Abstract
BACKGROUND AND PURPOSE:
The use of invasive cerebral angiography with CTA for active treatment of patients with suspected ischemic strokes has been increasing recently. This study aimed to identify the incidence of postcontrast acute kidney injury using baseline renal function when CTA and cerebral angiography were performed sequentially.
MATERIALS AND METHODS:
This retrospective observational study evaluated adults (18 years of age or older) with ischemic stroke who underwent CTA and cerebral angiography sequentially between 2010 and 2018. The incidence of postcontrast acute kidney injury was determined using the baseline estimated glomerular filtration rate. The value of the baseline estimated glomerular filtration rate at which the occurrence of postcontrast acute kidney injury increased was also determined.
RESULTS:
Postcontrast acute kidney injury occurred in 57/601 (9.5%) patients. Those with a baseline estimated glomerular filtration rate of <30 mL/min/1.73 m2 showed a higher incidence of acute kidney injury. Age, chronic kidney disease, medication (nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β blockers, statins, and insulin) use following contrast media exposure, and serum albumin affected the incidence of postcontrast acute kidney injury. The incidence of postcontrast acute kidney injury increased when the baseline estimated glomerular filtration rate was <43 mL/min/1.73 m2.
CONCLUSIONS:
Patients with low baseline renal function had the highest incidence of postcontrast acute kidney injury after CTA and cerebral angiography, but no fatal adverse effects were documented. Thus, patients suspected of having a stroke should be actively managed with respect to neurovascular function.
Acute ischemic stroke requires active management in the emergency setting to prevent long-term sequelae and curb mortality.1,2 The recently published 2018 American Heart Association/American Stroke Association guidelines for early treatment of stroke recommend endovascular recanalization after 6 hours of symptom onset, shifting the focus to a more active and invasive approach.3 The guidelines also recommend CTA to improve diagnosis, aid in the treatment of acute ischemic cerebral infarction, and assist patient selection in interventional radiology on the basis of the head and neck vascular status4,5—that is, once a CTA is performed, on the basis of the time of neurologic symptom onset and its severity, there is a high probability of active intervention with intra-arterial thrombolysis via cerebral angiography for early reperfusion.6-8
However, the incidence of postcontrast acute kidney injury (PC-AKI) is expected to increase in such conditions because of patient exposure to approximately 200 mL of contrast media in a short time.9,10 PC-AKI can lead to irreversible kidney damage and an increased risk of morbidity and mortality, particularly because no established treatments are available. Therefore, there is a need for emergency department (ED) personnel to perform evidence-based risk/benefit assessment for performing CTA and cerebral angiography in patients with suspected ischemic strokes.9-11
Previous studies have drawn conflicting conclusions on the risk factors associated with PC-AKI.12-14 Even though there are studies on PC-AKI after either CTA or cerebral angiography, studies that consider concomitant CTA and cerebral angiography performed within a short duration are lacking.10,11 In addition, there is scant literature on the occurrence of PC-AKI according to the baseline renal function. Furthermore, there are no clear guidelines on the use of contrast media in patients with renal disease.1,15-17
Therefore, this study aimed to identify the incidence of PC-AKI in terms of the baseline renal function in patients who underwent both CTA and cerebral angiography for suspected ischemic stroke in the ED. Furthermore, we aimed to present a reference value of the baseline of the estimated glomerular filtration rate (eGFR) that increases the likelihood of PC-AKI, to serve as a basis for decision-making in the ED.
MATERIALS AND METHODS
Ethics
The institutional review board of Yonsei University Health System Clinical Trial Center approved the study (approval number, 4–2019–0170) and waived the requirement for informed consent on the basis of the retrospective observational study design. The study was conducted according to the tenets of the Declaration of Helsinki as revised in 2013.
Study Design and Population
This retrospective study was conducted using data from patients enrolled in the Brain Salvage through Emergent Stroke Therapy critical pathway protocol implemented at our tertiary university hospital in Seoul, South Korea. The necessary data for the study were collected from the electronic medical records.
We evaluated data of adult patients (18 years of age or older) who visited the ED between October 2010 and September 2018 and underwent both CTA and cerebral angiography for differentiation and treatment of ischemic stroke, respectively. The exclusion criteria were the following: 1) CTA performed at another hospital or not performed, 2) death within 48 hours of CTA, 3) transfer or discharge within 48 hours of CTA, 4) CTA and cerebral angiography performed at an interval longer than 48 hours, 5) the presence of end-stage renal disease, and 6) no serum creatinine data within 72 hours of contrast media use.
Brain Salvage through Emergent Stroke Therapy Protocol
The Brain Salvage through Emergent Stroke Therapy critical pathway is a medical process protocol implemented by the ED to perform rapid thrombolytic therapy for patients with suspected acute ischemic stroke. This protocol identifies patients with symptoms indicative of stroke, such as single-sided weakness, speech disorder, gait disturbance, loss of consciousness, visual field symptoms, and sudden headache. Once the critical pathway is activated by an ED physician, a neurologist examines the patient and CTA is performed subsequently. Thrombolytic therapy through cerebral angiography is performed in the angiography suite as necessary.
CTA
All imaging was performed using a high-resolution CT scanner (Revolution EVO; GE Healthcare, Milwaukee, Wisconsin). For CTA, isotonic contrast material (iopamidol 755 mg/mL, Iopamiro 370; Bracco, Milan, Italy) was injected at a rate of 4 mL/s. The upper limit volume of the contrast material was 100 mL.
Cerebral Angiography
Cerebral angiography was performed on an angiography system (Allura Clarity FD 20/20; Philips Healthcare, Best, the Netherlands). For cerebral angiography, isotonic contrast material (iodixanol 652 mg/mL, Visipaque 320; GE Healthcare) was injected at a rate of 3–4 mL/s.
Definition of Exposure and Outcome
In this study, the eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (https://www.kidney.org/content/ckd-epi-creatinine-equation-2009), according to the current guidelines. The enrolled patients were then divided into 4 groups based on eGFR (milliliters/minute/1.73 m2) values that increase the risk of PC-AKI as follows: eGFR <30, eGFR 30–59, eGFR 60–89, and eGFR ≥90.18 Although there are diverse definitions of PC-AKI, we used the absolute increase in serum creatinine levels by ≥0.5 mg/dL or a relative increase in serum creatinine levels by ≥25% from baseline observed within 72 hours after contrast media exposure as proposed by Barrett and Parfrey.19
Statistical Analysis
Post hoc analyses were performed to determine the PC-AKI incidence according to the baseline eGFR groups. In addition, univariate logistic analysis was performed to determine the impact of each variable on PC-AKI occurrence. Statistically significant variables in the univariate logistic analysis were subjected to multivariable logistic regression analysis through a stepwise variable selection to determine independent variables. Subsequently, the baseline eGFR cutoff value was determined using the maximum area under the curve. All statistical analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, North Carolina), and P values < .05 were considered statistically significant.
RESULTS
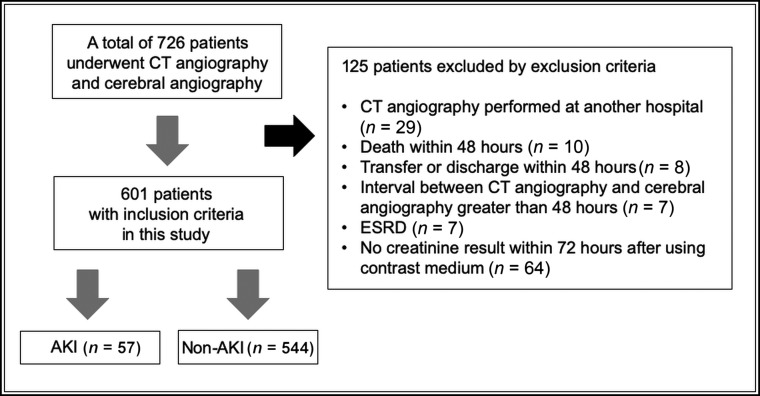
Of the 726 patients identified, 125 patients were excluded. Thus, 601 patients were included in the analysis. PC-AKI occurred in 57 (9.5%) patients (Fig 1). In total, 15 (2.5%) patients had an eGFR of <30 mL/min/1.73 m2; 97 (16.14%) patients, eGFR of 30–59 mL/min/1.73 m2; 302 (50.25%) patients, eGFR of 60–89 mL/min/1.73 m2; and 187 (31.11%) patients, eGFR of ≥90 mL/min/1.73 m2. The incidences of PC-AKI significantly differed among the baseline eGFR groups (P < .001) (Table 1). Post hoc analysis showed that the group with an eGFR of <30 mL/min/1.73 m2 had a higher PC-AKI incidence than the other groups, and the differences in incidences among the remaining groups were not significant (Table 2).
Fig 1.
Patient inclusion flowchart. ESRD indicates end-stage renal disease.
Table 1:
PC-AKI incidence based on baseline eGFRa
| Group | eGFR (mL/min/1.73 m2) | Non-AKI | PC-AKI | P |
|---|---|---|---|---|
| 1 | <30 | 8 (1.47) | 7 (12.28) | <.001 |
| 2 | 30–59 | 91 (16.73) | 6 (10.53) | |
| 3 | 60–89 | 269 (49.45) | 33 (57.89) | |
| 4 | ≥90 | 176 (32.35) | 11 (19.30) |
Data are number of patients and percentage.
Table 2:
Post hoc analysis for comparison of PC-AKI incidence between baseline eGFR groups
| Adjusted P | |||||
|---|---|---|---|---|---|
| 1 vs 2 | 1 vs 3 | 1 vs 4 | 2 vs 3 | 2 vs 4 | 3 vs 4 |
| .001 | .005 | <.001 | >.999 | >.999 | .349 |
In the univariate analyses, the significant risk factors (all P < .05) for PC-AKI were age older than 75 years, diabetes mellitus, chronic kidney disease, atrial fibrillation, use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers before CTA, medication (nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β blockers, statins, and insulin) use after contrast exposure, proteinuria, low hemoglobin and hematocrit levels, a high Δ neutrophil index, low platelet count, low serum albumin level, and low sodium concentration. In the multivariate model, age older than 75 years, chronic kidney disease, medication (nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β blockers, statins, insulin) use after contrast exposure, and serum albumin levels were found to significantly (all P < .05) influence the occurrence of PC-AKI (On-line Table).
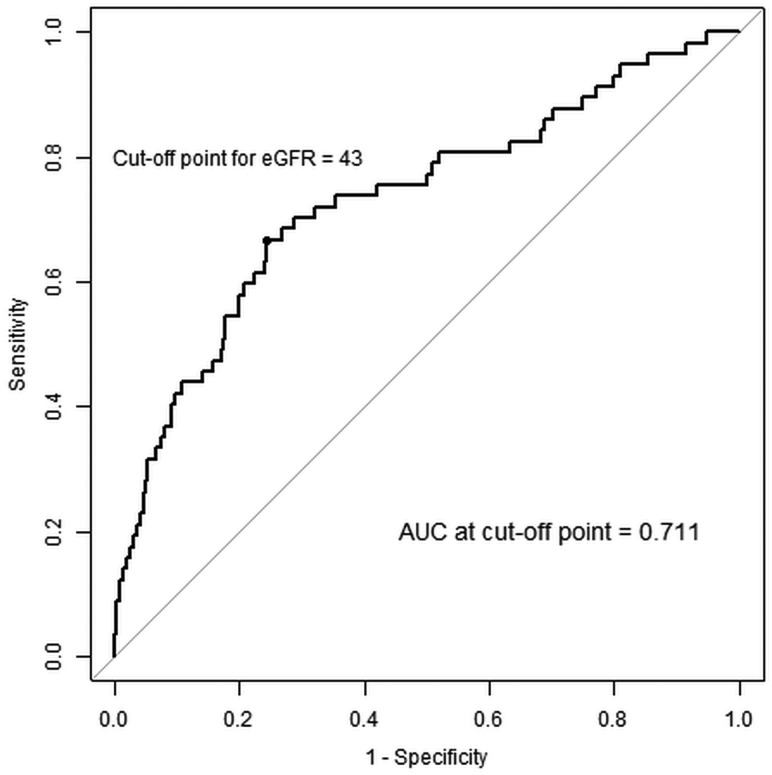
The maximum area under the curve value in the receiver operating characteristic curve analysis was obtained for the variables, and the baseline eGFR value corresponding to the maximum area under the curve value was identified. The resulting cutoff value of baseline eGFR was found to be 43 mL/min/1.73 m2 (Fig 2). This value retained significance in the univariate analysis.
Fig 2.
Receiver operating characteristic (ROC) curves showing the probability of PC-AKI. The ROC curve was drawn for PC-AKI prediction probabilities using significant variables from the multivariate analysis. Baseline eGFR: ROC curve drawn on the basis of the baseline eGFR variables. eGFR is in milliliter/minute/1.73 m2. AUC indicates area under the curve.
DISCUSSION
Patients with acute ischemic stroke who visit the ED have high short-term mortality rates (16%–23%).20,21 Even mild ischemic stroke is associated with poor prognosis during long-term follow-up.22 Therefore, rapid and accurate diagnosis and treatment of ischemic stroke are essential in the ED for such patients. Noncontrast brain CT has been used as a standard test in the evaluation of patients with acute stroke because of the short amount of time required, its convenience, and wide availability at many hospitals.23 The recently updated American Heart Association/American Stroke As-sociation guidelines recommend endovascular recanalization therapy for patients with suspected stroke arriving at the ED after 6 hours of symptom onset. In line with aggressive treatment, CTA is a tool optimized for cerebral angiographic treatment with the advantage of rapidly detecting cerebrovascular large-vessel occlusion with high accuracy in the emergency department.24 However, concomitant use of CTA and cerebral angiography exposes the patient to excessive contrast media in a short time. This can be burdensome to not only the patient but also the physicians. To date, studies on contrast media administration and acute kidney injury (AKI) incidence have reported diverse results according to the underlying diseases and general patient condition. Furthermore, studies that include cases with simultaneous contrast media use for diagnosis and treatment are lacking.
The task of accurately predicting contrast-induced kidney injury in patients with suspected stroke who require rapid management in the ED could likely delay the decision-making by neurologists and ED physicians.12-14 Therefore, presenting grounds for treatment that can be the criteria for judgment by ED physicians can be very important. Recently, Jia et al9 reported no significant difference in the incidence of PC-AKI between CTA only and with a combination of CTA and DSA. However, this study with a small sample size did not control for factors that affect AKI. In the present study, the PC-AKI incidence was compared among groups classified according to baseline eGFR without any adjustments. PC-AKI occurred in 7/15 (46.6%) patients in the group with an eGFR of <30 mL/min/1.73 m2, and the incidence of PC-AKI was also significantly higher in this group compared with the other groups. This result was similar to the results of other previous studies.25 However, this result was judged to be inaccurate because the patients’ underlying medical conditions were not considered. Therefore, the risk factors of AKI other than the contrast medium were determined and adjusted for to determine the cutoff value of eGFR at which the risk of AKI occurrence increases. We found that when CTA and cerebral angiography were performed consecutively, the probability of PC-AKI occurrence increased when the baseline eGFR was <43 mL/min/1.73 m2.
However, this result cannot be an absolute guideline to prevent examinations that are indispensable due to the risk of PC-AKI in patients with eGFR <43 mL/min/1.73 m2. In fact, continuous renal replacement therapy was performed in only 5/57 patients (8.7%) with PC-AKI, corresponding to only 0.8% of the 601 patients evaluated. All 5 patients fully recovered within 4 days of continuous renal replacement therapy. In addition, the renal function of the remaining patients who developed PC-AKI but did not undergo hemodialysis or continuous renal replacement therapy also recovered within several days. Therefore, in this study, even when PC-AKI occurred, its degree was not severe. Hence, the treatment decisions must be according to the neurologic symptoms and patient status. However, on the basis of the significant variables noted in the univariate analysis, physicians can predict patients at high risk of PC-AKI. Therefore, physicians will be able to perform sufficient IV hydration for these patients and plan an individual nephron-protective strategy for each patient.26,27 This process will enable performance of relevant tests after sufficiently explaining the predictable results to patients and their caregivers and is expected to be helpful in clinical practice.
The strength of this study is that data on various factors known to be related to PC-AKI were collected from patients treated using the Brain Salvage through Emergent Stroke Therapy critical pathway protocol that has been implemented consistently for a long time at a single institution. Many studies on the relationship between contrast media and renal diseases exist. However, to the best of our knowledge, this is the first study to identify the relationship between contrast media and AKI based on the baseline eGFR in patients who undergo CTA and cerebral angiography sequentially within a short interval. Furthermore, the cutoff value of eGFR for predicting PC-AKI was calculated with adjustment for various factors associated with PC-AKI. These results can be helpful for accurate treatment planning. Moreover, our findings will be valuable in the prevention of AKI because the cutoff values of eGFR presented here can be used to identify patients at high risk for PC-AKI, which, in turn, will enable physicians to provide individualized treatment.
However, our study has several limitations. Because this study was conducted at a single center, the results may have limited generalizability. Due to the retrospective design of the study, selection biases may have occurred because in some cases, laboratory data for creatinine levels after CTA and cerebral angiography were missing. Furthermore, additional biases owing to seriously ill patients who died within 48 hours after the examinations and those who were discharged or transferred within 48 hours for other reasons not included in the study may also exist. In our study, the postcontrast exposure to medication can also be thought of as a confounder because the more severe the disease state of the patient is, such as in patients with PC-AKI, the more medication is prescribed after contrast exposure. In addition, for patients with an extremely low baseline eGFR, cerebral angiography was performed after noncontrast brain CT without performing CTA, and there is a possibility that this feature may have affected the results to some extent. Finally, the area under the curve after adjustment for risk factors in this study was 0.7310, indicating that PC-AKI is influenced by more risk factors. Therefore, our findings need to be validated in future studies. Last, because PC-AKI is affected by many variables, AKI should ideally be diagnosed on the basis of clinically important adverse effects instead of a simple creatinine level elevation.
CONCLUSIONS
Among patients with acute ischemic stroke who underwent CTA and cerebral angiography in the ED, those with a baseline eGFR of <30 mL/min/1.73 m2 had a higher incidence of PC-AKI compared with those with other eGFR levels. After we adjusted for risk factors of PC-AKI, the risk of PC-AKI was high in the group with a baseline eGFR of <43 mL/min/1.73 m2. Hence, physicians could consider noncontrast CT and MR imaging/MRA testing for the patients in this group. However, because PC-AKI was not associated with fatal adverse effects in this study, it is recommended that patients suspected of having a stroke be actively managed with respect to neurovascular function on the basis of risk/benefit analyses.
ABBREVIATIONS:
- AKI
acute kidney injury
- ED
emergency department
- eGFR
estimated glomerular filtration rate
- PC-AKI
postcontrast acute kidney injury
References
- 1.Brinjikji W, Demchuk AM, Murad MH, et al. Neurons over nephrons: systematic review and meta-analysis of contrast-induced nephropathy in patients with acute stroke. Stroke 2017;48:1862–68 10.1161/STROKEAHA.117.016771 [DOI] [PubMed] [Google Scholar]
- 2.Demel SL, Grossman AW, Khoury JC, et al. Association between acute kidney disease and intravenous dye administration in patients with acute stroke: a population-based study. Stroke 2017;48:835–39 10.1161/STROKEAHA.116.014603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–110 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 4.Esteban JM, Cervera V. Perfusion CT and angio CT in the assessment of acute stroke. Neuroradiology 2004;46:705–15 10.1007/s00234-004-1235-x [DOI] [PubMed] [Google Scholar]
- 5.Wildermuth S, Knauth M, Brandt T, et al. Role of CT angiography in patient selection for thrombolytic therapy in acute hemispheric stroke. Stroke 1998;29:935–38 10.1161/01.str.29.5.935 [DOI] [PubMed] [Google Scholar]
- 6.van den Berg LA, Dijkgraaf MG, Berkhemer OA, et al. ; MR CLEAN Investigators. Two-year outcome after endovascular treatment for acute ischemic stroke. N Engl J Med 2017;376:1341–49 10.1056/NEJMoa1612136 [DOI] [PubMed] [Google Scholar]
- 7.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 8.Fransen PS, Berkhemer OA, Lingsma HF, et al. ; Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands Investigators. Time to reperfusion and treatment effect for acute ischemic stroke: a randomized clinical trial. JAMA Neurol 2016;73:190–96 10.1001/jamaneurol.2015.3886 [DOI] [PubMed] [Google Scholar]
- 9.Jia ZY, Wang SX, Zhao LB, et al. Risk of acute kidney injury with consecutive, multidose use of iodinated contrast in patients with acute ischemic stroke. AJNR Am J Neuroradiol 2019;40:652–54 10.3174/ajnr.A5959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall SL, Munich SA, Cress MC, et al. Risk of acute kidney injury associated with neuroimaging obtained during triage and treatment of patients with acute ischemic stroke symptoms. J Neurointerv Surg 2016;8:1231–34 10.1136/neurintsurg-2015-012118 [DOI] [PubMed] [Google Scholar]
- 11.Sharma J, Nanda A, Jung RS, et al. Risk of contrast-induced nephropathy in patients undergoing endovascular treatment of acute ischemic stroke. J Neurointerv Surg 2013;5:543–55 10.1136/neurintsurg-2012-010520 [DOI] [PubMed] [Google Scholar]
- 12.Tao Y, Dong W, Li Z, et al. Proteinuria as an independent risk factor for contrast-induced acute kidney injury and mortality in patients with stroke undergoing cerebral angiography. J Neurointerv Surg 2017;9:445–48 10.1136/neurintsurg-2016-012349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang TE, Bivard A, Levi C, et al. Multi-modal CT in acute stroke: wait for a serum creatinine before giving intravenous contrast? No! Int J Stroke 2015;10:1014–17 10.1111/ijs.12605 [DOI] [PubMed] [Google Scholar]
- 14.Lima FO, Lev MH, Levy RA, et al. Functional contrast-enhanced CT for evaluation of acute ischemic stroke does not increase the risk of contrast-induced nephropathy. AJNR Am J Neuroradiol 2010;31:817–21 10.3174/ajnr.A1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrlich ME, Turner HL, Currie LJ, et al. Safety of computed tomographic angiography in the evaluation of patients with acute stroke: a single-center experience. Stroke 2016;47:2045–50 10.1161/STROKEAHA.116.013973 [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm-Leen E, Montez-Rath ME, Chertow G. Estimating the risk of radiocontrast-associated nephropathy. J Am Soc Nephrol 2017;28:653–59 10.1681/ASN.2016010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald RJ, McDonald JS, Newhouse JH, et al. Controversies in contrast material-induced acute kidney injury: closing in on the truth? Radiology 2015;277:627–32 10.1148/radiol.2015151486 [DOI] [PubMed] [Google Scholar]
- 18.Molen A, Reimer P, Dekkers IA, et al. Post-contrast acute kidney injury, Part 1: definition, clinical features, incidence, role of contrast medium and risk factors. Eur Radiol 2018;28:2845–55 10.1007/s00330-017-5246-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett BJ, Parfrey PS. Prevention of nephrotoxicity induced by radiocontrast agents. N Engl J Med 1994;331:1449–50 10.1056/NEJM199411243312111 [DOI] [PubMed] [Google Scholar]
- 20.Feigin VL, Lawes CM, Bennett DA, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003;2:43–53 10.1016/S1474-4422(03)00266-7 [DOI] [PubMed] [Google Scholar]
- 21.Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin 2008;26:871–95, vii 10.1016/j.ncl.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 22.Prencipe M, Culasso F, Rasura M, et al. Long-term prognosis after a minor stroke: 10-year mortality and major stroke recurrence rates in a hospital-based cohort. Stroke 1998;29:126–32 10.1161/01.str.29.1.126 [DOI] [PubMed] [Google Scholar]
- 23.Mair G, Wardlaw JM. Imaging of acute stroke prior to treatment: current practice and evolving techniques. Br J Radiol 2014;87:20140216 10.1259/bjr.20140216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr 2001;25:520–28 10.1097/00004728-200107000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Davenport MS, Khalatbari S, Cohan RH, et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013;268:719–28 10.1148/radiol.13122276 [DOI] [PubMed] [Google Scholar]
- 26.Weisbord SD, Gallagher M, Jneid H, et al. ; PRESERVE Trial Group. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 2018;378:603–14 10.1056/NEJMoa1710933 [DOI] [PubMed] [Google Scholar]
- 27.Partovi S, Trischman T, Kang PS. Lessons learned from the PRESERVE trial. Br J Radiol 2018;91:20180092 10.1259/bjr.20180092 [DOI] [PMC free article] [PubMed] [Google Scholar]