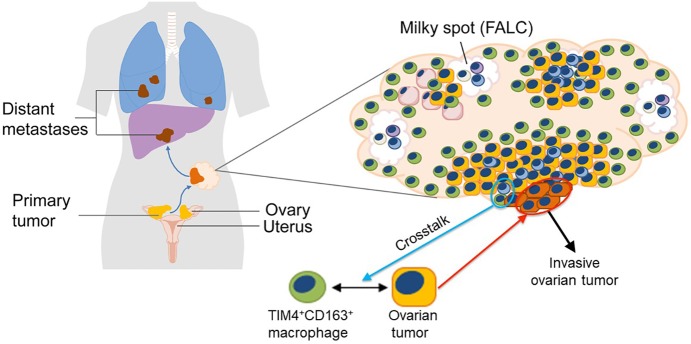
Omentum-resident macrophages of embryonic origin facilitate ovarian cancer metastasis
Abstract
The work by Etzerodt et al. in this issue of JEM (https://doi.org/10.1084/jem.20191869) identifies a distinct omentum-resident macrophage population of embryonic origin and demonstrates that these cells provide a niche for ovarian cancer metastasis and cancer stemness. This research opens up for many questions and therapeutic prospects.
In this issue of JEM, Etzerodt et al. report the identification of a unique subset of CD163+ Tim4+ tissue-resident macrophages (TRMs) in omentum that are maintained independently of bone marrow–derived monocytes and that have a causative role in the metastatic spread of ovarian cancer cells (OCCs) and in sustaining the ascitic population of cancer stem cells (CSCs).

Insights from Xiaojing Ma.
Ovarian cancer is progressively accompanied by the accumulation of malignant ascites in the peritoneal cavity containing large numbers of tumor-associated macrophages (TAMs) that are usually skewed to alternative polarization (M2) and that play an instrumental role in metastatic spread and therapy resistance (Reinartz et al., 2014; Yin et al., 2016). TAMs express cytokines, chemokines, growth factors, and proteases that can promote tumor growth, invasion, metastasis, immunosuppression (Okła et al., 2016), and cancer stemness (Ginestier et al., 2010). Therefore, infiltration of ovarian cancer with TAMs correlates well with disease progression and worse prognosis (Lan et al., 2013).
The dissemination of malignant ovarian cancer typically occurs in a “transcoelomic” process throughout the surfaces and organs of the abdominal and pelvic cavity covered by the peritoneum. The most frequent site for transcoelomic metastasis in high-grade serous ovarian cancer is the omentum, a specialized adipose tissue in the peritoneal cavity that is connected by a mesothelial layer to the spleen, stomach, transverse colon, and pancreas. Although it is primarily an adipose tissue, the omentum also contains clusters of leukocytes called “milky spots” consisting of T and B lymphocytes, macrophages, and dendritic cells that contribute to peritoneal immunity by collecting antigens, particulates, and pathogens from the peritoneal cavity and promoting a variety of immune responses, including inflammation, tolerance, and fibrosis. Crucially, the omentum collects metastasizing tumor cells and supports tumor growth by immunological and metabolic mechanisms in both mice and humans (Gerber et al., 2006; Rangel-Moreno et al., 2009). However, within the tumor microenvironment, TRMs and tumor-infiltrating TAMs display a high degree of phenotypic and ontogenetic heterogeneity. Particularly, the role of resident macrophages in tissue-specific tumor initiation and progression is unclear. Recent advances in molecular techniques that allow the fate-mapping of macrophages in vivo have revealed that most TRMs originate from embryonic precursors and can be maintained by local proliferation with little input from the bone marrow.
It is in this backdrop that Etzerodt et al. (2020) demonstrate here in a murine model of metastatic ovarian cancer (ID8) that there are four prominent groups of CD11b+, CD64+, F4/80+, CD169hi, Lyve+ macrophages in omentum, designated P1–P4, characterized by their expression level of CD163 and Tim4, i.e., CD163+ Tim4+ (P1), CD163+ Tim4− (P2), CD163−Tim4− (P3), and CD163− Tim4+ (P4). Using genetic fate-mapping and shielded chimera experiments, the researchers show that CD163+ Tim4+ omental resident macrophages are derived from embryonic progenitors and maintained in the omentum independently of bone marrow–derived monocytes, whereas the other three populations are most likely bone marrow derived and replenished from circulating monocytes. Further, the researchers show that omentectomy or specific depletion of CD163+ Tim4+ macrophages genetically or immunologically resulted in abrogated development of metastatic disease, whereas depletion of monocyte-derived CD163+ TIM4− TAMs had little impact on the invasiveness of the tumor. These studies suggest that omentum represents a critical premetastatic niche and that specific interactions between disseminated tumor cells and CD163+ Tim4+ TRMs in the omentum promotes the metastatic spread of ovarian cancer.
Ovarian cancer most frequently develops from the fallopian tube epithelium or ovarian surface epithelium. Tumor cells disseminate to the peritoneal cavity at early stages and most frequently colonize the omentum. Etzerodt et al. (2020) identify a subset of TRMs in omentum of embryonic origins that coexpress CD163 and Tim4. The study shows that these TRMs promote the malignant progression of OCCs and the acquisition of invasive properties in a paracrine manner leading to the metastatic spread of disease. The study further reveals that CD163+Tim4+ TRMs in omentum represent a critical premetastatic niche for OCCs and a potentially important therapeutic target to prevent disease progression. FALC, fat-associated lymphoid cluster. Adapted from Dr. T. Lawrence of King's College London.
Ascites-associated OCCs occur as single cells or multicellular spheroids and are likely to be responsible for peritoneal dissemination and to contribute to relapse of the disease (Liao et al., 2014). Further analyses by Etzerodt et al. (2020) showed that the acquisition of epithelial to mesenchymal transition (EMT) and CSC characteristics by ID8 cells, and the development of invasive disease, were dependent on the presence of CD163+ Tim4+ TRMs in the omentum. Not only did the specific depletion of the TRMs in Cd163-Csf1rDTR mice decrease the formation of malignant ascites, it also reduced the frequency of CSCs among the few ascitic tumor cells that did accumulate. Together, these studies add significantly to our understanding of TAM heterogeneity in the tumor microenvironment and the specific contribution of different macrophage subsets to disease progression.
It is somewhat perplexing that ID8 tumor progression had a severe effect on the number of the tumor-promoting TRMs in omentum (Fig. 5 A in Etzerodt et al., 2020). This was unexpected, since Zhu et al. (2017) showed in a genetically engineered orthotopic pancreatic ductal carcinoma (PDAC) model that pancreas-resident macrophages that originated from embryonic development expanded through in situ proliferation and promoted tumor progression (Zhu et al., 2017). Furthermore, colony stimulating factor 1 (CSF1) was more important for the survival of the embryonically derived macrophages than their bone marrow–derived counterparts. The reason for the difference in this aspect between the two tumor models is not obvious. It was noted that the majority of the Ki67+F4/80+ TRMs localized to fibrotic PDAC areas. Consistent with this observation, macrophages cultured on high-density collagen I gels had higher proliferation rates, suggesting that there could be a crosstalk between tumor fibrosis and macrophage proliferation, i.e., embryonically derived TRMs in PDAC tissues up-regulate proliferative programs, perhaps in response to fibrosis, to keep pace with tumor progression (Zhu et al., 2017). ID8 tumors, lacking strong fibrotic characteristics, may not transmit such proliferative signals to the omentum TRMs. If this is true, it would still leave the question unanswered: How are the omentum TRMs sustained during tumor progression? Another unresolved issue is what happens to distant metastasis to multiple vital organs within the abdomen following the depletion of CD163+ Tim4+ TRMs, since dissemination of OCCs, particularly high-grade serous ovarian cancer, can be attributed to both transcoelomic and hematogenous routes (Coffman et al., 2016; Pradeep et al., 2014). Obviously, one would also be interested in knowing the state of the T lymphocytes, particularly with respect to their activation status, in the omentum and peritoneum after the abolition of TRMs as increasing evidence suggests that T cell–mediated immunity is positively correlated with good prognosis in ovarian cancer patients (Santoiemma and Powell, 2015).
The work by Etzerodt et al. (2020) instinctively invites an immediate sequel: What is the molecular basis of the dialog between TRMs and OCCs leading to the up-regulation of JAK–STAT signaling, EMT, and CSC? RNA sequencing–based clustering analysis by the investigators revealed that the CD163+ Tim4+ omentum TRMs were enriched for genes associated with positive regulation of the JAK–STAT pathway. This included IL6, erythropoietin, and prolactin, all of which activate STAT3 and have been shown to promote EMT and the acquisition of CSC-like characteristics in ovarian cancer, whereas pathways related to angiogenesis, blood vessel development, and tissue remodeling were shared between the two CD163+ populations of omentum macrophages (P1 and P2). To definitively establish the role of the TRM-derived cytokines in orchestrating the CSC and metastatic activities of the OCCs in paracrine, experimental approaches such as adoptive transfer of TRMs that have been genetically or biochemically silenced in the expression of these cytokines can be considered.
One of the exciting prospects that this research has opened up to is the possibility to develop novel therapeutic strategies targeting omentum TRMs for ovarian cancer treatment since these resident cells play a vital role in establishing the metastatic forte and in conferring CSC-like capacity on ascitic OCCs. Matsui et al. (2010) developed a drug delivery system using oligomannose-coated liposomes, which were effectively taken up by mouse peritoneal macrophages to carry anticancer drugs to omental milky spots known as the initial metastatic sites in gastric cancer models. One could envision using this approach to deliver cytotoxic agents to reduce TRMs in ovarian cancer therapy. A similar but perhaps more “precision-guided” approach is to use a bi-specific blocking antibody, which binds to CD163 and Tim4 simultaneously on the same cell that coexpresses both molecules to target the omentum TRMs for elimination. Alternatively, the bi-specific antibody can target CD163 and CSF1R, which was avidly shown, using genetic tools, to be highly efficient in abrogating the TRMs and preventing disease progression (Fig. 6 in Etzerodt et al., 2020). Last but not least, since CSF1 is a critical survivor factor for the TRMs, targeted delivery of CSF1-antagonists can also be explored.
In summary, this work uncovers and highlights the importance of the unique subset of CD163+ Tim4+ omentum TRMs in ovarian cancer progression and provides some basic molecular understanding of the mechanisms in TRM-mediated metastasis and cancer stemness. It also points to the possibility of therapeutically targeting these cells and/or their molecular signaling pathways.
References
- Coffman L.G., et al. Transl. Res. 2016 doi: 10.1016/j.trsl.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzerodt A., et al. J. Exp. Med. 2020 doi: 10.1084/jem.20191869. [DOI] [Google Scholar]
- Gerber S.A., et al. Am. J. Pathol. 2006 doi: 10.2353/ajpath.2006.051222. [DOI] [Google Scholar]
- Ginestier C., et al. J. Clin. Invest. 2010 doi: 10.1172/JCI39397. [DOI] [Google Scholar]
- Lan C., et al. Technol. Cancer Res. Treat. 2013 doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- Liao J., et al. PLoS One. 2014 doi: 10.1371/journal.pone.0084941. [DOI] [Google Scholar]
- Matsui M., et al. Cancer Sci. 2010 doi: 10.1111/j.1349-7006.2010.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okła K., et al. Int. Rev. Immunol. 2016 doi: 10.1080/08830185.2016.1206097. [DOI] [PubMed] [Google Scholar]
- Pradeep S., et al. Cancer Cell. 2014 doi: 10.1016/j.ccr.2014.05.002. [DOI] [Google Scholar]
- Rangel-Moreno J., et al. Immunity. 2009 doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinartz S., et al. Int. J. Cancer. 2014 doi: 10.1002/ijc.28335. [DOI] [Google Scholar]
- Santoiemma P.P., and Powell D.J., Jr Cancer Biol. Ther. 2015 doi: 10.1080/15384047.2015.1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M., et al. J. Clin. Invest. 2016 doi: 10.1172/JCI87252. [DOI] [Google Scholar]
- Zhu Y., et al. Immunity. 2017 doi: 10.1016/j.immuni.2017.07.014. [DOI] [Google Scholar]