This study included 520 consecutive patients with a clinical diagnosis of acute ischemic stroke (49.4% men; mean age, 72 years) who underwent CTA to evaluate large-vessel occlusion of the proximal anterior circulation. CTA scans were retrospectively reviewed by a consensus panel of 2 neuroradiologists. The prevalence of large-vessel occlusion was 16% (84/520 patients); 20% (17/84) of large-vessel occlusions were missed atthe initial CTA evaluation. In multivariate analysis, non-neuroradiologists were more likely to miss large-vessel occlusion compared with neuroradiologists, and occlusions of the M2 segment were more likely to be missed compared with occlusions of the distal internal carotid artery and/or M1 segment. Calcified emboli were present in 4 of 17 (24%) initially missed or misinterpreted large-vessel occlusions.
Abstract
BACKGROUND AND PURPOSE:
It is currently not completely clear how well radiologists perform in evaluating large-vessel occlusion on CTA in acute ischemic stroke. The purpose of this study was to investigate potential factors associated with diagnostic error.
MATERIALS AND METHODS:
Five hundred twenty consecutive patients with a clinical diagnosis of acute ischemic stroke (49.4% men; mean age, 72 years) who underwent CTA to evaluate large-vessel occlusion of the proximal anterior circulation were included. CTA scans were retrospectively reviewed by a consensus panel of 2 neuroradiologists. Logistic regression analysis was performed to investigate the association between several variables and missed large-vessel occlusion at the initial CTA interpretation.
RESULTS:
The prevalence of large-vessel occlusion was 16% (84/520 patients); 20% (17/84) of large-vessel occlusions were missed at the initial CTA evaluation. In multivariate analysis, non-neuroradiologists were more likely to miss large-vessel occlusion compared with neuroradiologists (OR = 5.62; 95% CI, 1.06–29.85; P = .04), and occlusions of the M2 segment were more likely to be missed compared with occlusions of the distal internal carotid artery and/or M1 segment (OR = 5.69; 95% CI, 1.44–22.57; P = .01). There were no calcified emboli in initially correctly identified large-vessel occlusions. However, calcified emboli were present in 4 of 17 (24%) initially missed or misinterpreted large-vessel occlusions.
CONCLUSIONS:
Several factors may have an association with missing a large-vessel occlusion on CTA, including the CTA interpreter (non-neuroradiologists versus neuroradiologists), large-vessel occlusion location (M2 segment versus the distal internal carotid artery and/or M1 segment), and large-vessel occlusion caused by calcified emboli. Awareness of these factors may improve the accuracy in interpreting CTA and eventually improve stroke outcome.
Stroke is a leading cause of global mortality and disability.1 Randomized controlled trials have recently shown that endovascular thrombectomy (EVT) significantly reduces disability in patients with acute ischemic stroke caused by large-vessel occlusion (LVO) of the proximal anterior circulation.2-4 Therefore, EVT is currently considered the standard of care, and it is recommended that all potential EVT candidates (ie, patients with clinically suspected LVO [eg, Los Angeles Motor Scale score of ≥4] and presentation within 6 hours of symptom onset) are rapidly screened for LVO using CTA.5 This paradigm shift has a great impact on the workflow of radiology departments in stroke centers worldwide because they are required to provide rapid and accurate CTA evaluation with 24/7 coverage. In our hospital, which is one of the largest general hospitals in the Netherlands and a primary stroke center (ie, capable of administering intravenous thrombolytics but not EVT), CTA was introduced as a standard of care after the results from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) were published in January 2015.2
However, it is currently not completely clear how well radiologists perform in interpreting CTA in clinical practice. Unfamiliarity in reading CTA particularly among non-neuroradiologists, the crucial need for rapid diagnosis often during on-call hours, and the relatively small caliber of the M2 and A2 segments may lead to diagnostic error. Knowledge of potential factors associated with diagnostic error may be helpful to optimize accurate interpretation of CTA. Therefore, the purpose of our study was to investigate potential factors associated with diagnostic error in evaluating LVO on CTA in acute ischemic stroke.
MATERIALS AND METHODS
Patients
This retrospective study was approved by the institutional review board of our hospital (No. Z2019102), and patient consent was waived. Five hundred twenty consecutive patients with a clinical diagnosis of acute ischemic stroke (49% men; mean age, 72 years; range, 19–100 years) who underwent CTA to evaluate LVO of the proximal anterior circulation at Zuyderland Medical Center between January 2019 and August 2019 were included. Patients with suspected posterior circulation symptoms or occlusion were excluded from the study.
CTA Protocol
CT was performed using either 64-section CT scanners (Brilliance, 168 patients, Incisive, 43 patients, Philips Healthcare, Best, the Netherlands; or Somatom Definition AS, 302 patients, Siemens, Erlangen, Germany) or on a 64-section dual-source scanner (Somatom Definition Flash, 7 patients; Siemens). CTA was performed with 60 mL of iobitridol (Xenetix 300; Guerbet, Aulnay-sous-Bois, France) using a bolus-tracking technique (Philips scanners) or after a test bolus (Siemens scanners) at an injection speed of 5 mL/s. Scanning parameters were the following: collimation = 64 × 0.625 mm (Philips scanners) or 64 × 0.6 mm (Siemens scanners), 120 kV(peak) (Philips scanners) or 100 kVp (Siemens scanners), 250 mAs (Philips Brilliance) or 117 mAs (Philips Incisive) or 130 mAs (Siemens scanners), pitch = 0.391 (Philips Brilliance) or 0.60 (Philips Incisive) or 1.2 (Siemens scanners), and matrix size = 512 × 512. CTA images were reconstructed in the transverse plane with 0.67-mm section thickness and a 0.33-mm increment (Philips scanners) or with 1.0-mm section thickness and a 0.5-mm increment (Siemens scanners).
Initial CTA Interpretation
CTA scans were prospectively read and reported by either neuroradiologists (n = 4), non-neuroradiologists (n = 15), or senior radiology residents (n = 10) during office hours (8:00 am to 5:00 pm on weekdays) and on-call hours (5:00 pm to 8:00 am on weekdays, weekends, and official holidays). LVO was defined as the presence of a contrast filling defect in any of the following segments of the proximal anterior circulation: the distal intracranial carotid artery, M1 and M2 segments of the middle cerebral artery, and A1 and A2 segments of the anterior cerebral artery. Readers were able to interpret CTA in conjunction with noncontrast head CT, which was acquired just before CTA. CT images were analyzed on a PACS workstation with MIP and MPR capabilities.
Reference Standard
CTA scans were retrospectively reviewed for the presence or absence of LVO by a consensus panel of 2 neuroradiologists (R.M.K. and F.-J.H.H.). In case of LVO, whether it was caused by a calcified embolus was also recorded. Calcified emboli are considerably more attenuated (mean, 162 HU; range, 79–435 HU) than intraluminal thrombi (typical range, 50–70 HU) and are round or ovoid (not tubular or linear-like vascular wall calcifications).6 There were no disagreements between the 2 neuroradiologists.
Statistical Analysis
Statistical analyses were performed using MedCalc statistical software for Windows, Version 12.6.0 (MedCalc Software, Mariakerke, Belgium). Logistic regression analysis was performed to investigate the association among interpreters (neuroradiologists, non-neuroradiologists, or senior residents), time of CTA interpretation (on-call hours versus office hours), availability of specified clinical information (lateralizing symptoms/signs or suspected location of stroke reported on the request form for CTA), location of LVO (M2 segment versus distal internal carotid artery and/or M1 segment), and missed LVO at initial interpretation. Significant variables in univariate analysis (ie, predefined P value < .10) were considered for multivariate analysis.7
RESULTS
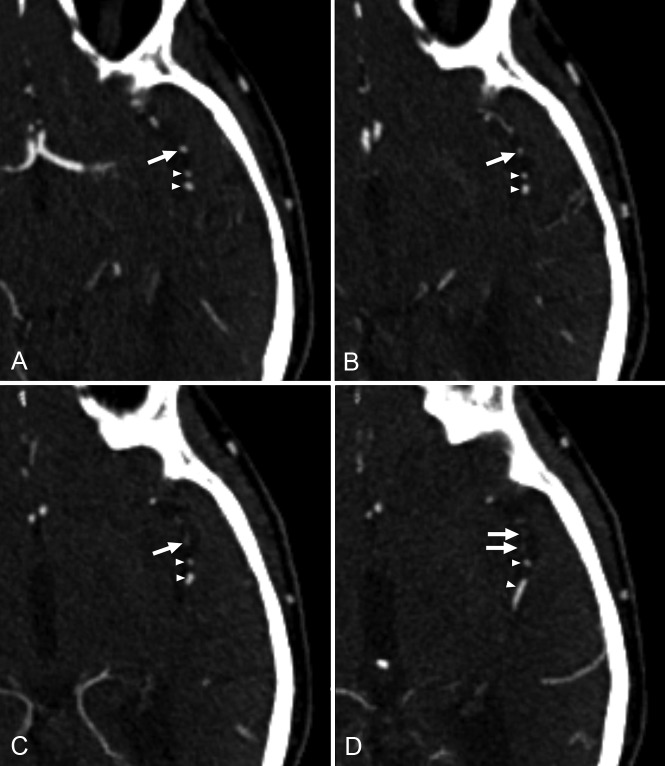
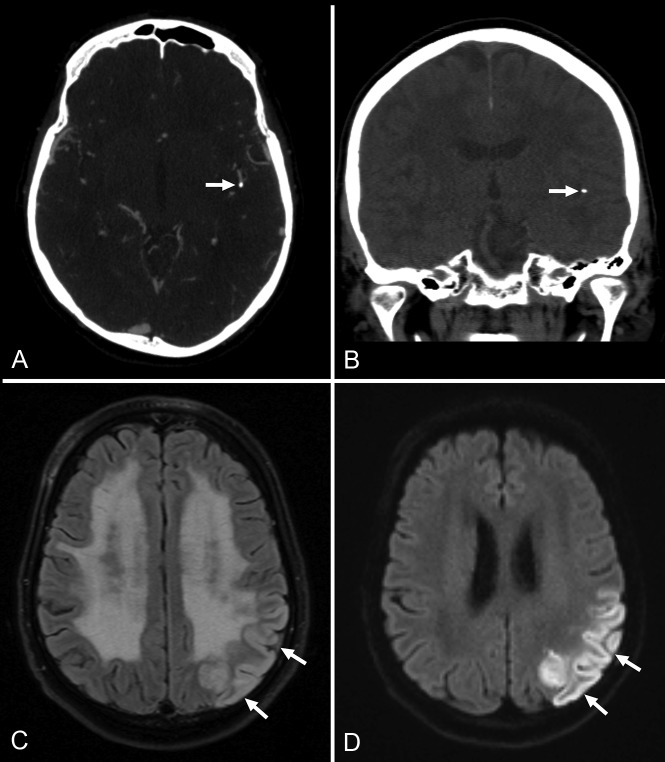
The prevalence of LVO was 16% (84/520 patients). The anatomic distribution of LVOs is shown in Table 1. Twenty percent of LVOs (17/84) were missed at initial CTA evaluation. In univariate analysis, non-neuroradiologists were more likely to miss LVOs compared with neuroradiologists, and occlusions of the M2 segment (Fig 1) were more likely to be missed compared with occlusions of the distal internal carotid artery and/or M1 segment (Table 2). The time of CTA interpretation and the availability of specified clinical information (lateralizing symptoms/signs or suspected location of stroke reported on the request form for CTA) were not significantly associated with missing LVO (Table 2). In multivariate analysis, the type of interpreter (non-neuroradiologists versus neuroradiologists, OR = 5.62; 95% CI, 1.06–29.85, P = .04) and the location of the LVO (M2 segment versus the distal internal carotid artery and/or M1 segment, OR = 5.69; 95% CI, 1.44–22.57, P = .01) remained significantly associated with missing the LVO at initial CTA evaluation (Table 3). In all correctly identified LVOs at initial CTA interpretation, there were no calcified emboli. However, calcified emboli were present in 4 of 17 (24%) initially missed or misinterpreted LVOs. In 3 patients, calcified emboli were missed (2 in the M1 segment, 1 in the M2 segment), whereas in 1 patient, a calcified embolus in the M2 segment was misinterpreted as clinically irrelevant calcification (Fig 2). In 16 patients with missed anterior circulation LVO, mRS scores after a median follow-up of 46.5 days (range, 6–163 days) were 1 (n = 5), 2 (n = 7), 3 (n = 1), 4 (n = 1), and 6 (n = 2). One patient with a missed anterior circulation LVO was lost to follow-up: This patient was transferred to a comprehensive stroke center because of complete hemiparesis with brain swelling requiring possible decompressive craniectomy.
Table 1:
Anatomic distribution of LVOs
| Location of LVO | No. (%) | No. of Missed LVOs at Initial CTA Evaluation (%) |
|---|---|---|
| Distal intracranial carotid artery | 1 (1.2%) | 0 (0%) |
| Distal intracranial carotid artery and M1 segment | 10 (11.9%) | 0 (0%) |
| Distal intracranial carotid artery and M2 segment | 1 (1.2%) | 0 (0%) |
| M1 segment | 31 (36.9%) | 3 (17.6%) |
| M2 segment | 40 (47.6%) | 14 (82.4%) |
| A1 segment | 1 (1.2%) | 0 (0%) |
| A2 segment | 0 | 0 (0%) |
Fig 1.
A 75-year-old male patient with acute ischemic stroke. At initial CTA evaluation, occlusion of 1 of the M2 segment branches of the left middle cerebral artery (arrows on all slices) was missed. Consecutive axial CTA slices in a caudocranial direction (A–D) show a contrast filling defect in a branch of the left M2 segment (arrows in C and D). Note that 2 adjacent branches of the left M2 segment show normal contrast filling on all slices (arrowheads).
Table 2:
Variables potentially associated with missing LVO at initial CTA evaluation; results of univariate logistic regression analysisa
| Variables | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Interpreter | ||
| Non-neuroradiologists (n = 33) vs neuroradiologists (n = 27) | 7.14 (1.43–35.57) | .02 |
| Senior residents (n = 24) vs neuroradiologists (n = 27) | 1.79 (0.27–11.71) | .55 |
| Senior residents (n = 24) vs non-neuroradiologists (n = 33) | 0.25 (0.06–1.02) | .05 |
| Time of CTA interpretation, on-call hours (n = 40) vs office hours (n = 44) | 1.89 (0.63–5.70) | .26 |
| Reporting of lateralizing symptoms/signs or suspected location of stroke on the request form for CTA; yes (n = 58) vs no (n = 26) | 0.91 (0.29–2.92) | .88 |
| Location of LVO; M2 segment (n = 40) vs distal internal carotid artery and/or M1 segment (n = 42) | 6.77 (1.79–25.57) | .005 |
Nos. in parentheses in column 1 represent the number of CTA scans.
Table 3:
Variables potentially associated with missing LVO at initial CTA evaluation; results of multivariate logistic regression analysisa
| Variables | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Interpreter | ||
| Non-neuroradiologists (n = 33) vs neuroradiologists (n = 27) | 5.62 (1.06–29.85) | .04 |
| Senior residents (n = 24) vs neuroradiologists (n = 27) | 1.63 (0.23–11.37) | .62 |
| Senior residents (n = 24) vs non-neuroradiologists (n = 33) | 0.29 (0.07–1.26) | .10 |
| Location of LVO; M2 segment (n = 40) vs distal internal carotid artery and/or M1 segment (n = 42) | 5.69 (1.44–22.57) | .01 |
Nos. in parentheses in column 1 represent the number of CTA scans.
Fig 2.
A 70-year-old male patient with acute ischemic stroke. At initial CTA evaluation, LVO due to a calcified embolus in the M2 segment of the left middle cerebral artery (arrows in CTA image, A; and in a noncontrast head CT image, B) was misinterpreted as clinically irrelevant calcification. Follow-up MR imaging (FLAIR image, C; and diffusion-weighted image, D) 1 day after CTA reveals infarction in the left middle cerebral artery territory (arrows).
DISCUSSION
In patients experiencing a typical large-vessel acute ischemic stroke, 1.9 million neurons are destroyed each minute that the stroke is untreated.8 Rapid and accurate detection of LVO on CTA is of crucial importance so that EVT can be performed as soon as possible to reduce disability. The prevalence of LVO in our study was 16%, which is comparable with prevalence values reported in the literature.9,10 Most striking, we found that as much as 20% of LVOs were missed or misinterpreted at initial CTA interpretation in clinical practice.
Errors and discrepancies are uncomfortably common, with an estimated day-to-day rate of 3%–5% of radiology studies reported, and even higher rates reported in many targeted studies.11 CTA evaluation for intracranial LVO appears to be no exception, with a total error rate of 3.3% (17 of all 520 CTA scans analyzed in this study). Potential factors associated with diagnostic error need to be uncovered and highlighted to prevent repetition and improve patient care. We found that non-neuroradiologists were more likely to miss LVOs compared with neuroradiologists. A plausible explanation could be that neuroradiologists are more experienced in evaluating CTA of the intracranial vasculature. We also found that occlusions in the M2 segment of the middle cerebral artery were more likely to be missed compared with occlusions in the distal internal carotid artery and/or M1 segment. The relatively smaller caliber, tortuous course, and variable branching pattern of the M2 segment12 may be potential causes of perception error. Radiologists should be aware of these causes and carefully scrutinize branches of the M2 segment. The use of MIP and/or MPR may be helpful.13,14 In addition, the use of wavelet-based reconstruction (which improves image quality),15 multiphase CTA,16,17 CT perfusion maps,17,18 angiographic volume perfusion CT reconstructions (4D CTA),18 and/or automated software19 may help to further improve the detection of LVO.
All 4 calcified emboli (2 in the M1 segment and 2 in the M2 segment) were either missed or misinterpreted at initial CTA evaluation in our study. The most probable reason for this diagnostic error is unfamiliarity with this entity. In a previous study, 27% of calcified emboli were misinterpreted and 9% were overlooked on noncontrast head CT.6 Once thought to be rare,20 calcified emboli are now considered more common than previously assumed.6 The prevalence in a former study in patients with stroke with acute LVO was 1.3%,21 whereas it was even higher in our study: 4.8% (4 of 84 patients with acute LVO). Removal of calcified emboli may be challenging, but successful recanalization can be achieved by mechanical thrombectomy.21,22 We believe that it is critical to interpret CTA in conjunction with thin-section noncontrast CT because calcified emboli may be more conspicuous on nonenhanced CT images. Furthermore, hyperdense thrombus may also be identified more easily using thin-section noncontrast CT.23 We speculated that LVOs may be more easily missed during on-call hours. However, our study findings do not support this hypothesis. Although the availability of specified clinical information (lateralizing symptoms/signs or suspected location of stroke reported on the request form for CTA) enables a more targeted search, we did not find evidence that its absence was associated with missing LVOs.
Our study has some potential limitations. First, we did not have confirmation of CTA findings with DSA. However, CTA using modern CT scanners provides equivalent information of the large intracranial arteries compared with DSA.24 Second, CTA evaluation may be subject to some degree of interobserver variation. However, the purpose of our study was to evaluate potential factors associated with diagnostic error rather than investigating interobserver variability. Moreover, retrospective review of CTA scans in a calm research setting does not reflect CTA evaluation in a usually busy clinical setting needing rapid diagnosis. Third, because of the retrospective nature of our study and the complexity, we could not investigate other potential sources of diagnostic error, including reading speed, fatigue, workload, and frequency of interruptions and distractions during CTA evaluation. Fourth, because A1 and A2 segment occlusions were scarce (only 1.2% of all LVOs) in our study, no conclusions can be drawn for these segments. However, A1 and A2 segment occlusions are overall less relevant from an incidence point of view (only 0.6% of all anterior circulation LVOs in the MR CLEAN trial).2
CONCLUSIONS
Twenty percent of LVOs were missed at initial CTA evaluation in clinical practice. Several factors may have an association with missing an LVO on CTA, including CTA interpreter (non-neuroradiologists versus neuroradiologists), LVO location (M2 segment versus distal internal carotid artery and/or M1 segment), and LVO caused by calcified emboli. Awareness of these factors may improve accuracy in interpreting CTA and eventually improve stroke outcome.
ABBREVIATIONS:
- EVT
endovascular thrombectomy
- LVO
large-vessel occlusion
References
- 1.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:459–80 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Majoie CB, Dippel DW; MR CLEAN Investigators. Endovascular therapy for ischemic stroke. N Engl J Med 2015;372:2363 10.1056/NEJMc1504715 [DOI] [PubMed] [Google Scholar]
- 3.van den Berg LA, Dijkgraaf MG, Berkhemer OA, et al. ; MR CLEAN Investigators. Two-year outcome after endovascular treatment for acute ischemic stroke. N Engl J Med 2017;376:1341–49 10.1056/NEJMoa1612136 [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators, Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 5.Almekhlafi MA, Kunz WG, Menon BK, et al. . Imaging of patients with suspected large-vessel occlusion at primary stroke centers: available modalities and a suggested approach. AJNR Am J Neuroradiol 2019;40:396–400 10.3174/ajnr.A5971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker BS, Shah LM, Osborn AG. Calcified cerebral emboli, a “do not miss” imaging diagnosis: 22 new cases and review of the literature. AJNR Am J Neuroradiol 2014;35:1515–19 10.3174/ajnr.A3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: logistic regression. Perspect Clin Res 2017;8:148–51 10.4103/picr.PICR_123_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saver JL. Time is brain–quantified. Stroke 2006;37:263–66 10.1161/01.STR.0000196957.55928.ab [DOI] [PubMed] [Google Scholar]
- 9.Zubkov AY, Uschmann H, Rabinstein AA. Rate of arterial occlusion in patients with acute ischemic stroke. Neurol Res 2008;30:835–38 10.1179/174313208X340969 [DOI] [PubMed] [Google Scholar]
- 10.Kummer BR, Gialdini G, Sevush JL, et al. . External validation of the Cincinnati prehospital stroke severity scale. J Stroke Cerebrovasc Dis 2016;25:1270–74 10.1016/j.jstrokecerebrovasdis.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady AP. Error and discrepancy in radiology: inevitable or avoidable? Insights Imaging 2017;8:171–82 10.1007/s13244-016-0534-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibo H, Carver CC, Rhoton AL Jr, et al. . Microsurgical anatomy of the middle cerebral artery. J Neurosurg 1981;54:151–69 10.3171/jns.1981.54.2.0151 [DOI] [PubMed] [Google Scholar]
- 13.Saba L, Sanfilippo R, Montisci R, et al. . Assessment of intracranial arterial stenosis with multidetector row CT angiography: a postprocessing techniques comparison. AJNR Am J Neuroradiol 2010;31:874–79 10.3174/ajnr.A1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ota H, Takase K, Rikimaru H, et al. . Quantitative vascular measurements in arterial occlusive disease. Radiographics 2005;25:1141–58 10.1148/rg.255055014 [DOI] [PubMed] [Google Scholar]
- 15.Kunz WG, Sommer WH, Havla L, et al. . Detection of single-phase CTA occult vessel occlusions in acute ischemic stroke using CT perfusion-based wavelet-transformed angiography. Eur Radiol 2017;27:2657–64 10.1007/s00330-016-4613-y [DOI] [PubMed] [Google Scholar]
- 16.Yu AY, Zerna C, Assis Z, et al. . Multiphase CT angiography increases detection of anterior circulation intracranial occlusion. Neurology 2016;87:609–16 10.1212/WNL.0000000000002951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne D, Sugrue G, Stanley E, et al. . Improved detection of anterior circulation occlusions: the “delayed vessel sign” on multiphase CT angiography. AJNR Am J Neuroradiol 2017;38:1911–16 10.3174/ajnr.A5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becks MJ, Manniesing R, Vister J, et al. . Brain CT perfusion improves intracranial vessel occlusion detection on CT angiography. J Neuroradiol 2019;46:124–29 10.1016/j.neurad.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Amukotuwa SA, Straka M, Smith H, et al. . Automated detection of intracranial large vessel occlusions on computed tomography angiography. Stroke 2019;50:2790–98 10.1161/STROKEAHA.119.026259 [DOI] [PubMed] [Google Scholar]
- 20.Kavanagh EC, Fenton DM, Heran MK, et al. . Calcified cerebral emboli. AJNR Am J Neuroradiol 2006;27:1996–99 [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrocky T, Piechowiak E, Cianfoni A, et al. . Thrombectomy of calcified emboli in stroke: does histology of thrombi influence the effectiveness of thrombectomy? J Neurointerv Surg 2018;10:345–50 10.1136/neurintsurg-2017-013226 [DOI] [PubMed] [Google Scholar]
- 22.Kwak HS, Park JS. Successful recanalization using the Embolus Retriever with Interlinked Cage for acute stroke due to calcified cerebral emboli. Interv Neuroradiol 2018;24:674–77 10.1177/1591019918784259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mair G, Boyd EV, Chappell FM, et al. ; IST-3 Collaborative Group. Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke 2015;46:102–07 10.1161/STROKEAHA.114.007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingebiel R, Kentenich M, Bauknecht HC, et al. . Comparative evaluation of 64-slice CT angiography and digital subtraction angiography in assessing the cervicocranial vasculature. Vasc Health Risk Manag 2008;4:901–07 10.2147/vhrm.s2807 [DOI] [PMC free article] [PubMed] [Google Scholar]