Abstract
Type Ia group B Streptococcus (GBS) is one of the major causes of fatal infections in neonates. Its extracellular capsular polysaccharide (CPS) is a useful target for the development of anti-type Ia GBS vaccines. To explore the structure-activity relationships of type Ia GBS CPS and design more effective vaccines, a dimer of the branched pentasaccharide repeating unit of this CPS was synthesized by a highly convergent strategy highlighted by constructing the key intermediate via one-pot iterative glycosylation and imposing two side chains in one step via dual glycosylation. This represented the first total synthesis of a dimer of the repeating unit of any GBS CPS reported so far and the strategy should be applicable to higher oligomers of this repeating unit. The synthetic dimer and its monomeric analog were coupled with CRM197 carrier protein to generate conjugates that were evaluated in mice. Immunological results revealed that both carbohydrate antigens could induce robust total and IgG antibody responses and the elicited antibodies were cross-reactive with both carbohydrate antigens. It was concluded that both the monomeric and the dimeric repeating units may be employed as haptens for anti-type Ia GBS vaccine development.
Graphical Abstract
A dimer of the repeating unit of Type Ia group B Streptococcus capsular polysaccharide was first synthesized, and related protein conjugates induced robust immune responses to carbohydrate antigens.
Introduction
Group B Streptococcus (GBS) remains the major cause of neonatal invasive and fatal infections, such as bacterial sepsis and meningitis.1 Thus far, ten different types of GBS have been identified, which are classified according to their extracellular capsular polysaccharide (CPS) structures.2, 3 Among them, serotypes Ia, Ib, and Ш cause about 80% of the invasive diseases, whilst type Ia is responsible for 36% of early-onset cases.4, 5 Intrapartum antibiotics prophylaxis (IAP) is commonly employed to control neonatal GBS infection in industrialized countries,6 but its efficacy has been challenged by the emergence of drug resistance along with the widespread use of antibiotics.7 As a result, vaccination has become an attractive strategy to control GBS infection.
CPSs as important bacterial virulence factors are useful targets for the design and development of antibacterial vaccines. However, typically CPSs are T-cell independent antigens and fail to elicit effective protection in infants and young children.8, 9 To address the issue, CPSs or their analogs are often coupled to immunogenic carrier proteins, such as tetanus toxoid (TT), diphtheria toxoid (DT) and CRM197, to form glycoconjugate vaccines that can provoke T-cell dependent immune responses against the carbohydrate hapten.10, 11 The strategy has witnessed great success; as a result, many antibacterial glycoconjugate vaccines have been licensed and used in clinic. Recently, a CPS and CRM197-based trivalent conjugate vaccine against GBS Ia, Ib, and Ш has been developed and explored in phase II clinical trial.4
Despite the great success of CPS-based conjugate vaccines, they have some inherent issues. For instance, CPSs derived from bacterial sources are structurally heterogeneous and, in the meantime, cannot completely avoid bacterial contaminants, thereby generating quality control problems and safety concerns.12 To address such problems, conjugate vaccines consisting of structurally defined, bacterial contaminant-free synthetic oligosaccharide haptens have become particularly attractive.13, 14 The first semi-synthetic oligosaccharide-based glycoconjugate vaccine, Quimi-hib, was developed in Cuba to prevent Haemophilus influenzae type b (Hib) infection and has been approved by several countries.13, 15, 16 Moreover, semi-synthetic glycoconjugate vaccines have provided the opportunity to clarify their structure-activity relationships for vaccine design optimization.16, 17
In an effort to comprehend the structure-activity relationships of GBS CPS oligosaccharides as haptens for the design and development of effective anti-GBS conjugate vaccines, we initiated the program to synthesize various CPS oligosaccharides and evaluate their immunogenicity. We have recently described the synthesis of repeating units of serotype Ia,18 II,19 III,20, 21 V22 and VII23 GBS CPSs, but all these reports, as well as previous synthetic study on type Ш GBS CPS,24 were focused on the monomer of repeating units. However, for structure-activity relationship analysis, it is necessary to have their derivatives, including their dimers and higher oligomers.
In the current work, we developed an efficient strategy for the synthesis of the dimer of type Ia GBS CPS repeating unit, which should be applicable to its more complex oligomers. Furthermore, we coupled the synthetic oligosaccharide to a carrier protein CRM197, evaluated immunologically the resultant conjugate, and compared it to the CRM197 conjugate of monomeric type Ia GBS CPS repeating unit to gain a better understanding of their structure-activity relationships.
Results and Discussion
Type Ia CPS is composed of a repeating disaccharide, →4)-β-D-Glc-(1→4)-β-D-Gal-(1→, that forms the main chain and a trisaccharide branch α-Neu5Ac-(2→3)-β-D-Gal-(1→4)-β-GlcNAc-(1→ linked to the main chain Gal unit 3-O-position (Figure 1). We recently reported the synthesis of the monomer 1 of this repeating unit with an aminoethyl group attached to the reducing end.18 In the current work, we planned to prepare its dimer 2 also with an aminoethyl group attached to the reducing end to facilitate conjugation with carrier proteins (Figure 1). Both 1 and 2 were coupled with CRM197 to form conjugate vaccines 1a and 2a for immunological evaluation, and their human serum albumin (HSA) conjugates 1b and 2b were also prepared and utilized as coating antigens to detect carbohydrate antigen-specific antibodies by enzyme-linked immunosorbent assay (ELISA).
Figure 1:
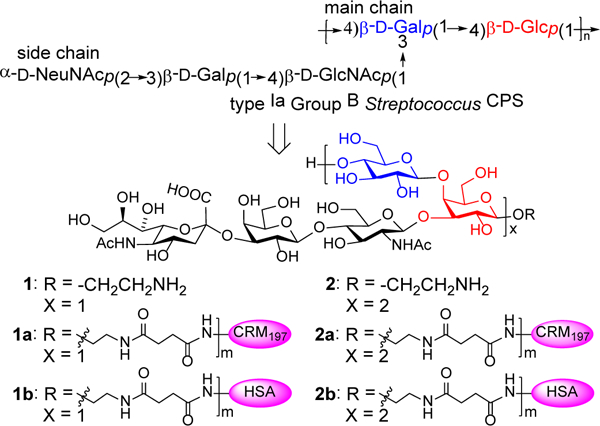
Structures of serotype Ia GBS CPS and the synthesized monomer 1 and dimer 2 of its repeating unit, as well as the protein conjugates 1a,b and 2a,b of 1 and 2.
Scheme 1 shows the synthetic plan for 2. We intended to construct the main chain oligosaccharide first and then introduce the two side chains in one pot. This would give a convergent synthesis that may be applicable to more complex oligomers of type Ia CPS repeating unit. Based on our previous observation,18 directly introducing the sialotrisaccharide branch to the main sugar chain might be difficult, thus we envisioned a [6+(2×2)] assembly strategy using sialodisaccharide 3 as a donor and diol 4 as an acceptor. In turn, the key intermediate 4 could be assembled by a convergent [2+3+1] strategy involving glycosylation of 8 with 7 and then glycosylation of the resultant pentasaccharide 6 with 5. Orthogonal protecting groups were utilized to temporarily block various positions in the synthetic intermediates to facilitate subsequent regiospecific deprotection and functionalization. In addition, the 4,6-O-positions of the terminal Glc residue in 4 were protected with a benzylidene group, which can be regiospecifically opened for exposing the 4-O-position25 to enable its further elongation for the synthesis of more complex oligomers of the repeating unit.
Scheme 1.
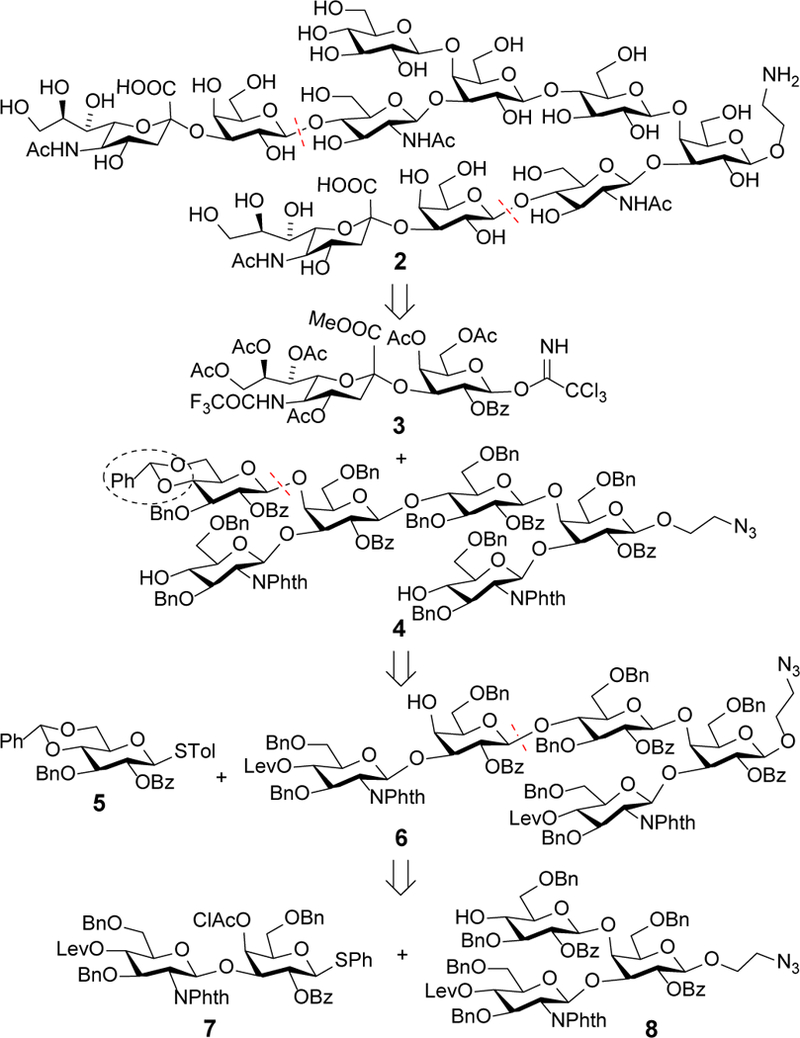
Synthetic plan for 2 via convergent [6+(2×2)] glycosylation
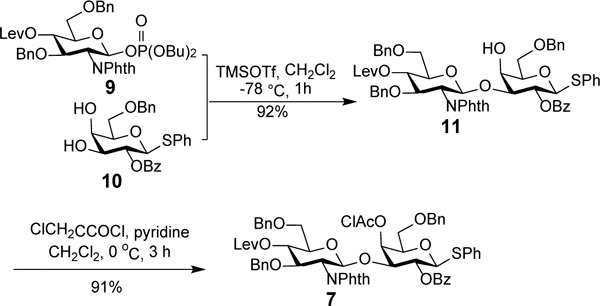
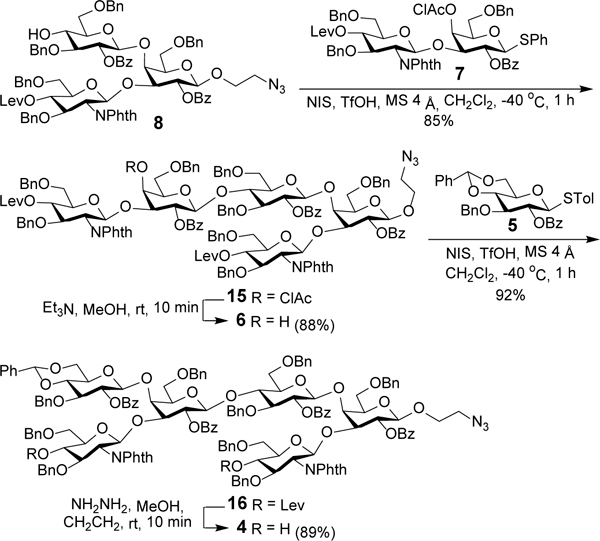
For the synthesis of disaccharide 7 (Scheme 2), to reduce the number of tedious protecting group manipulation and intermediate separation steps, 3,4-diol 10 was employed directly as the glycosyl acceptor to achieve regioselective 3-O-glycosylation, which took advantage of the higher reactivity of the Gal 3-OH group than its 4-OH group.26–30 Glycosylation of 10 with glycosyl phosphate 926, 30 as the glycosyl donor and trimethylsilyl triflate (TMSOTf) as a promoter at −78 °C was proved to be regio- and stereospecific to afford β-linked disaccharide 11 in an excellent isolated yield (92%). The stereospecificity of this glycosylation reaction was presumably determined by the neighboring group participation effect (NGPE). Thereafter, 11 was treated with chloroacetyl chloride (ClAcCl) to protect the free 4-OH group and provide disaccharide 7. The regio- and stereochemistries of the previous reaction were verified by the significant downfield shift (by 1.21 ppm) of the Gal residue H-4 signal as a result of chloroacetylation as well as the relatively large coupling constant between H-1’ and H-2’ (> 7.0 Hz) in the proton NMR spectra of both 11 and 7.
Scheme 2.
Regioselective glycosylation of 3,4-diol 10 to prepare 7
Encouraged by the excellent result of above glycosylation reaction in terms of yields and regio- and stereoselectivities, we envisioned a one-pot assembly for trisaccharide 8 (Scheme 3). Hence, diol 12 was glycosylated with sequential addition of glycosyl phosphate donors 9 (1.0 equiv) and 13 (1.0 equiv) in the presence of TMSOTf (0.2 equiv + 0.2 equiv), and both glycosylation reactions were started at −78 °C and finished at −40 °C for 1 h. Eventually, trisaccharide 14 was isolated in an overall yield of 88%, and the regio- and stereochemistries of the newly formed glycosidic bonds were characterized by 1D and 2D NMR spectra, as well as the large coupling constants between H-1’ and H-2’ (GlcN: JH1’-H2’ = 8.4 Hz; Glc: JH1’-H2’ = 7.9 Hz) in its proton NMR spectrum. Then, the t-butyldimethylsilyl (TBS) group protecting the Glc residue 4’-O-position in 14 was selectively removed with triethylamine-trihydrofluoride complex to produce trisaccharide 8.
Scheme 3.
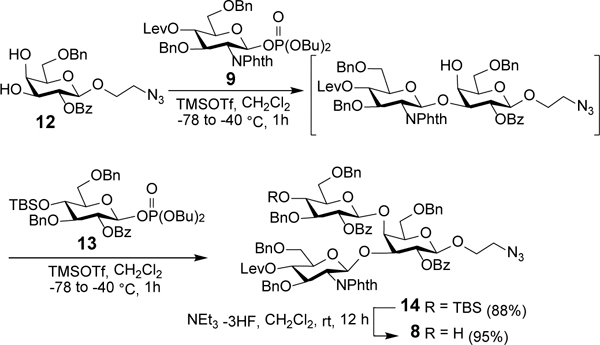
One-pot synthesis of trisaccharide 8
For the assembly of hexasaccharide 4 consisting of a repeating branched trisaccharide, worries about the potentially strong steric hindrance for a convergent [3+3] strategy compelled us to adopt a [3+2+1] strategy shown in Scheme 1. Glycosylation of 8 with disaccharide 7 under the influence of NIS/TfOH afforded 15 in a very good yield (85%). Next, the ClAc group in 15 was selectively removed, and the resultant 6 was glycosylated with 531 to afford hexasaccharide 16. After the two levulinoyl (Lev) groups in 16 were selectively removed with hydrazine acetate, hexasaccharide 4 was obtained as a dual glycosyl acceptor in an excellent overall yield. The stereochemistry of both glycosylation reactions was confirmed by the all β-glycosidic linkages in 6 (all JH1-H2 coupling constants > 7.8 Hz in its proton NMR spectrum). Furthermore, no α-anomer was isolated from the above reactions, indicating their high stereoselectivity probably as a result of the NGPE.
For the synthesis of sialodisaccharide 3 (Scheme 5), we chose to use trifluoroacetyl (TFA) group protected 1818 as the glycosyl donor since sialyation with TFA-protected donors was proved to give good yields and α-selectivity.18, 32 Glycosylation of 1718 with 18 afforded sialodisaccharide 19 that was directly applied to the next step of transformation, i.e., replacing the benzylidene group with two Ac groups upon acetic acid-promoted debenzylidenation and then acetylation to produce 20. The α-linkage of sialic acid in 19 was verified by its large C1-H3ax coupling constant (3J = 6.4 Hz) derived from single-frequency off-resonance decoupled (SFORD) NMR spectrum of 20. Next, we tried the glycosylation of 4 with 20 in the presence of NIS/TfOH, but the reaction gave mainly monoglycosylated product and only a low yield (8%) of the dual glycosylation product. To address the issue, 20 was converted into more reactive trichloroacetimidate 3 as glycosyl donor following an established procedure, including NIS-promoted hydrolysis of 20 and treatment of the resultant hemiacetal with trichloroacetonitrile and DBU. The reaction between 3 and 4 under the promotion of TMSOTf was smooth to give the desired dual glycosylation product 21 in a good isolated yield (58%). The stereochemistry of the newly formed glycosidic linkages was again verified by the 1H NMR spectrum of 21 (Gal: JH1-H2 = 8.6 and 8.0 Hz).
Scheme 5.
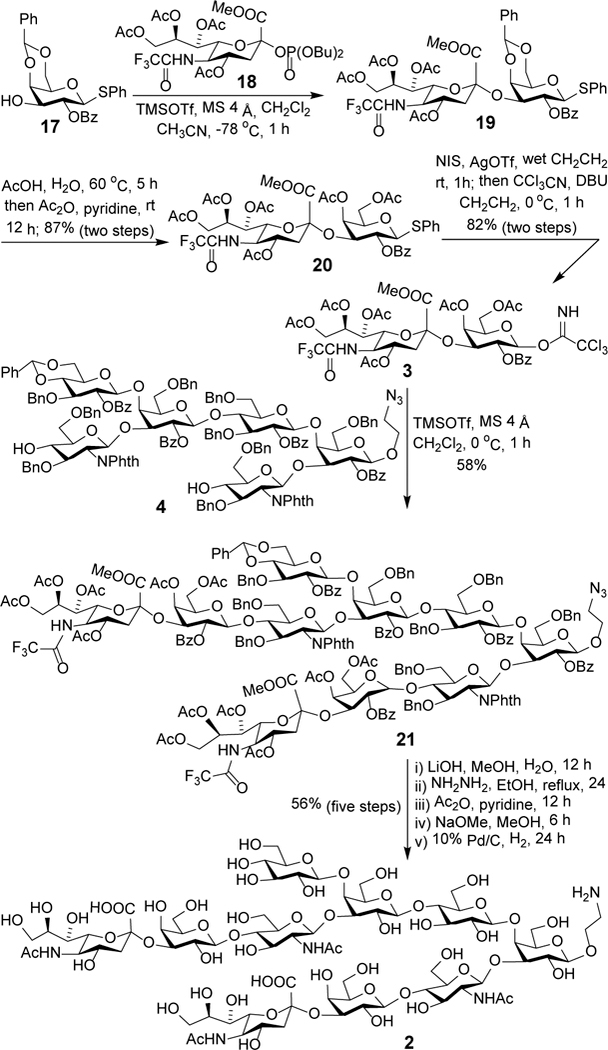
Synthesis of 2 via one-pot [6+(2×2)] glycosylation
Final global deprotection of 21 was achieved by an optimized five-step protocol. First, all of the Ac, Bz and TFA group as well as the methyl ester of carboxylic acids were removed by lithium hydroxide (LiOH)-catalyzed hydrolysis in methanol. Next, the two phthalyl groups were removed with hydrazine in ethanol under refluxing condition. Third, the freed amine groups were acetylated with acetyl anhydride in pyridine, which was followed by deacetylation using sodium methoxide in methanol to selectively remove all O-acetyl groups. Finally, Pd/C-catalyzed hydrogenolysis to remove the benzyl and benzylidene group with concomitant reduction of the azido group provided the deprotected decasaccharide 2 in a 56% overall yield in five steps after purification through size exclusion column chromatography using Sephadex G-25 with distilled water as the eluent. All of the deprotection steps were performed consecutively without purification of the intermediates but the reactions were closely monitored with TLC and MS to guarantee completion. The final product and all intermediates involved in this synthesis were carefully and fully characterized with 1D, 2D NMR and HR MS.
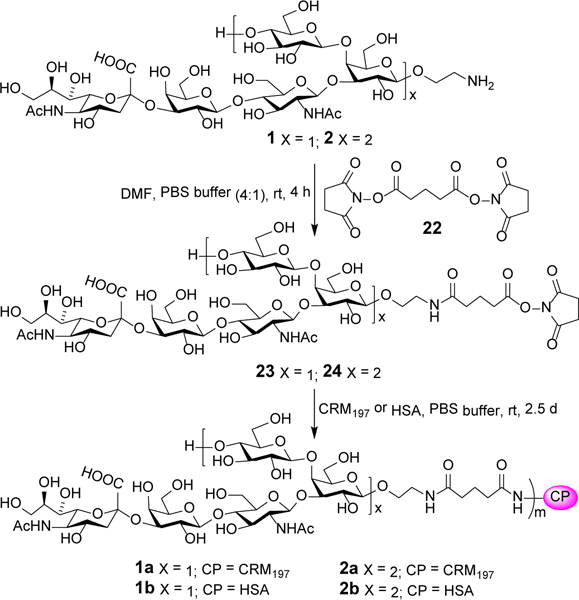
The linker utilized to conjugate oligosaccharides 2 and 1, which was synthesized by a reported method,18 with carrier proteins was the simple bifunctional glutaryl group, which was proved in our previous studies33–36 to be not only an efficient linker for glycan-protein conjugation but also practically nonimmunogenic, thus it would not induce significant undesired immune responses by itself. Consequently, oligosaccharides 1 and 2 were treated with a large excess (15 equiv) of a dual activated ester, disuccinimidyl glutarate (DSG, 22), to produce mono-activated esters 23 and 24, respectively. Then, 23 and 24 were coupled with CRM197 and HSA proteins through reactions of the activated ester in them with free amino groups on the surface of carrier proteins in phosphate-buffered saline (PBS) to afford conjugates 1a, 1b, 2a, and 2b. The carbohydrate loadings of these conjugates were analyzed by the Svennerholm method37 and were in the desired range (Table 1). The conjugation results were further confirmed by SDS-PAGE or MALDI MS analysis.
Table 1.
Carbohydrate loadings of glycoconjugates 1a,b and 2a,b
| Sample | CRM197 conjugates |
HSA conjugates |
||
|---|---|---|---|---|
| 1a | 2a | 1b | 2b | |
| Carbohydrate loading (%) | 7.8 | 8.5 | 6.3 | 9.4 |
Conjugates 1a and 2a were immunologically evaluated with female C57BL/6J mice (6–8 weeks of age). First, 1a and 2a were dissolved in 1× PBS and mixed thoroughly with complete Freund’s adjuvant (CFA) or incomplete Freund’s adjuvant (IFA) using a three-way syringe to form emulsions following the manufacturer’s instructions. On day 1, the CFA emulsion of each conjugate (0.1 mL, containing 3.0 μg of carbohydrate) was injected intramuscularly (i.m.) into a group of six mice for initial immunization. On day 14, the IFA emulsion of each conjugate (the same dose) was injected subcutaneously (s.c.) into the mice for boost immunization. Blood samples were collected from the mice on day 0 before the initial immunization and on day 21 after the boost immunization and used to prepare antisera for antibody titer analysis by ELISA.
The ELISA experiments were performed following standard protocols using HSA conjugates 1b and 2b as capture antigens to detect carbohydrate hapten-specific antibodies. PBS-diluted (1:1,000) alkaline phosphatase (AP)-linked goat anti-mouse kappa, IgG, and IgM antibodies were secondary antibodies utilized to detect total antibodies, total IgG antibodies, and IgM antibodies, respectively. Antibody titers were defined as the serum dilution numbers at which an optical density (OD) value of 0.10 was reached at 405 nm wavelength. Each ELISA experiment was repeated three times to obtain the mean titer.
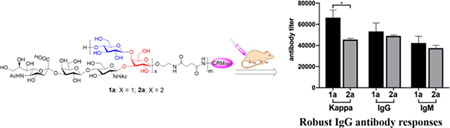
ELISA results with conjugates 1b and 2b as coating antigens to detect antibodies specific to the monomeric and dimeric repeating units (Figure 2), respectively, revealed that both 1a and 2a could induce significant levels of antigen-specific total, IgG, and IgM antibodies after only two times of immunization. These results suggested that conjugates 1a and 2a and the related oligosaccharides were very immunogenic since glycoconjugate vaccines reported in the literature needed usually at least three times of immunization to induce robust immune responses. The total antibody titer for the antiserum of 1a was slightly higher than that of 2a, but these two conjugates induced essentially the same levels of IgG and IgM antibody production, suggesting their similar immunogenicity and immunological properties. Moreover, the elicitation of IgG antibodies usually indicates the desired T-cell dependent immunity, as well as antibody class switch and maturation and long-term immune memory,35, 38, 39 thereby suggesting the promise of both oligosaccharides as haptens for the design and developments of conjugate vaccines.
Figure 2.
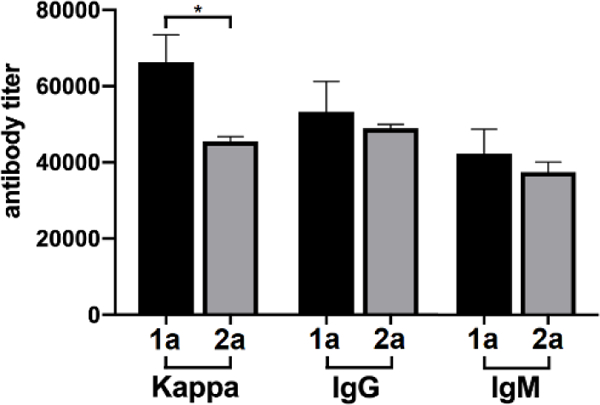
ELISA results of total (kappa), total IgG, and IgM antibody titers of pooled day 21 sera from mice immunized with CRM197 conjugates 1a and 2a, using HSA conjugates 1b and 2b as the capture antigens and AP-linked goat anti-mouse kappa, IgG, and IgM antibodies as the secondary antibodies to detect total, total IgG, and IgM antibodies specific to the monomer and dimer epitopes of the repeating unit of type Ia CPS, respectively. Antibody titer was defined as the serum dilution number at which the OD450 value reached 0.10 and was shown as the mean value of three parallel experiments for each serum. The error bar represented the standard error of mean (SEM). *p < 0.05 between two groups.
Next, we examined the cross-reactivity between the antisera and haptens. In this regard, ELISA plates coated with conjugates 1b and 2b were utilized to probe overall total and total IgG antibody titers of both pooled antisera. The results, as showed Figure 2, demonstrated that each antiserum had essentially the same reactivity with both oligosaccharide haptens. Thus, antibodies elicited by each conjugate showed extensive cross-reactivity with the other hapten.
In conclusion, an efficient and convergent method was developed for the first total synthesis of a dimer of the branched repeating unit of GBS Ia CPS. This synthesis is highlighted by constructing the key trisaccharide intermediate 8 via one-pot reiterative glycosylation and introducing both side chains in one step through dual glycosylation of the hexasaccharide diol acceptor 4. The synthetic dimer and its corresponding monomer were successfully coupled with CRM197 protein to generate glycoconjugates that were evaluated as vaccines in mice. Immunological results showed that both conjugates induced robust IgG and IgM antibody responses. Furthermore, it was also demonstrated that the antiserum of each glycoconjugate had similar reactivity with both carbohydrate haptens, indicating the extensive mutual recognition and cross-reactivity of the elicited antibodies with both the monomeric and the dimeric haptens and thereby the potential of utilizing synthetic monomeric repeating unit of GBS Ia CPS for vaccine development.
Supplementary Material
Figure 3.
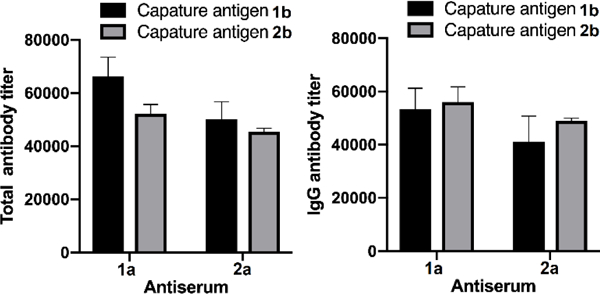
ELISA results to demonstrate the reactivity of each antiserum with both oligosaccharides using HSA conjugates 1b and 2b as coating antigens to detect total (A) and total IgG (B) antibodies in each pooled antiserum. The mean antibody titer of three parallel experiments was presented for each sample, and the error bar showed the SEM.
Scheme 4.
Synthesis of hexasaccharide 4
Scheme 6.
Conjugation of oligosaccharides with carrier proteins
Acknowledgements
This research project is financially supported by NIH/NCI (R01CA095142).
REFERENCES
- 1.Heath PT, Culley FJ, Jones CE, Kampmann B, Le Doare K, Nunes MC, Sadarangani M, Chaudhry Z, Baker CJ and Openshaw PJM, Lancet Infect. Dis., 2017, 17, e223. [DOI] [PubMed] [Google Scholar]
- 2.Cieslewicz MJ, Chaffin D, Glusman G, Kasper D, Madan A, Rodrigues S, Fahey J, Wessels MR and Rubens CE, Infect. Immun., 2005, 73, 3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berti F, Campisi E, Toniolo C, Morelli L, Crotti S, Rosini R, Romano MR, Pinto V, Brogioni B, Torricelli G, Janulczyk R, Grandi G and Margarit I, J. Biol. Chem., 2014, 289, 23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berardi A and Ferrari F, Lancet Infect.Dis., 2016, 16, 871. [DOI] [PubMed] [Google Scholar]
- 5.Vornhagen J, Waldorf KMA and Rajagopal L, Trends Microbiol., 2017, 25, 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, Hadler JL, Danila R, Cieslak PR and Schuchat A, New Engl. J. Med., 2000, 342, 15. [DOI] [PubMed] [Google Scholar]
- 7.Tyers M and Wright GD, Nat. Rev. Microb, 2019, 17, 141. [DOI] [PubMed] [Google Scholar]
- 8.Astronomo RD and Burton DR, Nat. Rev. Drug Discov., 2010, 9, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei MM, Wang YS and Ye XS, Med. Res. Rev., 2018, 38, 1003. [DOI] [PubMed] [Google Scholar]
- 10.Micoli F, Costantino P and Adamo R, Fems Microbiol. Rev., 2018, 42, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pon RA and Jennings HJ, in Carbohydrate-Based Vaccines and Immunotherapies, eds. Guo Z and Boons G-J, John Wiley & Sons, Inc., Hoboken, 2009, 117. [Google Scholar]
- 12.Astronomo RD and Burton DR, Nat. Rev. Drug Discov., 2010, 9, 308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamo R, Acc. Chem. Res., 2017, 50, 1270. [DOI] [PubMed] [Google Scholar]
- 14.Harale KR, Rout JK, Chhikara MK, Gill DS and Misra AK, Org. Chem. Front., 2017, 4, 2348. [Google Scholar]
- 15.Verez-Bencomo V, Fernandez-Santana V, Hardy E, Toledo ME, Rodriguez MC, Heynngnezz L, Rodriguez A, Baly A, Herrera L, Izquierdo M, Villar A, Valdes Y, Cosme K, Deler ML, Montane M, Garcia E, Ramos A, Aguilar A, Medina E, Torano G, Sosa I, Hernandez I, Martinez R, Muzachio A, Carmenates A, Costa L, Cardoso F, Campa C, Diaz M and Roy R, Science, 2004, 305, 522. [DOI] [PubMed] [Google Scholar]
- 16.Anish C, Schumann B, Pereira CL and Seeberger PH, Chem. Biol, 2014, 21, 38. [DOI] [PubMed] [Google Scholar]
- 17.Zhang GL, Wei MM, Song CC, Ma YF, Zheng XJ, Xiong DC and Ye XS, Org. Chem. Front., 2018, 5, 2179. [Google Scholar]
- 18.Mondal PK, Liao G, Mondal MA and Guo Z, Org. lett., 2015, 17, 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao L, Zhang H, Li Y, Gu G, Cai F, Guo Z and Gao J, J. Org. Chem., 2018, 83, 5920. [DOI] [PubMed] [Google Scholar]
- 20.Demchenko AV and Boons GJ, J. Org. Chem., 2001, 66, 2547. [DOI] [PubMed] [Google Scholar]
- 21.Cattaneo V, Carboni F, Oldrini D, De Ricco R, Donadio N, Ros IMY, Berti F and Adamo R, Pure Appl. Chem., 2017, 89, 855. [Google Scholar]
- 22.Gao J and Guo Z, Org Lett, 2016, 18, 5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Shao L, Wang X, Zhang Y, Guo Z and Gao J, Org. lett., 2019, DOI: 10.1021/acs.orglett.9b00653. [DOI] [PubMed] [Google Scholar]
- 24.Zou W, Brisson JR, Yang QL, van der Zwan M and Jennings HJ, Carbohyd. Res., 1996, 295, 209. [DOI] [PubMed] [Google Scholar]
- 25.Zhou ZF, Mondal M, Liao GC and Guo ZW, Org. Biomol. Chem., 2014, 12, 3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanashima S, Akai S and Sato K, Tetrahedron Lett., 2008, 49, 5111. [Google Scholar]
- 27.Crich D and Wu B, Org. Lett, 2008, 10, 4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B and Jiang HY, Tetrahedron Lett., 2011, 52, 6035. [Google Scholar]
- 29.Chuang HY, Ren CT, Chao CA, Wu CY, Shivatare SS, Cheng TJ, Wu CY and Wong CH, J. Am. Chem. Soc., 2013, 135, 11140. [DOI] [PubMed] [Google Scholar]
- 30.Kurimoto K, Yamamura H and Miyagawa A, Carbohyd. Res., 2015, 401, 39. [DOI] [PubMed] [Google Scholar]
- 31.Liao G, Burgula S, Zhou Z and Guo Z, Eur. J. Org. Chem., 2015, 2015, 2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Meo C, Demchenko AV and Boons G-J, J. Org. Chem., 2001, 66, 5490. [DOI] [PubMed] [Google Scholar]
- 33.Liao G, Zhou Z, Burgula S, Liao J, Yuan C, Wu Q and Guo Z, Bioconjugate Chem., 2015, 26, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao G, Zhou Z, Liao J, Zu L, Wu Q and Guo Z, ACS Infect. Dis., 2016, 2, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, Liao G, Mandal SS, Suryawanshi S and Guo Z, Chem. Sci., 2015, 6, 7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao GC, Zhou ZF and Guo ZW, Chem. Commun., 2015, 51, 9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svennerholm L, Biochi.Biophys. Acta, 1957, 24, 604. [DOI] [PubMed] [Google Scholar]
- 38.Song C, Zheng X-J, Liu C-C, Zhou Y and Ye X-S, Oncotarget, 2017, 8, 47330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kieber-Emmons T, Luo P, Qiu J, Chang TY, Insug O, Blaszczyk-Thurin M and Steplewski Z, Nat. Biotechnol., 1999, 17, 660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


