Abstract
Purpose
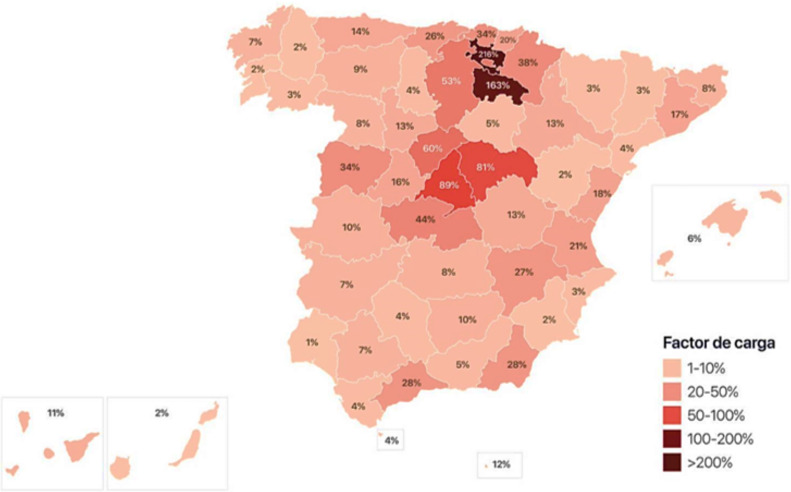
Community transmission of SARS-CoV-2 was detected in Spain in February 2020, with 216% intensive care unit (ICU) capacity expanded in Vitoria by March 18th, 2020.
Methods
We identified patients from the two public hospitals in Vitoria who were admitted to ICU with confirmed infection by SARS-CoV-2. Data reported here were available in April 6th, 2020. Mortality was assessed in those who completed 15-days of ICU stay.
Results
We identified 48 patients (27 males) with confirmed SARS-CoV-2. Median [interquartile range (IQR)] age of patients was 63 [51–75] years. Symptoms began a median of 7 [5–12] days before ICU admission. The most common comorbidities identified were obesity (48%), arterial hypertension (44%) and chronic lung disease (37%). All patients were admitted by hypoxemic respiratory failure and none received non-invasive mechanical ventilation. Forty-five (94%) underwent intubation, 3 (6%) high flow nasal therapy (HFNT), 1 (2%) extracorporeal membrane oxygenation (ECMO) and 22 (46%) required prone position. After 15 days, 14/45 (31%) intubated patients died (13% within one week), 10/45 (22%) were extubated, and 21/45 (47%) underwent mechanical ventilation. Six patients had documented super-infection. Procalcitonin plasma above 0.5 μg/L was associated with 16% vs. 19% (p = 0.78) risk of death after 7 days.
Conclusion
This early experience with SARS-CoV-2 in Spain suggests that a strategy of right oxygenation avoiding non-invasive mechanical ventilation was life-saving. Seven-day mortality in SARS-CoV-2 requiring intubation was lower than 15%, with 80% of patients still requiring mechanical ventilation. After 15 days of ICU admission, half of patients remained intubated, whereas one third died.
Keywords: COVID-19, Pneumonia, Procalcitonin, ARDS
1. Introduction
Since the initial identification of SARS-CoV-2 infections in Wuhan, it has been important to identify characteristics beyond China with implications in management [1], [2]. Reports describing intensive care unit (ICU) patients with SARS-CoV-2 out of China are still limited [3], [4]. The clinical course of adult inpatients in Wuhan has been reported [5] with a high mortality rate and a risk of death above 90% in presence of high (0.05 μg/L) procalcitonin (PCT) plasma [5]. Because most patients in China and Italy received non-invasive ventilation (NIV), information is needed on patients following a strategy of early intubation without inducing potential ventilator-induced lung injury. Moreover, in view of the limitation of resources, it is also important to improve insight on 7-day mortality and identify different phenotypes for personalised management [6]. In Vitoria (capital of Alava), a city in the Basque Country that experienced the acceleration curve before other Spanish provinces (in relation to a massive contagion in a funeral) with 216% ICU capacity being already effective on March 18th, 2020 (see comparison with Madrid (89%), Barcelona (17%) and other provinces in Fig. 1 ), anticipating the effect in other regions.
Fig. 1.
Percentages of ICU beds occupied on March 18th, 2020 in Spain.
This present study aimed at reporting the epidemiology of the first patients admitted with SARS-CoV-2 in Spanish ICUs, twenty days after the first admission in a hospital located at ground zero (Fig. 1) in Spain. Secondary objectives were: to compare with the first ICU admissions during the 2009 influenza pandemic in Spain and the first reported series of ICU patients with SARS-Cov-2 in Wuhan and USA. Because of mortality reports in Wuhan [5] suggesting a close association, we assessed correlation between PCT plasma at ICU admission and 7-day mortality.
2. Methods
2.1. Study design
All consecutive SARS-CoV-2 adult patients (≥ 18 years old) from the University Hospital Araba (Vitoria, Spain) between March 4th, 2020 and March 31th, 2020 were included. All patients that required hospitalisation with a COVID-19 diagnosis according to WHO interim guidance [7] were included. Patients were followed until ICU discharge or death from March 4th, 2020 (first patient admission) to March 31th, 2020. All clinical data were collected prospectively by the investigators. PCT plasma levels samples were obtained within 24 ICU admissions.
Non-invasive ventilation was not indicated in this cohort. Intubation was clinically indicated in presence of respiratory alkalosis with progressive hyperventilation when delivering high oxygen concentrations.
2.2. Data collection
Basic epidemiological, clinical, laboratory, microbiology, treatment, and outcome data were extracted (HB) and standardised in a CRF, modified from the ISARIC CRF. The study protocol was approved by the IRB and informed consent was waived (ref. 2020-022). Patient data were censored at 15 days of follow up, on April 6th, 2020 and survival data were estimated at 7-day and 15-day after ICU admission.
2.3. Laboratory procedures
Clinical specimens for SARS-CoV-2 identification were obtained in accordance with Centres of Disease Control guidelines. Methods for laboratory confirmation of SARS-CoV-2 polymerase chain reaction (PCR) were performed at the hospital laboratory.
2.4. Definitions
A confirmed case was defined by a positive result on a reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay of a nasopharyngeal swab or respiratory specimen. Comorbidities were identified from hospital charts. Definitions used in this article have been reported elsewhere [8].
2.5. Biomarkers measurement
Procalcitonin plasma levels were determined with the Assay Alinity I ®, Abbott, EEUU. The assay has a detection limit of 0.02 μg/L with a probability of 95%, sensitivity of 0.06 μg/L (upper-reference-range 0.5 μg/L in healthy subjects). Determination of PCT plasma levels was performed within 24 hours after ICU admission.
2.6. Statistical analysis
Considering the rapid spread of the COVID-19 pandemic, the aim of the study was to report a fast overview of the situation in one of the first cities to be impacted by the outbreak. Therefore, no sample size was calculated.
Continuous variables were described as medianswith interquartile range (IQR) or mean with standard deviation (SD), and categorical variables were presented as counts and percentages. Pairwise comparisons for categorical variables were performed by using the Pearson's χ2 test. Comparisons between continuous variables used Student‘s t-test and Mann–Whitney test according to their distribution. Statistical significance was considered if the P-value was less than 0.05. Statistical analyses were performed with SPSS Statistics version 25.0 software (IBM).
Association between survival and PCT plasma levels was estimated using Kaplan–Meier curves. Hazard ratios (HR) and 95% confidence intervals (CI) were computed using the long rank test. Statistical significance was considered if the P-value was less than 0.01. Statistical analyses were performed with GraphPad prism 6 software.
3. Results
From March 4th to March 24th, 2020, 48 patients with laboratory-confirmed COVID-19 infection (median age 63 years [51–75], 27 males, no pregnant women) were admitted and included. No patients were admitted from a nursing home. Patient characteristics are shown in Table 1 . Obesity (48%), arterial hypertension (44%) and chronic lung disease (38%) were the three most associated comorbidities, whereas diabetes mellitus, hypothyroidism and history of tobacco use were reported in 19% of admissions.
Table 1.
Baseline characteristics of the population with confirmed SARS-CoV-2, compared with series in Seattle (Arentz et al.), Wuhan (Yang et al.) and pandemic 2009 influenza in Spain (Rello et al.).
| Study | Current study Spain | Arentz et al., 2020 USA [13] | Yang et al., 2020 China [11] | Rello et al., 2009 Spain [4] |
|---|---|---|---|---|
| Illness | SARS-CoV-2 pneumonia | SARS-CoV-2 pneumonia | SARS-CoV-2 pneumonia | Influenza A (H1N1) |
| Total patients with confirmed illness | 48 | 21 | 52 | 32 |
| Age, mean (SD or range) | 63 (12) | 70 (43–92) | 60 (13) | 40 (14) |
| Sex | ||||
| Male | 27 (56%) | 11 (52%) | 35 (67%) | 21 (66%) |
| Female | 21 (44%) | 10 (48%) | 17 (33%) | 11 (34%) |
| Days from onset symptoms to ICU admission, median (IQR) | 7 (5–12) | – | 10 (7–13) | 3 (2–6) |
| APACHE II score, mean (SD) | 15 (5) | – | 17 (1) | 14 (6) |
| SOFA score, mean (SD) | 7 (3) | – | – | 7 (3) |
| Signs and symptomsa | ||||
| Fever | 48 (100%) | 11 (52%) | 51 (98%) | 706 (96%) |
| Cough | 35 (74%) | 11 (48%) | 40 (77%) | 647 (88%) |
| Dyspnoea | 42 (88%) | – | 33 (63%) | - |
| Malaise | 21 (44%) | – | 18 (35%) | 221 (30%) |
| Myalgia | 2 (4%) | – | 6 (12%) | 508 (69%) |
| Headache | – | – | 3 (6%) | 434 (59%) |
| Rhinorrhea | – | – | 3 (6%) | – |
| Vomiting | – | – | 2 (4%) | – |
| Arthralgia | – | – | 1 (2%) | – |
| Chest pain | – | – | 1 (2%) | – |
| Sore throat | – | – | – | 427 (58%) |
| Sudden onset symptoms | – | – | – | 338 (46%) |
| Shortness of breath | – | 17 (76%) | – | – |
| Treatment | ||||
| Antibacterial agents | 42 (88%) | – | 49 (94%) | 32 (100%) |
| beta-lactams plus fluoroquinolones | – | – | – | 20 (63%) |
| beta-lactams plus macrolides | – | – | – | 6 (19%) |
| beta-lactams plus linezolid | – | – | – | 5 (16%) |
| levofloxacin | 17 (35%) | – | – | 1 (3%) |
| ceftriaxone | 22 (46%) | – | – | – |
| azithromycin | 10 (21%) | – | – | – |
| linezolid | 9 (19%) | – | – | – |
| other beta-lactams | 15 (31%) | – | – | – |
| Antiviral agents | 45 (94%) | – | 23 (44%) | 21 (66%) |
| Oseltamivir standard dose (75 mg bid) | – | – | 18 (35%) | 32 (100%) |
| Oseltamivir high dose (150 mg bid) | – | – | – | 10 (31%) |
| Ganciclovir | – | – | 14 (27%) | – |
| Lopinavir | – | – | 7 (14%) | – |
| Kaletra | 45 (94%) | – | – | – |
| Others | – | |||
| Steroids | 17 (35%) | – | 30 (58%) | 11 (34%) |
| Immunoglobulin | - | – | 28 (54%) | – |
| Tocilizumab | 2 (4%) | – | – | – |
| Chloroquine | 45 (94%) | – | – | – |
| Interferon | 41 (85%) | – | – | |
| Vasoconstrictor agents | – | 14 (67%) | 18 (35%) | 20 (63%) |
| Renal replacement therapy | – | – | 9 (17%) | 7 (22%) |
| Prone position ventilation | – | 8 (50%) | 6 (12%) | 8 (33%) |
| MV | 45 (94%) | 15 (71%) | 37 (71%) | 24 (75%) |
| Invasive | – | – | 22 (42%) | 16 (67%) |
| Non-invasive | – | – | 29 (56%) | 8 (33%) |
| HFNC | – | – | 33 (64%) | – |
| ECMO | 1 (2%) | – | 6 (12%) | – |
| Comorbidities/Complications | ||||
| Obesity | 23 (48%) | – | – | 10 (31%) |
| BMI 30 to 40 | 15 (31%) | – | – | 6 (19%) |
| BMI > 40 | 7 (15%) | – | – | 4 (13%) |
| Arterial hypertension | 21 (44%) | – | – | 1 (3%) |
| Hyperglycaemia | – | – | 18 (35%) | – |
| Acute kidney injury | – | – | 15 (29%) | – |
| Liver dysfunction | – | – | 15 (29%) | – |
| Cardiac injury | – | – | 12 (23%) | – |
| HAP | – | – | 7 (13%) | – |
| Gastrointestinal haemorrhage | – | – | 2 (4%) | – |
| Pneumothorax | – | – | 1 (2%) | – |
| Bacteremia | – | – | 1 (2%) | – |
| Urinary tract infection | 1 (2%) | – | ||
| Diabetes mellitus | 9 (19%) | 7 (33%) | – | – |
| Smoker | 9 (19%) | – | – | 1 (3%) |
| Hypothyroidism | 9 (19%) | – | – | – |
| Heart disease | 5 (10%) | – | – | – |
| Immunosuppression | 3 (6%) | 3 (14%) | – | 1 (3%) |
| Asthma | – | 2 (9%) | – | – |
| COPD | 18 (38%) | 7 (33%) | – | 5 (16%) |
| Pregnancy | – | – | – | 4 (13%) |
| Chronic renal failure | – | – | – | 2 (6%) |
| HIV | – | – | – | 1 (3%) |
| Neuromuscular disease | – | – | – | 1 (3%) |
| Haematologic disease | – | – | – | 1 (3%) |
| Congestive heart failure | – | 9 (43%) | – | 1 (3%) |
| Rheumatologic disease | – | 1 (5%) | – | – |
| Obstructive sleep apnoea | – | 6 (29%) | – | – |
| Chronic kidney disease | – | 10 (48%) | – | – |
| History of solid organ transplant | 1 (2%) | 2 (10%) | – | – |
| Cirrhosis | – | 1 (5%) | – | – |
| ARDS | 48 (100%) | 20 (95%) | 35 (67%) | |
| Pathogens identified | ||||
| Pseudomonas aeruginosa | 3 (6%) | – | 1 (2%) | 3 (9%) |
| Aspergillus flavus | – | – | 1 (2%) | 1 (3%) |
| Aspergillus fumigatus | – | – | 1 (2%) | – |
| Klebsiella pneumoniae | – | – | 1 (2%) | – |
| Serratia marcescens | – | – | 1 (2%) | – |
| Invasive candidiasis | – | – | 1 (2%) | – |
| Enterococcus faecium | 1 (2%) | – | – | – |
| Haemophilus influenzae | 1 (2%) | – | – | – |
| MRSA | 1 (2%) | – | – | – |
| Mortality at 28-days | 16 (36%) | 11 (52%) | 32 (62%) | 16 (50%) |
| Length of MV for survivors, median (IQR) | – | – | – | 10 (1–21) |
APACHE: acute physiology and chronic health evaluation II; ARDS: acute respiratory distress syndrome; BMI: body mass index; COPD: chronic obstructive pulmonary disease; ECMO: Extracorporeal membrane oxygenation; HAP: hospital-acquired pneumonia; HIV: positive human immunodeficiency virus; HFNC: High-flow nasal cannula; ICU: intensive care unit; IMV: invasive mechanical ventilation; IQR: interquartile range; MRSA: Methicillin-resistant Staphylococcus aureus; MV: mechanical ventilation; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SD: standard deviation; SOFA: sequential organ failure assessment.
Rello et al., reported signs and symptoms from a total of 735 cases of influenza A (H1N1) were confirmed in Spain in 2009.
The median time to onset of symptoms prior to ICU admission was 7 days [5–12 days] (Table 1). The most common symptoms at ICU admission were fever (100%), dyspnoea (88%), cough (73%) and malaise (44%). Myalgias (4%) were very uncommon. Ninety-four percent of patients received antiviral treatment with lopinavir/ritonavir and hydroxychloroquine, plus interferon beta (85%). Empirical antibiotic agents were administrated to 42 patients, whereas super-infection was identified in 6 patients.
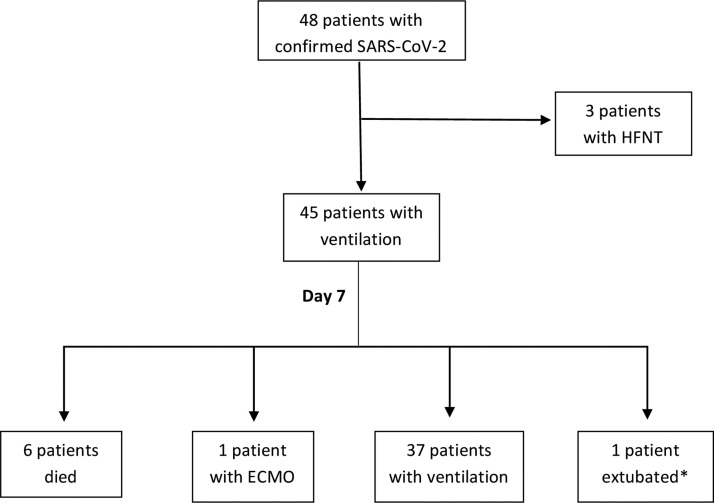
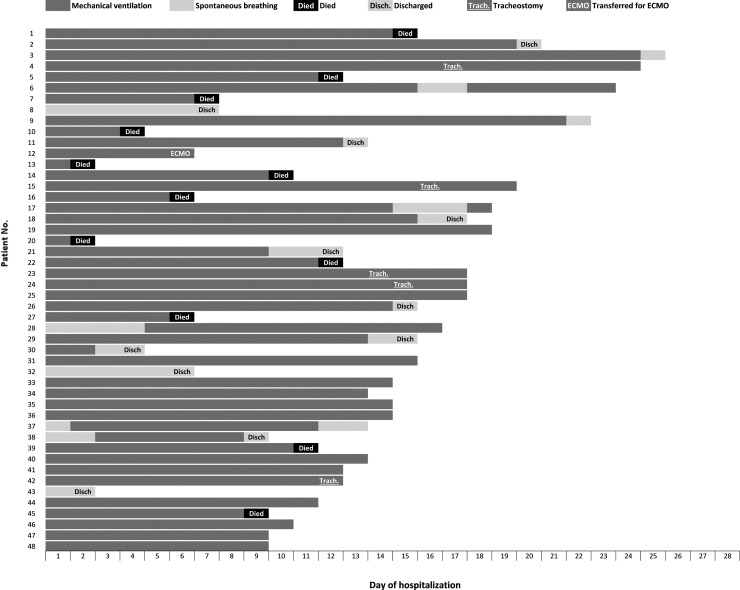
All patients were admitted with hypoxemic respiratory failure and none received non-invasive mechanical ventilation. Forty-five (94%) underwent intubation and 3 (6%) high-flow oxygen nasal therapy (HFNT). Tracheostomy was performed in five (11%) ventilated patients. Prone position was performed in 22 patients (49% of mechanically ventilated patients) and none received inhaled nitric oxide or prostacyclin. One patient was transferred to a referral centre for veno-venous extracorporeal membrane oxygenation (ECMO) therapy. One myocarditis was documented. A flow chart (Fig. 2 ) is detailing outcomes on day-7 post intubation. Outcomes for individual patients are summarised in Fig. 3 .
Fig. 2.
Flowchart of detailing outcomes at day-7 post ICU admission.
Fig. 3.
Outcomes for individual patients included in the manuscript (assessed April 6th, 2020).
After 7 days of ICU admission, among 45 intubated patients, six (13%) died, one (2%) underwent ECMO and one (2%) was extubated. Among 45 intubated, 37 (82%) still underwent mechanical ventilation. After 15 days, 14 (31%) intubated patients died (77% above 65 years), 21 (47%) remained with mechanical ventilation and 10 (22%) were extubated. Thirteen (27%) of the 48 patients admitted to the ICU were discharged alive.
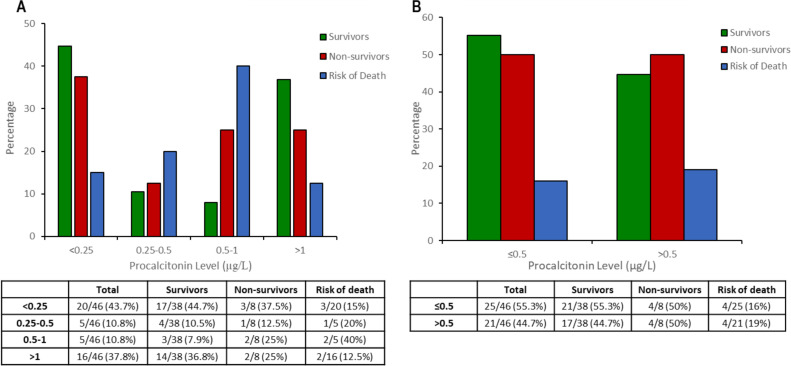
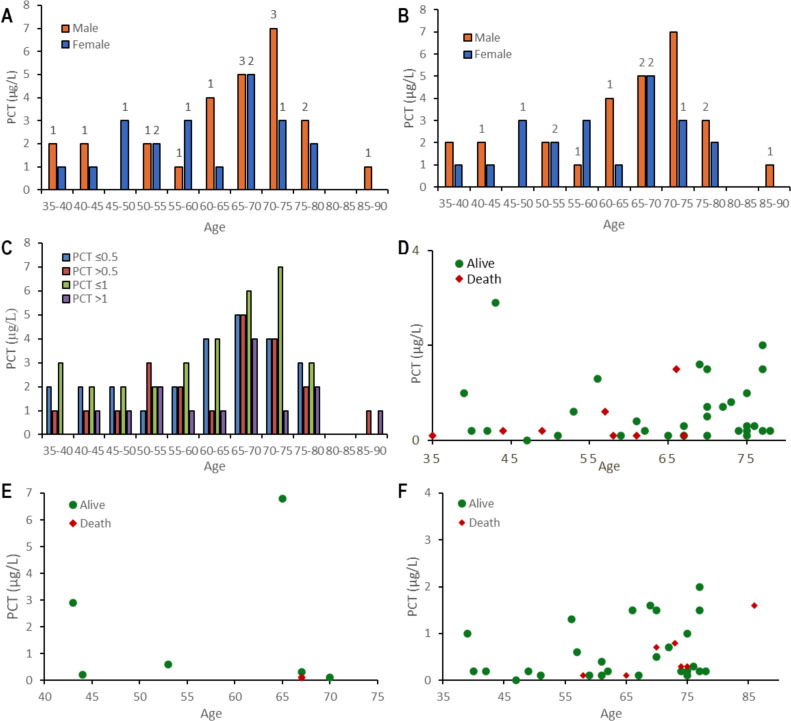
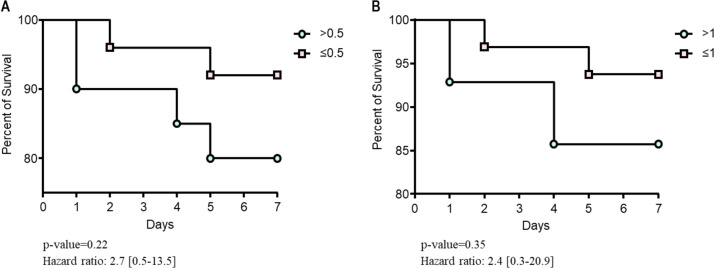
Six patients had super-infection documented, with median PCT plasma levels 0.2 μg/L [0.1–2.9] vs.0.4 μg/L [0.1–1.5] in non-infected ones (p = 0.22). PCT plasma levels details with different breakpoints are showed in Table 2 . PCT plasma levels above 0.5 μg/L were 16%vs.19.0% (p > 0.20) for survivors and non-survivors, respectively. PCT plasma levels details are showed in Fig. 4 and 5. No significant differences were identified, using a breakpoint of 1 μg/L (Table 2 and Fig. 4, Fig. 5 ). A Kaplan–Meier survival curve (Fig. 6 ) is detailing overall survival rates and correlations with initial PCT plasma (P-value = 0.22).
Table 2.
PCT plasma levels at different breakpoints of the population with confirmed SARS-CoV-2 pneumonia.
| All | ≤ 0.5 PCT μg/L | > 0.5 PCT μg/L | ≤ 1 PCT μg/L | > 1 PCT μg/L | |
|---|---|---|---|---|---|
| Total patients with confirmed illness | 48a | 25 (52%) | 21 (44%) | 32 (67%) | 14 (30%) |
| Age, median (IQR) | 67 (53–74) | 65 (51–75) | 68 (54–73) | 66 (52–74) | 67 (54–73) |
| Sex | |||||
| Male | 27 (56%) | 11 (44%) | 14 (67%) | 17 (53%) | 8 (57%) |
| Female | 21 (44%) | 14 (56%) | 7 (33%) | 15 (47%) | 6 (43%) |
| Days from onset symptoms to ICU admission, median (IQR) | 7 (5–12) | 7 (5–13) | 8 (6–12) | 7 (5–13) | 8 (5–13) |
| APACHE II score, median (IQR) | 15 (12–19) | 15 (13–18) | 17 (12–19) | 15 (12–19) | 17 (12–18) |
| SOFA score, median (IQR) | 7 (4–8) | 6 (4–8) | 7 (4–11) | 6 (3–8) | 8 (4–11) |
| Signs and symptoms | |||||
| Fever | 48 (100%) | 25 (100%) | 21 (100%) | 32 (100%) | 14 (100%) |
| Cough | 35 (73%) | 20 (80%) | 14 (67%) | 24 (75%) | 10 (71%) |
| Dyspnoea | 42 (88%) | 23 (92%) | 18 (86%) | 30 (94%) | 11 (79%) |
| Malaise | 21 (44%) | 8 (32%) | 11 (52%) | 12 (38%) | 7 (50%) |
| Myalgia | 2 (4%) | 1 (4%) | 1 (5%) | 2 (6%) | – |
| Treatment | |||||
| Antibacterial agents | 42 (88%) | 20 (80%) | 20 (95%) | 27 (84%) | 13 (93%) |
| levofloxacin | 17 (35%) | 4 (16%) | 12 (57%) | 8 (25%) | 8 (57%) |
| ceftriaxone | 22 (46%) | 9 (36%) | 11 (52%) | 14 (44%) | 6 (43%) |
| azithromycin | 10 (21%) | 4 (16%) | 5 (24%) | 6 (19%) | 3 (21%) |
| linezolid | 9 (19%) | 7 (28%) | 2 (10%) | 7 (22%) | 2 (14%) |
| other beta-lactams | 15 (31%) | 11 (44%) | 4 (19%) | 11 (35%) | 4 (29%) |
| Others | |||||
| Kaletra | 45 (94%) | 25 (100%) | 19 (90.5%) | 31 (97%) | 13 (92.9%) |
| Steroids | 17 (35%) | 11 (44%) | 6 (28.6%) | 12 (38%) | 5 (36%) |
| Tocilizumab | 2 (4%) | 2 (8%) | – | 2 (6%) | – |
| Chloroquine | 45 (94%) | 25 (100%) | 19 (90.5%) | 31 (97%) | 13 (93%) |
| Interferon | 41 (85%) | 24 (96%) | 16 (76.2%) | 29 (91%) | 11 (79%) |
| MV | 45 (94%) | 23 (92%) | 20 (95.2%) | 30 (94%) | 13 (93%) |
| ECMO | 1 (2%) | 1 (4%) | – | 1 (3%) | – |
| Comorbidities | |||||
| Obesity | 23 (48%) | 12 (48%) | 10 (48%) | 16 (50%) | 6 (43%) |
| BMI 30 to 40 | 15 (31%) | 9 (36%) | 6 (28%) | 11 (34%) | 3 (21%) |
| BMI > 40 | 7 (15%) | 3 (12%) | 4 (19%) | 4 (13%) | 3 (21%) |
| Arterial hypertensionb | 21 (44%) | 7 (28%) | 13 (62) | 11 (34%) | 9 (64%) |
| Lung disease | 18 (37%) | 9 (36%) | 8 (38%) | 11 (34%) | 6 (43%) |
| Smoker | 9 (19%) | 5 (20%) | 4 (19%) | 7 (22%) | 2 (14%) |
| Heart disease | 5 (10%) | 2 (8%) | 3 (14%) | 3 (9%) | 6 (43%) |
| Diabetes mellitus | 9 (19%) | 3 (12%) | 5 (24%) | 4 (13%) | 4 (28%) |
| Hypothyroidism | 9 (19%) | 6 (24%) | 3 (14%) | 6 (19%) | 3 (21%) |
| Immunosuppression | 3 (6%) | 2 (8%) | 1 (5%) | 3 (10%) | – |
| Pathogens identified | |||||
| Pseudomonas aeruginosa | 3 (6%) | 3 (12%) | – | 3 (9%) | – |
| E. faecium | 1 (2%) | – | 1 (5%) | 1 (3%) | – |
| H. influenza | 1 (2%) | – | 1 (5%) | – | 1 (7%) |
| MRSA | 1 (2%) | 1 (4%) | – | 1 (3%) | – |
| Mortality at 3-days | 3 (6%) | 1 (4%) | 2 (9%) | 2 (6%) | 1 (7%) |
| Mortality at 7-days | 6 (15%) | 2 (8%) | 4 (19%) | 4 (12.5%) | 2 (14.2%) |
APACHE: acute physiology and chronic health evaluation II; BMI: body mass index; ECMO: Extracorporeal membrane oxygenation; ICU: intensive care unit; IMV: invasive mechanical ventilation; IQR: interquartile range; MRSA: Methicillin-resistant Staphylococcus aureus; MV: mechanical ventilation; NR: not reported; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SD: standard deviation; SOFA: sequential organ failure assessment
Only forty-seven patients had PCT value.
P < 0.05 for breakpoint PCT at 0.5 and P = 0.06 for breakpoint PCT at 1.
Fig. 4.
Patients distribution with SARS-CoV-2 pneumonia according to survivors or non-survivors by PCT levels (A) four breakpoints of PCT levels (B) breakpoint of PCT level at 0.5.
Fig. 5.
Patients’ distribution with SARS-CoV-2 pneumonia by PCT plasma levels and age: (A) by sex and PCT μg/L > 0.5; (B) by sex and PCT μg/L > 1; (C) by PCT μg/L ≤ 0.5vs. > 0.5 and ≤ 1 vs. > 0; (D) Alive and death by PCT μg/L; (E) Alive and death by PCT of patients with super-infection; (F) Alive and death by PCT of patients without super-infection. In figures A and B, the numbers indicate the total patients with positive PCT.
Fig. 6.
Kaplan-Meier with 95% confidence interval (CI) representing mortality at 7 days based on PCT values. (A) PCT breakpoint at 0.5; (B) PCT breakpoint at 1. HFNT: High-flow nasal therapy; ECMO: extracorporeal membrane oxygenation.
4. Discussion
This study describes 48 critically ill patients with COVID-19 and severe acute respiratory failure in Vitoria, Spain, from March 4th, 2020 to March 31th, 2020. Patients received HFNT or intubation, but non-invasive mechanical ventilation was not applied. Two weeks after ICU admission, three out of ten intubated patients have died, and half of the patient cohort required prolonged ventilatory support. Two out of ten intubated patients were extubated (and discharged), most at the second week of ventilation. PCT plasma levels (threshold 0.5 μg/L) failed to predict mortality. Our findings suggest that an oxygenation strategy emphasising optimisation of oxygenation, intubation based on clinical criteria of hyperventilation and avoiding ventilator-induced lung injury associated with non-invasive mechanical ventilation would be life-saving in a significant proportion of patients.
Table 1 compares current findings with first series of SARS-CoV2 in the ICU in China, USA, and 2009 pandemic influenza in Spain. Clinical presentation is consistent with a recent systematic review [9], lymphocytopenia and coagulation alterations being common at hospital admission, with some important differences documented when compared with pandemic influenza in 2009 (Table 1), as reported elsewhere [2], and also with the first ICU series reported from Wuhan [10]. Obesity was the most common comorbidity in our report, suggesting differences in western countries regarding Wuhan reports (11), followed by hypertension and chronic respiratory diseases. The low prevalence of immunocompromised (solid organ transplants or HIV) and pregnant women compared with severe influenza-infected patients may be associated with the interaction of coronavirus with innate immunity. Fei Zhou et al. [5] reported a risk of death above 90% in patients with high procalcitonin, which is not consistent with our findings. This can be due to different laboratory techniques, super-infection rates or degree of acute lung injury (no NIV was applied in our cohort). Although more information on PCT is required [12], our findings suggest that no prognostic information can be inferred. The earliest extubation occurred three days after initiation of mechanical ventilation, but nine subsequent patients were extubated within the next week (Fig. 3). These figures may be different depending of SARS-CoV-2 phenotype, prior strategy of oxygenation (HFNT, continous positive airway pressure (CPAP) or NIV), and strategies of management post-ventilation (levels of positive end-expiratory pressure (PEEP), use of inhaled nitric oxide, etc). Further research is required to identify how to improve management. Most patients were admitted to the ICU after a few days of fever above 38.5 °C, arriving at the emergency department (ED) severely dehydrated and hyperventilating. Hypovolaemia leads to increased dead space and pulmonary hypoperfusion, needing to be corrected. High PEEP and furosemide may lead to unnecessary requirement of vasopressors and induce acute kidney injury, and thus in need of continuous veno-venous haemofiltration program (CVVHF).
This early report of characteristics of SARS-CoV-2 influenza in Spain is of interest, as most information currently available is coming from large cohorts in China, or short case series from Italy or USA [5], [6], [11], [13]. An important characteristic is that in the current cohort, no patients were exposed to prior NIV, which was commonly performed using a facial mask in Wuhan or a helmet in Italy, with a protocol of earlier intubation based on hyperventilation unable to maintain SatvO2 above 90%. Patients in this cohort were intubated a median of 7 days after onset, which is later than in pandemic influenza but earlier than in Wuhan. Zhou et al. [5] reported 97% mortality in intubated patients during a median time (IQR) of 18.5 (15 to 19) days. Seven-day mortality was estimated to be lower than 15% in our cohort and 10 patients were extubated within the second week, which means that the prognosis is better with different strategies of oxygenation. In contrast with reports from China or Seattle suggesting a severe acute respiratory distress syndrome (ARDS), a strategy of early intubation disclosed that SARS-CoV-2 does not lead to a typical ARDS. In our experience, two thirds of our patients have initial lung compliance ≥ 40 ml/cm H20 post intubation being consistent with a preliminary report with 16 patients by Gattinoni et al. [6], suggesting that ARDS is a consequence of acute lung injury associated with delayed intubation or super-infection. Thus, NIV seems not recommended and early high PEEP (above 10 cm H20 is probably not the right ventilatory strategy) may be harmful. Our experience suggests avoiding spontaneous ventilation early in the ED or ward may be harmful. Thus, SARS-CoV-2 patients can be maintained with high-flow oxygen nasal therapy (HFNT) or high-concentration oxygen reservoir if they do not present extreme hyperventilation. Early on this disease, non-intubated patients may benefit from prone position before intubation. Three of our patients were managed like this without intubation and were discharged early. Recruitment manoeuvres should be contraindicated and the benefit of prone position in intubated patients and protective ventilation should be restricted to those developing acute lung injury. These findings suggest that hypoxemic vasoconstriction is the main early mechanism and patients can benefit of inhaled prostacyclin or nitric oxide (before developing tachyphylaxis).
Lastly, although we did not document pulmonary embolisms in our cohort (autopsies were not allowed), laboratory tests are consistent with endothelial injury and micro-thrombosis. Zhou et al. [5] reported serum ferritin with a median above 1400 ng/L among 54 non-survivors in a context of hyperinflammatory states. These patients should receive sCD25 measurements and a bone marrow aspirate to rule out systemic haemophagocytic lymphocytosis, which should be treated with 500 mg/kg gamma globulins/day and dexamethasone 10 mg/12 h for 4 days. The same authors also reported D-dimer above 1 ug/ml among 81% of non-survivors and our findings are consistent with these observations. Although no difference on overall 28-day mortality was found between heparin users and non-users (30.3% vs. 29.7%, p = 0.91) in a report among 449 patients with severe SARS-CoV-2 infection in China [14], the 28-day mortality was significantly reduced in those receiving low molecular weight heparin with a D-dimer > 6 fold the upper limit of normal (32% vs. 52%, P = 0.01) or a Sepsis-Induced Coagulopathy (SIC) score [15] ≥ 4 (40% vs. 64%, P = 0.02).
Our study has several limitations. More than half of the cohort remained in the ICU at the time of censoring on April 6th, 2020 and further outcomes assessment have to be performed. Our sample size is small, because we focused on critically ill, and data cannot be extrapolated to patients hospitalised in medical wards. However, it is an early report illustrative of the epidemiology in South Europe, which can be compared with Wuhan and initial reports of pandemic influenza A H1N1pdm 2009 in Spain (Table 1). We already expanded the ICU capacity above two-fold in March 18thand data cannot be generalisable to patients with another acceleration phase or with different available resources, but may serve to develop contingency plans in other geographical regions. Procalcitonin technique of determination may influence values and data may not be comparable when using other methods, such as KRIPTOR® to determine plasma values. Similarly, the strategy of early intubation, without prior NIV trial would means that data cannot be extrapolated to sites with other management strategies. Lastly, pulmonary compliance and driving pressure was not recorded in the study protocol, limiting identification of phenotypes and extrapolation to other sites.
5. Conclusion
This early experience with SARS-CoV-2 in Spain suggests that the right oxygenation is life-saving. Seven-day mortality in SARS-CoV-2 requiring intubation was lower than 15%, with 80% of patients still requiring prolonged mechanical ventilation. PCT plasma levels do not predict survival. After 15 days of ICU admission, half of patients remained intubated, whereas one third were non-survivors. Our clinical observations provide useful insights that can help to improve management and outcomes.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
This work was funded in part by CIBERES, Instituto Salud Carlos III, Madrid, Spain (CB06-06-036 and fondos FEDER).
Compliance with ethical standards
The study was approved by the Clinical Research Ethics Committee of Araba Hospital (2020-0022) and consent was waived due to the observational nature of the study.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to privacy (patients’ data) but are available from the corresponding author on reasonable request.
Authors’ contributions
The study was designed by JR. HB enrolled patients and it is responsible for the integrity of data. An analysis of data was performed by ST, HB and JM. JR and ST wrote the first draft of the manuscript. All authors contributed scientifically in the subsequent versions. All authors read and approved the final manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.accpm.2020.04.001.
Appendix A. Supplementary data
References
- 1.Jansson M., Liao X., Rello J., Strengthening I.C.U. health security for a coronavirus epidemic. Intensive Crit Care Nurs. 2020;57:102812. doi: 10.1016/j.iccn.2020.102812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rello J., Tejada S., Userovici C., Arvaniti K., Pugin J., Waterer G. Coronavirus disease 2019 (COVID-19): a critical care perspective beyond China. Anaesth Crit Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.03.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen I.G., Crespo A.M., White D.B. Potential legal liability for withdrawing or withholding ventilators during COVID-19: assessing the risks and identifying needed reforms. JAMA. 2020 doi: 10.1001/jama.2020.5442. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically Ill patients in the Seattle Region - Case Series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. AJRCCM. 2020 doi: 10.1164/rccm.202003-0817LE. [2020 Epub Ahead Print} [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health, Organisation Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance, V., 1.2. World Health Organ. 2020 https://www.who.int/ [Google Scholar]
- 8.Rello J., Rodríguez A., Ibañez P., Socias L., Cebrian J., Marques A. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101623. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. 2020 doi: 10.1016/S2213-2600(20)30079-5. Epub Ahead Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. Epub aHead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 doi: 10.1111/jth.14817. Epub Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iba T., Levy J.H., Warkentin T.E. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy (patients’ data) but are available from the corresponding author on reasonable request.