Abstract
Objectives. To examine long-term gonorrhea prevalence trends from a sentinel surveillance population of young people at elevated risk for gonorrhea.
Methods. We analyzed annual cross-sectional urogenital gonorrhea screening data from 191 991 women (2000–2017) and 224 348 men (2003–2017) 16 to 24 years of age entering the National Job Training Program, a US vocational training program. We estimated prevalence among women using an expectation-maximization algorithm incorporated into a logistic regression to account for increases in screening test sensitivity; log-binomial regression was used to estimate prevalence among men.
Results. The adjusted gonorrhea prevalence among women followed a U-shaped curve, falling from 2.9% to 1.6% from 2000 through 2011 before rising to 2.7% in 2017. The prevalence among men declined from 1.4% to 0.8% from 2003 through 2017. In the case of both women and men, the prevalence was highest across all study years among those who were Black or American Indian/Alaska Native and those who resided in the South or Midwest.
Conclusions. Trends among National Job Training Program enrollees suggest that gonorrhea prevalence is rising among young women while remaining low and steady among young men.
Gonorrhea is the second most commonly reported sexually transmitted disease (STD) in the United States. Most infections are asymptomatic, and untreated infection may lead to severe reproductive sequelae, including pelvic inflammatory disease and infertility among women and epididymitis among men. The burden is highest among adolescents and young adults 15 to 24 years of age, non-Hispanic Blacks, and those residing in the South.1–3 Gonorrhea control programs, including those offering screening and treatment of asymptomatic infections, are important for reducing the risk of sequelae, as well as interrupting ongoing transmission and reducing prevalence and incidence.
National notifiable surveillance of reported gonorrhea cases is helpful in monitoring trends in diagnosed gonorrhea and assessing the impact of control programs over time. National gonorrhea case rates among adolescents and young adults have increased steadily since 2014, potentially indicating an increase in gonorrhea incidence.1 However, case rates may also increase as a result of a number of biases. Case rates, which are estimated by dividing the total number of reported cases by the total population size, may rise because of increased screening coverage (including increased screening at extragenital sites); screening with newer, more sensitive tests; or improved reporting (e.g., electronic laboratory reporting). In addition, case rates are influenced by changes in the risk composition of those who are screened (i.e., case mix), as case counts will likely rise if an increasing proportion of high-risk individuals are screened.4 The influence of these potential biases on case rate trends is difficult to ascertain and complicates interpretation of trends.
Prevalence trends derived from sentinel surveillance can be a useful counterpart to case rate trends because prevalence (referring to cases in which all participants are screened) is not subject to bias resulting from screening coverage or reporting. One sentinel program for the surveillance of gonorrhea in the United States is the National Job Training Program (NJTP), a vocational training program for socioeconomically disadvantaged young adults run by the Department of Labor. The NJTP has included gonorrhea screening at program entry since 2000 for young women and 2003 for young men, and the program has maintained consistent eligibility criteria.5 NJTP entrants represent a relatively stable population of economically disadvantaged young adults in which gonorrhea prevalence can be measured. Gonorrhea prevalence among women and men entering the NJTP between 2004 and 2009 declined modestly,6 but long-term trends accounting for potential bias due to changes in case mix and screening tests have not been reported.
We examined gonorrhea prevalence trends among young women entering the NJTP during 2000 to 2017 and young men entering the program during 2003 to 2017, accounting for potential bias associated with changes in screening tests and case mix, to provide minimally biased prevalence estimates over time in a sentinel population at elevated risk for gonorrhea.
METHODS
US residents 16 to 24 years of age who meet low-income criteria and face barriers to employment are eligible for the NJTP.5 The NJTP includes gonorrhea and chlamydia screening for all enrollees within 48 hours of entering the program. The majority of gonorrhea testing for the program is performed by a national contract laboratory, although NJTP centers may use local laboratories for testing; local testing data were not available for analysis. Treatment and follow-up care are provided by NJTP centers. We included non-Hispanic White, non-Hispanic Black, Hispanic, and American Indian/Alaska Native (AI/AN) enrollees residing in the Northeast, South, Midwest, and West for whom gonorrhea screening test results and information on type of diagnostic test (women only) were available.
During 2000 to 2006, women entering the NJTP were screened with a cervical swab; the Gen-Probe PACE 2 DNA hybridization probe (Gen-Probe Inc, San Diego, CA) was used in this screening. After 2006, women were screened through either a vaginal swab or a urine sample via the BD ProbeTec ET strand displacement assay (Becton-Dickinson, Sparks, MD). Screening for men began in 2003. Most men were screened via a urine sample with the BD ProbeTec assay, although some were screened with the Gen-Probe PACE 2 probe in the early study years.
Evaluation of Possible Biases
Our methods closely followed those used to estimate chlamydia prevalence in the NJTP.7 We explored potential sources of bias that might need to be addressed in analyses of prevalence trends. First, we examined possible bias resulting from missing gonorrhea test results, as only test results from the national laboratory were available for analysis. In addition, we did not have information on which centers used the national contract laboratory for gonorrhea testing. Because all chlamydia testing is performed by the national contract laboratory and reported as part of sentinel surveillance, a comparable number of chlamydia and gonorrhea test results would indicate that all gonorrhea screening was performed at the national contract laboratory rather than a local laboratory.
We compared the number of gonorrhea screening test results with the number of chlamydia screening test results per month as a proxy for completeness of data on gonorrhea and identified each center as being at least 50%, 75%, or 90% complete.6 We then examined gonorrhea positivity including centers at each level of completeness. We observed no meaningful differences by level of completeness (Figure A, available as a supplement to the online version of this article at http://www.ajph.org), and we present results from centers that tested at least 50% of enrollees for chlamydia and gonorrhea at the national laboratory.
Second, we investigated possible changes in case mix over time. Gonorrhea prevalence and case rates vary by race/ethnicity and region, with young adults who are Black or living in the South having the highest burden.1,3 We examined potential bias due to case mix by assessing longitudinal changes in the demographic characteristics of NJTP enrollees. We examined stacked bar charts of race/ethnicity, region of residence, and age group (16–19 years and 20–24 years) to assess the relative proportion of each factor over time. We also modeled the proportion of each level of race/ethnicity, region, and age group over time using log-binomial models to assess trends. We observed no meaningful variations in the relative proportions of race/ethnicity, region, or age group (Figures B and C, available as supplements to the online version of this article at http://www.ajph.org). The stable distribution of race/ethnicity, region, and age suggested that potential case mix bias could be excluded from further consideration.
Finally, we sought to account for time-varying misclassification among women because the quality of screening tests for women improved over the study period. We generated pairs of sensitivity and specificity estimates for each screening test and sample type through targeted meta-analyses of the existing literature. We searched PubMed and Scopus using medical subject heading terms and keywords related to gonorrhea screening and diagnostic accuracy. We included studies that reported the diagnostic accuracy of the Gen-Probe PACE 2 and BD ProbeTec ET among women and from which counts of true positive, true negative, false positive, and false negative tests could be extracted or calculated (Figure D, available as a supplement to the online version of this article at http://www.ajph.org). We used bivariate generalized linear mixed-effects models with a logit link to generate summary sensitivity and specificity estimates and 95% confidence intervals (CIs). We did not estimate sensitivity and specificity for men because nearly all men were screened with the BD ProbeTec ET, a highly sensitive and specific test.8,9
Gonorrhea Prevalence Trends
We analyzed annual cross-sectional gonorrhea screening data from female NJTP enrollees during 2000 to 2017 and male NJTP enrollees during 2003 to 2017. Data for 2014 were not available as a result of administrative challenges. Because screening is part of enrollment and coverage is high, test positivity at enrollment (number positive/number tested) was used as a proxy for prevalence.
We modeled gonorrhea prevalence trends among women using an expectation-maximization algorithm incorporated into a maximum-likelihood regression to account for misclassification due to imperfect screening test sensitivity and specificity (as calculated through our meta-analyses).10 In this approach, expectation maximization is used to estimate a logistic regression model when the outcome is measured with uncertainty; the approach allows test sensitivity and specificity to vary across observations according to the test type and sample type used for screening.
Gonococcal infection status (positive or negative) was the dependent variable in our models, and study year (continuous) was the independent variable. Several variable year specifications were examined, and year was specified as restricted cubic spline terms with 4 knots placed at the 5th, 35th, 65th, and 95th percentiles based on the Akaike information criterion and visual inspection. We used logistic regression models rather than log binomial models because of problems with log binomial model convergence. Parameter estimates from logistic regression models were used to calculate predicted gonorrhea probability (prevalence). Ninety-five percent confidence intervals were obtained via bootstrapping (n = 200).
Gonorrhea prevalence and 95% confidence intervals among men were estimated through log binomial regression without correction for imperfect test sensitivity and specificity. The unadjusted prevalence among men was low, and men were overwhelmingly screened with the BD ProbeTec ET. Very few men were screened with the Gen-Probe PACE 2. Correcting for imperfect but highly sensitive and specific tests had a negligible influence on prevalence.
We estimated gonorrhea prevalence trends among all female and male NJTP entrants and examined trends by race/ethnicity, region, and age group (16–19 years and 20–24 years) to evaluate differences by subgroup.
Sensitivity Analyses
Substantial race/ethnicity data were missing for 2013 and the distribution of known race/ethnicity deviated from other years (Figures B and C), so we explored potential bias from missing data. We examined unadjusted prevalence trends without excluding observations with missing data on race/ethnicity and region and found no differences in the shape of prevalence trends (Figure E, available as a supplement to the online version of this article at http://www.ajph.org). We also explored the influence of random error in Gen-Probe PACE 2 and BD ProbeTec ET sensitivity and specificity estimates among women. To account for random error, we modeled gonorrhea prevalence using the upper and lower bounds of the 95% confidence intervals around the summary sensitivity and specificity estimates. Analyses were performed with Stata version 12 (StataCorp LLC, College Station, TX).
RESULTS
The sensitivity and specificity of screening tests among women increased over the study period as a result of improvements in test technology (Table A, available as a supplement to the online version of this article at http://www.ajph.org). Sensitivity was 88.4% (95% CI = 84.1, 91.6) for the Gen-Probe PACE 2, 89.6% (95% CI = 85.0, 93.0) for BD ProbeTec ET urine samples, and 95.1% (95% CI = 89.3, 97.8) for BD ProbeTec ET vaginal swabs.
Gonorrhea Prevalence Among Women
During 2000 to 2017 (excluding 2014), 359 984 women were screened for gonorrhea or chlamydia (or both) by the national contract laboratory upon entering the NJTP. We excluded data from NJTP centers where less than 50% of women who were screened for chlamydia had gonorrhea test results (n = 116 813; 32%) to minimize bias from missing gonorrhea results. We further excluded women with missing or other race/ethnicity (n = 30 450; 13%) and missing data on region (n = 4677; 2%), test result (n = 13 440; 6%), or type of screening test (n = 2613; 1%). The final analytic sample included 191 991 women.
Most female entrants were Black (60%) and 16 to 19 years of age (66%). Almost half of women were from the South (44%), and few reported symptoms (1.5%). Approximately 18% of women were tested with the Gen-Probe PACE 2, and the remainder were tested with the BD ProbeTec ET via a urine sample (66%) or vaginal swab (16%). Unadjusted prevalence values were 2.6% with the Gen-Probe PACE 2, 2.1% with the BD ProbeTec ET for urine samples, and 2.5% with the BD ProbeTec ET for vaginal swabs (Table 1).
TABLE 1—
Characteristics of Women and Men Entering the National Job Training Program and Unadjusted Gonorrhea Prevalence: United States, 2000–2017
| Women |
Men |
|||
| Characteristic | Tested, No. (%) | Positive, No. (Unadjusted Prevalencea) | Tested, No. (%) | Positive, No. (Unadjusted Prevalencea) |
| Age, y | ||||
| 16–19 | 126 271 (65.8) | 3 308 (2.6) | 148 122 (66.0) | 1 600 (1.1) |
| 20–24 | 65 720 (34.2) | 1 059 (1.6) | 76 226 (34.0) | 783 (1.0) |
| Race/ethnicity | ||||
| Non-Hispanic Black | 115 395 (60.1) | 3 660 (3.2) | 123 804 (55.2) | 2 198 (1.8) |
| Non-Hispanic White | 40 799 (21.3) | 349 (0.9) | 64 234 (28.6) | 85 (0.1) |
| Hispanic | 30 967 (16.1) | 278 (0.9) | 31 510 (14.0) | 78 (0.3) |
| American Indian/Alaska Native | 4 830 (2.5) | 82 (1.7) | 4 800 (2.1) | 22 (0.5) |
| Region | ||||
| Midwest | 38 687 (20.2) | 1 087 (2.8) | 40 594 (18.1) | 538 (1.3) |
| Northeast | 35 309 (18.4) | 470 (1.3) | 33 555 (15.0) | 224 (0.7) |
| South | 84 628 (44.1) | 2 527 (3.0) | 113 437 (50.6) | 1 512 (1.3) |
| West | 33 367 (17.4) | 283 (0.9) | 36 762 (16.4) | 109 (0.3) |
| Symptoms at entrance | ||||
| Yes | 2 962 (1.5) | 88 (3.0) | 2 023 (0.9) | 53 (2.5) |
| No | 189 029 (98.5) | 4 279 (2.3) | 222 325 (99.1) | 2 330 (1.1) |
| Screening test (sample type) | ||||
| Gen-Probe PACE 2 DNA hybridization probe (swab for women, urine for men) | 34 456 (17.8) | 896 (2.6) | 447 (0.2) | 25 (5.6) |
| BD ProbeTec ET strand displacement assay (urine) | 125 909 (65.6) | 2 684 (2.1) | 223 901 (99.8) | 2 358 (1.1) |
| BD ProbeTec ET strand displacement assay (swab) | 31 626 (16.5) | 787 (2.5) | ||
| Year | ||||
| 2000 | 4 063 (2.1) | 140 (3.4) | ||
| 2001 | 2 961 (1.5) | 108 (3.6) | ||
| 2002 | 3 348 (1.7) | 88 (2.6) | ||
| 2003 | 8 641 (4.5) | 184 (2.1) | 5 116 (2.3) | 95 (1.9) |
| 2004 | 8 925 (4.6) | 229 (2.6) | 11 423 (5.1) | 146 (1.3) |
| 2005 | 10 491 (5.5) | 241 (2.3) | 13 871 (6.2) | 221 (1.6) |
| 2006 | 11 930 (6.2) | 331 (2.8) | 16 227 (7.2) | 222 (1.4) |
| 2007 | 14 052 (7.3) | 352 (2.5) | 18 753 (8.4) | 230 (1.2) |
| 2008 | 14 903 (7.8) | 333 (2.2) | 19 864 (8.9) | 209 (1.0) |
| 2009 | 15 704 (8.2) | 258 (1.6) | 19 222 (8.6) | 165 (0.8) |
| 2010 | 17 441 (9.1) | 355 (2.0) | 19 274 (8.6) | 181 (0.9) |
| 2011 | 17 730 (9.2) | 294 (1.7) | 19 453 (8.7) | 179 (0.9) |
| 2012 | 15 119 (7.9) | 277 (1.8) | 18 425 (8.2) | 176 (1.0) |
| 2013b | 3 291 (1.7) | 52 (1.6) | 3 858 (1.7) | 24 (0.6) |
| 2014b | ||||
| 2015 | 15 320 (8.0) | 372 (2.4) | 19 605 (8.7) | 183 (0.9) |
| 2016 | 14 811 (7.7) | 397 (2.7) | 19 536 (8.7) | 167 (0.9) |
| 2017 | 13 261 (6.9) | 356 (2.7) | 19 721 (8.8) | 185 (0.9) |
Note. Women, n = 191 991; men, n = 224 348.
Unadjusted prevalence was calculated as the total number of positive tests divided by the total number tested. Unadjusted estimates in this table differ slightly from the unadjusted estimates derived from maximum likelihood regression models presented in the text and Figure 1.
Data on race/ethnicity were missing for a substantial number of women and men in 2013, and these observations were excluded from the analyses. Screening was conducted in 2014, but the data were not available for analysis.
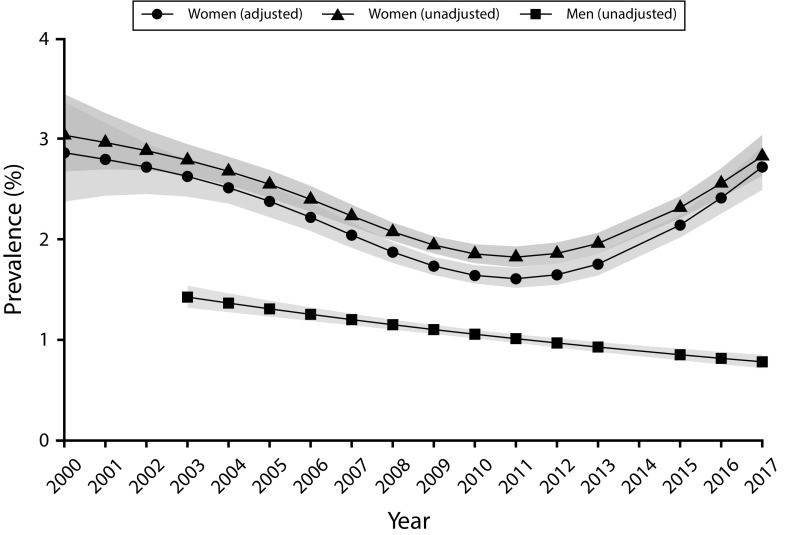
There was a U-shaped trend in model-estimated unadjusted and adjusted gonorrhea prevalence over the study period, and the prevalence was high overall. The unadjusted prevalence fell from 3.0% in 2000 to 1.8% in 2011 before rising to 2.9% in 2017 (Figure 1 and Figure F, available as a supplement to the online version of this article at http://www.ajph.org). After misclassification associated with different screening tests had been taken into account, the adjusted prevalence was slightly lower than the unadjusted prevalence, falling from 2.9% in 2000 to 1.6% in 2011 before rising to 2.7% in 2017 (a relative increase of nearly 70%).
FIGURE 1—
Adjusted and Unadjusted Gonorrhea Prevalence and 95% Confidence Intervals Among Women Entering the National Job Training Program From 2000 to 2017 and Unadjusted Prevalence Among Men From 2003 to 2017: United States
Note. Women, n = 191 991; men, n = 224 348. Adjusted prevalence estimates for women accounted for the use of increasingly sensitive and specific screening tests over time. Unadjusted prevalence estimates were not corrected for time-varying outcome misclassification. Prevalence estimates for men were not adjusted because highly sensitive and specific tests were used throughout the period of the study. No point estimates are shown for 2014 because screening data in that year were not available for analyses.
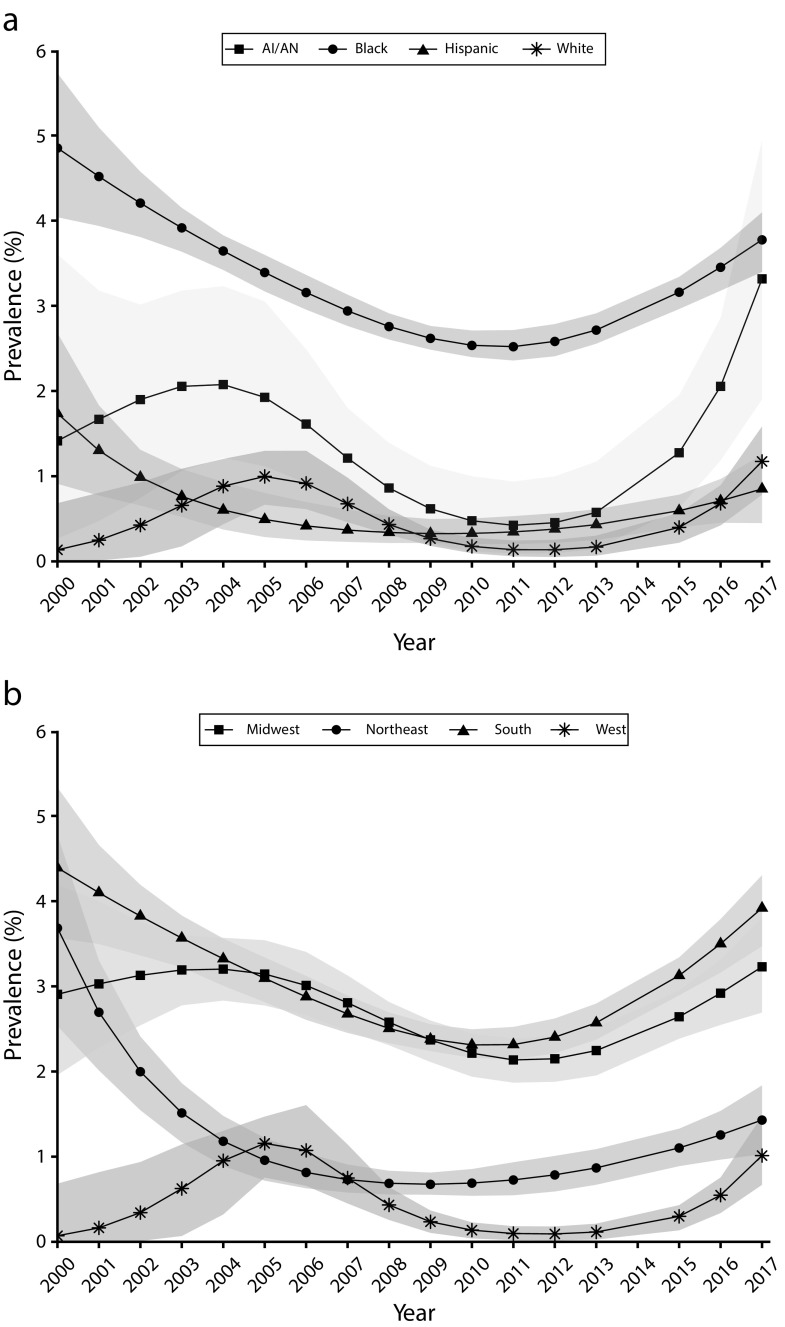
Gonorrhea prevalence trends among women varied by race/ethnicity, although the prevalence among all racial/ethnic groups rose steadily during 2011 to 2017. Non-Hispanic Black women had the highest gonorrhea prevalence across the study period, and the prevalence in this group followed a U-shaped curve. The adjusted prevalence among non-Hispanic Black women declined steadily from 4.9% to 2.5% during 2000 to 2011 and then rose steadily to 3.8% in 2017 (Figure 2). Adjusted prevalence trends among AI/AN women generally declined early on (reaching 0.4% in 2011) but climbed sharply after 2011 to 3.3% in 2017; however, estimates for these women were imprecise. The adjusted prevalence among Hispanic and non-Hispanic White women remained under 2% across all study years but began rising modestly in 2011.
FIGURE 2—
Adjusted Gonorrhea Prevalence Among Women Entering the National Job Training Program From 2000 Through 2017 by (a) Race/Ethnicity and (b) Region: United States
Note. AI/AN = American Indian/Alaska Native. n = 191 991. Prevalence estimates were adjusted for changes in screening test sensitivity and specificity.
There were regional differences in gonorrhea prevalence trends, with the prevalence being consistently higher among women residing in the South and Midwest than among those in the West and Northeast. The adjusted prevalence among women in the South and Midwest generally declined during 2000 to 2011 (from 4.4% to 2.3% in the South and from 2.9% to 2.1% in the Midwest) before increasing through 2017 (to 3.9% in the South and 3.2% in the Midwest; Figure 2). The adjusted prevalence among women in the Northeast and West was under 2% for most study years, although the adjusted prevalence in the Northeast declined sharply in early study years before leveling off.
Younger women 16 to 19 years of age had a higher prevalence of gonorrhea than did older women 20 to 24 years of age throughout the study period. During 2000 to 2011, the adjusted gonorrhea prevalence among younger women dropped from 3.4% to 1.9%, and the adjusted prevalence among older women dropped from 1.4% to 1.1% (Figure G, available as a supplement to the online version of this article at http://www.ajph.org). The prevalence in both age groups increased after 2011, rising to 3.2% among younger women and 1.9% among older women in 2017.
Sensitivity analyses examining random error influences on screening test sensitivity and specificity estimates showed that the adjusted prevalence from 2000 to 2010 was relatively stable when modeled with the lower 95% confidence limits of sensitivity and specificity estimates (Figure H, available as a supplement to the online version of this article at http://www.ajph.org). The adjusted prevalence decreased from 2000 to 2010 when modeled with the upper limits. The adjusted prevalence increased during 2011 to 2017 when modeled with both the lower and upper limits.
Gonorrhea Prevalence Among Men
During 2003 to 2017 (excluding 2014), 479 279 men were screened for gonorrhea or chlamydia (or both) by the national contract laboratory upon entering the NJTP. We excluded data from NJTP centers where less than 50% of men who were screened for chlamydia had gonorrhea test results (n = 160 902; 34%) to minimize bias from missing gonorrhea results. We further excluded men with missing or other race/ethnicity (n = 33 631; 11%) and missing data on region (n = 4515; 2%) and test result (n = 55 883; 20%). Our final sample included 224 348 men.
Most men were 16 to 19 years of age (66%), non-Hispanic Black (55%), and from the South (51%). Fewer than 1% reported symptoms. More than 99% of men were screened with the BD ProbeTec ET via a urine specimen (unadjusted prevalence: 1.1%), and the remainder (0.2%) were screened with the Gen-Probe PACE 2 (unadjusted prevalence: 5.6%). The prevalence among men decreased steadily over the study period, from 1.4% in 2003 to 0.8% in 2017 (Table 1, Figure 1, and Figure F).
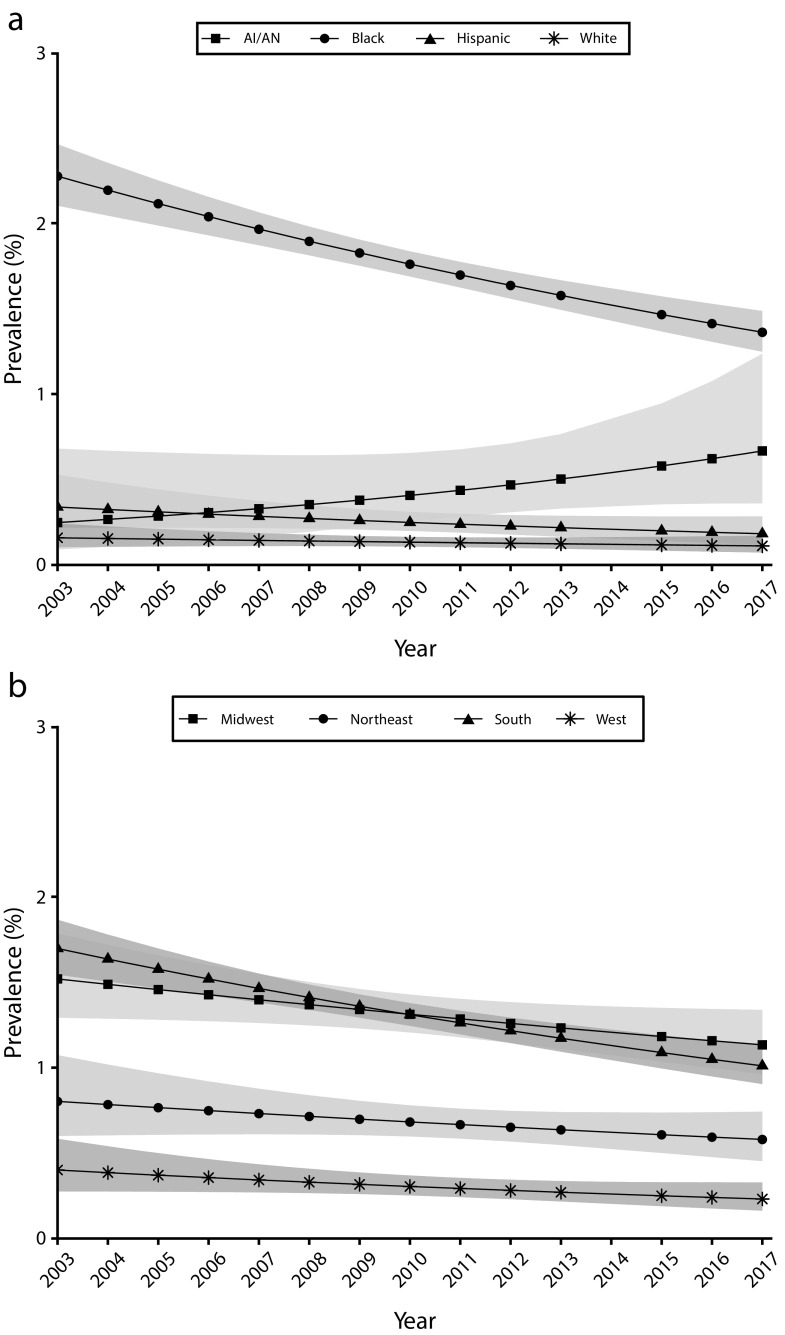
There were differences in prevalence trends by race/ethnicity; non-Hispanic Black men had the highest prevalence throughout the study period. The prevalence among non-Hispanic Black men decreased from 2.3% to 1.4% during 2003 to 2017, the prevalence among AI/AN men increased from 0.3% to 0.7%, and the prevalence among Hispanic and non-Hispanic White men was steady at approximately 0.2% and 0.1%, respectively (Figure 3).
FIGURE 3—
Unadjusted Gonorrhea Prevalence Among Men Entering the National Job Training Program From 2003 Through 2017 by (a) Race/Ethnicity and (b) Region: United States
Note. AI/AN = American Indian/Alaska Native. n = 224 348. Prevalence estimates were not adjusted for screening test accuracy because highly sensitive and specific tests were used throughout the period of the study.
The prevalence among men also differed by region and was highest in the South and Midwest. The prevalence in the South declined from 1.7% to 1.0%, whereas the prevalence in the Midwest declined more modestly from 1.5% to 1.1%. The prevalence in the Northeast and West was stable over the study period (at approximately 0.6% and 0.3%, respectively; Figure 3).
Differences in gonorrhea prevalence trends among men by age group were minor. The prevalence among men 16 to 19 years of age decreased from 1.5% to 0.7% during 2003 to 2017, whereas the prevalence among men 20 to 24 years of age hovered around 1.0% throughout the study period (Figure I, available as a supplement to the online version of this article at http://www.ajph.org).
DISCUSSION
Long-term gonorrhea prevalence trends from sentinel surveillance of the NJTP augment national case rate trends, which can be difficult to interpret as a result of biases that are challenging to measure and control. After accounting for time-varying misclassification due to imperfect screening tests among women and ruling out bias attributable to case mix, we found that the gonorrhea prevalence among women entering the NJTP declined during 2000 to 2011 before rising steadily to near 2000 levels in 2017. The prevalence among men entering the NJTP declined modestly during 2003 to 2017. Non-Hispanic Black enrollees and enrollees residing in the South and Midwest had the highest prevalence of gonorrhea throughout the study period.
The rising gonorrhea prevalence among women in the second half of our study period may point to a need for increased prevention efforts targeting economically disadvantaged young women. Increasing prevalence was observed among women overall and across race/ethnicity, region, and age categories. Increases among non-Hispanic Black and AI/AN women, women in the South and Midwest, and younger women are particularly concerning given that the prevalence in these groups is already high and the reproductive sequelae of gonorrhea can be severe for women. Continued monitoring of gonorrhea prevalence and the increasing trends in these groups will be important for understanding gonorrhea epidemiology among women, tailoring control efforts, and preventing adverse sequelae.
The gonorrhea prevalence among men was low and decreased over time. This low prevalence, particularly relative to women, is unsurprising because urogenital infections among men are more likely to be symptomatic and may have prompted men entering the NJTP to have sought treatment prior to enrollment. The decreasing trend among men appears to be driven by a declining prevalence among non-Hispanic Black men; the prevalence in this group was highest over the study period but decreased over time, whereas the prevalence in other racial/ethnic groups was lower but generally remained stable. The trend among non-Hispanic Black men is encouraging but unexpected because case rates among Black men have increased in recent years.1
In addition, the prevalence among non-Hispanic Black men was generally more than 10 times as high as the prevalence among non-Hispanic White men over the course of the study. This racial/ethnic disparity has been previously documented,1,2,6 but our results suggest a reduction in this disparity over time in the sentinel study population. Continued monitoring of prevalence by race/ethnicity within the NJTP and among other populations is needed to understand whether these trends will persist.
Our study is one of the first to our knowledge to examine long-term gonorrhea prevalence trends in the United States. Estimates from national probability surveys have been too imprecise and unstable as a result of small sample sizes and low gonorrhea prevalence to draw meaningful conclusions about trends.2 The NJTP has a sufficient sample size and prevalence to examine trends, and previously estimated trends for women and men entering the NJTP showed a decline over a 5-year period.6 Our analysis, which spanned 18 years for women and 15 years for men, showed that the gonorrhea prevalence among men declined throughout the study period and that the prevalence among women declined and then subsequently increased.
We also carefully examined bias due to case mix by considering temporal trends in factors previously associated with gonorrhea, including race/ethnicity, socioeconomic status, age, and region of residence.1,3 The NJTP entrance criteria for socioeconomic status and age were unchanged over the course of the study period, and we did not observe meaningful longitudinal variation in race/ethnicity or region. We could not assess whether other important characteristics, such as sexual behavior, changed over time.
Finally, we addressed outcome misclassification due to imperfect and changing screening tests separately for women and men to generate minimally biased prevalence estimates. For women, we conducted meta-analyses to estimate plausible screening test sensitivities and specificities. We used those estimates in an expectation-maximization algorithm incorporated into a logistic regression to account for misclassification. As a result, our prevalence estimates among women are slightly lower than were previously reported estimates.6 However, we were unable to account for any potential heterogeneity in sensitivity or specificity estimates by age. Misclassification among men was minimal and did not have a meaningful impact on point estimates or trend interpretations because nearly all men were screened with a highly accurate test.
Limitations
Our results are generalizable to young people of disadvantaged socioeconomic standing who may enter a job training program; however, our findings may not reflect trends in the general population. In addition, our study does not provide insight into why urogenital gonorrhea prevalence may be increasing among women or decreasing among men entering the NJTP. Sexual activity (e.g., age of sexual debut and condom use) and prevalence within one’s sexual network may be important influential factors, but data on these factors were not collected. Both sexual activity and condom use among adolescents have generally declined over the past decade, which may have influenced trends among NJTP enrollees.11 Further research into the relative contribution of changes in sexual behaviors to prevalence trends could help inform prevention interventions.
Changes in structural factors such as health care access and use may also affect prevalence, as women and men who access care are more likely to be screened and treated than are those who do not access care and thus are less likely to have prevalent infections. For example, asymptomatic women may be screened for STDs during wellness or prenatal care visits, and factors that influence these visits (e.g., changes in cervical cancer screening guidelines and increased contraception uptake) may also influence STD screening rates. Such a relationship was observed among women enrolled in Medicaid; a slowed increasing trend in chlamydia screening rates among women from 2004 through 2013 corresponded to a decrease in Papanicolaou testing and adolescent pregnancy.12 Access to screening among women and men is also limited by reductions in public funding for STD clinics, which may result in reduced clinical services or increased patient copays.13 Examining multiple factors that influence gonorrhea screening and prevalence trends is an important area for continued study.
In addition, our prevalence estimates are based on urogenital test results. Gonococcal infections can also occur in the pharynx and rectum, and our estimates likely underestimate the true burden of infection. This may be particularly relevant among men. Other sentinel surveillance data that include information on all anatomic sites suggest increasing diagnoses beginning in 2011 among men, primarily gay, bisexual, and other men who have sex with men.1,14,15 Modestly decreasing or stable trends in urogenital infections observed among male NJTP enrollees may not fully reflect trends in the gonorrhea epidemic in this sentinel population. Data from programs that screen all anatomic sites and collect information on sexual behaviors would be useful to better characterize the overall burden of gonorrhea and inform interpretation of trends.
Public Health Implications
Gonorrhea prevalence trends from sentinel surveillance may be subject to fewer biases than case rate trends, allowing for improved interpretation and understanding of changes in gonorrhea epidemiology. We examined long-term gonorrhea prevalence trends among NJTP enrollees. Diverging trends among men and women suggest that screening and prevention efforts may be adequate for men but may need to be targeted and strengthened to reverse the increasing prevalence among women in this high-risk sentinel population. There were also racial/ethnic and regional disparities in the present study population. Continued monitoring of trends within the NJTP and other high-risk populations will be helpful for understanding whether trends will persist.
ACKNOWLEDGMENTS
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
HUMAN PARTICIPANT PROTECTION
No protocol approval was needed for this study because data collection was determined to be part of routine surveillance and not research.
REFERENCES
- 1.Sexually Transmitted Disease Surveillance 2017. Atlanta, GA: Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 2.Torrone EA, Johnson RE, Tian LH et al. Prevalence of Neisseria gonorrhoeae among persons 14 to 39 years of age, United States, 1999 to 2008. Sex Transm Dis. 2013;40(3):202–205. doi: 10.1097/OLQ.0b013e31827c5a71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller WC, Ford CA, Morris M et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291(18):2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 4.Miller WC. Epidemiology of chlamydial infection: are we losing ground? Sex Transm Infect. 2008;84(2):82–86. doi: 10.1136/sti.2007.028662. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Labor. US Department of Labor Job Corps annual report. Available at: https://s3-us-west-2.amazonaws.com/jobcorps.gov/2017-04/Job_Corps-py08report.pdf. Accessed February 10, 2020.
- 6.Bradley H, Satterwhite CL. Prevalence of Neisseria gonorrhoeae infections among men and women entering the National Job Training Program—United States, 2004–2009. Sex Transm Dis. 2012;39(1):49–54. doi: 10.1097/OLQ.0b013e318231cd5d. [DOI] [PubMed] [Google Scholar]
- 7.Learner ER, Torrone EA, Fine JP et al. Chlamydia prevalence trends among women and men entering the National Job Training Program from 1990 through 2012. Sex Transm Dis. 2018;45(8):554–559. doi: 10.1097/OLQ.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Pol B, Ferrero DV, Buck-Barrington L et al. Multicenter evaluation of the BDProbeTec ET system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39(3):1008–1016. doi: 10.1128/JCM.39.3.1008-1016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaydos CA, Cartwright CP, Colaninno P et al. Performance of the Abbott RealTime CT/NG for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2010;48(9):3236–3243. doi: 10.1128/JCM.01019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146(2):195–203. doi: 10.1093/oxfordjournals.aje.a009251. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Youth Risk Behavior Survey data. Available at: https://www.cdc.gov/healthyyouth/data/yrbs/pdf/trendsreport.pdf. Accessed February 10, 2020.
- 12.Tao G, Kreisel K, Gift TL. Impact of significant decreases over time in the proportion of sexually active Medicaid women who had Papanicolaou testing or were pregnant on trends of overall chlamydia testing rates. Sex Transm Dis. 2018;45(11):e90–e93. doi: 10.1097/OLQ.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 13.Leichliter JS, Heyer K, Peterman TA et al. US public sexually transmitted disease clinical services in an era of declining public health funding: 2013–14. Sex Transm Dis. 2017;44(8):505–509. doi: 10.1097/OLQ.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson Jones ML, Chapin-Bardales J, Bizune D et al. Extragenital chlamydia and gonorrhea among community venue-attending men who have sex with men—five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(14):321–325. doi: 10.15585/mmwr.mm6814a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenger MR, Pathela P, Anschuetz G et al. Increases in the rate of Neisseria gonorrhoeae among gay, bisexual and other men who have sex with men—findings from the Sexually Transmitted Disease Surveillance Network 2010–2015. Sex Transm Dis. 2017;44(7):393–397. doi: 10.1097/OLQ.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]