Abstract
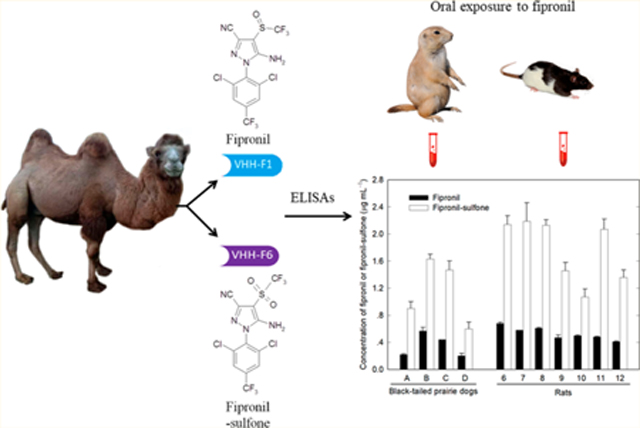
The insecticide fipronil can be metabolized to its sulfone in mammalian species. Two camel single-domain antibodies (VHHs) F1 and F6, selective to fipronil and fipronil-sulfone, respectively, were generated and used to develop enzyme linked immunosorbent assays (ELISAs) for the detection of the two compounds in the sera of black-tailed prairie dogs and rats. The limits of detection of fipronil and fipronil-sulfone in the rodent sera by the corresponding ELISAs were 10 and 30 ng mL−1, and the linear ranges were 30–1000 and 75–2200 ng mL−1. ELISAs showed a good recovery for fipronil and fipronil-sulfone cospiked in the control sera of the black-tailed prairie dogs (90–109%) and rats (93–106%). The VHH-based ELISAs detected fipronil and fipronil-sulfone in the sera of the rodents that received a repeated oral administration of fipronil. The average concentration of fipronil-sulfone was approximately 3.2-fold higher than fipronil in the prairie dog sera (1.15 vs 0.36 μg mL−1) and rat sera (1.77 vs 0.53 μg mL−1). ELISAs agreed well with a liquid chromatography–mass spectrometry method for the quantification of both fipronil and fipronil-sulfone in real serum samples. Fipronil-sulfone was identified as the predominant metabolite of fipronil in the black-tailed prairie dog and rat sera.
Graphical Abstract
Fipronil is a broad-spectrum phenylpyrazole insecticide which disrupts insect normal neural signal transmission by targeting the γ-aminobutyric acid (GABA)-gated chloride channel and at sufficient doses, causes excessive neural excitation, severe paralysis, and death of insects.1,2 Due to its effectiveness on a large number of insects, fipronil is an active ingredient in flea/tick control products for pets and home cockroach traps as well as field insect control for rice, corn, golf courses, and commercial turf. Nevertheless, there is an increasing concern about the possible off-target harm to ecosystems and human health associated with the indiscriminate use of fipronil.3,4 The 2017 fipronil eggs contamination is an incident in Europe and Asia involving the illegal use of fipronil in poultry farms.
Fipronil has recently shown promise as an oral drug in the control of insect blood-feeding on different animals, such as sand flies on roof rats,5 sand flies and mosquitos on cattle,6,7 fleas and ticks on rat,8,9 and fleas on great gerbil10 and black-tailed prairie dog.11,12 In this form of treatment fipronil can be applied at low concentrations with the route of administration posing less risk to nontarget insect and animal species. The predominant pathway of fipronil metabolism is S-oxidation into the corresponding sulfone which is mainly catalyzed by the CYP3A4 cytochrome P450 isoform.13 Fipronil was metabolized into fipronil-sulfone in several mammalian species such as rat,14 human,15 livestock species,16 and dog.17 Fipronil-sulfone was suspected to be more toxic than fipronil in different species.18,19 Furthermore, fipronil-sulfone persisted much longer in blood than fipronil itself.20,21 All these data on fipronil-sulfone highlight the need to pay more attention to the toxicological impact of this metabolite and better characterize fipronil metabolic pathways in different species.
Fipronil metabolism studies have been aided with well-established instrumental analytical techniques, such as gas chromatography–mass or tandem mass spectrometry (GC–MS or GC–MS/MS)16,22 and liquid chromatography–MS or tandem MS (LC–MS or LC–MS/MS),14,23–25 allowing precise simultaneous quantification of multianalytes. Recently, a simple, rapid and sensitive enzyme linked immunosorbent assay (ELISA) was developed to analyze fipronil and its metabolites in human urine and rat serum samples.26 Two polyclonal antibodies (pAbs) with different specificity to fipronil and its metabolites were produced. However, because both pAbs have high cross-reactivity (CR) with fipronil-sulfone (60–71%) compared to fipronil, the resulting assays do not discriminate well these two compounds and make their simultaneous quantification unavailable.26 For the purpose of developing an immunoassay discriminating between fipronil and fipronil-sulfone, antibodies with higher selectivity to the targets are essential.
Camelid single-domain antibodies (VHHs),27 also known as nanobodies, are of increasing interest in the analysis of small-molecule compounds from various matrices.28 VHHs have several advantages over conventional antibodies such as small size, high solubility, high thermal stability, and low-cost production.28–30 In addition, phage display technologies allow the potential selection of VHHs with novel characteristics that are not present in conventional antibodies. Although VHHs against a diverse group of environmental chemicals have been generated and used to develop sensitive immunoassays for a wide variety of applications,28,31–33 they have rarely been applied to the biomonitoring of pesticide metabolism.
There is great promise in the use of blood/serum-based biomarkers as indicators of adverse health outcomes in risk assessment, environmental exposure, and translational research. Fipronil-sulfone is the main metabolite of fipronil and plays a major role in the toxicological properties of fipronil in several mammals. Black-tailed prairie dogs are highly susceptible to sylvatic plague which is primarily maintained and transmitted in nature by fleas acting as vectors and rodents serving as the main hosts.34 Fipronil can potentially work as an oral systemic insecticide to reduce flea population density on black-tailed prairie dogs, and subsequently plague transmission, among mammalian hosts.11,12 Thus, the potential toxic effects of fipronil on prairie dogs are under investigation. The specific objective of this study was to generate VHHs selective to fipronil or fipronil-sulfone and develop sensitive VHH-based ELISAs to investigate the disposition of these compounds in the serum of rodents (prairie dog and rat) following a repeated oral administration of fipronil. This investigation would provide the first-hand information for understanding the serum metabolic fate of fipronil in prairie dogs as well as rats and assessing the fipronil safety on the rodents. Furthermore, the high throughput screening approach could be further developed for possible applications in human biomonitoring to evaluate the risks of human exposure to fipronil.
MATERIALS AND METHODS
Information about materials, chemicals, and reagents is detailed in the Supporting Information (SI).
Generation of VHHs against Fipronil and Fipronil-Sulfone.
The animal experiments were approved by the China Agricultural University Animal Care and Use Committee. The haptens of fipronil H1–H5 (Figure 1) were available from Vasylieva et al.26 H2 and H3 coupled to the carrier protein thyroglobulin (Thy) were used as immunogens. All haptens coupled with bovine serum albumin (BSA) and conalbumin (CON) were used as coating antigens. A three-year old male Bactrian camel was injected subcutaneously with an emulsified mixture of H2-Thy, H3-Thy, and Freund’s incomplete adjuvant five times biweekly (see Table S1). The procedure of construction of phage displayed VHH library is similar to that described by Kim et al.31 except that one more reverse primer was used in this study, 5′-CATGCCATGACTCGCGGCCGGCCTGGCCCTTGCATACTTCATTCGTTC-CTG-3′.
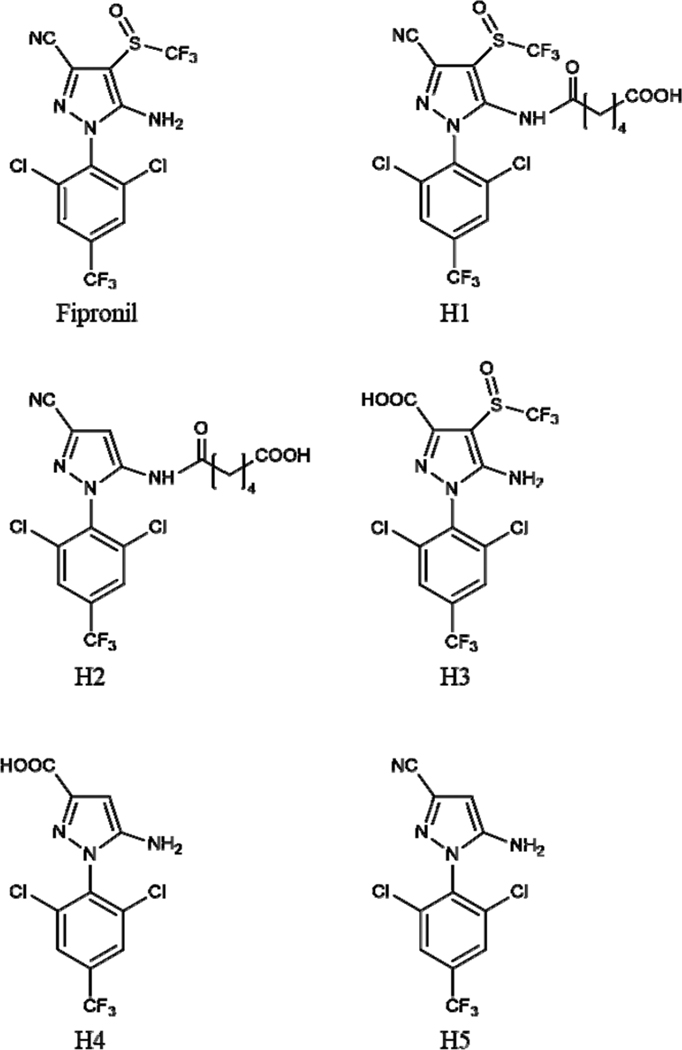
Figure 1.
Structures of fipronil and the haptens (H1–H5).
H2-BSA and H3-BSA were used as panning antigens to isolate VHHs against fipronil and fipronil-sulfone from the constructed library by the method detailed in the SI. Two optimal VHHs F1 and F6 specific for fipronil and fipronil-sulfone, respectively, were identified and used for the ELISA development according to the protocol available from our previous study.32
Selectivity of VHHs.
The selectivity of VHHs F1 or F6 was evaluated by determining the CR of ELISAs for fipronil, fipronil-sulfone and their structural analogs based on the half-maximum signal inhibition concentration (IC50). For VHH F1, CR (%) = [IC50(fipronil)/IC50(tested compound)] × 100. For VHH F6, CR (%) = [IC50(fipronil-sulfone)/IC50(tested compound)] × 100.
Serum Sample Preparation and Analysis.
Prairie dog sera were available from a trial for evaluating fipronil potential toxic effects on wild black-tailed prairie dogs (Cynomys ludovicianus) summarized in the SI. Sera were collected from 4 prairie dogs orally administrated with fipronil and 2 control prairie dogs that were untreated with fipronil. Rat sera from an investigation of the neurotoxic effects of fipronil in rodents were provided by Dr. Strynar from the United States Environmental Protection Agency (EPA). Details on rat experiments are available from McMahen et al.24 All sera were stored at −20 °C until analysis.
Control sera free of fipronil and its metabolites identified by LC–MS were used for the recovery and precision study. Equal amounts of fipronil and fipronil-sulfone were cospiked into serum samples with each compound at three levels: 100, 400, and 2000 ng mL−1. An aliquot of serum (50 μL) was diluted 50-fold with PBS to reach a volume of 2.5 mL, followed by the addition of 7.5 mL of ethyl acetate. The mixture was vortexed for 1 min and then ultrasonicated for 5 min. The ethyl acetate layer was collected (around 7.5 mL) and divided into 4.5 mL and 3 mL aliquots. The 4.5 mL aliquot was evaporated under a gentle stream of nitrogen and the residue was reconstituted in PBS (≥1.5 mL) containing 5% methanol. The extract was directly subjected to ELISAs. The 3 mL aliquot was evaporated in the same way as above and the residue was dissolved in 1 mL of methanol. This extract was passed through a 0.22-μm filter prior to LC–MS. The LC–MS conditions are detailed in Tables S2–S5.
RESULTS AND DISCUSSION
Selection of Haptens for Camel Immunization.
The procedures to elicit heavy chain antibodies (HCAbs) in camelids are, in principle, similar to those in use to raise conventional antibodies in other animals. In the generation of antibodies specific for small-molecules, it is generally accepted that a suitable hapten for immunization should preserve the main chemical features of the target compound. In a previous study,26 immunizing haptens (H1–H4) were designed to expose structural determinants CN, SOCF3 and NH2 of the fipronil molecule (Figure 1) to raise antibodies selective to these particular patterns of analyte. Two rabbit pAbs (#2265 and #2268) showing high binding affinity to fipronil and its metabolites were induced by immunogens H2-Thy and H3-Thy, respectively, whereas antibodies induced by H1-Thy and H4-Thy showed lower binding affinity to fipronil.
Considering the efficacy of haptens H2 and H3 in the production of rabbit pAbs, immunizing camel with these two compounds seems promising for inducing the HCAbs specific for fipronil and its metabolites. Camels have higher contents of HCAbs in the sera than those of llama or alpaca35 and thereby were selected for immunization. To stimulate a diverse range of immune response and make the immunization cost-effective as well, an individual camel was injected simultaneously with H2-Thy and H3-Thy for the generation of desired VHHs. After the fifth injection, the antisera showed a strong humoral response to the haptens H2 and H3 (titers ≥1.0 × 106) and high binding affinity to fipronil, with inhibitions ≥70% in the presence of 100 ng mL−1 fipronil (see Table S6).
Selection of Phage-VHH Clones for Fipronil and Fipronil-Sulfone.
The resulting library with a size of ∼1.8 × 108 colony forming units was estimated to contain VHHs selective to the particular determinants CN, SOCF3, and NH2 (Figure 1). H2-BSA was expected to capture VHHs selective to the CN group, while H3-BSA targeted to VHHs selective to the SOCF3 and NH2 groups. After the fourth round of panning, 34 clones in total showing strong binding affinity to H2-BSA or H3-BSA (OD > 1.5) in the absence of fipronil, and high inhibition (>50%) in the presence of 100 ng mL−1 fipronil by phage ELISAs were identified as positive clones. All positive clones were sequenced and only 8 clones with unique VHH sequences, 5 clones (F1–F5) panned from H2-BSA, and 3 clones (F6–F8) panned from H3-BSA, were identified (see Figure S1). The main features of VHHs that distinguish them from VHs of conventional IgGs are the occurrence of amino acids, F, E/G, R, and G/A in the framework region 2 (FR2) at positions 37, 44, 45, and 47, respectively. The variable domain complementarity determining region 3 (CDR3) possesses 17–23 amino acids. The canonical disulfide bond connects FR1 and FR3 in the two β-sheets of the immunoglobulin domain. A cysteine residue particularly in the CDR1 region and the cysteine residue in CDR3 probably form an additional disulfide bridge,36 which is helpful to keep the protein structure stable but may lead to the constraints of VHH expression and purification. According to the sequences of the hinge regions,27 clones F1, F2, F3, F6, and F7 belong to subfamily 3, whereas clones F4, F5, and F8 belong to subfamily 2.
Since the purpose of this study was to select VHHs specific to fipronil or fipronil-sulfone, the binding activity of each positive clone to these compounds was further evaluated by phage ELISAs (Table 1). It is noteworthy that clones panned from either H2-BSA or H3-BSA bound weakly to another. The clones F1–F5 showed higher sensitivity to fipronil than fipronil-sulfone while the clones F6–F8 showed reverse sensitivity. Among the various clone-coating antigen pairs, F1/H2-BSA and F6/H3-BSA exhibit the highest sensitivity to fipronil (IC50 = 1.8 ng mL−1) and fipronil-sulfone (IC50 = 4.5 ng mL−1), respectively.
Table 1.
Sensitivity of Positive Phage Clones to Fipronil and Fipronil-Sulfone by Phage ELISAs Based on the Coating Antigen H2-BSA or H3-BSA
| coating H2-BSA |
coating H3-BSA |
||||
|---|---|---|---|---|---|
| positive phage clones | IC50 of fipronil (ng mL−1) | IC50 of fipronil-sulfone (ng mL−1) | IC50 of fipronil (ng mL−1) | IC50 of fipronil-sulfone (ng mL−1) | |
| panned from H2-BSA | F1 | 1.8 | 10 | NDa | ND |
| F2 | 8.2 | 36 | ND | ND | |
| F3 | 12 | 60 | ND | ND | |
| F4 | 20 | 92 | ND | ND | |
| F5 | 15 | 67 | ND | ND | |
| panned from H3-BSA | F6 | ND | ND | 17 | 4.5 |
| F7 | ND | ND | 50 | 12 | |
| F8 | ND | ND | 48 | 10 | |
ND, not detectable due to OD < 0.3.
Screening Pairs of VHH and Coating Antigen.
VHHs F1 and F6 were expressed and purified for the development of VHH-based ELISAs. The size and purity of VHHs were verified on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with one major band at MW ≈ 15 kDa (see Figure S2). In competitive immunoassays the sensitivity of the assay can be greatly increased when the hapten in coating antigens is different from that in immunogens. Consequently, VHHs F1 and F6 were tested for the recognition of different haptens (H1–H5) conjugated with BSA or CON (see Table S7). F1 can recognize H1, H2, and H5 well, while F6 shows good recognition of H3 and modest recognition of H4. It is clear that F1 and F6 have different recognition patterns toward CN, SOCF3, and NH2 groups. The pairs of VHH/coating antigen showing good recognition were then tested in a full competitive ELISA for fipronil or fipronil-sulfone (see Table S8). Among the successful combinations of VHH/coating antigen, F1/H2-BSA and F6/H3-CON pairs have the highest sensitivity to fipronil (IC50 = 4.2 ng mL−1) and fipronil-sulfone (IC50 = 11 ng mL−1), respectively, with sufficiently high maximal-signal (A0) (see Table S8). Since F1 and F6 were panned from H2-BSA and H3-BSA, respectively, both F1/H2-BSA and F6/H3-CON were considered homologous.
Optimization of VHH-Based ELISAs.
The F1/H2-BSA assay for fipronil and the F6/H3-CON assay for fipronil-sulfone were optimized by evaluating the effects of methanol, pH and ionic strength (NaCl) on the values of IC50 and A0,26 prior to their application to real samples. As both assays showed similar pattern of response to the variables, only the procedure of the F1/H2-BSA ELISA optimization is detailed here. The F1/H2-BSA ELISA was significantly affected by methanol >5% (v/v) because the IC50 values increased dramatically (see Figure S3A). The optimal assay sensitivity to fipronil (IC50 = 4.4 ng mL−1; A0 = 1.26) was observed in the buffer containing 5% methanol. The binding capacity of F1 shifted obviously with the assay buffer pH increasing from 4.5 to 9.5 and the highest sensitivity to fipronil was observed at pH 7.5 (see Figure S3B). This assay had reasonable sensitivities to fipronil in 0.4–3.2% NaCl (w/v) with IC50 values varying in a narrow range of 3.9–7.4 ng mL−1 and the best sensitivity appeared in 0.8% NaCl (see Figure S3C).
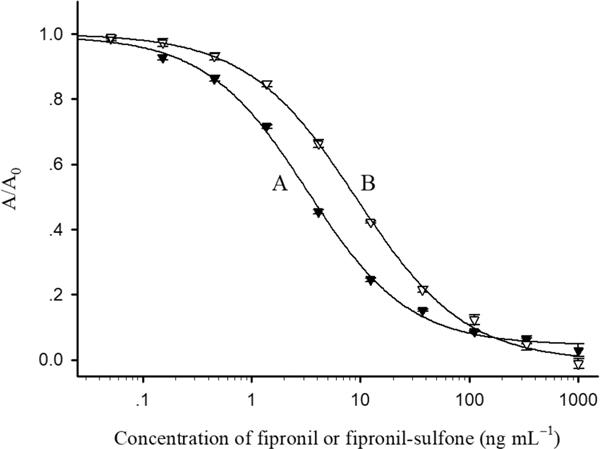
The typical calibration curves of tow ELISAs for fipronil and fipronil-sulfone were generated under the optimized conditions (PBS containing 5% methanol and 0.8% NaCl, pH 7.5) (Figure 2). The F1/H2-BSA ELISA for fipronil had an IC50 of 3.6 ng mL−1, a limit of detection (LOD, IC10) of 0.2 ng mL−1 and a linear range of 0.6–20 ng mL−1 (IC20–IC80), while the F6/H3-CON ELISA for fipronil-sulfone had an IC50 of 9.3 ng mL−1, a LOD of 0.6 ng mL−1 and a linear range of 1.5–44 ng mL−1. The sensitivities of the VHH-based ELISAs are slightly lower than those of conventional antibody-based immunoassays.26 This is because the antigen-binding site of camelid HCAbs or VHHs, with just three CDRs in their variable domains, has a more limited structural diversity than that of conventional antibodies.29 Moreover, unlike conventional antibodies, VHHs do not have the ideal surface, i.e., a pocket, in which a small-molecule can be bound with high affinity.37,38 Despite of the decrease of binding affinity of VHHs to fipronil and fipronil-sulfone compared with that of pAbs reported previously,26 the monoclonal nature of the VHHs enabled them to discriminate the two compounds.
Figure 2.
Calibration curves of the F1/H2-BSA ELISA for fipronil (A) and the F6/H3-CON ELISA for fipronil-sulfone (B) in PBS under the optimized conditions (5% methanol and 0.8% NaCl, pH 7.5). The concentrations of all VHHs and coating antigens were 0.1 and 0.3 μg mL−1, respectively. The data are the average of six replicates performed on different plates.
Selectivity of VHHs.
The selectivity of F1 and F6 was evaluated by comparing the IC50 values of fipronil and fipronil-sulfone with that of fipronil analogs, respectively (Table 2). F1 was panned by using hapten H2 linking to BSA through the amine moiety while exposing the CN group and missing the SOCF3 group. F1 showed high selectivity to the functional group CN in the compounds 2–6 (Table 2), giving CR in the range of 12–27% compared to fipronil (compound 1). Nonetheless, F1 had a lower CR (4.5%) with fipronil-hydroxy (compound 12) despite of the presence of CN. The OH substituent adjacent to CN might drastically reduce F1 binding affinity to fipronil-hydroxy, recently identified to be a new urinary biomarker of exposure to fipronil in rats.39 In contrast, compounds 7–11 missing the CN group poorly competed with the coating antigen H2-BSA, and their CRs were ≤2.5%. Compared with VHH F1, antisera #2265 derived from H2-Thy in the previous study demonstrated higher selectivity to CN, having CRs with compounds 2–6 in the range of 38–101%.26
Table 2.
CR of Two ELISAs for Fipronil, Fipronil-Sulfone, and Their Structural Analogs
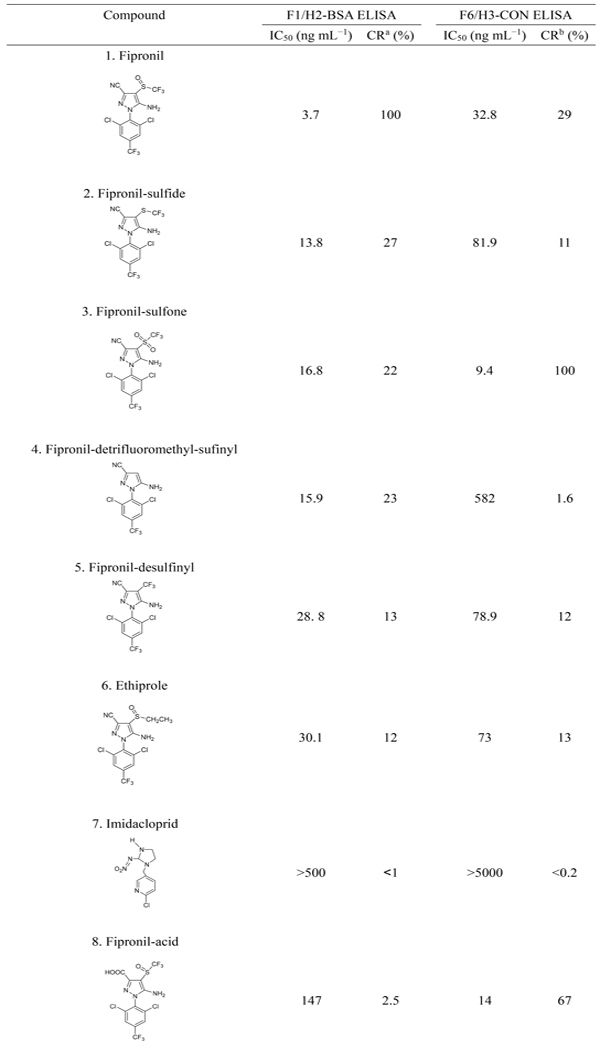 | |||
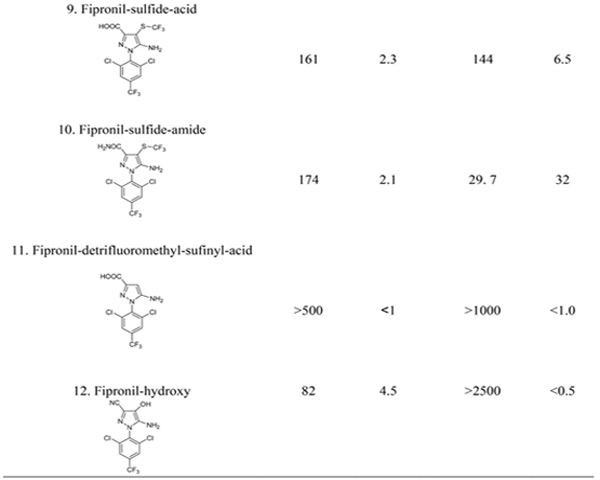 |
CR (%)= [IC50(fipronil)/IC50 (tested compound)] × 100
CR (%)= [IC50(fipronil-sulfone)/IC50 (tested compound)] × 100
The selectivity of VHH F6 to fipronil and the metabolites significantly differs from F1 (Table 2). F6 was panned with H3 (fipronil-acid) attached to BSA through CONH linkage while exposing the groups SOCF3 and NH2. Obviously, F6 showed greater selectivity to SO2CF3 than SOCF3, giving 29% CR with fipronil (compound 1) compared to fipronil-sulfone (compound 3). It is not surprising that this VHH had a CR of 67% with fipronil-acid (compound 8), working as both the immunizing and the panning hapten. The similarity of CONH in H3-BSA to CONH2 in compound 10 resulted in the high recognition of F6 to this compound (CR = 32%). In addition, F6 was partially selective to the substituent SCF3 (compounds 2 and 9), CF3 (compound 5), and SOCH2CH3 (compound 6), with CRs in a range of 6.5–13%. The substituent OH (compound 12) or missing SOCF3 (compound 4 and 11) was poorly recognized by F6.
These results suggest that panning haptens had a significant impact on the selectivity of VHHs. The resulting VHHs have different selectivity to fipronil and its metabolites from that of the pAbs #2265 and #2268, which showed high CRs of 60% and 71% with fipronil-sulfone compared to fipronil, respectively.26 Since the VHHs are more specific to fipronil and fipronil-sulfone than the conventional antibodies, they are suitable for the simultaneous quantification of these two compounds generally coexisting in the blood of rodents exposed to fipronil.
Matrix Effects.
The F1/H2-BSA and F6/H3-CON ELISAs were applied to quantify fipronil and fipronil-sulfone from rodent sera, respectively. Simple dilution is commonly used to minimize the matrix effect on immunoassays. However, due to the complex serum matrix, significant interference was still observed from the serum of both prairie dogs and rats after 100-fold dilution by PBS (data not shown). Therefore, simple extraction was conducted for the serum samples and the effect of matrix on assay performance was evaluated with the extracts of control sera. Prairie dog and rat serum matrices had similar effects on the competition curve. When assays were performed in extract solutions constituted by adding ≥1.5 mL of PBS (5% methanol) into the residue, the sensitivity did not change dramatically in comparison to that of the assay performed in PBS (5% methanol) without matrix (see Figure S4, shown as prairie dog serum extract only), indicating minimal matrix effects. After the pretreatment of sera via 50-fold dilution, solvent extraction and reconstitution of extract residue in at least 1.5 mL of PBS, the F1/H2-BSA ELISA for fipronil in sera has an IC50 of 180 ng mL−1, a LOD of 10 ng mL−1 and a linear range of 30–1000 ng mL−1; and the F6/H3-CON ELISA for fipronil-sulfone in sera has an IC50 of 465 ng mL−1, a LOD of 30 ng mL−1 and a linear range of 75–2200 ng mL−1.
Recovery of Fipronil and Fipronil-Sulfone from Sera.
To mimic the real world samples, fipronil and fipronil-sulfone were simultaneously added into control sera for the recovery study. Because F1 and F6 recognize fipronil and fipronil-sulfone with different affinity (Table 2), the results by the F1/H2-BSA or the F6/H3-CON ELISA are actually the combined responses of two compounds and could be reported as fipronil and fipronil-sulfone equivalents. The levels of fipronil and fipronil-sulfone equivalents could be converted to their real individual concentrations according to the response of VHHs to fipronil and fipronil-sulfone by multiplying each compound concentration by the value of CR and then summing up. Namely the levels of fipronil equivalent by the F1/H2-BSA ELISA = fipronil concentration × 100% + fipronil-sulfone concentration × 22%; and the levels of fipronil-sulfone equivalent by the F6/H3-CON ELISA = fipronil concentration × 29% + fipronil-sulfone concentration × 100%. The equations can be transformed as the following: fipronil concentation = fipronil equivalent by ELISA × 1.068–fipronil-sulfone equivalent by ELISA × 0.235; and fipronil-sulfone concentation = fipronil-sulfone equivalent by ELISA × 1.068–fipronil equivalent by ELISA × 0.31 (Table 3). On the basis of the corrected concentrations, the recoveries of fipronil and fipronil-sulfone in prairie dog sera by ELISAs were in a range of 90–103% and 97–109%, respectively, and in rat sera, 95–106% and 93–101%, respectively (Table 3). The precision of two ELISAs for fipronil and fipronil-sulfone were satisfactory, with coefficients of variation (CVs) of 1.8–7.9% and 1.1–7.2%, respectively.
Table 3.
Recovery of Fipronil and Fipronil-Sulfone Co-spiked in Control Sera Determined by ELISAs
| levels spiked in sera (ng mL−1) |
levels detected by ELISAa (ng mL−1), n = 3, mean ± SD |
recovery (CV)d, % |
||||
|---|---|---|---|---|---|---|
| species | fipronil | fipronil-sulfone | fipronil equivalents (fipronil concentration)b |
fipronil-sulfone equivalents (fipronil-sulfone concentration)c |
fipronil | fipronil-sulfone |
| black-tailed prairie dog | 100 | 100 | 127(103) ± 6.5 | 139(109) ± 7.2 | 103 (5.1) | 109 (5.2) |
| 400 | 400 | 459(369) ± 11 | 517(410) ± 17 | 92 (2.1) | 102 (3.6) | |
| 2000 | 2000 | 2232(1805) ± 55 | 2462(1937) ± 36 | 90 (2.8) | 97 (1.1) | |
| rat | 100 | 100 | 128(106) ± 10 | 132(101) ± 9.7 | 106 (7.9) | 101 (7.2) |
| 400 | 400 | 476(387) ± 12 | 517(405) ± 13 | 97 (2.5) | 101 (2.5) | |
| 2000 | 2000 | 2309(1901) ± 38 | 2406(1854) ± 31 | 95 (1.8) | 93 (1.2) | |
Fipronil and fipronil-sulfone levels were detected by the F1/H2-BSA ELISA and the F6/H3-CON ELISA, respectively.
Fipronil concentation = Fipronil equivalent by ELISA × 1.068 – Fipronil-sulfone equivalent by ELISA × 0.235.
Fipronil-sulfone concentation = Fipronil-sulfone equivalent by ELISA × 1.068 – Fipronil equivalent by ELISA × 0.31.
Coefficient of variation.
In another series of experiments, a LC–MS method was conducted to analyze the same samples as the above. Fipronil and fipronil-sulfone concentrations determined by LC–MS were close to theoretical spiked values, showing recoveries of 86–94% and 87–101% from prairie dog sera, respectively; and 81–97% and 86–98% from rat sera, respectively (see Table S9).
Analysis of Real Sample.
Serum samples from 6 prairie dogs and 12 rats were first analyzed by LC–MS to identify fipronil and the possible metabolites in CR study (Table 2). Neither fipronil nor its metabolites were detectable in the sera of control animals. In the sera of animals dosed with fipronil, except for unmetabolized fipronil, no additional metabolites other than fipronil-sulfone were found (see Table S10). It is notable that the concentrations of fipronil-sulfone were approximately 3.5-fold higher than those of fipronil, showing an average of 1.13 vs 0.32 μg mL−1 in prairie dog sera and 1.75 vs 0.49 μg mL−1 in rat sera. The results are consistent with those of previous studies.14,24
As described in the SI, the 4 fipronil-treated prairie dogs were indexed to have consumed 1.92–3.46 mg (<di>x</di> = 2.64 ± 0.73 mg) of fipronil and appeared in good health during the 5day trial. Remarkably different concentrations of fipronil or fipronil-sulfone were observed in the sera of animals with similar treatment (see Table S10), i.e., prairie dog pairs A/B and C/D, most probably due to the difference of fipronil consumption and metabolism of individuals. There are no data on the metabolic pathway of fipronil in prairie dog available from the literature. The preliminary results of this test suggested fipronil-sulfone was the predominant metabolite in the serum of this species.
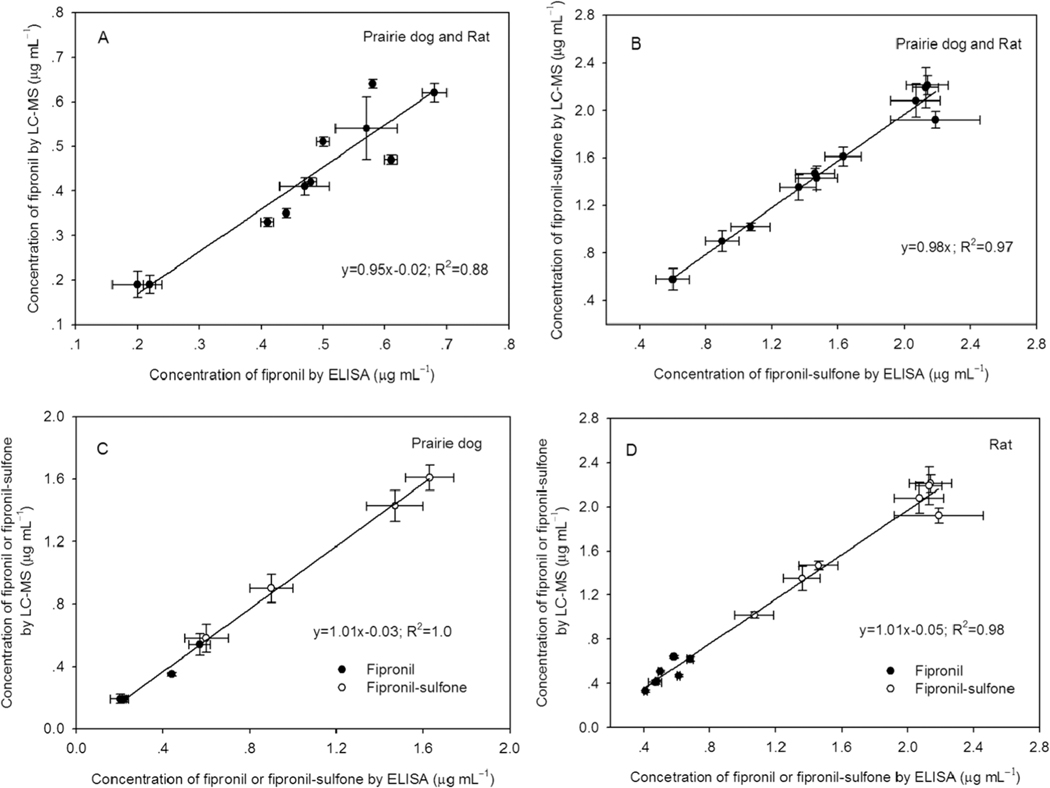
Subsequently, two ELISAs were employed to quantify fipronil and fipronil-sulfone in the 18 serum samples. Both fipronil and fipronil-sulfone equivalents were found in the sera of fipronil-dosed rodents but not in the control sera (see Table S10). As expected, ELISAs gave higher levels of fipronil and fipronil-sulfone equivalents than those of fipronil and fipronil-sulfone by LC–MS because compounds are cross-reactive in both ELISAs. For further comparison of two types of methods, fipronil and fipronil-sulfone equivalents by ELISAs were converted to their individual concentrations by the way mentioned previously (see Table S10). The concentrations of fipronil-sulfone by the F6/H3-CON ELISA are approximately 3.2-fold higher than those of fipronil by the F1/H2-BSA ELISA, showing an average of 1.15 vs 0.36 μg mL−1 in prairie dog sera and 1.77 vs 0.53 μg mL−1 in rat sera, which are in good agreement with the results from LC–MS. ELISAs demonstrated satisfactory correspondence to the LC–MS technique while two types of methods showed a better correlation for the quantification of fipronil-sulfone (R2 = 0.97) than for fipronil (R2 = 0.88) (Figure 3, A and B). An excellent correlation was observed in different matrices as the R2 values were 1.0 and 0.98 in prairie dog sera and rat sera, respectively (Figure 3, C and D). The VHH-based ELISAs appear to be a promising method for quantification of fipronil and fipronil-sulfone in serum with a simple sample preparation, thus reducing analysis time, especially in the case of a large screening campaign, and reducing the cost of analysis.
Figure 3.
Correlation between ELISA and LC-MS for fipronil and fipronil-sulfone in real serum samples. (A) fipronil in sera of black-tailed prairie dogs and rats; (B) fipronil-sulfone in sera of black-tailed prairie dogs and rats; (C) fipronil and fipronil-sulfone in sera of black-tailed prairie dogs; and (D) fipronil and fipronil-sulfone in sera of rats.
CONCLUSIONS
Two resulting VHH-based ELISAs demonstrated high efficiency in monitoring fipronil and fipronil-sulfone in the sera of black-tailed prairie dogs and rats. Fipronil-sulfone was initially confirmed by LC–MS as the unique metabolite in the sera of the rodents dosed with fipronil and the metabolite concentration was higher than the parent fipronil. For the first time fipronil-sulfone was identified as the major metabolite of fipronil in the serum of black-tailed prairie dogs. These results were reproduced by the VHH-based ELISAs. Both the ELISAs and LC–MS produced comparable results for fipronil and fipronil-sulfone in real serum samples, whereas ELISAs showed advantages of high efficiency and cost-effectiveness over LC–MS. Compared to pAb-based ELISAs with broad selectivity for fipronil and its metabolites,26 VHH-based ELISAs are more suitable for discrimination detection of fipronil and fipronil-sulfone in sera of rodents exposed to fipronil.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the Key Project of Intergovernmental International Scientific and Technological Innovation Cooperation, 2016YFE0108900, the National Key Research and Development Program of China, 2016YFD0800606, and the National Institute of Environmental Health Sciences Superfund Research Program, P42ES04699, U.S.A. Research on black-tailed prairie dogs was supported by U.S. Fish and Wildlife Service. The photos of black-tailed prairie dog and rat were provided by Dr. Dean Biggins from USGS and Dr. Strynar from EPA, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The findings and conclusions in this article are those of authors and do not necessarily represent the views of U. S. Fish and Wildlife Service.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.8b04653.
Reagents, selection of VHHs against fipronil and fipronil-sulfone, oral administration of fipronil for black-tailed prairie dogs, collection of black-tailed prairie dog serum, analysis of fipronil and its metabolites by LC–MS-MRM, ELISA data, and additional references (Tables S1–S10, Figures S1–S4) (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Gant DB; Chalmers AE; Wolff MA; Hoffman HB; Bushey DF Rev. Toxicol 1998, 2 (1), 147–156. [Google Scholar]
- (2).Aajoud A; Ravanel P; Tissut M J. Agric. Food Chem 2003, 51 (5), 1347–1352. [DOI] [PubMed] [Google Scholar]
- (3).Tingle CCD; Rother JA; Dewhurst CF; Lauer S; King WJ Rev. Environ. Contam. Toxicol 2003, 176, 1–66. [DOI] [PubMed] [Google Scholar]
- (4).Bonmatin J-M; Giorio C; Girolami V; Goulson D; Kreutzweiser DP; Krupke C; Liess M; Long E; Marzaro M; Mitchell EAD; Noome DA; Simon-Delso N; Tapparo A Environ. Sci. Pollut. Res 2015, 22 (1), 35–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ingenloff K; Garlapati R; Poché D; Singh MI; Remmers JL; Poché RM Med. Vet. Entomol 2013, 27 (1), 10–18. [DOI] [PubMed] [Google Scholar]
- (6).Poché RM; Garlapati R; Singh MI; Poché DMJ J. Med. Entomol 2013, 50 (4), 833–837. [PubMed] [Google Scholar]
- (7).Poché RM; Burruss D; Polyakova L; Poché DM; Garlapati RB Malar. J 2015, 14, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Leirs H; Larsen KS; Lodal J Med. Vet. Entomol 2001, 15 (3), 299–303. [DOI] [PubMed] [Google Scholar]
- (9).Borchert JN; Poche RM US Patent 2011, 7943 (160), B2. [Google Scholar]
- (10).Poché DM; Torres-Poché Z; Yeszhanov A; Poché RM; Belyaev A; Dvořák V; Sayakova Z; Polyakova L; Aimakhanov B PLoS Neglected Trop. Dis 2018, 12 (7), No. e0006630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Poché DM; Hartman D; Polyakova L; Poché RM J. Vector Ecol 2017, 42 (1), 171–177. [DOI] [PubMed] [Google Scholar]
- (12).Eads DA; Biggins DE; Bowser J; Broerman K; Livieri TM; Childers E; Dobesh P; Griebel RL Vector-Borne Zoonot. 2018. [DOI] [PubMed] [Google Scholar]
- (13).Tang J; Usmani KA; Hodgson E; Rose RL Chem.-Biol. Interact 2004, 147 (3), 319–329. [DOI] [PubMed] [Google Scholar]
- (14).Lacroix MZ; Puel S; Toutain PL; Viguié C J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 2010, 878 (22), 1934–1938. [DOI] [PubMed] [Google Scholar]
- (15).Mohamed F; Senarathna L; Percy A; Abeyewardene M; Eaglesham G; Cheng R; Azher S; Hittarage A; Dissanayake W; Sheriff MH; Davies W; Buckley NA; Eddleston M J. Toxicol., Clin. Toxicol 2004, 42 (7), 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Le Faouder J; Bichon E; Brunschwig P; Landelle R; Andre F; Le Bizec B Talanta 2007, 73 (4), 710–717. [DOI] [PubMed] [Google Scholar]
- (17).Hainzl D; Cole LM; Casida JE Chem. Res. Toxicol 1998, 11 (12), 1529–1535. [DOI] [PubMed] [Google Scholar]
- (18).Roques BB; Lacroix MZ; Puel S; Gayrard V; Picard-Hagen N; Jouanin I; Perdu E; Martin PG; Viguié C Toxicol. Sci 2012, 127 (1), 29–41. [DOI] [PubMed] [Google Scholar]
- (19).Romero A; Ramos E; Ares I; Castellano V; Martínez M; Martínez-Larrañaga MR; Anadón A; Martínez MA Toxicol. Lett 2016, 252, 42–49. [DOI] [PubMed] [Google Scholar]
- (20).Leghait J; Gayrard V; Toutain PL; Picard-Hagen N; Viguié C Toxicol. Lett 2010, 194 (3), 51–57. [DOI] [PubMed] [Google Scholar]
- (21).Cravedi JP; Delous G; Zalko D; Viguié C; Debrauwer L Chemosphere 2013, 93 (10), 2276–2283. [DOI] [PubMed] [Google Scholar]
- (22).Wu J; Lu J; Lu H; Lin Y; Wilson PC Sci. Total Environ 2015, 518–519, 139–147. [DOI] [PubMed] [Google Scholar]
- (23).He S-W; Zhao Y-G; Zhang Y; Jin M-C; Zhu YJ Chromatogr. A 2018, 1553, 16–24. [DOI] [PubMed] [Google Scholar]
- (24).McMahen RL; Strynar MJ; Dagnino S; Herr DW; Moser VC; Garantziotis S; Andersen EM; Freeborn DL; McMillan L; Lindstrom AB Environ. Int 2015, 78, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).McMahen RL; Strynar MJ; McMillan L; DeRose E; Lindstrom AB Sci. Total Environ 2016, 569–570, 880–887. [DOI] [PubMed] [Google Scholar]
- (26).Vasylieva N; Ahn KC; Barnych B; Gee SJ; Hammock BD Environ. Sci. Technol 2015, 49 (16), 10038–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hamers-Casterman C; Atarhouch T; Muyldermans S; Robinson G; Hammers C; Songa EB; Bendahman N; Hammers R Nature 1993, 363 (6428), 446–448. [DOI] [PubMed] [Google Scholar]
- (28).Bever CS; Dong J-X; Vasylieva N; Barnych B; Cui Y; Xu Z-L; Hammock BD; Gee SJ Anal. Bioanal. Chem 2016, 408 (22), 5985–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gonzalez-Sapienza G; Rossotti MA; Rosa S. T.-d. Front. Immunol 2017, 8, 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ingram JR; Schmidt FI; Ploegh HL Annu. Rev. Immunol 2018, 36, 695–715. [DOI] [PubMed] [Google Scholar]
- (31).Kim HJ; Mccoy MR; Majkova Z; Dechant JE; Gee SJ; Rosa S. T.-d.; González-Sapienza GG; Hammock BD. Anal. Chem 2012, 84 (2), 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Wang J; Bever CS; Majkova Z; Dechant JE; Yang J; Gee SJ; Xu T; Hammock BD Anal. Chem 2014, 86 (16), 8296–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zhang Y-Q; Xu Z-L; Wang F; Cai J; Dong J-X; Zhang J-R; Si R; Wang C-L; Wang Yu.; Shen Y-D; Sun Y-M; Wang H. Anal. Chem 2018, 90 (21), 12886–12892. [DOI] [PubMed] [Google Scholar]
- (34).Gage KL; Kosoy MY; Roelle JE; Miller BJ; Godbey JL; Biggins DE Recent Trends in Plague Ecology 2006, 213–232. [Google Scholar]
- (35).Blanc MR; Anouassi A; Abed MA; Tsikis G; Canepa S; Labas V; Belghazi M; Bruneau G Biotechnol. Appl. Biochem 2009, 54 (4), 207–212. [DOI] [PubMed] [Google Scholar]
- (36).Wesolowski J; Alzogaray V; Reyelt J; Unger M; Juarez K; Urrutia M; Cauerhff A; Danquah W; Rissiek B; Scheuplein F; et al. Med. Microbiol. Immunol 2009, 198 (3), 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Fanning SW; Horn JR Protein Sci. 2011, 20 (7), 1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Makvandi-Nejad S; Fjallman T; Arbabi-Ghahroudi M; MacKenzie CR; Hall JC J. Immunol. Methods 2011, 373 (1–2), 8–18. [DOI] [PubMed] [Google Scholar]
- (39).Vasylieva N; Barnych B; Wan D; El-Sheikh E-SA; Nguyen HM; Wulff H; McMahen R; Strynar M; Gee SJ; Hammock BD Environ. Int 2017, 103, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.