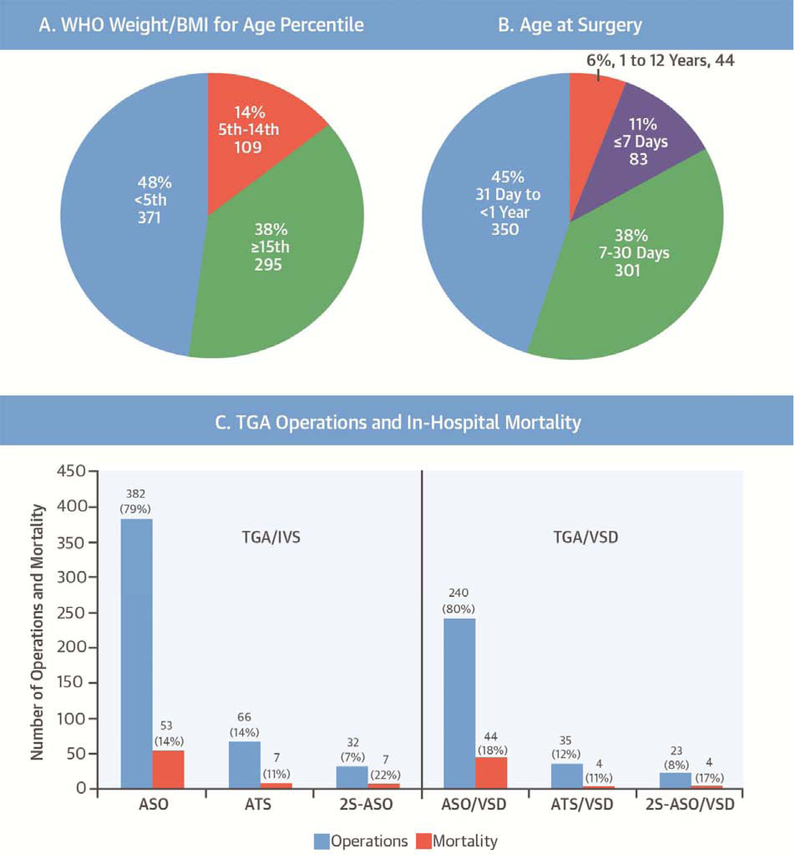
It is encouraging that surgery for TGA is performed in this setting and that overall survival is 85% (Central Illustration). Key differences and notable similarities between developing world and North American/European settings were suggested by this study. In the developing world, pre-operative comorbid conditions and infectious illnesses were common. Age at surgery differed considerably, and atrial baffling and 2-stage procedures were frequently performed in the developing world, while septostomy was utilized in a comparatively very small group of cases. Conversely, challenges related to nutrition and center volume appeared to be shared with resource-replete environments.
CENTRAL ILLUSTRATION. Transposition of the Great Arteries in the Developing World: Access and Outcomes.
A. WHO weight/BMI-for-age percentile. Results displayed as number and percentage of all operations. B. Age at surgery. Results displayed as number and percentage of all operations. C. Operations performed and mortality. Results displayed as number and percentage of operations for each type of anatomy (TGA/IVS or TGA/VSD). Mortality displayed as number and percentage of specific operations. IQIC, International Quality Improvement Collaborative for Congenital Heart Surgery in Developing World Countries; WHO, World Health Organization; BMI, body mass index; TGA, transposition of the great arteries; IVS, intact ventricular septum; VSD, ventricular septal defect; ASO, arterial switch operation; ATS, atrial switch operation; 2S, 2-stage.
In an era when many reports have suggested a 30-day mortality of <3% in North America and Europe (7), the fact that 15% of patients in this study experienced mortality is an important reminder of room for improvement in developing world settings. The reasons for this relatively high mortality rate among the varied group described in this study are undoubtedly multifactorial. However, this discrepancy is less disconcerting when one considers that mortality from ASO was similarly high in the early experience in developed countries (13). For example, in 1986, Quaegebeur and colleagues reported an overall mortality rate of 12.1% for ASO and 18% for ASO/VSD (14), similar to those reported among surgeries in the current study. A learning curve for the operative care of children with TGA is one historical certainty.
It must be noted, however, that many centers participating in this study have considerable experience with TGA operations. The IQIC database does not capture data regarding the number of years a center has been performing a specific surgery, nor does it capture data regarding individual surgeon experience. Nevertheless, findings in this study regarding surgical volume were similar to those identified using Society for Thoracic Surgery (STS) data, in that a rate of <10 ASO operations per year was associated with increased mortality. Analysis of STS data also identified individual surgeon volume as an important factor associated with increased mortality (15,16).
Operations in this study were clearly performed at a later age than in the developed world. Reasons were heterogeneous on both a regional and patient level. Differences in prenatal diagnosis, cardiac anatomic details, preoperative health, and critical care and resuscitative expertise all likely contributed to choices surrounding age at operation.
Recent data from developed countries suggested that increasing numbers of infants with TGA are diagnosed prenatally, with rates ranging from 18% to 50% (17–20). Sparse data from developing countries exist, but the experience of the authors of this study and others suggested that prenatal diagnosis of congenital anomalies is rare, usually <5% (21).
Lack of prenatal diagnosis is further complicated by limited access to pulse oximetry in many regional facilities and limited training in detection of CHD, as pointed out by Sidi and colleagues (22). Local geography might pose additional challenges to patient care. In Vietnam, for example, there are 5 centers with facilities that perform CHD surgery. Long transportation times, often exceeding several hours, combined with limited or no access to prostaglandin, may further contribute to patient deterioration (23). Local customs might also affect timing of patient care, as some infants in Vietnam are deliberately kept from strong light exposure (24), possibly further delaying the detection of cyanosis.
This study identified major medical illnesses and major infections as commonly occurring in this patient group. Reasons for this are speculative and specific details regarding the type and duration of illness or infection are lacking. One can infer that among those successfully initiated on prostaglandin, some received excessive doses with resultant apnea, requiring assisted ventilation and hemodynamic support. Some infants may require transport to units high above sea level, which can inhibit pulmonary blood flow. Contributing to these significant challenges, a number of countries simply do not have an adequate number of pediatric cardiologists (1).
Altogether, infants presenting with such preoperative challenges may require significant resuscitation, which can delay surgery from several days to weeks. Other patients may have required previous cardiopulmonary resuscitation and little or no information about their neurological status may be available. Finally, many patients present with active infection. One can reasonably infer that a later age at presentation and/or the degree of illness at presentation, rather than an intentional delay in surgical repair, is at least partly responsible for the timing of repairs (25).
It is interesting to note that few patients underwent preceding septostomy. Practices at institutions in the United States and Europe vary, but many centers frequently perform septostomy prior to surgical repair (7). By contrast, a preceding septostomy for patients with TGA was relatively rarely performed (16%) in this study. Some centers reported ASO within the first 24 h of life, essentially obviating the need for septostomy (26). This certainly does not reflect the low rate reported in this study, as most patients in this group had surgery after 1 month of life. This provokes important questions about the preoperative status of some of these patients. Some centers do not have ready access to catheterization equipment, facilities, or expertise (27), suggesting that those presenting for ASO in this study reflect a significant survivorship bias. This is supported by the lack of association between increased septostomy use and survival identified by this study. Simply put, those a priori lacking adequate atrial septal defects, a patent ductus arteriosus, or other physiologically favorable attributes may not survive to operation.
In addition, pediatric critical care in developing countries lags behind care in developed countries as pointed out by Smith and colleagues (28). The finding of a 50% intensive care unit mortality rate in their study was ascribed to delayed presentation, poor access to health care resources, inadequate staffing, and inadequately trained individuals with limited diagnostic and interventional capabilities.
The types of repair reported in this study are of interest. The large majority of patients underwent primary ASO, but 20% underwent ATS or 2-stage ASO. Our analysis did not reveal institutional preference, age difference, or a survival benefit for a particular surgical approach, which raises important questions as to why the physiologically preferable ASO was not performed. Perhaps this reflected inexperience with surgical management of complex coronary patterns (intramural and single coronary, for example), known to still carry a higher mortality at repair in developed countries (29). Nonetheless, it seems unlikely that coronary artery patterns alone could explain the high number of ATS and 2-stage ASO.
Furthermore, previous experience with atrial baffling procedures has borne the fact that the right ventricle is suboptimal as the systemic pumping chamber in the long term (30), and with no difference in survival and no clear institutional preference, this study closely resembled the early experience reported by the Congenital Heart Surgeons Society (13), in that surgeon preference might be related to anatomy. As experience is gained, ASO should be the preferred approach and recent studies would suggest that training and uniform acquisition of surgical technique in due course is feasible (15).
Among all types of surgical repair, the patients who underwent 2-stage ASO for TGA/IVS experienced the highest mortality. This difference did not achieve statistical significance, possibly due to the relatively low number of such patients. This study could not identify reasons for this particular approach; nonetheless, the utilization of the 2-stage ASO strategy raised interesting questions in light of reports, some very recent, which suggest that primary arterial switch can safely be performed at 1 month of age and beyond, even in the presence of a deconditioned left ventricle (31). Given the high rate of mortality among the 2-stage ASO patients, this might be a potential target for reducing mortality, particularly among those patients younger than 1 month at the time of planned surgery. Furthermore, as attention in this setting inevitably gravitates from in-hospital survival toward long-term neurodevelopmental outcomes, increasing focus will be placed on the timing and type of surgery.
Low WHO weight/BMI-for-age percentile was significantly associated with mortality. Associations between weight at surgery and mortality have also been identified using STS data. Curzon and colleagues found that for a number of operations, including ASO, lower weight at surgery was a risk factor for mortality (32). While this was notably similar to the findings in our study, the interplay between preoperative condition, later age at surgery, nutritional status, and mortality in the developing settings is complex and merits additional investigation. Certainly, the importance of preoperative nutritional status in these settings cannot be overstated; improving nutrition for these particularly sensitive patients is an important goal.
STUDY LIMITATIONS.
This study had several important limitations. The IQIC database includes many data elements regarding patients and outcomes; however, ensuring accurate data collection, despite annual data audits, is challenging. In addition, data regarding patient characteristics have intrinsic limitations. Major noncardiac abnormalities and prematurity were probably clinically evident; yet, it is unlikely that genetic testing was performed for all patients. It is therefore possible a larger patient proportion than that reported had 1 or more of these preoperative risk factors.
Furthermore, this study only collected data on TGA operative outcomes during a 4-year period. It is possible that these 4 years were not representative of the collaborative as a whole, especially as new centers joined IQIC. Importantly, this study reflected data from 26 centers from regions throughout the world and there are undoubtedly region-specific differences that cannot be adequately delineated by this study. Furthermore, TGA surgery outcomes among IQIC participating centers might not reflect all such surgeries throughout the developing world. Importantly, while this study explored effects of center volume on outcomes, data regarding the amount of center and surgeon experience were not obtained.
Details regarding the type and timing of infectious illness, specific types of major medical illnesses, and important anatomic details (coronary artery and atrial septum anatomy, for example) were not captured. Additional details regarding prenatal diagnosis, delay in postnatal diagnosis, transport times, availability of resuscitative medications (including prostaglandin), preoperative status, availability and utilization of extracorporeal membrane oxygenation, and surgeon preferences might help shed light on choices surrounding operations and outcomes. Importantly, this study does not address patients with TGA who passed away prior to receiving operative care.
CONCLUSIONS
Surgery for TGA is increasingly available in the developing world. Survival is encouraging at 85%, but gains can be made. Important questions regarding the preoperative status, surgical strategy, timing of surgery, and post-operative care remain.
PERSPECTIVES.
Competency in Systems-Based Practice:
The pre-operative health status, frequency of antecedent septostomy, age at operation, and surgical approach for patients with transposition of the great arteries (TGA) undergoing corrective surgery in resource-limited health settings differ from those in North America and Europe. The overall in-hospital survival is 85% in these developing nations, with multiple factors responsible for mortality, including poor nutrition and low surgical center volume are associated with higher rates of in-hospital mortality.
Translational Outlook:
Such quality improvement programs as the International Quality Improvement Collaborative for Congenital Heart Surgery in Developing Countries (IQIC), may identify opportunities to improve outcomes for patients undergoing surgical correction of TGA and other forms of congenital heart disease in resource-limited health settings.
Acknowledgements:
Dr. Schidlow is supported by the National Institutes of Health (T32-HL007572). The authors would like to gratefully acknowledge the IQIC centers that contributed data for this study and the entire collaborative’s ongoing efforts toward improving outcomes for children with congenital heart disease.
ABBREVIATIONS AND ACRONYMS
- ASO
arterial switch operation
- ATS
atrial switch
- BMI
body mass index
- CHD
congenital heart disease
- IQIC
International Quality Improvement Collaborative
- IVS
intact ventricular septum
- TGA
transposition of the great arteries
- VSD
ventricular septal defect
- WHO
World Health Organization
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bernier P-L, Stefanescu A, Samoukovic G, Tchervenkov CI. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2010;13:26–34. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JI. The global burden of congenital heart disease. Cardiovasc J Afr 2013;24:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquali SK, Li JS, Burstein DS, et al. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics 2012;129:e370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman JI, Kaplan S The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890–900. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell SC, Korones SB, Berendes HW. Congenital Heart Disease in 56,109 Births Incidence and Natural History. Circulation 1971;43:323–32. [DOI] [PubMed] [Google Scholar]
- 6.Campbell M. Incidence of cardiac malformations at birth and later, and neonatal mortality. Br Heart J 1973;35:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villafañe J, Lantin-Hermoso MR, Bhatt AB, et al. D-Transposition of the Great Arteries. J Am Coll Cardiol 2014;64:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Linde D, Konings EEM, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241–7. [DOI] [PubMed] [Google Scholar]
- 9.Brown JW, Park HJ, Turrentine MW. Arterial switch operation: factors impacting survival in the current era. Ann Thorac Surg 2001;71:1978–84. [DOI] [PubMed] [Google Scholar]
- 10.Sarris GE, Chatzis AC, Giannopoulos NM, et al. The arterial switch operation in Europe for transposition of the great arteries: a multi-institutional study from the European Congenital Heart Surgeons Association. J Thorac Cardiovasc Surg 2006;132:633–9. [DOI] [PubMed] [Google Scholar]
- 11.Khairy P, Clair M, Fernandes SM, et al. Cardiovascular outcomes after the arterial switch operation for D-transposition of the great arteries. Circulation 2013;127:331–9. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins KJ, Castaneda AR, Cherian KM, et al. Reducing Mortality and Infections After Congenital Heart Surgery in the Developing World. Pediatrics 2014;134:e1422–30. [DOI] [PubMed] [Google Scholar]
- 13.Castaneda AR, Trusler GA, Paul MH, Blackstone EH, Kirklin JW. The early results of treatment of simple transposition in the current era. J Thorac Cardiovasc Surg 1988;95:14–28. [PubMed] [Google Scholar]
- 14.Quaegebeur JM, Rohmer J, Ottenkamp J, et al. The arterial switch operation. An eight-year experience. J Thorac Cardiovasc Surg 1986;92:361–84. [PubMed] [Google Scholar]
- 15.Karamlou T, Jacobs ML, Pasquali S, et al. Surgeon and Center Volume Influence on Outcomes After Arterial Switch Operation: Analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg 2014;98:904–11. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in outcomes for benchmark operations: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann Thorac Surg 2011;92:2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobar-Diaz MC, Freud LR, Bueno A, et al. Prenatal Diagnosis of Transposition of the Arteries over a 20-Year Period: Improved but Imperfect. Ultrasound Obstet Gynecol 2015;45:678–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet D, Coltri A, Butera G, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation 1999;99:916–18. [DOI] [PubMed] [Google Scholar]
- 19.Bartlett JM, Wypij D, Bellinger DC, et al. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics 2004;113:e335–40. [DOI] [PubMed] [Google Scholar]
- 20.Friedberg MK, Silverman NH, Moon-Grady AJ, et al. Prenatal detection of congenital heart disease. J Pediatr 2009;155:26–31, 31 e1. [DOI] [PubMed] [Google Scholar]
- 21.Kouame B, N′guetta-Brou I, Kouame GY, et al. Epidemiology of congenital abnormalities in West Africa: Results of a descriptive study in teaching hospitals in Abidjan: Cote d′Ivoire. African J Paediatr Surg 2015;12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mocumbi AO, Lameira E, Yaksh A, Paul L, Ferreira MB, Sidi D. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol 2011;148:285–88. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett JM, Wypij D, Bellinger DC, et al. Challenges in the management of congenital heart disease in Vietnam: A single center experience. Ann Pediatr Cardiol 2015;8:44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le LT, Partridge JC, Tran BH, et al. Care practices and traditional beliefs related to neonatal jaundice in northern Vietnam: a population-based, cross-sectional descriptive study. BMC Pediatr 2014;14:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisoi AK, Ahmed T, Malankar DP, et al. Midterm outcome of primary arterial switch operation beyond six weeks of life in children with transposition of great arteries and intact ventricular septum. World J Pediatr Congenit Heart Surg 2014;5:219–25. [DOI] [PubMed] [Google Scholar]
- 26.Nevvazhay T, Chernogrivov A, Biryukov E, et al. Arterial switch in the first hours of life: No need for rashkind septostomy? Eur J Cardiothorac Surg 2012;42:520–3. [DOI] [PubMed] [Google Scholar]
- 27.Kumar RK, Tynan MJ. Catheter interventions for congenital heart disease in Third World countries. Pediatr Cardiol 2005;26:241–9. [DOI] [PubMed] [Google Scholar]
- 28.Smith ZA., Ayele Y, McDonald P. Outcomes in critical care delivery at Jimma University Specialised Hospital, Ethiopia. Anaesth Intensive Care 2013;41:363–8. [DOI] [PubMed] [Google Scholar]
- 29.Pasquali SK, Hasselblad V, Li JS, Kong DF, Sanders SP. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: a meta-analysis. Circulation 2002;106:2575–80. [DOI] [PubMed] [Google Scholar]
- 30.Lange R, Hörer J, Kostolny M, et al. Presence of a ventricular septal defect and the Mustard operation are risk factors for late mortality after the atrial switch operation: Thirty years of follow-up in 417 patients at a single center. Circulation 2006;114:1905–13. [DOI] [PubMed] [Google Scholar]
- 31.Ma K, Hua Z, Yang K, et al. Arterial switch for transposed great vessels with intact ventricular septum beyond one month of age. Ann Thorac Surg 2014;97:189–95. [DOI] [PubMed] [Google Scholar]
- 32.Curzon CL, Milford-Beland S, Li JS, et al. Cardiac surgery in infants with low birth weight is associated with increased mortality: Analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg 2008;135:546–51. [DOI] [PubMed] [Google Scholar]