Resident microglia are the primary source of myeloid cells present at β-amyloid deposits.
Abstract
In this issue of JEM, Reed-Geaghan et al. (https://doi.org/10.1084/jem.20191374) address the long-standing question about the primary source of myeloid cells located at β-amyloid deposits. Using genetic labeling experiments, the authors identify resident microglia as the only myeloid cells present at β-amyloid deposits.
Monocyte-derived macrophage (MDM) infiltration has been demonstrated after injuries and neurodegenerative diseases such as stroke (Planas, 2018) and multiple sclerosis (Ajami et al., 2011). However, a possible role for myeloid cell infiltration in Alzheimer’s disease (AD) has been intensively debated in light of divergent findings. In this issue of JEM, Reed-Geaghan et al. shed new light on the topic, demonstrating that resident microglia are the unique myeloid cell type at the site of β-amyloid deposition.

Insights from Nàdia Villacampa and Michael T. Heneka.
Microglia arise from progenitors in the yolk sac that populate the developing nervous system at embryonic stages. During adulthood, maintenance and local expansion of microglia are mostly due to self-renewal (Ajami et al., 2007). Due to their myeloid origin, microglia and MDMs are morphologically indistinguishable and share identification markers depending on their activation status. Microglia that are located at sites of β-amyloid deposition highly express CD45, which has been, up to now, the most commonly used criterion to differentiate infiltrating MDM (so-called CD45high) from resident microglia (associated with CD45low/med), e.g., by FACS. As such, this technique is prone to mistakes depending on the quality of staining performed. New tools and strategies, which help to distinguish resident microglia from MDM, regardless of their activation status, are therefore crucial for our understanding and to advance the field of neuroinflammation.
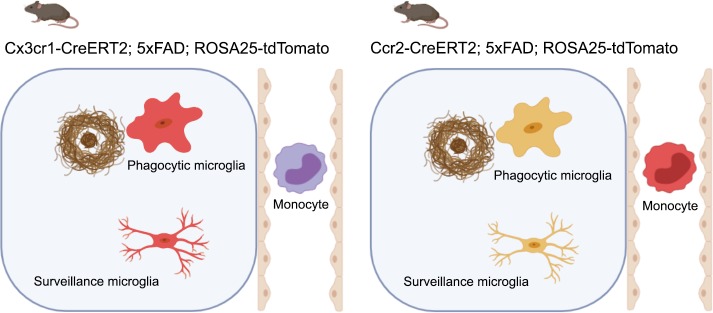
In this work, Reed-Geaghan et al. (2020) used genetic labeling strategies to accurately identify the ontogeny of myeloid cells associated with β-amyloid deposits. They employed selective expression of the fluorescent marker tdTomato via tamoxifen-inducible Cx3cr1-CreERT2 to exclusively label resident microglia in the brain, or Ccr2-CreERT2 to label circulating Ly6Chi monocytes, allowing for their identification between both cell types. Then, these transgenic lines were crossed into 5xFAD mice, a murine model of AD, where β-amyloid deposition rapidly starts at 2 mo of age. Previous attempts to describe the nature of myeloid cells located at β-amyloid deposits included the use of bone marrow chimeras, which, due to irradiation, led to substantial changes in the central nervous system (CNS) and increased “non-physiological” monocyte infiltration from peripheral sources (Mildner et al., 2011). In the present study, the authors definitively demonstrate that plaque-associated myeloid cells exclusively arise from brain-resident microglia in the absence of potentially confounding experimental conditions.
Schematic figure showing that cells around plaques and in brain parenchyma were tdTomato-positive using the resident microglial driver Cx3cr1-CreERT2, while using the monocyte driver Ccr2-CreERT2, no tdTomato cell is observed within the CNS. Created with BioRender.com.
Does this study close the case for MDM-based new therapeutic approaches? Former studies showed that these cells can have a protective role in AD. For example, adoptive transfer of monocytes from young wild-type mice reduced amyloid deposition and improved memory function in APPswDI mice (Hohsfield and Humpel, 2015). Using genetically engineered monocytes to express neprilysin decreased amyloid deposition in APP/PS1 mice (Lebson et al., 2010). In addition, monocytes overexpressing angiotensin-converting enzyme, another amyloid-β peptide degrading enzyme, led to reductions in soluble amyloid and to pronounced preservation of synaptic and cognitive function (Koronyo-Hamaoui et al., 2020). Although Reed-Geaghan et al. (2020) demonstrated no infiltration of MDM in nonmanipulated AD mice, one may argue that adoptive transfer of monocytes may have promoted infiltration of these cells in the CNS due to a transfer-related additional factor, which in case of whole-body irradiation most likely is the transient radiation-induced opening of the blood–brain barrier. In addition, one dimension of its beneficial effect could be due to the removal of vascular β-amyloid from within the lumen of veins (Michaud et al., 2013). This hypothesis could indeed be tested using the murine models employed in the present work. Although it remains unclear to date how peripheral MDMs exert a protective role in the context of AD without entering the brain parenchyma, blood enrichment with genetically altered monocytes remains a highly interesting therapeutic strategy in AD that should be further explored.
The next challenging step will be to identify the ontogeny of β-amyloid–associated myeloid cells in the human AD brain. In the era of RNA-sequencing methods, spatial transcriptomics may provide useful information to further decipher the nature of these cells (Salmén et al., 2018). These new techniques present several advantages: (i) frozen and fixed tissues can be used, almost a requisite in case of human sample collection; (ii) compared with single cell suspension techniques, spatial resolution is intact, and cells close and distant to the β-amyloid deposit can be analyzed; and (iii) as the final readout is the whole transcriptome, it is not required to identify RNA targets beforehand. Such strategies will not only help to identify the cells surrounding β-amyloid accumulations in the human brain, but also unravel their genetic signature and possibly key signal transduction pathways that can be targeted by future therapeutic strategies.
References
- Ajami B., et al. Nat. Neurosci. 2007 doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Ajami B., et al. Nat. Neurosci. 2011 doi: 10.1038/nn.2887. [DOI] [Google Scholar]
- Hohsfield L.A., and Humpel C. PLoS One. 2015 doi: 10.1371/journal.pone.0121930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronyo-Hamaoui M., et al. Brain. 2020 doi: 10.1093/brain/awz364. [DOI] [Google Scholar]
- Lebson L., et al. J. Neurosci. 2010 doi: 10.1523/JNEUROSCI.0329-10.2010. [DOI] [Google Scholar]
- Michaud J.P., et al. Cell Reports. 2013 doi: 10.1016/j.celrep.2013.10.010. [DOI] [Google Scholar]
- Mildner A., et al. J. Neurosci. 2011 doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [Google Scholar]
- Planas A.M. Stroke. 2018 doi: 10.1161/STROKEAHA.118.021474. [DOI] [Google Scholar]
- Reed-Geaghan E.G., et al. J. Exp. Med. 2020 doi: 10.1084/jem.20191374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmén F., et al. Nat. Protoc. 2018 doi: 10.1038/s41596-018-0045-2. [DOI] [PubMed] [Google Scholar]