Abstract
In 2018, the world commemorated the centennial of the 1918 influenza A(H1N1) pandemic, the deadliest pandemic in recorded history; however, little mention was made of the 50th anniversary of the 1968 A(H3N2) pandemic. Although pandemic morbidity and mortality were much lower in 1968 than in 1918, influenza A(H3N2) virus infections have become the leading cause of seasonal influenza illness and death over the last 50 years, with more than twice the number of hospitalizations from A(H3N2) as from A(H1N1) during the past six seasons. We review the emergence, progression, clinical course, etiology, epidemiology, and treatment of the 1968 pandemic and highlight the short- and long-term impact associated with A(H3N2) viruses. The 1968 H3N2 pandemic and its ongoing sequelae underscore the need for improved seasonal and pandemic influenza prevention, control, preparedness, and response efforts.
In the United States, 1968 is remembered for the military conflict in Vietnam, assassinations of prominent leaders, and widespread public demonstrations, as well as significant scientific achievements such as heart transplant surgeries and manned space flights. Remembered less frequently is the 1968 H3N2 pandemic. Although the estimated morbidity and mortality of this pandemic was only a small fraction of that associated with the 1918 H1N1 pandemic, the ongoing impact of influenza A(H3N2) virus on public health has been profound. The A(H3N2) subtype virus that emerged in 1968 was associated with increased influenza morbidity and mortality globally through 1972. Since then, this subtype has circulated as a seasonal influenza A virus associated with more severe annual epidemics than those caused by influenza A(H1N1) and influenza B viruses. In this review, we reflect on the 1968 H3N2 pandemic, the continuing public health challenges from A(H3N2) virus, and the need for better prevention and control of seasonal and pandemic influenza.
THE 1968 PANDEMIC
There are typically two influenza seasons in Hong Kong—January through March or April and July through August—but an unusual and sudden increase of patients with influenza-like illness (ILI) presented to government clinics there on July 13, 1968.1 With 500 000 ILI cases in July, the outbreak was the largest in Hong Kong since the 1957 H2N2 pandemic.2 The National Influenza Center at the University of Hong Kong isolated the new influenza A(H3N2) virus on July 17 and sent it immediately to the World Influenza Center in London. Additional specimens were sent to the International Influenza Center for the Americas in Atlanta, Georgia (a component of the National Communicable Disease Center, now the US Centers for Disease Control and Prevention [CDC]). Confirmation that the virus strain was a distinct antigenic variant of contemporary influenza viruses prompted a World Health Organization (WHO) warning on August 16.3 At this time, the virus became available to research and vaccine production laboratories.4 Spread was confirmed in August when isolates of the same virus were identified in Singapore, Taiwan, the Philippines, Vietnam, and Malaysia. Thailand, India, the Northern Territory of Australia, and Iran experienced outbreaks in September.5 Air travel by an estimated 160 million persons during the pandemic6 facilitated rapid transmission worldwide.
On September 2, a respiratory specimen from a Marine who had just returned to San Diego, California, from Vietnam produced the first US isolate.7 Before leaving Vietnam, the Marine had shared a bunker with a friend recently returned from Hong Kong. An additional 22 ILI cases occurred in San Diego among students and contacts from the Marine Corps Drill Instructors School, with the A(H3N2) virus isolated from 9 of 21 respiratory specimens. Concurrently, military physicians reported outbreaks in Hawaii and Alaska among personnel recently returned from southeast Asia. On September 6, National Communicable Disease Center officials requested cooperation from all state health officers, epidemiologists, and laboratory directors for “monitoring the importation of the virus and in conducting surveillance for influenza.”8
Public health investigations reported in the Morbidity and Mortality Weekly Report identified influenza A2/Hong Kong virus (subsequently referred to as influenza A(H3N2) virus) in travelers to the United States from Asia.9 Increased surveillance in the United States continued over the next year, expanding upon systems implemented for the 1957 pandemic and including reports on school and workplace absenteeism, school closings, hospital admissions, and outpatient visits, as well as reported cases and outbreaks. Initially, cases occurred primarily among persons returning from Asia.10
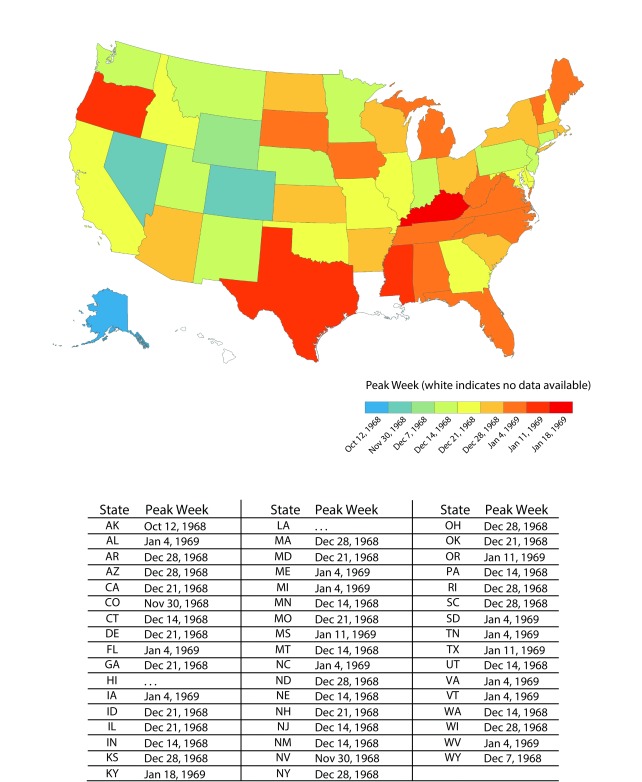
US influenza activity increased dramatically in October. The first reported civilian outbreak in the continental United States was identified in Needles, California, with more than one third of its population reporting ILI. ILI reports in Colorado increased from 62 cases for the week ending November 2 to 670 for the week ending November 9,11 a week in which other western states and Hawaii also reported outbreaks.12 The first outbreaks in eastern states occurred the next week. All 50 states experienced increased school absenteeism during the pandemic; 23 faced school and college closures and 31 saw elevated worker absenteeism. The peak week of influenza activity for most states fell between December 14 and January 11, with pandemic activity generally starting in the western United States and moving eastward13 (Figure 1).
FIGURE 1—
Peak Week of Pandemic Influenza Activity, United States, 1968 Influenza A(H3N2) Pandemic
Source. National Center for Communicable Diseases. Influenza—Respiratory Diseases Surveillance. National Communicable Disease Center, June 30, 1969. Report No.: 85.
Newspaper articles chronicled the widespread college closures, slowdowns in business and industry, and threats to Christmas mail deliveries. In December, the Apollo 8 astronauts were vaccinated to protect them from pandemic influenza in advance of their December 21 moon-orbiting flight, and President Johnson was hospitalized with a respiratory infection that his aides said “could be called the flu.” National concerns were reflected in a December 19 New York Times editorial describing the pandemic as “one of the worst in the nation’s history,” bemoaning the “amount of discomfort and distress suffered by the millions who have already been hit,” and the potential for “billions of dollars” associated with treatment and lost productivity.14
A second, less severe pandemic wave of illness in the United States occurred late in the following season (1969–1970). Over these two seasons, 70% of excess pneumonia and pandemic influenza deaths in the United States occurred during 1968–1969 season. This was unlike the experience in most countries, where the initial 1968 wave tended to be less severe but was followed by an increasingly severe wave in 1969–1970.15
CLINICAL COURSE
The majority of A(H3N2) virus infections resulted in clinically mild, uncomplicated upper respiratory tract disease. Predominant findings among uncomplicated pandemic cases included malaise, fever, myalgia, cough, headache, coryza, and sore throat. During the initial outbreak in Hong Kong, symptoms were mostly mild without observable excess mortality.16 In a British Royal Air Force study, half of those with serological evidence of infection had no recorded illness.17 All 965 cases reported by the University of Singapore Health Service had a mild clinical course with no hospitalizations, although some experienced atypical symptoms including severe anorexia, nausea, and ocular pain.18 A survey of nearly 7000 US high school students indicated that the median duration of illness was five days,19 although cough and prostration in some cases persisted as long as three weeks.20
However, severe disease did occur. Among pediatric inpatients and outpatients in Washington, DC, influenza A virus infections occurred more frequently during A(H3N2) activity (1968–1976) than during influenza A(H2N2) virus circulation (1957–1968) and were associated with more hospitalizations for pneumonia, bronchiolitis, bronchitis, and croup.21 Some outbreak areas reported complications including pneumonia, myocarditis, and pericarditis. Pulmonary complications in adults included localized primary viral or secondary bacterial pneumonia, or diffuse bilateral pneumonia, occurring early or late in the clinical course, mostly among persons with underlying comorbid conditions.22 Grady Hospital in Atlanta, Georgia reported a threefold increase in staphylococcal pneumonia admissions during the pandemic compared with the previous season. Hospital admissions began increasing in December 1968, and 74% of pneumonia patients hospitalized between January 1 and 5, 1969, had serological evidence of pandemic virus infection.23 Autopsy findings in fatal cases included acute bronchopneumonia, diffuse hemorrhagic or necrotizing pneumonitis, bronchitis, or bronchiolitis; in some cases, bacterial cultures of sputum or lung samples yielded Staphylococcus aureus, Pseudomonas aeruginosa, or other bacterial pathogens.24
ETIOLOGY
The 1968 pandemic was caused by influenza A/Hong Kong/1968 (H3N2) virus. This pandemic virus contained two genes derived from a low-pathogenicity avian influenza A virus and six genes from the A(H2N2) virus that had been circulating among people since its emergence to cause the 1957 H2N2 pandemic.25 The hemagglutinin (HA) gene contained two mutations in its receptor binding site from the closest avian viruses, altering its receptor binding specificity, from preferential binding for a2,3-linked sialic acids (viral receptors predominant in birds) to a2,6-linked sialic acids (viral receptors predominant in humans).26 However, studies suggest that these binding preferences alone do not determine influenza A entry to human airway cells.27 Additional changes identified in the HA1 subunit of the HA, based on their location, may have affected the HA receptor-binding and fusion activities28 and facilitated human-to-human transmission, enabling the virus to spread quickly.
EPIDEMIOLOGY
The clinical attack rate varied. Milwaukee, Wisconsin, public health authorities reported an overall illness attack rate of 43%.29 A study among inmates of Georgia State Prison chronicled a 40% illness attack rate, and a study of residents of a California retirement community revealed that 10% were symptomatic.30 This may have reflected some preexisting immunity in older age groups, suggested by serological studies revealing antibodies to the virus prior to the pandemic in large percentages of adults aged older than 65 years.31 However, unlike the 1957 H2N2 pandemic, in which 86% of index cases were school age, index cases in 1968 were equally divided among school-aged children and adults.32 Overall, children aged 10 to 14 years comprised the age group with the highest clinical attack rate (40%).33 In 2010, Jackson et al. used a variety of published data sets to estimate that the first-wave basic reproduction number (R0) was between 1.06 and 2.06 and the second-wave R0 was between 1.21 and 3.58.34 A subsequent 2014 meta-analysis of R0 studies revealed an overall median point estimate of 1.8 for the 1968 pandemic H3N2 virus.35 Complications and exacerbation of underlying disease conditions, such as diabetes, cardiac failure, and chronic obstructive pulmonary disease, contributed to excess mortality.36 In the United States, the pandemic resulted in an estimated 100 000 deaths.37 As with other 20th-century pandemics, large proportions (half in 1968) of pneumonia and influenza deaths occurred in persons younger than 65 years.38
COUNTERMEASURES
Medical advances in the 1960s, primarily the advent of antiviral medications and expansion of influenza vaccine options, provided a stronger arsenal to combat this pandemic than had been available during the 1957 pandemic.
ANTIVIRALS
The first opportunity to assess the effect of antiviral use during a pandemic occurred in 1968. In 1966, the Food and Drug Administration had approved amantadine for chemoprophylaxis of influenza A virus infection—a controversial decision both criticized39 and supported.40 Studies had shown that amantadine inhibited infections of group A influenza viruses by blocking or slowing virus entry into cells.41 The prophylactic effectiveness of amantadine had been demonstrated in some double-blinded randomized controlled trials with experimentally induced influenza and during naturally occurring influenza outbreaks. However, sample sizes in these studies tended to be small and study results were unconvincing to some.42 Studies assessing the effects of amantadine treatment also produced inconsistent results. Clinical findings in some showed amantadine to be effective in reducing the severity and duration of illness when given within 48 hours of symptom development of influenza A, whereas others failed to demonstrate effectiveness. During the pandemic, a multicenter double-blinded randomized controlled trial in Japan demonstrated a statistically significant reduction in fever duration among laboratory-confirmed pandemic influenza patients treated with amantadine.43 Also, in a study of prison inmates, all indicators of a therapeutic effect of amantadine approached or achieved statistical significance.44
A 1983 Mayo Clinic Symposium on Antimicrobial Agents affirmed the use of amantadine for influenza, reporting prophylactic effectiveness as well as “some” therapeutic effect when amantadine was used early in the course of influenza A virus infection.45 A subsequent Clinical Evidence review46 reported that amantadine was likely to be beneficial in reducing the duration of symptoms when used in early treatment of influenza A in adults. During the 2005–2006 influenza season, high prevalence of amantadine resistance among circulating influenza A viruses prompted changes in recommendations for its use,47 and amantadine has not been recommended for treatment or chemoprophylaxis of influenza A virus infections since then.
Despite the controversies around influenza prophylaxis and treatment during the pandemic, researchers identified a remarkable remission of Parkinson’s symptoms in a woman being treated with amantadine to prevent influenza in 1969. This prompted studies that demonstrated significant improvement in Parkinson’s symptoms with amantadine use,48 and amantadine has been used to treat Parkinson’s disease since that time.
VACCINE
The Division of Biologics Standards of the National Institutes of Health provided the A/Hong Kong/1968 (H3N2) vaccine virus to manufacturers in August 1968. However, before manufacturers had completed studies to determine its feasibility for producing a pandemic vaccine, a new vaccine virus became available. This virus, the Aichi strain from Japan, demonstrated superior vaccine production potential and was supplied to manufacturers on September 9, 1968, 49 and incorporated into a monovalent pandemic influenza vaccine.
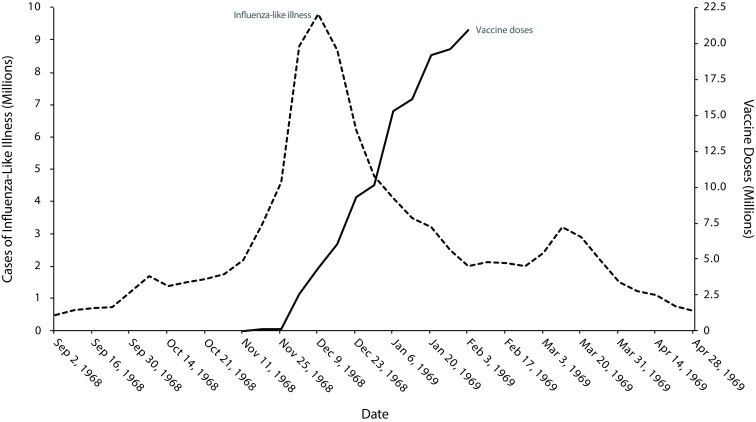
Vaccine manufacturers released a first lot of 110 000 pandemic vaccine doses on November 15, 1968. Subsequently, 15 million doses became available by the pandemic’s peak50 in January 196951 (Figure 2). After the peak, vaccine demand waned. For most of the population, the pandemic vaccine was “too little and too late,”52 and the effect of the vaccine on reducing pandemic spread was questionable. In January 1969, manufacturers began to phase out production, and unused vaccine remained. Some vaccine became available for use in the southern hemisphere.53
FIGURE 2—
Estimated Influenza-Like Illness and Vaccine Availability, United States, 1968 Influenza A(H3N2) Pandemic
Source. Influenza-like estimates are from the Department of Health Education and Welfare, “Acute Conditions.” Vaccine availability is from Murray, “Production and Testing in the USA of Influenza Virus Vaccine.”
Virologists had been actively exploring methods to improve influenza vaccine technology since the 1957 influenza pandemic, and the 1968 pandemic provided an opportunity to assess these technologies. For example, a split vaccine utilizing only those components of the virus essential to stimulate humoral immunity was authorized in the United States54; it performed comparably to whole virus vaccines, with fewer adverse reactions in adults55 and children.56 The principle of reassortment, a process in which two or more influenza A viruses exchange genetic material, had been established in 1960 with the combination of a low-yielding A(A2) strain and a standard laboratory strain (A/Puerto Rico[PR]/8).57 In 1968, successful reassortment of the Aichi strain with a standard laboratory strain (A/PR/8) by simultaneous inoculation of chick embryos produced a combined virus with the desired antigenicity of a pandemic virus and the growth characteristics of the standard laboratory strain.58 Reassortment using the A/PR/8 strain has since become standard practice and is used in current vaccine preparation.
To evaluate pandemic H3N2 vaccine effectiveness, officials used a variety of methods. One study compared respiratory illness rates in two Michigan communities.59 Schoolchildren in one community received pandemic vaccine while the other served as an unvaccinated comparison community. Throughout the study, respiratory illness rates in the vaccinated community remained considerably lower than in the comparison community. During the 10 weeks of the season when the Hong Kong A(H3N2) virus was isolated from community residents, the excess rate of self-reported respiratory illness in the unvaccinated community was triple that of the vaccinated community. The authors concluded that vaccination of school-age children resulted in a markedly lower rate of respiratory illness in the entire community. However, Glezen et al. reached a much different conclusion after vaccinating infants and children with the pandemic vaccine. Their results demonstrated that although the vaccine was safe and acceptable for children, there was no evidence that a single dose provided adequate protection.60
In another study of the pandemic vaccine’s efficacy, researchers concluded that the attack rate in persons receiving high doses of the pandemic vaccine was at least 50% lower than in those who received the seasonal A(H2) vaccine. In addition, high-dose vaccine recipients who did become ill experienced milder illness, with fewer and shorter fevers and less need for bed rest.61 However, the authors cautioned that the standard dose of the pandemic vaccine was not effective, and multiple doses were not feasible given the timing of the pandemic and existing production parameters. A 2007 Cochrane review of influenza vaccines concluded that one-dose or two-dose monovalent pandemic vaccines used in 1968 demonstrated 65% protection against ILI, 93% protection against illness from influenza A(H3N2) virus infection, and 65% protection against hospitalizations.62 However, their review used a solitary study to assess all outcomes except for ILI, which was assessed using four studies, limiting generalizability of the results.
AFTER THE PANDEMIC
Walter Dowdle, who in 1968 was director of the National Communicable Disease Center Laboratory Program in the WHO International Influenza Center for the Americas, declared that all that had been learned since the 1957 H2N2 pandemic had been applied to the 1968 H3N2 pandemic.63 For example, in 1968, the US Advisory Committee on Immunization Practices published recommendations for pandemic vaccine use in August, well before the United States experienced increased pandemic influenza activity, identifying priority vaccination groups to be targeted when pandemic vaccine became available.64 During the 1957 pandemic, priority vaccination groups were identified only after widespread outbreaks.65 The 1968 pandemic also demonstrated the utility of communication networks and US surveillance systems that provided several large study populations for vaccine trials.66
However, the 1968 pandemic also highlighted areas for improvement. Specifically, the 1968 pandemic showed the need for WHO to incorporate a better influenza forecasting system and to pursue further research to improve prevention and control of influenza.67 Precise virological information had been promptly shared during the pandemic, but epidemiological data varied widely between countries in both quantity and quality, because of diverse reporting methods, systems of medical care, and availability and use of laboratory diagnostic services. This prompted WHO officials to introduce a standard surveillance reporting form for National Influenza Centers, which had been implemented by many and had begun to work well by the fall of 1969.68 Global collaboration was also highlighted in October 1969, when the National Communicable Disease Center, WHO, and Emory University hosted an international working conference on Hong Kong pandemic influenza.69 At the conference, Alexander Langmuir, director of epidemiology at the National Communicable Disease Center, emphasized the need for improved influenza surveillance, specifically the collection of more quantitative data regarding disease incidence, age- and sex-specific attack rates, character and severity of complications, socioeconomic factors influencing mortality, and vaccine distribution and use.70
For the past 50 years, the CDC has worked toward these goals; many improvements, particularly in surveillance, have been achieved and were used during the 2009 H1N1 pandemic. US influenza surveillance now collects data from an extensive surveillance network of outpatient and inpatient care settings, as well as virological characterization using next-generation sequencing of all viruses received at the CDC. Surveillance systems now also monitor mortality and influenza vaccination coverage. Pediatric influenza deaths are currently included in the Council of State and Territorial Epidemiologists list of nationally notifiable conditions to be reported to the CDC. A wide range of seasonal influenza vaccines is available. Influenza diagnostic tests and antiviral medications with different mechanisms of action and routes of administration are now available in many clinical settings for the diagnosis and treatment of seasonal influenza.
The illnesses and deaths in 1968–1969 were notable but did not approximate the impact of the A(H1N1) virus that emerged in 1918. However, the cumulative impact of A(H3N2) virus infections over the last 50 years has been substantial. The average estimated number of annual hospitalizations during the past six seasons for A(H3N2) virus (675 000) was more than twice that of A(H1N1)pdm09 virus (330 000).71 The 2017–2018 H3N2-predominant influenza season was particularly severe, with record hospitalization rates recorded. On the basis of data from the National Center for Health Statistics (NCHS), the proportion of deaths attributed to pneumonia and influenza that season peaked at 10.8%, the highest percentage recorded since NCHS data were first used for routine influenza surveillance.72 Whereas A(H1N1) virus was the scourge in the early part of the 20th century, A(H3N2) virus has been the predominant cause of influenza disease burden in the early 21st century.
Several factors have contributed to the higher impact of A(H3N2) viruses over the last 50 years. First, A(H3N2) viruses have undergone antigenic change at a much higher rate than influenza A(H1N1) viruses.73 Frequent changes to the hemagglutinin protein have allowed A(H3N2) viruses to evade human immune responses both through (1) conformational changes around important antigenic sites, notably the receptor binding pocket, and (2) increased glycosylation of the hemagglutinin protein shielding the antigenic sites of the virus from antibody binding.74
Secondly, A(H3N2) virus has had a disproportionate impact on older adults. Persons aged 65 years and older have a higher rate of comorbidities that increase their risk for influenza complications, and this group experiences higher mean hospitalization rates during influenza seasons in which H3 viruses predominate than in seasons in which H1 viruses predominate.75 Contributing factors may include waning immunity and decline in vaccine-derived immune protection.76 In addition, older adults may respond less effectively to A(H3N2) virus infections because of immunological imprinting, also referred to as “original antigenic sin.”77 This suggests that persons first infected by A(H1N1) virus (i.e., 1918–1957) are protected from severe H1N1 disease but are less protected against severe illness with A(H3N2) virus infection.
Third, when A(H3N2) viruses are propagated in eggs, they change conformation and can lose sites of glycosylation, causing them to differ from the circulating A(H3N2) viruses. This likely contributes to the lower vaccine effectiveness observed for A(H3N2) viruses, especially in older adults,78 highlighting the need for improving the effectiveness of seasonal influenza vaccines through increased antigen content, addition of adjuvants, and ultimately through development of more broadly protective and longer lasting “universal” vaccines.
Since their emergence, influenza A(H3N2) viruses have caused substantial cumulative morbidity and mortality worldwide during seasonal influenza epidemics, greatly exceeding their impact in the first years of the pandemic beginning in 1968. More than 50 years later, A(H3N2) continues to adapt to evade host immunity and cause higher numbers of hospitalizations and deaths than influenza A(H1N1) and B viruses. New therapies and vaccine technologies have been developed, but further improvements in the prevention and control of influenza are still needed and will be critical in preparing for the next influenza pandemic.
ACKNOWLEDGMENTS
We thank our colleagues in the Influenza Division (particularly Krista Kniss), the National Center for Immunization and Respiratory Diseases, and the CDC Library for their support and encouragement in preparing the manuscript.
Note. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Department of Health and Human Services.
Note. For further reading, please see the list of additional references, available as a supplement to the online version of this article at http://www.ajph.org.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interests to declare.
ENDNOTES
- 1.Chang W. K. National Influenza Experience in Hong Kong, 1968. Bulletin of the World Health Organization 41. 1969;(3):349–351. [PMC free article] [PubMed] [Google Scholar]
- 2.Cockburn W. C., Delon P. J., Ferreira W. “Origin and Progress of the 1968–69 Hong Kong Influenza Epidemic. Bulletin of the World Health Organization 41. 1969;(3):345–348. [PMC free article] [PubMed] [Google Scholar]
- 3. Chang, “National Influenza Experience.”.
- 4. Cockburn et al., “Origin and Progress.”.
- 5. Ibid.
- 6.Grais R. F., Ellis J. H., Glass G. E. Assessing the Impact of Airline Travel on the Geographic Spread of Pandemic Influenza. European Journal of Epidemiology 18. 2003;(11):1065–1072. doi: 10.1023/a:1026140019146. [DOI] [PubMed] [Google Scholar]
- 7.Sharrar R. G. “National Influenza Experience in the USA, 1968–69. Bulletin of the World Health Organization 41. 1969;(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- 8. Ibid, p. 361.
- 9.National Communicable Disease Center. Presumptive A2/Hong Kong Influenza. MMWR. Morbidity and Mortality Weekly Report 17. 1968;(37):338–339. [Google Scholar]
- 10. Sharrar, “National Influenza Experience.”.
- 11.National Communicable Disease Center. “Influenza,” MMWR. Morbidity and Mortality Weekly Report 17. 1968;(46):425–426. [Google Scholar]
- 12. Sharrar, “National Influenza Experience.”.
- 13.National Communicable Disease Center. United States Surveillance Summary 1968–1969. MMWR. Morbidity and Mortality Weekly Report 18. 1969;(85):217–219. [Google Scholar]
- 14. B. Gwertzman, “Johnson in Hospital With Cold and Fever.” New York Times, December 19, 1968, p. 1.
- 15.Viboud C., Grais R. F., Lafont B. A., Miller M. A., Simonsen L. Group Multinational Influenza Seasonal Mortality Study, “Multinational Impact of the 1968 Hong Kong Influenza Pandemic: Evidence for a Smoldering Pandemic. Journal of Infectious Diseases 192. 2005;(2):233–248. doi: 10.1086/431150. [DOI] [PubMed] [Google Scholar]
- 16. Chang, “National Influenza Experience.”.
- 17.Miller D. L., Reid D., Daimond J. R., Pereira M. S., Chakraverty P. “Hong Kong Influenza in the Royal Air Force 1968–70. Journal of Hygiene (London) 71. 1973;(3):535–547. doi: 10.1017/s0022172400046520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadri Z. N. “An Outbreak of ‘Hong Kong ‘Flu’ in Singapore, I. Clinical Study,” Singapore. Medical Journal 11. 1970;(1):30–32. [PubMed] [Google Scholar]
- 19.Davis L. E., Caldwell G. G., Lynch R. E., Bailey R. E., Chin T .D. Hong Kong Influenza: The Epidemiologic Features of a High School Family Study Analyzed and Compared With a Similar Study During the 1957 Asian Influenza Epidemic. American Journal of Epidemiology 92. 1970;(4):240–247. doi: 10.1093/oxfordjournals.aje.a121203. [DOI] [PubMed] [Google Scholar]
- 20. Sharrar, “National Influenza Experience.”.
- 21.Kim H. W., Brandt C. D., Arrobio J. O., Murphy B., Chanock R. M., Parrott R. H. “Influenza A and B Virus Infection in Infants and Young Children During the Years 1957–1976. American Journal of Epidemiology 109. 1979;(4):464–479. doi: 10.1093/oxfordjournals.aje.a112704. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay M. I., Jr, Herrmann E. C., Jr, Morrow G. W., Jr, Brown A. L., Jr Hong Kong Influenza: Clinical, Microbiologic, and Pathologic Features in 127 Cases. JAMA 214. 1970;(10):1825–1832. doi: 10.1001/jama.214.10.1825. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzmann S. W., Adler J. L., Sullivan R. J., Jr, Marine W. M. “Bacterial Pneumonia During the Hong Kong Influenza Epidemic of 1968–1969. Archives of Internal Medicine 127. 1971;(6):1037–1041. [PubMed] [Google Scholar]
- 24.Feldman P. S., Cohan M. A., Hierholzer W. J., Jr Fatal Hong Kong Influenza: A Clinical, Microbiological and Pathological Analysis of Nine Cases. Yale Journal of Biology and Medicine 45. 1972;(1):49–63. [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaoka Y., Krauss S., Webster R. G. Avian-to-Human Transmission of the Pb1 Gene of Influenza a Viruses in the 1957 and 1968 Pandemics. Journal of Virology 63. 1989;(11):4603–4608. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Poucke S., Doedt J., Baumann J. et al. Role of Substitutions in the Hemagglutinin in the Emergence of the 1968 Pandemic Influenza Virus. Journal of Virology 89. 2015;(23):12211–12216. doi: 10.1128/JVI.01292-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis A. S., Chertow D. S., Kindrachuk J. et al. 1918 Influenza Receptor Binding Domain Variants Bind and Replicate in Primary Human Airway Cells Regardless of Receptor Specificity. Virology 493. 2016:238–246. doi: 10.1016/j.virol.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Poucke et al., “Role of Substitutions in the Hemagglutinin.”.
- 29.Piraino F. F., Brown E. M., Krumbiegel E. R. “Outbreak of Hong Kong Influenza in Milwaukee, Winter of 1968–69. Public Health Reports 85. 1970;(2):140–150. [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenbaum S. C., Mostow S. R., Dowdle W. R., Coleman M. T., Kaye H. S. Studies With Inactivated Influenza Vaccines Purified by Zonal Centrifugation. 2. Efficacy. Bulletin of the World Health Organization 41. 1969;(3):531–535. [PMC free article] [PubMed] [Google Scholar]
- 31.Masurel N. Relation Between Hong Kong Virus and Former Human A2 Isolates and the a-Equ12 Virus in Human Sera Collected Before 1957. Lancet . 1969;(7601):907–910. doi: 10.1016/s0140-6736(69)92544-6. [DOI] [PubMed] [Google Scholar]
- 32. Davis et al., “Hong Kong Influenza: The Epidemiologic Features of a High School Family Study.”. [DOI] [PubMed]
- 33.Cox N. J., Subbarao K. Global Epidemiology of Influenza: Past and Present. Annual Review of Medicine 51. 2000;(1):407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 34.Jackson C., Vynnycky E., Mangtani P. Estimates of the Transmissibility of the 1968 (Hong Kong) Influenza Pandemic: Evidence of Increased Transmissibility Between Successive Waves. American Journal of Epidemiology 171. 2010;(4):465–478. doi: 10.1093/aje/kwp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biggerstaff M., Cauchemez S., Reed C., Gambhir M., Finelli L. Estimates of the Reproduction Number for Seasonal, Pandemic, and Zoonotic Influenza: A Systematic Review of the Literature. BMC Infectious Diseases 14. 2014;(1):480. doi: 10.1186/1471-2334-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen-Van-Tam J. S., Hampson A. W. The Epidemiology and Clinical Impact of Pandemic Influenza. Vaccine 21. 2003;(16):1762–1768. doi: 10.1016/s0264-410x(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 37.Glezen W. P. Emerging Infections: Pandemic Influenza. Epidemiologic Reviews 18. 1996;(1):64–76. doi: 10.1093/oxfordjournals.epirev.a017917. [DOI] [PubMed] [Google Scholar]
- 38.Simonsen L., Clarke M. J., Schonberger L. B., Arden N. H., Cox N. J., Fukuda K. Pandemic Versus Epidemic Influenza Mortality: A Pattern of Changing Age Distribution. Journal of Infectious Diseases. 178. 1998;(1):53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 39.Sabin A. B. Amantadine Hydrochloride. Analysis of Data Related to Its Proposed Use for Prevention of A2 Influenza Virus Disease in Human Beings. JAMA 200. 1967;(11):943–950. doi: 10.1001/jama.200.11.943. [DOI] [PubMed] [Google Scholar]
- 40.AMA Council on Drugs, “The Amantadine Controversy. JAMA 201. 1967;(6):112–113. [Google Scholar]
- 41.Davies W. L., Grunert R.R., Haff R.F. et al. Antiviral Activity of 1-Adamantanamine (Amantadine) Science 144. 1964;(3620):862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 42. Sabin, “Amantadine Hydrochloride. Analysis of Data.”.
- 43.Kitamoto O. “Therapeutic Effectiveness of Amantadine Hydrochloride in Naturally Occurring Hong Kong Influenza–Double-Blind Studies. Japanese Journal of Tuberculosis and Chest Diseases 17. 1971;(1):1–7. [PubMed] [Google Scholar]
- 44.Knight V., Fedson D., Baldini J., Douglas R. G., Couch R. B. Amantadine Therapy of Epidemic Influenza a(2) (Hong Kong) Infection and Immunity 1. 1970;(2):200–204. doi: 10.1128/iai.1.2.200-204.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermans P. E., Cockerill F. R., 3rd “Antiviral Agents,” Mayo Clinic Proceedings 58. 1983;(4):217–222. [PubMed] [Google Scholar]
- 46.Uyeki T., Winquist A. “Influenza,” Clinical Evidence. 2001;(6):550–556. [Google Scholar]
- 47.Centers for Disease Control and Prevention. “High Levels of Adamantane Resistance Among Influenza a (H3n2) Viruses and Interim Guidelines for Use of Antiviral Agents—United States, 2005–06 Influenza Season. MMWR. Morbidity and Mortality Weekly Report 55. 2006;(2):44–46. [PubMed] [Google Scholar]
- 48.Schwab R. S., England A. C., Jr, Poskanzer D. C., Young R. R. “Amantadine in the Treatment of Parkinson’s Disease. JAMA 208. 1969;(7):1168–1170. [PubMed] [Google Scholar]
- 49.Murray R. “Production and Testing in the USA of Influenza Virus Vaccine Made From the Hong Kong Variant in 1968–69. Bulletin of the World Health Organization 41. 1969;(3):495–496. [PMC free article] [PubMed] [Google Scholar]
- 50. “Acute Conditions: Incidence and Associated Disability United States—July 1968–June 1969,” in National Center for Health Statistics, ed., Data From the National Health Interview Survey (Rockville, MD: Department of Health, Education and Welfare, 1969) [PubMed]
- 51. Murray, “Production and Testing in the USA of Influenza Virus Vaccine.”. [PMC free article] [PubMed]
- 52.Hilleman M. R. The Roles of Early Alert and of Adjuvant in the Control of Hong Kong Influenza by Vaccines. Bulletin of the World Health Organization 41. 1969;(3):623–628. [PMC free article] [PubMed] [Google Scholar]
- 53. Murray, “Production and Testing in the USA of Influenza Virus Vaccine.”. [PMC free article] [PubMed]
- 54.Barberis I., Myles P., Ault S. K., Bragazzi N. L., Martini M. History and Evolution of Influenza Control Through Vaccination: From the First Monovalent Vaccine to Universal Vaccines. Journal of Preventive Medicine and Hygiene 57. 2016;(3):E115–E120. [PMC free article] [PubMed] [Google Scholar]
- 55.Brandon F. B., Cox F., Quinn E., Timm E. A., McLean I. W., Jr Influenza Immunization: Clinical Studies With Ether-Split Subunit Vaccines. Bulletin of the World Health Organization 41. 1969;(3):629–637. [PMC free article] [PubMed] [Google Scholar]
- 56.Davenport F. M., Hennessy A. V., Brandon F. M., Webster R. G., Barrett C. D., Jr, Lease G. O. Comparisons of Serologic and Febrile Responses in Humans to Vaccination With Influenza A Viruses or Their Hemagglutinins. Journal of Laboratory and Clinical Medicine 63. 1964:5–13. [PubMed] [Google Scholar]
- 57.Kilbourne E. D., Murphy J. S. Genetic Studies of Influenza Viruses. I. Viral Morphology and Growth Capacity as Exchangeable Genetic Traits. Rapid in Ovo Adaptation of Early Passage Asian Strain Isolates by Combination With Pr8. Journal of Experimental Medicine 111. 1960;(3):387–406. doi: 10.1084/jem.111.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kilbourne E. D. Future Influenza Vaccines and the Use of Genetic Recombinants. Bulletin of the World Health Organization 41. 1969;(3):643–645. [PMC free article] [PubMed] [Google Scholar]
- 59.Monto A. S., Davenport F. M., Napier J. A., Francis T., Jr Effect of Vaccination of a School-Age Population Upon the Course of an A2-Hong Kong Influenza Epidemic. Bulletin of the World Health Organization 41. 1969;(3):537–542. [PMC free article] [PubMed] [Google Scholar]
- 60.Glezen W. P., Loda F. A., Denny F. W. A Field Evaluation of Inactivated, Zonal-Centrifuged Influenza Vaccines in Children in Chapel Hill, North Carolina, 1968–69. Bulletin of the World Health Organization 41. 1969;(3):566–569. [PMC free article] [PubMed] [Google Scholar]
- 61. Schoenbaum et al., “Studies With Inactivated Influenza Vaccines.”.
- 62.Jefferson T. O., Rivetti D., Di Pietrantonj C., Rivetti A., Demicheli V. Vaccines for Preventing Influenza in Healthy Adults. Cochrane Database of Systematic Reviews. 2007;(2):CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 63.AMA Editorial Board. What the Hong Kong Flu Has Taught. JAMA 207. 1969;(11):2016–2017. [Google Scholar]
- 64.National Communicable Disease Center. Recommendation of the Public Health Service Advisory Committee on Immunization Practices. MMWR. Morbidity and Mortality Weekly Report 17. 1968;(35):323. [Google Scholar]
- 65.Henderson D. A., Courtney B., Inglesby T. V., Toner E., Nuzzo J. B. “Public Health and Medical Responses to the 1957–58 Influenza Pandemic. Biosecurity and Bioterrorism 7. 2009;(3):265–273. doi: 10.1089/bsp.2009.0729. [DOI] [PubMed] [Google Scholar]
- 66.Etheridge E. Sentinel for Health. Berkeley: University of California Press; 1992. [Google Scholar]
- 67.Blakely D. E. M. Mass Mediated Disease: A Case Study Analysis of Three Flu Pandemics and Public Health Policy. Lanham, MD: Lexington Books; 2006. [Google Scholar]
- 68. Cockburn et al., “Origin and Progress.”.
- 69. Etheridge, Sentinel for Health.
- 70.Langmuir A. D., Housworth J. A Critical Evaluation of Influenza Surveillance. Bulletin of the World Health Organization 41. 1969;(3):393–398. [PMC free article] [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention. Disease Burden of Influenza,” https://www.cdc.gov/flu/about/burden/index.html (accessed November 12, 2019)
- 72.Garten R., Blanton L., Elal A. I. A. et al. “Update: Influenza Activity in the United States During the 2017–18 Season and Composition of the 2018–19 Influenza Vaccine,” MMWR. Morbidity and Mortality Weekly Report 67. 2018;(22):634–642. doi: 10.15585/mmwr.mm6722a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang H., Carney P. J., Chang J. C., Guo Z., Villanueva J. M., Stevens J. Structure and Receptor Binding Preferences of Recombinant Human A(H3N2) Virus Hemagglutinins. Virology 477. 2015:18–31. doi: 10.1016/j.virol.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belongia E. A., McLean H. Q. Influenza Vaccine Effectiveness: Defining the H3n2 Problem. Clinical and Infectious Diseases 69. 2019;(10):1817–1823. doi: 10.1093/cid/ciz411. [DOI] [PubMed] [Google Scholar]
- 75.Budd A. P., Beacham L., Smith C. B. et al. Birth Cohort Effects in Influenza Surveillance Data: Evidence That First Influenza Infection Affects Later Influenza-Associated Illness. Journal of Infectious Diseases 220. 2019;(5):820–829. doi: 10.1093/infdis/jiz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henry C., Zheng N. Y., Huang M. et al. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host & Microbe 25. 2019;(3):357–366.e6. doi: 10.1016/j.chom.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monto A. S., Malosh R. E., Petrie J. G., Martin E. T. The Doctrine of Original Antigenic Sin: Separating Good From Evil. Journal of Infectious Diseases 215. 2017;(12):1782–1788. doi: 10.1093/infdis/jix173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belongia E. A., Simpson M. D., King J. P. et al. Variable Influenza Vaccine Effectiveness by Subtype: A Systematic Review and Meta-Analysis of Test-Negative Design Studies. Lancet Infectious Diseases 16. 2016;(8):942–951. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]