To the Editor: During the time of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, questions arise regarding patients being treated with immunomodulatory therapies. In particular, is there an increased risk of acquiring the infection or experiencing a worse outcome from SARS-CoV-2?
Although this exact question is presently unanswerable, we can look at safety data from clinical trials to try to understand patient susceptibility to different infections. Others have addressed this in the context of biologic1 or classical small molecule therapy,2 but the risk of Janus kinase inhibitor (JAKi) treatment has not been addressed. In light of the growing off-label use of JAKis in dermatology in addition to pharmaceutical industry-sponsored clinical trials of JAKis for alopecia areata, atopic dermatitis, vitiligo, and other conditions, dermatologists need data to better understand the risks of JAKi treatment so they can best manage and counsel their patients during this unique time.
We analyzed and collated adverse events data from JAKi clinical trials. In particular, we focused on infections and pulmonary toxicities observed across the different United States Food and Drug Administration-approved JAKi for their Food and Drug Administration-approved indications. When available, data from phase II or III clinical trials for dermatologic indications was included. Table I summarizes the rates of various infections, including upper respiratory infections, nasopharyngitis, and influenza, for JAKi-treated groups vs placebo groups. Overall, rates of infectious events are only mildly increased in JAKi-treated patients. We also collated pulmonary toxicities of JAKis to identify potential risks of worsening severe respiratory disease from SARS-CoV-2, and such toxicities are all but absent.
Table I.
Rate of infections with Janus kinase inhibitors in randomized, double-blind, placebo-controlled trials over 8 to 24 weeks' duration
| JAK inhibitor specificity | JAK inhibitor name | DX | Treatment groups (No. patients) | Total infections, No. (%) | Serious infections, No. (%) | URI, No. (%) | UTI, No. (%) | NP, No. (%) | HSV, No. (%) | Zoster, No. (%) | Influenza, No. (%) | Reported pulmonary toxicity∗ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JAK1/3 | Tofacitinib | RA | Placebo (n = 122) Tofacitinib 5 mg bid (n = 243) Tofacitinib 10 mg bid (n = 245) Study duration 12 weeks (Fleischmann et al, N Engl J Med, 2012;367(6):495-507.) |
NR | 0 0 1 (<1) |
6 (5) 11 (5) 8 (3) |
3 (3) 4 (2) 10 (4) |
2 (2) 4 (2) 5 (2) |
NR | One case | 4 (3) 2 (1) 4 (2) |
CR: PAH (Habib et al, J Clin Rheumatol, 2018.) |
| UC | OCTAVE 1 Placebo (n = 122) Tofacitinib 10 mg bid (n = 476) (∼45%-50% on steroids) Study duration 8 weeks OCTAVE 2 Placebo (n = 112) Tofacitinib 10 mg bid (n = 429) (∼45%-50% on steroids) Study duration 8 weeks (Sandborn et al, N Engl J Med, 2017;376(18):1723-1736.) |
19 (16) 111 (23) 17 (15) 78 (18) |
0 6 (1) 0 1 (<1) |
NR NR |
0 0 0 0 |
9 (7) 34 (7) 4 (4) 21 (5) |
NR NR |
1 (<1) 3 (<1) 0 2 (<1) |
NR NR |
|||
| JAK1/2 | Baricitinib | AD | BREEZE-AD1 Placebo (n = 249) Baricitinib 1 mg qd (n = 127) Baricitinib 2 mg qd (n = 123) Baricitinib 4 mg qd (n = 125) Study duration 16 weeks BREEZE-AD2 Placebo (n = 244) Baricitinib 1 mg qd (n = 124) Baricitinib 2 mg qd (n = 123) Baricitinib 4 mg qd (n = 123) Study duration 16 weeks (Simpson et al, Br J Dermatol, March 2020, bjd.18898.) |
NR NR |
NR NR |
6 (2) 1 (1) 3 (2) 4 (3) 5 (2) 6 (5) 5 (4) 4 (3) |
4 (2) 1 (1) 2 (2) 4 (3) 3 (1) 0 0 2 (2) |
26 (10) 22 (17) 12 (10) 12 (10) 30 (12) 13 (11) 16 (13) 10 (8) |
3 (1) 7 (6) 4 (3) 9 (7) 11 (5) 6 (5) 7 (6) 5 (4) |
1 case Blinded 2 cases blinded |
NR NR |
NR |
| RA | RA-BEAM phase 1 Placebo (n = 488) Baricitinib 4 mg qd (n = 487) Adalimumab 40 mg q2wk (n = 330) (100% on MTX) Study duration 24 weeks (Taylor et al, N Engl J Med, 2017;376(7) 652-662.) |
134 (27) 176 (36) 110 (36) |
7(1) 5 (1) 2 (<1) |
14 (3) 15 (3) 13 (4) |
0 1 (<1) 0 |
0 1 (<1) 0 |
NR |
2 (<1) 7 (1) 4 (1) |
4 (<1) 12 (2) 5 (2) |
|||
| Ruxolitinib | MF | COMFORT I Ruxolitinib 15 or 20 mg bid (n = 155) Placebo (n = 151) Study duration 24 weeks (Verstovsek et al, J Hematol Oncol, 2017;10 (1):55.) |
NR |
NR |
34 (22) 15 (10) |
31 (20) 7 (5) |
14 (9) 9 (6) |
6 (4) 2 (1) |
16 (10) 1 (1) |
8 (5) 0 |
CR: ARDS (Kerget et al, Respir Med Case Rep, 2017;22:243-245; †Herman et al, Ann Am Thorac Soc, 2014;11(7):1145-1148; †Beauverd, Samii, Int J Hematol, 2014;100(5):498-501.) †CR: Pleural effusion (Tefferi and Pardanani, Mayo Clin Proc, 2011;86(12):1188-1891.) ‡CR: PAH (Low et al, Haematologica, 2015;100(6):e244-245.) |
|
| JAK1 | Upadacitinib | AD | Placebo (n = 40) Upadacitinib 7.5 mg qd (n = 42) Upadacitinib 15 mg qd (n = 42) Upadacitinib 30 mg qd (n = 42) Study duration 16 weeks (Guttman-Yassky et al, J Allergy Clin Immunol, 2020;145(3):877-884.) |
8 (20) 22 (52) 18 (43) 17 (41) |
0 2 (5) 1 (2) 0 |
4 (10) 7 (17) 5 (12) 5 (12) |
NR | 1 (3) 2 (5) 4 (10) 3 (7) |
NR | 0 0 0 0 |
0 3 (7) 0 0 |
NR |
| RA | SELECT-BEYOND Placebo (n = 169) Upadacitinib 15 mg qd (n = 164) Upadacitinib 30 mg qd (n = 165) (∼70% of all groups on MTX, 50% on steroids) Study duration 12 weeks (Genovese et al, Lancet, 2018;391(10139):2513-2124.) |
51 (30) 54 (33) 55 (33) |
0 1 (1) 4 (2) |
13 (8) 13 (8) 10 (6) |
10 (6) 15 (9) 9 (5) |
11 (7) 7 (4) 9 (5) |
NR |
1 (1) 1 (1) 4 (2) |
0 0 0 |
NR |
AD, Atopic dermatitis; ARDS, acute respiratory distress syndrome; bid, twice daily; CR, case report; DX, diagnosis; HSV, herpes simplex virus; JAK, Janus kinase; MF, myelofibrosis; MTX, methotrexate; NP, nasopharyngitis; NR, not reported; PAH, pulmonary arterial hypertension; q2wk, every other week; qd, once daily; RA, rheumatoid arthritis; URI, upper respiratory infection; UTI, urinary tract infection; Zoster, varicella-zoster virus.
Indicates adverse events as a result of abrupt discontinuation of Janus kinase therapy.
Exacerbation of pre-existing condition.
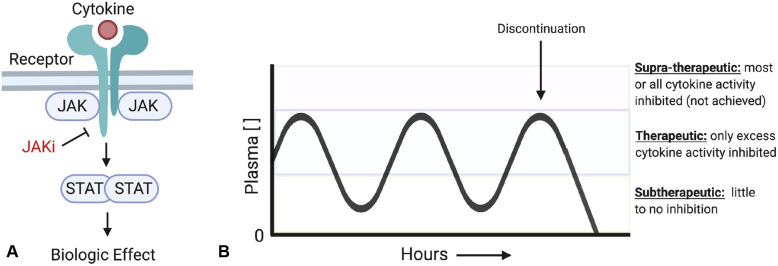
To understand the infection data, an understanding of the mechanism and pharmacokinetics of JAKis is helpful (Fig 1 , A). Cytokines can drive autoimmunity when their activity is exaggerated. JAKis, which are taken orally 1 to 2 times per day, largely impact pathogenically elevated cytokine activity, with relative sparing of normal cytokine activity because drug concentrations are subtherapeutic for part of the day (Fig 1, B).3 Therefore, the immune response to infection is grossly intact.
Fig 1.
Janus kinase (JAK) inhibitors (JAKi) block the activity of cytokines. (A) Greater than 50 cytokines signal via the JAK-signal transducer and activator of transcription proteins (STAT) pathway and rely entirely on the kinase activity of JAK proteins to transmit their signals. JAK inhibitors block the activity of activated JAK proteins downstream of cytokine receptor signaling and thus prevent downstream activation of STAT proteins. (B) JAK inhibitors are oral medications dosed 1 to 2 times per day. The levels of drug in the plasma fluctuate throughout the day. During peak plasma levels a portion, but not all, of a particular cytokine's activity is inhibited. In practice, in this therapeutic range, pathologically elevated cytokine activity is targeted while normal cytokine function is relatively spared. Throughout the day, the plasma concentration is also frequently subtherapeutic. The specific range varies for individual cytokines and the specificity of the JAK inhibitor. Upon cessation of the drug, the effect dissipates rapidly.
Discontinuation of JAKis in the setting of initial infection, such as with SARS-CoV-2, may be beneficial given the role of JAK-signal transducer and activator of transcription proteins (STAT)-dependent type I (α/β) and type II (γ) interferons in antiviral immunity. The biologic effects of JAKis dissipate rapidly with cessation of the drug, given their short half-lives. The potential role of JAKi treatment for patients with cytokine release syndrome of severe SARS-CoV-2 infection is more complex and an area of active investigation.
While anecdotal, we are aware of 3 patients (2 women and 1 man) in their 20s in our care who are taking JAKis for alopecia areata, of whom 2 have tested positive for SARS-CoV-2, and 1 very likely has it (per symptoms). All 3 have had uneventful courses and are recovering after cessation of treatment.
In this time of the SARS-CoV-2 pandemic, we must be as informed as possible regarding the risks of the treatments we prescribe our patients. Of course, shared decision making reigns supreme, but without data we, as physicians, will be unable to provide our patients the guidance they rely on us for.
Footnotes
Funding sources: This work was supported by the Ranjini and Ajay Poddar Fund for Dermatologic Diseases Research (Dr King). Dr Damsky is supported by the Dermatology Foundation.
Conflicts of interest: Dr Damsky has received research funding from Pfizer, but it did not support this work, and is a consultant for Eli Lilly. Dr King is an investigator for Concert Pharmaceuticals Inc, Eli Lilly and Company, and Pfizer Inc, is a consultant to and/or has served on advisory boards for Aclaris Therapeutics, Arena Pharmaceuticals, Bristol-Meyers Squibb, Concert Pharmaceuticals Inc, Dermavant Sciences, Eli Lilly and Company, and Pfizer Inc, and is on speaker's bureau for Pfizer Inc, Regeneron, and Sanofi Genzyme. Dr Peterson has no conflicts of interest to declare.
IRB approval status: Not applicable.
Reprints not available from the authors.
References
- 1.Lebwohl M., Rivera-Oyola R., Murrell D.F. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82(5):1217–1218. doi: 10.1016/j.jaad.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price K.N., Frew J.W., Hsiao J.L., Shi V.Y. COVID-19 and immunomodulator/immunosuppressant use in dermatology. J Am Acad Dermatol. 2020;82(5):e173–e175. doi: 10.1016/j.jaad.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark J.D., Flanagan M.E., Telliez J.-B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57(12):5023–5038. doi: 10.1021/jm401490p. [DOI] [PubMed] [Google Scholar]